Significance
Human papillomaviruses (HPVs) are major human carcinogens. It is widely assumed that HPV-positive tumor cells must sustain viral E6/E7 oncogene expression to continuously block the tumor-suppressive senescence response of the host cell. Consequently, E6/E7 are considered attractive therapeutic targets for immunotherapy or for functional inhibition. Here we show that hypoxic conditions, as often found in HPV-positive cancers, allow the cells to induce a dormant state in which E6/E7 is down-regulated but induction of senescence is avoided. Instead, a reversible growth arrest is induced that can be overcome by reoxygenation. As a consequence, hypoxic HPV-positive cancer cells are protected against chemotherapy as well as against virus-specific therapeutic approaches, and may serve as reservoirs for cancer recurrence on reoxygenation.
Keywords: human papillomavirus, tumor virus, cervical cancer, hypoxia, mTOR
Abstract
Oncogenic human papillomaviruses (HPVs) are closely linked to major human malignancies, including cervical and head and neck cancers. It is widely assumed that HPV-positive cancer cells are under selection pressure to continuously express the viral E6/E7 oncogenes, that their intracellular p53 levels are reconstituted on E6/E7 repression, and that E6/E7 inhibition phenotypically results in cellular senescence. Here we show that hypoxic conditions, as are often found in subregions of cervical and head and neck cancers, enable HPV-positive cancer cells to escape from these regulatory principles: E6/E7 is efficiently repressed, yet, p53 levels do not increase. Moreover, E6/E7 repression under hypoxia does not result in cellular senescence, owing to hypoxia-associated impaired mechanistic target of rapamycin (mTOR) signaling via the inhibitory REDD1/TSC2 axis. Instead, a reversible growth arrest is induced that can be overcome by reoxygenation. Impairment of mTOR signaling also interfered with the senescence response of hypoxic HPV-positive cancer cells toward prosenescent chemotherapy in vitro. Collectively, these findings indicate that hypoxic HPV-positive cancer cells can induce a reversible state of dormancy, with decreased viral antigen synthesis and increased therapeutic resistance, and may serve as reservoirs for tumor recurrence on reoxygenation.
Oncogenic human papilloma viruses (HPVs) are some of the most important known cancer risk factors and are closely linked to the development of every 20th human cancer worldwide, including prevalent cancers in the oropharynx and anogenital region (1, 2). Best characterized is their causative role for cervical cancer, which alone accounts for more than 500,000 new cancer cases and more than 250,000 cancer deaths per year worldwide (3). Cervical cancer cells virtually always contain the DNA of high-risk HPV types, such as HPV16 and HPV18. Maintenance of the malignant phenotype of HPV-positive cancer cells is considered to require sustained expression of the viral E6/E7 oncogenes (1, 2). Inhibition of E6/E7 expression leads to the rapid induction of cellular senescence (4–6), a central tumorsuppressive pathway, resulting in an irreversible growth arrest (7). This indicates that the viral oncogenes maintain the growth of HPV-positive cancer cells by blocking cellular senescence. However, their potential to induce senescence on E6/E7 inhibition also shows that this pathway is not irreversibly destroyed in HPV-positive cancer cells.
These considerations are not only fundamental for our mechanistic concepts of HPV-linked cell transformation, but also have important therapeutic implications. The development of specific E6/E7 inhibitors could provide a rational strategy for targeting HPV-positive neoplasias (8, 9) as a tumor-specific prosenescence therapy (10, 11). Furthermore, the concept that continuous E6/E7 expression is essential for the growth of HPV-positive tumor cells implies that the two viral proteins represent attractive targets for immunotherapy, because E6/E7 synthesis cannot be down-regulated as an evasion mechanism (12, 13).
Many cancers are characterized by low O2 concentrations (14–16). Hypoxia, usually defined as tissue O2 concentration <1.5% (17), is considered to play a major role in tumor development and progression. Clinically, hypoxia can increase the resistance to chemotherapy and radiotherapy and is a negative prognostic marker for many cancers, including HPV-positive tumors (15, 16, 18–20). Notably, although O2 availability is known to affect tumor cell biology (14–16), most functional studies of the HPV E6/E7 oncogenes in cervical cancer cells have been performed under standard cell culture conditions at 21% O2. In contrast, cervical cancers often exhibit strongly reduced O2 content, with a heterogenous distribution of more- and less-oxygenated regions and a median O2 concentration of 1.2% (16, 21).
These considerations raise the question of whether our current concepts about the interactions of the viral oncogenes with the host cell are mirrored under hypoxic conditions. In the present work, we found that hypoxic HPV-positive cancer cells strongly down-regulate E6/E7 expression, but this is not linked to a reconstitution of p53. Notably, and in sharp contrast to their phenotype under normoxia, we found that hypoxic HPV-positive cancer cells do not senesce despite efficient E6/E7 repression. Instead, the cells switch to a dormant state, characterized by E6/E7 down-regulation and a reversible growth arrest. On reoxygenation, the dormant cells restore E6/E7 expression and resume proliferation. Mechanistically, we found that senescence induction on E6/E7 repression under normoxia is critically dependent on intact mechanistic target of rapamycin (mTOR) signaling. Hypoxic HPV-positive cancer cells escape from this regulation owing to the concomitant impairment of the mTOR pathway via the inhibitory REDD1/TSC2 axis. These results provide surprising insight into the functional cross-talk between the HPV oncogenes and the host cell machinery, which also has implications for the clinical behavior of HPV-positive cancers.
Results
E6/E7 and p53 Expression in Hypoxic HPV-Positive Cancer Cells.
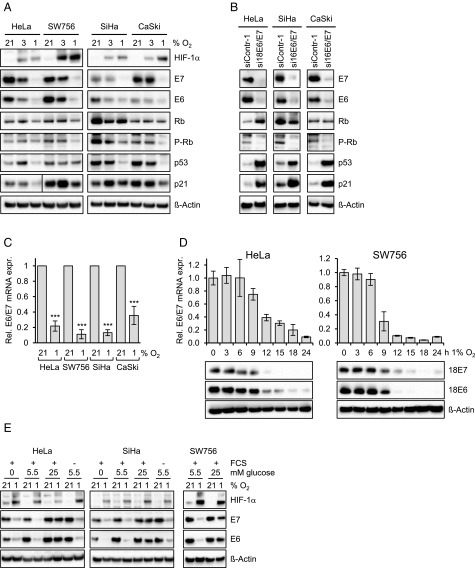
HPV18-positive (HeLa, SW756) and HPV16-positive (SiHa, CaSki) cervical cancer cells were cultured at 21% O2 (“normoxia”), 3% O2, or 1% O2 (“hypoxia”). Interestingly, although a reduction of E6 and E7 protein expression was detectable at 3% O2, we observed a dramatic drop at 1% O2 in all HPV-positive cell lines (Fig. 1A). The efficacy of hypoxic E6/E7 repression was comparable to the experimental down-regulation of E6/E7 by potent RNA interference (RNAi) under normoxia (Fig. 1B). E6/E7 repression under hypoxia was also detectable at the mRNA level (Fig. 1C), as shown by qRT-PCR analyses measuring the amounts of all three transcript classes coding for HPV16 or HPV18 E6/E7 (5). Kinetic analyses indicated that the down-regulation of E6/E7 mRNA and protein expression started at ∼9–12 h under hypoxia (Fig. 1D).
Fig. 1.
Repression of HPV E6/E7 oncogene expression under hypoxia. (A) HPV18-positive HeLa and SW756 cells and HPV16-positive SiHa and CaSki cells were cultured for 24 h at the indicated O2 concentrations. Shown are immunoblots of HIF-1α (hypoxia-linked marker), HPV16/18 E6, HPV16/18 E7, total Rb, phosphorylated Rb (P-Rb; Ser807/811), p53, and p21 protein expression. β-actin served as a loading control. (B) Normoxic cells were transfected with E6/E7-targeting siRNAs (si16E6/E7 or si18E6/E7) or control siRNA (siContr-1), and protein expression was analyzed by immunoblotting. (C) E6/E7 mRNA expression under normoxia (21% O2) or hypoxia (1% O2). Indicated are the mean E6/E7 transcript levels of at least five independent experiments measured by qRT-PCR after 24 h under hypoxia, for each cell line relative to the corresponding E6/E7 transcript levels under normoxia (set at 1.0). SDs are indicated. Asterisks above columns indicate statistically significant differences from normoxic cells (***P < 0.001). (D) Time course of hypoxia-linked E6/E7 repression. (Upper) Measurements of transcript levels by qRT-PCR. SDs of technical replicates are indicated (n = 3). (Lower) Accompanying analyses of protein levels by immunoblot. β-actin served as a loading control. (E) HPV-positive cancer cells were grown in medium containing 0 mM, 5.5 mM, or 25 mM glucose. The presence (+) or absence (−) of FCS in the medium is indicated. Cells were cultured under normoxia (21% O2) or hypoxia (1% O2). Shown are immunoblots of HPV16/18 E6, HPV16/18 E7, and HIF-1α levels. β-actin served as a loading control.
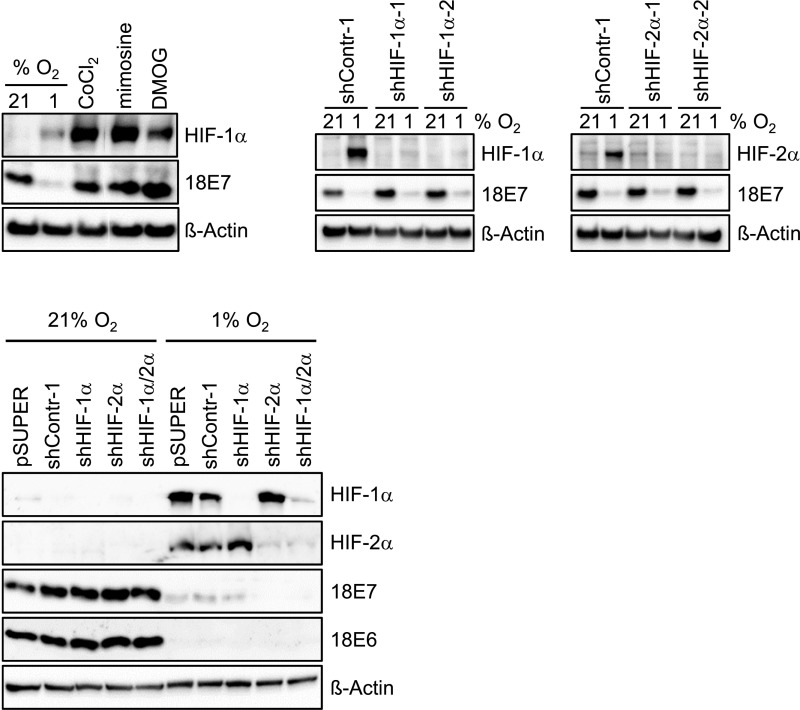
To gain insight into the underlying molecular mechanism, we analyzed candidate pathways that can mediate the cellular response to hypoxia. The hypoxia-induced factors HIF-1α and HIF-2α are major coordinators of this process, by modulating the transcription of a broad array of target genes (22). We did not find experimental evidence linking HIF-1α or HIF-2α induction to E6/E7 repression, however, given that HIF mimetics do not repress E6/E7 under normoxia and that silencing of HIF-1α or HIF-2α, alone or in combination, does not affect hypoxia-induced E6/E7 down-regulation (Fig. S1).
Fig. S1.
E6/E7 repression is not linked to HIF-1α or HIF-2α expression. (Upper, Left) Immunoblot analyses investigating the effects of the HIF agonists CoCl2, mimosine, or DMOG on E7 levels in normoxic HeLa cells. (Upper, Center and Right) Analyses of the effects of shRNAs blocking HIF-1α (shHIF-1α-1, shHIF-1α-2) or HIF-2α (shHIF-2α-1, shHIF-2α-2) expression, respectively, on hypoxia-linked E7 repression. (Lower) Analyses of the effects of pooled shRNAs blocking HIF-1α (shHIF-1α) or HIF-2α (shHIF-2α), applied either alone or in combination, on hypoxia-linked E6 and E7 repression. β-actin served as a loading control.
Hypoxia also can modulate gene expression by affecting carbohydrate metabolism via HIF-dependent (22) or HIF-independent (23, 24) routes. Therefore, we cultured HPV-positive cells in medium devoid of glucose (0 mM), medium containing physiological serum glucose levels (5.5 mM; 100 mg/dL), and medium containing unphysiologically high glucose concentrations (25 mM; 450 mg/dL). We also tested whether E6/E7 repression depends on serum in the medium. Neither glucose nor serum was required for hypoxic E6/E7 repression (Fig. 1E). Of note, however, the inhibitory effect of hypoxia on E6/E7 expression was strongly impaired at 25 mM glucose (Fig. 1E), indicating that a high glucose supply can efficiently counteract hypoxia-mediated E6/E7 repression.
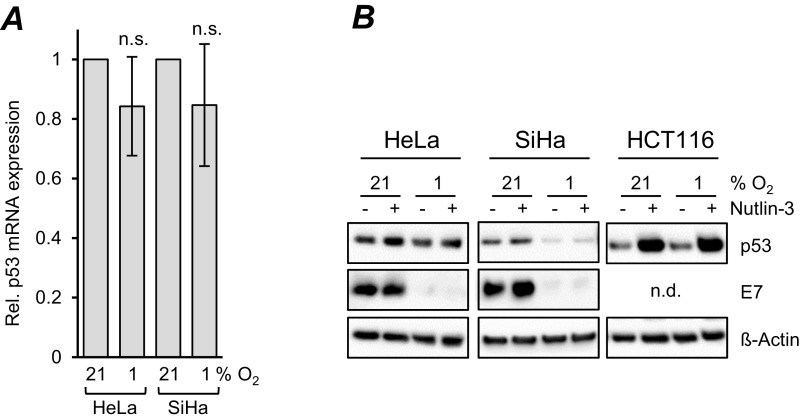
The HPV E6 and E7 oncoproteins target the p53 and pRb tumor suppressor proteins, respectively, for inactivation (2). RNAi-mediated E6/E7 repression under normoxia resulted in decreased amounts of phosphorylated pRb (Fig. 1B). This regulation was similar in three of four tested cervical cancer cell lines when E6/E7 amounts were down-regulated under hypoxia; the sole exception was HeLa cells, in which phosphorylated pRb levels remained largely unaffected (Fig. 1A). Of note, however, we found profound differences in the regulation of p53. RNAi-mediated E6/E7 repression under normoxia resulted in strong quantitative increases in p53 protein, as expected from its interference with E6-mediated p53 degradation (25), as well as in p21 induction, representing a transcriptional target gene for p53 (Fig. 1B). In contrast, despite efficient E6/E7 repression, p53 protein levels did not increase in any of the investigated HPV-positive cancer cell lines under hypoxia, but remained unaffected or even decreased (Fig. 1A). We observed only marginal and nonsignificant effects on p53 mRNA levels (Fig. S2A). Treatment with Nutlin-3, a potent inhibitor of the MDM2/p53 interaction (26), did not markedly increase p53 protein levels in HPV-positive cancer cells, in contrast to the response of HPV-negative HCT116 colon cancer cells, which showed a strong up-regulation of p53 concentrations under the same experimental conditions (Fig. S2B). Consistent with the lack of p53 induction in hypoxic HPV-positive cancer cells, p21 expression was not increased (Fig. 1A).
Fig. S2.
p53 regulation under hypoxia. (A) HeLa and SiHa cells were cultivated under normoxia (21% O2) or hypoxia (1% O2). Shown are the mean p53 transcript levels measured by qRT-PCR after 24 h under hypoxia for each cell line relative to the corresponding p53 transcript levels under normoxia (set at 1.0). SDs of biological replicates are indicated (n = 4), n.s., statistically not significant. (B) HPV18-positive HeLa, HPV16-positive SiHa, and HPV-negative HCT116 cells were cultivated for 24 h under normoxia (21% O2) or hypoxia (1% O2), in either the absence (−) or the presence (+) of 10 μM Nutlin-3. Shown are immunoblots of p53 and HPV16/18 E7 protein levels. β-actin served as a loading control. n.d., not determined.
Hypoxic E6/E7 Repression Does Not Result in Senescence.
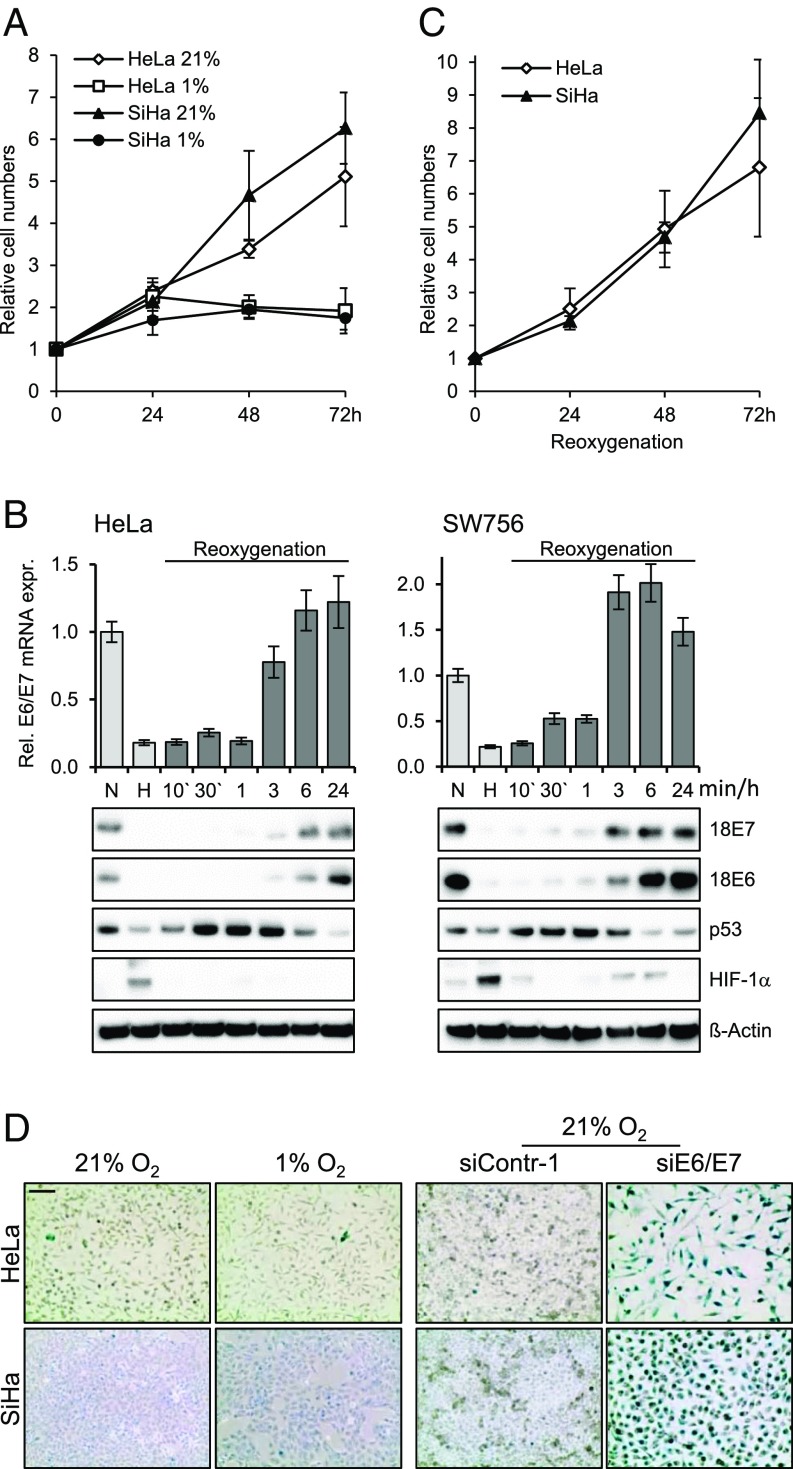
We next examined the phenotypic consequences of hypoxia-induced E6/E7 repression. We found that hypoxia inhibits the proliferation of HPV-positive cancer cells, with cell numbers reaching a plateau after 24 h (Fig. 2A). Of note, viral E6/E7 expression was reinduced in HPV-positive cancer cells on reoxygenation, at both the mRNA and protein levels (Fig. 2B). During these time course experiments, p53 protein levels showed a biphasic regulation, increasing soon after reoxygenation but decreasing again at later time points when E6 protein levels began to increase (Fig. 2B). The reactivation of E6/E7 expression was linked to the reinduction of cell proliferation (Fig. 2C).
Fig. 2.
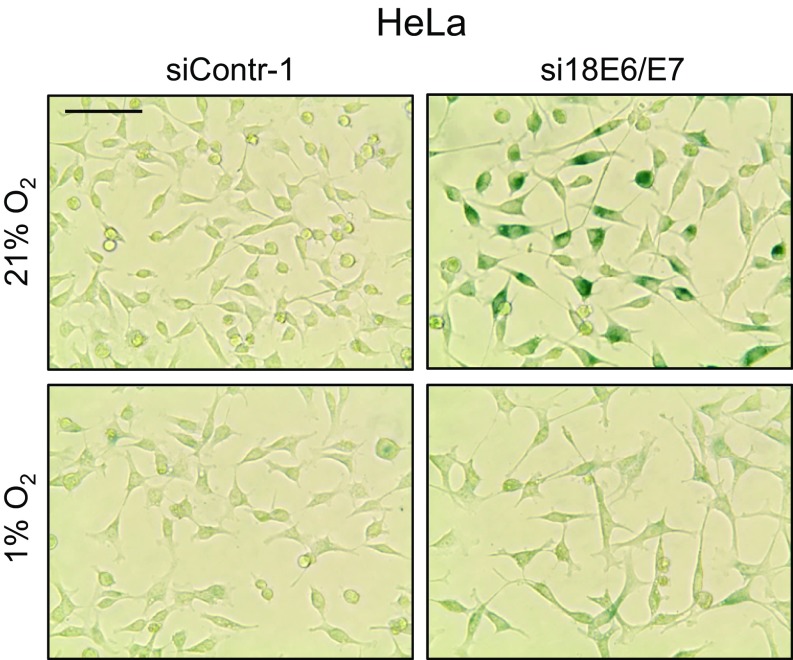
Hypoxia-induced growth inhibition and E6/E7 repression are reversible on reoxygenation. (A) HeLa and SiHa cells were cultured for the indicated time periods at 21% O2 or at 1% O2, and relative cell numbers were determined. For each cell line, initial cell numbers (time point 0) were set at 1.0. SDs of biological replicates are indicated (n = 4). (B, Upper) qRT-PCR analyses of E6/E7 mRNA levels under normoxia (N; 21% O2) and hypoxia (H; 1% O2) (bright columns), and on reoxygenation of hypoxic cells for the indicated time periods (dark columns). SDs of technical replicates are indicated (n = 3). (B, Lower) Accompanying immunoblot analyses of E6, E7, p53, and HIF-1α levels. β-actin served as a loading control. (C) Cells were incubated for 72 h at 1% O2. Subsequently (time point 0), the cells were cultured at 21% O2 for the indicated time periods, and cell numbers were determined. SDs of biological replicates are indicated (n = 3). (D, Left) Cells were cultured under normoxia (21% O2) or hypoxia (1% O2) for 72 h and the stained for expression of the senescence marker SA-β-Gal. (Scale bar: 200 µm.) (D, Right) Cells were cultured under normoxia, endogenous E6/E7 expression was silenced by RNAi, and cells were stained for SA-β-Gal expression at 72 h after transfection.
The reversibility of the hypoxia-linked growth arrest of HPV-positive cancer cells indicates that the cells do not senesce, although E6/E7 is efficiently repressed. Concordantly, and in contrast to RNAi-mediated E6/E7 repression under normoxia (Fig. 2D, Right), we observed that hypoxic HPV-positive cancer cells lacked the typical morphological signs of senescence (e.g., cell enlargement, flattening, long cytoplasmic projections) and did not stain positive for the senescence marker senescence-associated β-galactosidase (SA-β-Gal) (Fig. 2D, Left). HPV-positive cells treated with E6/E7 inhibitory siRNAs in combination with hypoxia also escaped from senescence (Fig. S3).
Fig. S3.
E6/E7 inhibitory siRNAs do not induce senescence in hypoxic HPV-positive cancer cells. HeLa cells were transfected with E6/E7 inhibitory siRNAs (si18E6/E7) or control siRNA (siContr-1) and cultivated under normoxia (21% O2) or hypoxia (1% O2). After 72 h, cells were stained for expression of the senescence marker SA-β-Gal. (Scale bar: 200 µm.)
Senescence Induction on E6/E7 Inhibition Requires mTOR Signaling, Which Is Impaired Under Hypoxia.
To decipher the mechanism underlying this differential regulation in hypoxic and normoxic HPV-positive cancer cells, we first addressed the question of which cellular pathway is critical for senescence induction on E6/E7 repression under normoxia. The mTOR pathway emerged as a possible candidate, given that mTOR signaling can support senescence induction and can be inhibited by hypoxia in some, but not all, cell types (27).
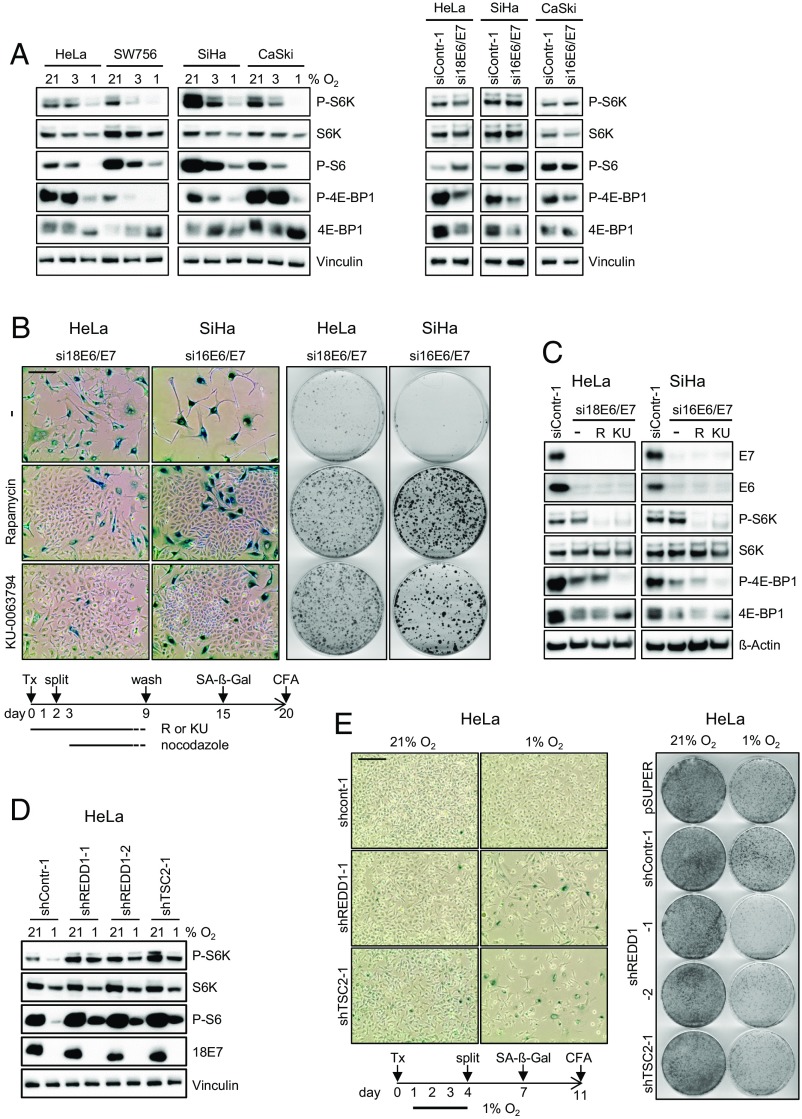
We found that hypoxia inhibits mTOR signaling in HPV-positive cancer cells, as indicated by the quantitative reduction of the phosphorylated forms of substrates of the mTOR pathway, including p70-S6 kinase (P-S6K), S6 ribosomal protein (P-S6), and eukaryotic initiation factor 4E-binding protein 1 (P-4E-BP1) (Fig. 3A, Left). The effect is glucose-sensitive and can be counteracted by a high glucose (25 mM) supply (Fig. S4A). The hypoxic impairment of mTOR signaling is not a peculiarity of HPV-positive cancer cells, and was also detected in a panel of HPV-negative cancer cells under the same experimental conditions (Fig. S4B). In contrast to the situation in hypoxic cells, when E6/E7 is inhibited under normoxia by RNAi, P-S6K and P-S6 concentrations were not diminished, with P-S6 amounts even increasing in HeLa and SiHa (Fig. 3A, Right). Only P-4E-BP1 levels were somewhat reduced (Fig. 3A, Right). Collectively, these findings raise the possibility that mTOR activity is critical for senescence induction on E6/E7 inhibition in normoxic HPV-positive cancer cells.
Fig. 3.
Senescence induction on E6/E7 repression in HPV-positive cancer cells depends on mTOR signaling, which is impaired under hypoxia. (A, Left) Cells were cultured under different O2 concentrations, as indicated. Immunoblot analyses of phosphorylated S6K (P-S6K), total S6K (S6K), phosphorylated S6 (P-S6), phosphorylated 4E-BP1 (P-4E-BP1) and total 4E-BP1 (4E-BP1). Vinculin served as a loading control. (A, Right) Immunoblot analyses of mTOR substrates on RNAi-mediated E6/E7 repression in normoxic HPV-positive cancer cells. (B, Left) Senescence assays (SA-β-Gal staining), in the absence (−) or the presence of the mTOR inhibitors rapamycin or KU-0063794. (Scale bar: 200 µm.) (B, Right) Colony-formation assays of HPV-positive cancer cells, in the absence (−) or presence of rapamycin or KU-0063794. Colonies were visualized with crystal violet. Scheme below: Treatment protocol, further detailed in the text. Tx, transfection. (C) Normoxic HPV-positive cancer cells were transfected with E6/E7-inhibitory siRNAs in either the absence (−) or presence of rapamycin (R) or KU-0063794 (KU). The levels of HPV16/18 E6 and E7, P-S6K, S6K, P-4E-BP1, and 4E-BP1 were determined by immunoblotting. siContr-1, control siRNA. β-actin served as a loading control. (D) HeLa cells expressing shREDD1-1, shREDD1-2, or shTSC2-1 were cultured at 21% or 1% O2 for 24 h, after which levels of P-S6K, S6K, P-S6 and HPV18 E7 were determined by immunoblotting. Vinculin served as a loading control. (E) HeLa cells were transfected with shRNA expression vectors for shREDD1-1, shREDD1-2, or shTSC2-1 and cultured under normoxia or hypoxia. (Left) Senescence assays (SA-β-Gal staining) of cells subsequently cultured under normoxia. (Scale bar: 200 µm.) (Right) Corresponding CFAs. Control cells were transfected with empty vector (pSUPER) or with a vector expressing shContr-1. Scheme below: Treatment protocol, further detailed in the text. Tx, transfection.
Fig. S4.
mTOR signaling in hypoxic cells. (A) HeLa cells were cultivated for 16 h under normoxia (21% O2) or hypoxia (1% O2) in cell culture medium containing either 5.5 mM glucose or 25 mM glucose. Shown are immunoblots of HIF-1α, HPV18 E7, P-S6K, S6K, P-S6, and P-4E-BP1 levels. β-actin served as a loading control. (B) A panel of HPV-negative cells was cultivated for 24 h under normoxia (21% O2) or hypoxia (1% O2). Immunoblot analyses of P-S6K (s.e., short exposure; l.e., long exposure), S6K, P-S6, P-4E-BP1, and 4E-BP1 levels. β-actin served as a loading control.
To directly address this question, we silenced E6/E7 expression in normoxic HPV-positive cancer cells by transient transfection with siRNAs in either the absence or the presence of the chemical mTOR inhibitors KU-0063794 and rapamycin. To exclude the possibility that untransfected cells (in which E6/E7 is not silenced by RNAi) can form colonies, we also treated cells with nocodazol to eliminate cells that were not arrested (28, 29). SA-β-Gal analyses indicated that HPV-positive cancer cells, in which E6/E7 expression was silenced alone, became senescent (Fig. 3B, Upper Left). In contrast, the concomitant treatment with either mTOR inhibitor resulted in the outgrowth of cells that do not stain for SA-β-Gal (Fig. 3B, Center Left and Lower). These findings show that mTOR inhibitors enable the escape of HPV-positive cancer cells from senescence, despite efficient E6/E7 repression (Fig. 3C).
This idea is further corroborated by results of colony-formation assays (CFAs) following transient transfection with E6/E7-inhibitory siRNAs. We found that the concomitant exposure to rapamycin or KU-0063794 led to a strong increase in the colony-formation capacity of HPV-positive cancer cells (Fig. 3B, Right). The mTOR inhibitors did not reinduce HPV E6 or E7 expression (Fig. 3C) and induced the expected effects on the phosphorylation of mTOR substrates (30, 31), with P-4E-BP1 being largely resistant to rapamycin treatment and KU-0063794 leading to reductions in P-4E-BP1 and P-S6K (Fig. 3C). These results show that mTOR signaling is critical for the induction of senescence on E6/E7 repression in normoxic HPV-positive cancer cells.
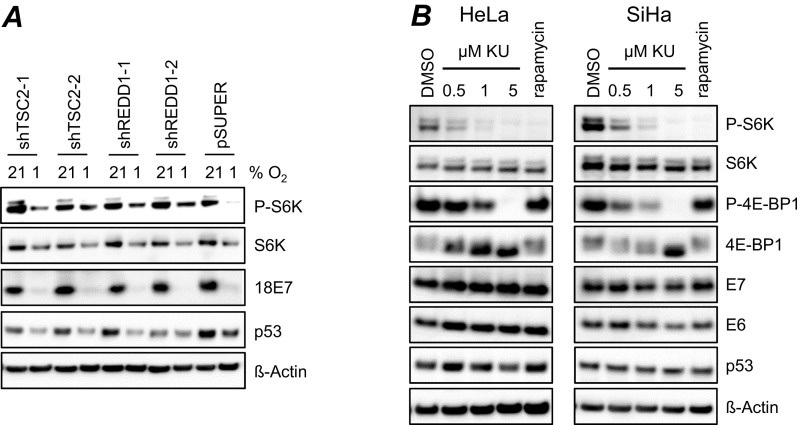
We next investigated whether the lack of senescence induction observed in hypoxic HPV-positive cancer cells is due to impaired mTOR signaling. Many different pathways have been implicated in the weakening of mTOR signaling under hypoxia (32), including stimulation of the HIF-1 target gene REDD1 (regulated in development and DNA damage responses 1), which interferes with mTOR signaling by activating the mTOR inhibitor TSC2 (tuberous sclerosis complex 2) (33, 34). We observed that hypoxia leads to the up-regulation of REDD1 expression in HeLa cells (Fig. S5A), raising the possibility that the mTOR-inhibitory REDD1/TSC2 axis plays a critical role in the blocking of senescence in hypoxic HPV-positive cancer cells. We generated shRNAs that blocked TSC2 or REDD1 expression (Fig. S5B) and found that they stimulated mTOR signaling under hypoxia, as shown by the increased amounts of P-S6K and P-S6 at 1% O2 (Fig. 3D and Fig. S5C), whereas E7 oncoprotein expression was not reinduced (Fig. 3D). Under our experimental conditions, p53 levels did not increase in HPV-positive cancer cells on stimulation of mTOR signaling at 1% O2 (Fig. S6A) and did not decrease on inhibition of mTOR signaling at 21% O2 (Fig. S6B).
Fig. S5.
REDD1 expression under hypoxia and RNAi-mediated repression of REDD1 and TSC2. (A) HeLa cells were grown under normoxia (21% O2) or under hypoxia (1% O2) for 24 h, and REDD1 transcript levels were determined by qRT-PCR. Indicated are relative REDD1 mRNA levels, with REDD1 expression under normoxia set at 1.0. Asterisks above the column indicate statistically significant differences from normoxic cells (**P < 0.01). (B) HeLa cells were transfected with pSUPER vectors expressing shRNAs targeting REDD1 (shREDD1-1, shREDD1-2) or TSC2 (shTSC2-1, shTSC2-2) mRNAs. Indicated are relative REDD1 (Left) and TSC2 (Right) transcript levels at 72 h after transfection compared with cells expressing control shRNA shContr-1 (set at 1.0). SDs of biological replicates are indicated. n ≥3. Asterisks above columns indicate statistically significant differences from control shRNA-treated cells (**P < 0.01; ***P < 0.001). (C) Reconstitution of mTOR signaling on shTSC2-2 expression in hypoxic HeLa cells (experimental details in Fig. 3D). (D) Senescence assays on expression of shTSC2-2 in normoxic and hypoxic HeLa cells (experimental details in Fig. 4E, Left). (E) Senescence assays on expression of shTSC2-2 in etoposide-treated normoxic and hypoxic HeLa cells (experimental details in Fig. 5E, Left). (Scale bar: 200 µm.)
Fig. S6.
mTOR modulation and p53 levels. (A) HeLa cells expressing shTSC2-1, shTSC2-2, shREDD1-1, or shREDD1-2 were cultured at 21% or 1% O2 for 24 h. The levels of P-S6K, S6K, HPV18 E7, and p53 were determined by immunoblotting. pSUPER, empty expression vector; β-actin served as a loading control. (B) Normoxic HPV-positive cancer cells were treated for 24 h with 0.5, 1.0, or 5 μM KU-0063794 (KU) or 50 nM rapamycin. Shown are immunoblots of P-S6K, S6K, P-4E-BP1, 4E-BP1, HPV16/18 E6/E7, and p53 protein levels. DMSO served as a solvent control; β-actin, as a loading control.
Of note, stimulation of the mTOR pathway in hypoxic HPV-positive cancer cells led to the emergence of cells staining positive for SA-β-Gal (Fig. 3E, Left and Fig. S5D), indicating the induction of senescence. Accordingly, the cells showed decreased colony-formation capacity when switched to normoxic culture conditions (Fig. 3E, Right). Collectively, these results indicate that hypoxic HPV-positive cancer cells, in which E6/E7 expression is down-regulated, evade senescence owing to the concomitant impairment of mTOR signaling that occurs, at least in part, via stimulation of the inhibitory REDD1/TSC2 axis.
Hypoxia Blocks Chemotherapy-Induced Senescence in HPV-Positive Tumor Cells.
There is increasing evidence that, along with apoptosis, senescence induction in tumor cells is a major mechanism through which chemotherapeutic agents can exert their anticancer effects (10, 35). Therefore, we also tested the impact of chemotherapeutics on the senescence regulation of HPV-positive cancer cells, dependent on their O2 supply.
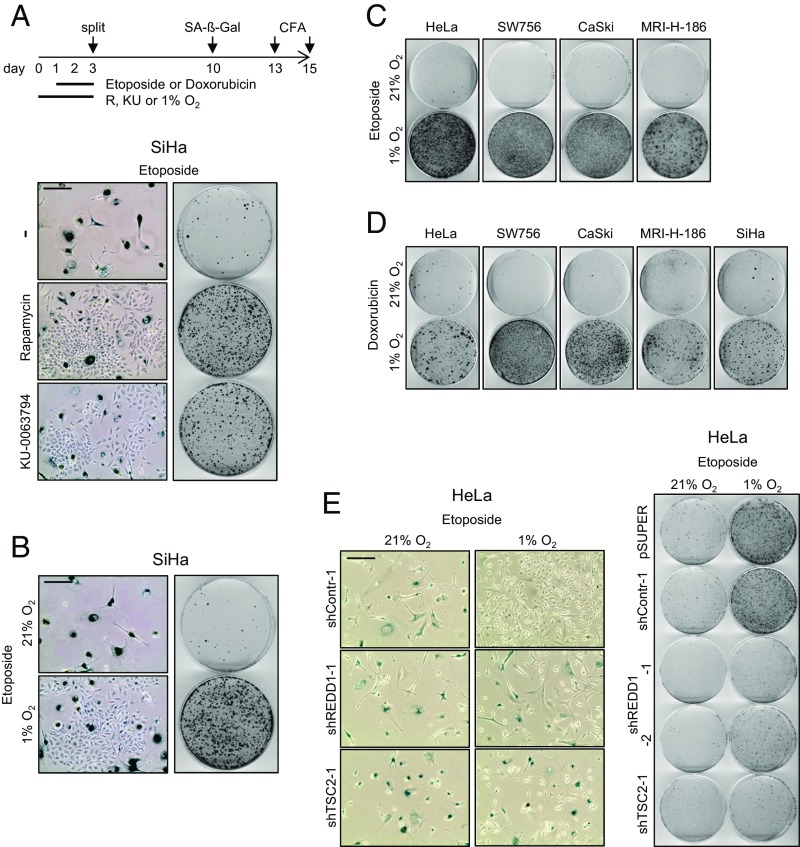
As shown in Fig. 4A, Left, treatment of normoxic HPV16-positive SiHa cells with etoposide efficiently induced senescence. Concomitant treatment with rapamycin or KU-0063794 allowed evasion of the cells from etoposide-induced senescence, as indicated by the outgrowth of SA-β-Gal–negative cells. This finding is corroborated by CFAs showing that the simultaneous application of mTOR inhibitors enhanced the colony-forming capacity of HPV-positive cells (Fig. 4A, Right) compared with etoposide treatment alone.
Fig. 4.
CT-induced senescence in HPV-positive cancer cells depends on mTOR signaling and is counteracted by hypoxia. (A) SiHa cells were treated under normoxia with etoposide, in either the absence (−) or the presence of rapamycin or KU-0063794. (Left) Senescence assays (SA-β-Gal staining). (Scale bar: 200 µm.) (Right) CFAs. Scheme above: Treatment protocol for Fig. 4 A–D, further detailed in the text. (B) SiHa cells were treated for 48 h with etoposide under normoxia or hypoxia, and subsequently cultured under normoxia. (Left) Senescence assays (SA-β-Gal staining). (Scale bar: 200 µm.) (Right) CFAs. (C and D) Normoxic and hypoxic HPV16- and HPV18-positive cancer cell lines were exposed to etoposide (C) or doxorubicin (D) at 21% or 1% O2, and subsequently analyzed by CFAs under normoxia. (E) HeLa cells were transfected with shRNA expression vectors for shREDD1-1, shREDD1-2, or shTSC2-1 and cultured under normoxia or hypoxia. (Left) Senescence assays (SA-β-Gal staining) of cells subsequently cultured under normoxia. (Scale bar: 200 µm.) (Right) Corresponding CFAs. Control cells were transfected with empty vector (pSUPER) or with a vector expressing shContr-1. Treatment protocol corresponds to the scheme indicated in Fig. 3E, but with additional etoposide treatment at days 2–4.
We next analyzed whether hypoxia can exert a similar detrimental effect on CT-induced senescence in HPV-positive cancer cells as was noted for chemical mTOR inhibitors. We observed that hypoxia allowed the cells to escape from etoposide-induced senescence (Fig. 4B, Left) and resulted in increased colony-formation capacity (Fig. 4B, Right). This response is consistent for all HPV-positive cancer cell lines examined (Fig. 4C) and not specific for etoposide, but was also detected for another tested chemotherapeutic drug, doxorubicin (Fig. 4D).
We then investigated whether the evasion of hypoxic HPV-positive tumor cells from etoposide-induced senescence is due to impaired mTOR signaling. We found that REDD1- or TSC2-inhibitory shRNAs reduced the outgrowth of SA-β-Gal–negative cells under hypoxia (Fig. 4E, Left and Fig. S5E), indicating that fewer hypoxic HPV-positive cells can evade etoposide-induced senescence when mTOR signaling is stimulated. Consistently, etoposide treatment of hypoxic cells, in which mTOR signaling is increased, results in a reduced colony-formation capacity when the cells are switched to normoxic culture conditions (Fig. 4E, Right). Taken together, these results indicate that intact mTOR signaling is crucial not only for senescence induction in HPV-positive cancer cells on endogenous E6/E7 repression, but also for their response toward external prosenescent stimuli, such as chemotherapeutic drugs.
Inverse Correlation Between Expression of HPV E7 and Carbonic Anhydrase IX in Cervical Cancer.
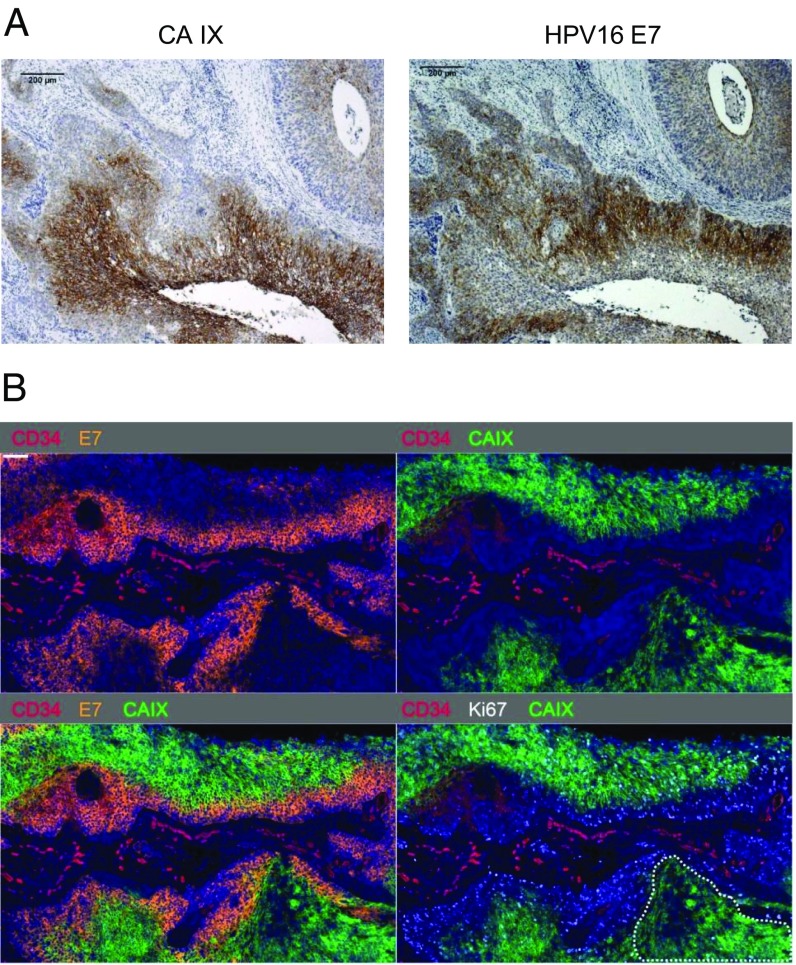
Finally, we investigated the in vivo expression of the HPV E7 oncoprotein in relation to the hypoxia-linked marker carbonic anhydrase IX (CA IX) (36) in HPV16-positive squamous cell cervical cancer specimens (n = 17) by immunohistochemistry. We observed prominent tumor areas with strong CA IX staining in 9 of the 17 cancers. Notably, and consistent with the in vitro data presented above, we found that these tumor areas invariably exhibited an inverse correlation between HPV16 E7 and CA IX expression levels (Fig. 5A).
Fig. 5.
Negative correlation between E7 and CA IX expression in tissue specimens from patients with cervical cancer. (A) Representative immunohistochemical analysis of an HPV16-positive cervical cancer, stained for the expression of the hypoxia-linked marker CA IX and HPV16 E7. (Scale bar: 200 µm.) (B) Multiplex immunofluorescence staining of the tumor depicted in Fig. 5A. Nuclei were counterstained with DAPI (blue). (Upper, Left) Expression of CD34 (red) and E7 (orange). (Upper, Right) Expression of CD34 (red) and CA IX (green). (Lower, Left) Expression of CD34 (red), E7 (orange) and CA IX (green). (Lower, Right) Expression of CD34 (red), CA IX (green) and Ki67 (white). (Scale bar: 100 µm.) Note the autofluorescence of red blood cells in the red channel. See also Fig. S7.
To allow a more detailed investigation of the relative spatial distribution of the antigens of interest, we prepared multicolor immunofluorescence stains for CA IX, E7, CD34 (staining microvascular endothelium), and Ki-67 (proliferation marker), using DAPI as a counterstain for cell nuclei. Again, we regularly detected an inverse correlation between CA IX and E7 protein expression (Fig. 5B). Costaining for CD34 revealed that E7 is expressed predominantly in the vicinity of blood vessels, whereas CA IX is predominantly expressed more distant from blood vessels (Fig. 5B). Moreover, in all investigated cervical cancer specimens, we detected CA IX-positive/E7-negative regions in which expression of Ki-67 is strongly reduced or absent (dotted lines in Fig. 5B; another example of a cervical cancer specimen from a different patient is provided in Fig. S7), indicating the presence of hypoxic tumor areas with little or no cell proliferation.
Fig. S7.
Multiplex immunofluorescence analysis of an HPV16-positive cervical cancer specimen. (Upper, Left) Expression of CD34 (red) and E7 (orange). (Upper, Right) Expression of CD34 (red) and CA IX (green). (Lower, Left) Expression of CD34 (red), E7 (orange), and CA IX (green). (Lower, Right) Expression of CD34 (red), CA IX (green), and Ki-67 (white). Dotted lines surround CA IX-positive/E7-negative regions in which the expression of Ki-67 is strongly reduced or absent. (Scale bar: 100 µm.)
Discussion
In this study, we found that hypoxic HPV-positive cancer cells can convert to a state of dormancy in which they efficiently shut off E6/E7 expression, induce a proliferative halt, but do not senesce. The cells are reactivated when they regain access to improved O2 supply, viral oncogene expression is reinduced, and proliferation is resumed. This phenotype differs fundamentally from the behavior of HPV-positive cancer cells under normoxic conditions, where E6/E7 repression results in rapid induction of senescence. Mechanistically, we show that these discrepancies can be attributed to differences in mTOR signaling. We found that the senescence response to E6/E7 repression in normoxic HPV-positive cancer cells requires intact mTOR signaling, which is impaired in hypoxic HPV-positive cancer cells via the mTOR inhibitory REDD1/TSC2 axis. The ability of HPV-positive cancer cells to induce senescence under hypoxia can be restored by experimental reactivation of mTOR signaling. Collectively, these findings indicate that hypoxic conditions allow HPV-positive cancer cells to withdraw from the selection pressure to continuously express E6/E7 without undergoing an irreversible growth arrest, suggesting that our current conception of the cross-talk between the HPV oncogenes and the host cell is likely too simplistic. The evasion from senescence by hypoxic HPV-positive cancer cells could be crucial for the pathogenesis of HPV-positive cancers, in that it allows escape from a major tumor-suppressive defense mechanism of the cell (7).
Our findings in this study have clinical implications as well (Fig. 6). The reversibility of hypoxia-induced growth inhibition may contribute to cancer recurrence when dormant HPV-positive cancer cells regain access to an increased O2 supply, which can occur, for example, following tumor shrinkage after therapy (37) or on neovascularization (38). Moreover, the hypoxia-induced proliferative halt of HPV-positive cancer cells likely provides them with increased resistance to CT, which preferentially attacks proliferating cells. In line with these considerations, hypoxia is linked to a worsened clinical prognosis, as well as to an increased therapeutic resistance of HPV-positive cancers (15, 16, 20).
Fig. 6.
Implications of hypoxia-linked alterations in HPV-positive cancer cells. Hypoxia results in E6/E7 repression and inhibition of cellular proliferation of HPV-positive cancer cells. The concomitant interference with mTOR signaling by hypoxia allows the cells to evade senescence. The growth inhibition under hypoxia contributes to the resistance of HPV-positive cancer cells toward CT. The ability of hypoxic HPV-positive cancer cells to block E6/E7 expression without undergoing senescence also provides therapeutic resistance toward strategies aiming at E6/E7 inhibition. The repression of E6/E7 antigen synthesis, together with the immunosuppressive effects of hypoxia, support evasion of hypoxic HPV-positive cancer cells from the host’s immune response and protects against immunotherapeutic approaches targeting E6/E7. Owing to the reversibility of hypoxia-linked growth inhibition, dormant hypoxic HPV-positive cells could serve as a reservoir for tumor recurrence on reoxygenation.
Furthermore, it is conceivable that the hypoxic E6/E7 repression reduces the synthesis and, subsequently, the presentation of viral antigens on the cell surface. In concert with the general immunosuppressive effects of hypoxia (39), this may help HPV-positive cancer cells evade the patient’s immune system in hypoxic tumor regions. The latter scenario could also represent a major obstacle for immunotherapeutic approaches targeting E6/E7-derived antigens, providing a possible molecular explanation as to why the success of immunotherapy for the treatment of HPV-positive cancers has been rather limited to date (13, 40).
Our findings also raise concerns about the strategy to block E6/E7 expression as a prosenescent therapeutic approach, in that E6/E7 inhibitors could be ineffective in hypoxic HPV-positive cancer cells where E6/E7 expression is shut down and mTOR signaling is impaired. However, these considerations do not preclude a therapeutic use of E6/E7 inhibitors, because they may be effective in better-oxygenated regions of HPV-positive tumors and could reduce tumor size. Moreover, our data show that the regrowth of dormant HPV-positive cancer cells on reoxygenation is linked to the reinduction of viral E6/E7 oncogene expression, which may then allow targeting of these cells by E6/E7 inhibitors.
The critical significance of the mTOR pathway in HPV-positive cells is not limited to E6/E7 repression acting as a prosenescent stimulus. We found that mTOR inhibitors can also interfere with the prosenescent activity of CT. Currently, mTOR inhibitors are under investigation in clinical studies as anticancer agents and are showing growth-inhibiting effects in preclinical models, including xenografts of HPV-positive cancer cells (41–43). The antitumorigenic effects of mTOR inhibitors in the clinic have often been unsatisfying when given as a monotherapy, however (44, 45). Increasingly, mTOR inhibitors are being used in combination with CT, to sensitize tumor cells toward chemotherapeutic agents (46). Given that the prosenescent activity of CT is considered important for its anticancer effects (10, 35), this use is difficult to reconcile with our data and data of others (47) showing that mTOR inhibitors can block CT-induced senescence. A possible explanation for this discrepancy is that senescence induction by CT could also have undesired effects, in that senescent cells within the tumor microenvironment (including stromal fibroblasts) secrete protumorigenic factors [Senescence-Associated Secretory Phenotype (SASP)] (7). Indeed, recent studies have indicated that the CT-sensitizing effect of mTOR inhibitors is linked to their ability to interfere with secretion of major components of the SASP of stromal cells (48, 49). Thus, the biological effects of mTOR inhibitors are likely to be complex, and their efficacy in cancer therapy may be determined by the balance between potentially protumorigenic (blocking the senescence response of cancer cells) and antitumorigenic (cytostasis, interference with the SASP, inhibition of angiogenesis) (50) responses.
Our findings also provide a foundation for future studies in several related areas. First, what is the mechanism that prevents the reconstitution of p53 under hypoxia-induced E6/E7 repression? Under normoxia, the p53 levels in HPV-positive cancer cells are dependent on E6-mediated proteolytic p53 degradation (51). Our results indicate that this control mechanism is uncoupled under hypoxia, in that E6 repression no longer results in increased p53 levels. Hypoxic HPV-positive cells do not appear to switch from E6-dependent to MDM2-dependent p53 degradation, given that treatment with Nutlin-3 did not appreciably reincrease p53 levels. In addition, p53 activities may be further blunted by the impairment of p53 transactivation function under hypoxia (52, 53).
Second, it will be interesting to further decipher in detail the molecular mechanism of hypoxia-linked E6/E7 repression. We have shown that high glucose concentrations (25 mM), which can be achieved in the blood of individuals with severe uncontrolled diabetes, efficiently counteract hypoxic E6/E7 repression. The basis of glucose-linked effects on gene expression is complex and incompletely understood, but can involve epigenetic mechanisms as well as specific transcription factors, including MondoA/ChREBP-Mlx, NF-κB, c-Myc, and SP1 (54, 55). Third, it will be important to study how E6/E7 repression under hypoxia influences viral antigen presentation on HPV-positive cancer cells and thereby may support their escape from immune defense mechanisms of the host.
In conclusion, the results of the present study show that the cross-talk between the HPV oncogenes and the host cell machinery, as well as the resulting phenotypic consequences in HPV-positive cancer cells, are profoundly dependent on cellular oxygenation levels. The ability of hypoxic HPV-positive cancer cells to induce a dormant state, reversibly blocking viral oncogene expression and cellular proliferation without undergoing senescence, is likely to affect the clinical behavior of HPV-positive cancers and should be considered in the ongoing development of novel treatment strategies, such as immunotherapy or targeted E6/E7 inhibition.
Materials and Methods
Cell Culture, Transfections, Treatments, and Reagents.
HPV18-positive HeLa and SW756 cervical carcinoma cells, and HPV16-positive SiHa, CaSki, and MRI-H-186 cervical carcinoma cells, as well as HPV-negative C33A cervical cancer cells, spontaneously immortalized HaCaT keratinocytes, HepG2 hepatoma cells, U2OS osteosarcoma cells, HCT116 and RKO colon cancer cells, and MCF7 breast cancer cells, were obtained from the tumor bank of the German Cancer Research Center or from the American Tissue Culture Collection. Cells were certified negative for mycoplasma contamination by PCR, and their identities were verified by multiplex human cell line authentication. Authenticated cells were frozen in aliquots, and after thawing, cells were used in experiments for a maximum of 4 wk.
Cells were cultured under normoxia (21% O2, 5% CO2), reduced oxygen (3% O2, 5% CO2), or hypoxia (1% O2, 5% CO2) in DMEM (HeLa, SW756, SiHa, CaSki, C33A, HepG2, U2OS, and MCF7), RPMI (MRI-H186 and RKO) or McCoy’s 5A medium (HCT116), supplemented with 10% FCS (Life Technologies), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma-Aldrich). The standard cell culture medium contained 100 mg/dL glucose (5.5 mM).
The following chemicals were used for treatment: rapamycin (AdipoGen); nocodazole (Merck); etoposide and doxorubicin (Enzo Life Science); CoCl2, mimosine, DMOG, and KU-0063794 (Sigma-Aldrich); and Nutlin-3 (Cayman Chemical). siRNAs were chemically synthesized (Life Technologies) or expressed as shRNAs from vector pSUPER as described previously (56). The si/shRNA target sequences were as follows: HPV18 E6/E7-1: 5′-CCACAACGUCACACAAUGU-3′; HPV18 E6/E7-2: 5′-CAGAGAAACACAAGUAUAA-3′; HPV18 E6/E7-3: 5′-UCCAGCAGCUGUUUCUGAA-3′; HPV16 E6/E7-1: 5′-CCGGACAGAGCCCAUUACA-3′; HPV16 E6/E7-2: 5′-CACCUACAUUGCAUGAAUA-3′; HPV16 E6/E7-3: 5′-CAACUGAUCUCUACUGUUA-3′; HIF-1α-1: 5′-CUAACUGGACACAGUGUGU-3′; HIF-1α-2: 5′-CUGAUGACCAGCAACUUGA-3′; HIF-2α-1: 5′-GCGACAGCUGGAGUAUGAA-3′; HIF-2α-2: 5′-CAGCAUCUUUGAUAGCAGU-3′; REDD1-1: 5′-GAAGCTGTACAGCTCGGAA-3′; REDD1-2: 5′-GGAACAGCTGCTCATTGAG-3′; TSC2-1: 5′-GCTCATCAACAGGCAGTTC-3′ (57); TSC2-2: 5′-CGACGAGTCAAACAAGCCA-3′ (57); control siRNA, siContr-1: 5′-CAGUCGCGUUUGCGACUGG-3′, containing at least four mismatches to all known human genes. To minimize potential off-target effects, three different siRNAs, each targeting all three HPV18 or HPV16 E6/E7 transcript classes, were pooled at equimolar concentrations (referred to in the text as si18E6/E7 and si16E6/E7, respectively), as detailed previously (5). Synthetic siRNAs were transfected with DharmaFECT I (Thermo Fisher Scientific) at a final siRNA concentration of 10 nM, and pSUPER plasmids were transfected by calcium phosphate coprecipitation (56).
Immunoblot Analyses.
Cellular protein was prepared and analyzed by immunoblotting as described previously (5). The following primary antibodies were used: anti–β-actin (A2228; Sigma-Aldrich); anti-HPV18 E7 (E7C); anti-HPV16 E7 (NM2, kind gift from Martin Müller, German Cancer Research Center); anti-HPV18 E6 (AVC 399) and anti-HPV16 E6 (AVC 843) (kind gift from Johannes Schweizer, Arbor Vita Corporation); anti-p53 (sc-126) and anti-vinculin (sc-73614) (Santa Cruz Biotechnology); anti-p21 (556431) and anti–HIF-1α (610959) (BD Biosciences); anti–HIF-2α (NB100-122; Novus Biologicals); and anti-Rb (9309), anti–phospho-Rb (Ser807/811) (9308), anti–phospho-S6 (Ser235/236) (2211), anti–4E-BP1 (9452), anti–phospho-4E-BP1 (Ser65) (9451), anti-p70S6K (9202), and anti–phospho-p70S6K (Thr389) (9234) (Cell Signaling Technology). The following HRP-conjugated secondary antibodies were used: anti-mouse IgG (W402), anti-chicken IgY (G1351), and anti-goat IgG (V8051) (Promega), and anti-rat IgG (112035003; Dianova).
RNA Extraction and qRT-PCR.
All qRT-PCR analyses were performed at least three times, in duplicate. For time courses, representative experiments (qRT-PCR performed in triplicate) are depicted along with corresponding protein analyses. RNA extraction and qRT-PCR conditions are detailed elsewhere (5). Forward (fwd) and reverse (rev) primer sequences (Eurofins MWG) were as follows: 18E6/E7 fwd: 5′-ATGCATGGACCTAAGGCAAC-3′; 18E6/E7 rev: 5′-AGGTCGTCTGCTGAGCTTTC-3′; 16E6/E7 fwd: 5′-CAATGTTTCAGGACCCACAGG-3′; 16E6/E7 rev: 5′-CTCACGTCGCAGTAACTGTTG-3′; REDD1 fwd: 5′-CCTCACCATGCCTAGCCTTT-3′; REDD1 rev: 5′-GTAAGCCGTGTCTTCCTCCG-3′; TSC2 fwd: 5′-TCCTCGACCAGATCCCATCA-3′; TSC2 rev: 5′-GCCATGCTCATTGGACAGGA-3′; 18S RNA fwd: 5′-CATGGCCGTTCTTAGTTGGT-3′; 18S RNA rev: 5′-ATGCCAGAGTCTCGTTCGTT-3′. Relative RNA quantification was performed using the comparative Ct (2−ΔΔCt) method (58). Data are presented as the fold change in gene expression, normalized to a reference gene (18S RNA), and relative to a calibrator sample (5). Statistical significance was determined by the two-tailed Student’s t test. P values of **P < 0.01 and ***P < 0.001 were considered significant.
CFAs, Senescence Assays, and Cell Counts.
For CFAs on RNAi-mediated E6/E7 repression (Fig. 3B), cells were transfected with E6/E7-inhibitory siRNAs, in the absence or presence of mTOR inhibitors. At 2 d after transfection, cells were split and further grown in the absence or presence of mTOR inhibitors. Starting 24 h after splitting, cells were also treated with nocodazole for 6 d, to eliminate nontransfected proliferating cells while sparing growth-arrested cells, following the protocol established by Leontieva et al. (27, 28). Then cells were cultured in drug-free medium, fixed, and stained with formaldehyde-crystal violet at 20 d after transfection. For analyzing the effects of CT by CFAs (Fig. 4 A–D), cells were cultured under hypoxia for 24 h and subsequently treated with 5–10 μM etoposide, depending on the cell line, or with 0.2 μM doxorubicin. The cells were grown for another 48 h under hypoxia, split, and incubated for 10–12 d under normoxia in drug-free medium. Colonies were fixed and stained with formaldehyde-crystal violet. Control cells were treated accordingly, but consistently kept under normoxia. For the analyses of mTOR inhibitors on the cellular response toward etoposide (Fig. 4A), cells were cultured in the absence or presence of 50 nM rapamycin or 1 μM KU-0063794 for 24 h, then also treated with etoposide for 48 h, split, and further processed as described above. For senescence assays, cells were treated as described for the CFAs, and stained for SA-β-Gal activity, as detailed previously (5), after the indicated time periods. All CFAs and senescence assays were performed independently at least three times, with consistent results. Viable cell numbers were determined by a standard trypan blue technique, using a Countess cell counter (Thermo Fisher Scientific). Cell count experiments were performed at least three times, in duplicate.
Immunohistochemistry and Multiplex Immunofluorescence Staining.
Conventional immunohistochemistry was performed using heat-induced epitope retrieval, and DakoEnvision technology was used as described previously (59). Multicolor immunofluorescence staining was carried out using a modification of the method published by Toth and Mezey (60). In brief, specimens were dewaxed in two changes of fresh xylene and then rehydrated in a descending alcohol series. Retrieval of antigenic binding sites was performed by heating specimens in appropriate buffers (Tris/EDTA 10/1 mM, pH 9.0; citrate pH 6.0) in a steamer (FS10; Braun) for 40 min. For the detection of HPV16 E7, a mouse monoclonal antibody, clone hrHPV-E7 5B4K2, was used. All other primary antibodies were purchased from Abcam (CA IX, ab108352; Ki67, ab16667; CD34, ab81289) and detected using polymer-based, HRP-conjugated anti-rabbit or anti-mouse SuperPicture reagents (Thermo Fisher Scientific). Tyramide conjugates of Alexa Fluor 488 (CA IX), Alexa Fluor 546 (CD 34) (Thermo Fisher Scientific), and Cy5 (PerkinElmer) were used for visualization. Tyramide conjugation of ATTO 425 (ATTO-TEC; Siegen) for Ki-67 visualization was carried out according to the method described by Hopman et al. (61). Quenching of residual peroxidase activity between successive rounds of antigen detection was achieved by reheating tissue specimens in the appropriate retrieval buffers. Nuclei were counterstained with DAPI, and slides were covered with a coverslip using ProLong Gold mounting medium (Thermo Fisher Scientific) and dried overnight. Digital images of the specimens were acquired using a fluorescence-enabled digital Slidescanner equipped with a LED light source and appropriate filter sets at a magnification of 0.325 µm/pixel (Pannoramic Confocal; 3D Histech).
All patients provided written informed consent to use their biopsy material for further molecular analyses to be conducted in the Jena University Hospital and in collaboration with academic partners. This study was approved by the Ethics Committee of the Friedrich-Schiller University Jena (reference nos. 0175–02/00 and 2174–12/07).
Acknowledgments
We thank Julia A. Braun, Dr. Annette Kopp-Schneider, and Dr. Martin Scheffner for discussions. This work was supported by the Wilhelm Sander-Stiftung (Grant 2015.137.1) and the Deutsche Krebshilfe (Grant 112132).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.M. is a Guest Editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1615758114/-/DCSupplemental.
References
- 1.zur Hausen H. Papillomaviruses and cancer: From basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 2.Howley PM, Schiller JT, Lowy DR. Papillomaviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 6th Ed. Lippincott, Williams & Wilkins; Philadelphia: 2013. pp. 1662–1703. [Google Scholar]
- 3.American Cancer Society . Cancer Facts and Figures 2015. American Cancer Society; Atlanta, GA: 2015. [Google Scholar]
- 4.Goodwin EC, et al. Rapid induction of senescence in human cervical carcinoma cells. Proc Natl Acad Sci USA. 2000;97(20):10978–10983. doi: 10.1073/pnas.97.20.10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honegger A, et al. Dependence of intracellular and exosomal microRNAs on viral E6/E7 oncogene expression in HPV-positive tumor cells. PLoS Pathog. 2015;11(3):e1004712. doi: 10.1371/journal.ppat.1004712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells SI, et al. Papillomavirus E2 induces senescence in HPV-positive cells via pRB- and p21(CIP)-dependent pathways. EMBO J. 2000;19(21):5762–5771. doi: 10.1093/emboj/19.21.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellner K, Münger K. Human papillomaviruses as therapeutic targets in human cancer. J Clin Oncol. 2011;29(13):1785–1794. doi: 10.1200/JCO.2010.28.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoppe-Seyler F, Hoppe-Seyler K. Emerging topics in human tumor virology. Int J Cancer. 2011;129(6):1289–1299. doi: 10.1002/ijc.26087. [DOI] [PubMed] [Google Scholar]
- 10.Nardella C, Clohessy JG, Alimonti A, Pandolfi PP. Pro-senescence therapy for cancer treatment. Nat Rev Cancer. 2011;11(7):503–511. doi: 10.1038/nrc3057. [DOI] [PubMed] [Google Scholar]
- 11.Acosta JC, Gil J. Senescence: A new weapon for cancer therapy. Trends Cell Biol. 2012;22(4):211–219. doi: 10.1016/j.tcb.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Nizard M, et al. Immunotherapy of HPV-associated head and neck cancer: Critical parameters. OncoImmunology. 2013;2(6):e24534. doi: 10.4161/onci.24534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stern PL, et al. Therapy of human papillomavirus-related disease. Vaccine. 2012;30(Suppl 5):F71–F82. doi: 10.1016/j.vaccine.2012.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8(12):967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Höckel M, Vaupel P. Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93(4):266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 16.Vaupel P, Mayer A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26(2):225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 17.Vaupel P. 2009. Pathophysiology of solid tumors. The Impact of Tumor Biology on Cancer Treatment and Multidisciplinary Strategies, eds Molls M, Vaupel P, Nieder C, Anscher MS (Springer, Berlin, Heidelburg), pp 51–92.
- 18.Bachtiary B, et al. Overexpression of hypoxia-inducible factor 1alpha indicates diminished response to radiotherapy and unfavorable prognosis in patients receiving radical radiotherapy for cervical cancer. Clin Cancer Res. 2003;9(6):2234–2240. [PubMed] [Google Scholar]
- 19.Birner P, et al. Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res. 2000;60(17):4693–4696. [PubMed] [Google Scholar]
- 20.Sørensen BS, et al. Radiosensitivity and effect of hypoxia in HPV-positive head and neck cancer cells. Radiother Oncol. 2013;108(3):500–505. doi: 10.1016/j.radonc.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Vaupel P, Höckel M, Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal. 2007;9(8):1221–1235. doi: 10.1089/ars.2007.1628. [DOI] [PubMed] [Google Scholar]
- 22.Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014;9:47–71. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- 23.Chai TF, et al. Hypoxia-inducible factor independent down-regulation of thioredoxin-interacting protein in hypoxia. FEBS Lett. 2011;585(3):492–498. doi: 10.1016/j.febslet.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, et al. HIF- and non–HIF-regulated hypoxic responses require the estrogen-related receptor in Drosophila melanogaster. PLoS Genet. 2013;9(1):e1003230. doi: 10.1371/journal.pgen.1003230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 26.Vassilev LT, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303(5659):844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 27.Leontieva OV, Blagosklonny MV. Hypoxia and gerosuppression: The mTOR saga continues. Cell Cycle. 2012;11(21):3926–3931. doi: 10.4161/cc.21908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leontieva OV, Demidenko ZN, Gudkov AV, Blagosklonny MV. Elimination of proliferating cells unmasks the shift from senescence to quiescence caused by rapamycin. PLoS One. 2011;6(10):e26126. doi: 10.1371/journal.pone.0026126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leontieva OV, et al. Hypoxia suppresses conversion from proliferative arrest to cellular senescence. Proc Natl Acad Sci USA. 2012;109(33):13314–13318. doi: 10.1073/pnas.1205690109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell type-specific repression of mRNA translation. Proc Natl Acad Sci USA. 2008;105(45):17414–17419. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang SA, et al. mTORC1 phosphorylation sites encode their sensitivity to starvation and rapamycin. Science. 2013;341(6144):1236566. doi: 10.1126/science.1236566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8(11):851–864. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- 33.Brugarolas J, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18(23):2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reiling JH, Hafen E. The hypoxia-induced paralogs Scylla and Charybdis inhibit growth by down-regulating S6K activity upstream of TSC in Drosophila. Genes Dev. 2004;18(23):2879–2892. doi: 10.1101/gad.322704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ewald JA, Desotelle JA, Wilding G, Jarrard DF. Therapy-induced senescence in cancer. J Natl Cancer Inst. 2010;102(20):1536–1546. doi: 10.1093/jnci/djq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olive PL, et al. Carbonic anhydrase 9 as an endogenous marker for hypoxic cells in cervical cancer. Cancer Res. 2001;61(24):8924–8929. [PubMed] [Google Scholar]
- 37.Seiwert TY, Salama JK, Vokes EE. The concurrent chemoradiation paradigm—general principles. Nat Clin Pract Oncol. 2007;4(2):86–100. doi: 10.1038/ncponc0714. [DOI] [PubMed] [Google Scholar]
- 38.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 39.Noman MZ, et al. Hypoxia: A key player in antitumor immune response. Am J Physiol Cell Physiol. 2015;309(9):C569–C579. doi: 10.1152/ajpcell.00207.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skeate JG, Woodham AW, Einstein MH, Da Silva DM, Kast WM. Current therapeutic vaccination and immunotherapy strategies for HPV-related diseases. Hum Vaccin Immunother. 2016;12(6):1418–1429. doi: 10.1080/21645515.2015.1136039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molinolo AA, et al. mTOR as a molecular target in HPV-associated oral and cervical squamous carcinomas. Clin Cancer Res. 2012;18(9):2558–2568. doi: 10.1158/1078-0432.CCR-11-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coppock JD, et al. Improved clearance during treatment of HPV-positive head and neck cancer through mTOR inhibition. Neoplasia. 2013;15(6):620–630. doi: 10.1593/neo.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grabiner BC, et al. A diverse array of cancer-associated MTOR mutations are hyperactivating and can predict rapamycin sensitivity. Cancer Discov. 2014;4(5):554–563. doi: 10.1158/2159-8290.CD-13-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fruman DA, Rommel C. PI3K and cancer: Lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13(2):140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiarini F, Evangelisti C, McCubrey JA, Martelli AM. Current treatment strategies for inhibiting mTOR in cancer. Trends Pharmacol Sci. 2015;36(2):124–135. doi: 10.1016/j.tips.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Lim HJ, Crowe P, Yang JL. Current clinical regulation of PI3K/PTEN/Akt/mTOR signalling in treatment of human cancer. J Cancer Res Clin Oncol. 2015;141(4):671–689. doi: 10.1007/s00432-014-1803-3. [DOI] [PubMed] [Google Scholar]
- 47.Leontieva OV, Blagosklonny MV. DNA-damaging agents and p53 do not cause senescence in quiescent cells, while consecutive re-activation of mTOR is associated with conversion to senescence. Aging (Albany NY) 2010;2(12):924–935. doi: 10.18632/aging.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herranz N, et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol. 2015;17(9):1205–1217. doi: 10.1038/ncb3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laberge RM, et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol. 2015;17(8):1049–1061. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guba M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: Involvement of vascular endothelial growth factor. Nat Med. 2002;8(2):128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 51.Hengstermann A, Linares LK, Ciechanover A, Whitaker NJ, Scheffner M. Complete switch from Mdm2 to human papillomavirus E6-mediated degradation of p53 in cervical cancer cells. Proc Natl Acad Sci USA. 2001;98(3):1218–1223. doi: 10.1073/pnas.031470698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koumenis C, et al. Regulation of p53 by hypoxia: Dissociation of transcriptional repression and apoptosis from p53-dependent transactivation. Mol Cell Biol. 2001;21(4):1297–1310. doi: 10.1128/MCB.21.4.1297-1310.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Achison M, Hupp TR. Hypoxia attenuates the p53 response to cellular damage. Oncogene. 2003;22(22):3431–3440. doi: 10.1038/sj.onc.1206434. [DOI] [PubMed] [Google Scholar]
- 54.Meugnier E, Rome S, Vidal H. Regulation of gene expression by glucose. Curr Opin Clin Nutr Metab Care. 2007;10(4):518–522. doi: 10.1097/MCO.0b013e3281298fef. [DOI] [PubMed] [Google Scholar]
- 55.Havula E, Hietakangas V. Glucose sensing by ChREBP/MondoA-Mlx transcription factors. Semin Cell Dev Biol. 2012;23(6):640–647. doi: 10.1016/j.semcdb.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 56.Leitz J, et al. Oncogenic human papillomaviruses activate the tumor-associated lens epithelial-derived growth factor (LEDGF) gene. PLoS Pathog. 2014;10(3):e1003957. doi: 10.1371/journal.ppat.1003957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korotchkina LG, et al. The choice between p53-induced senescence and quiescence is determined in part by the mTOR pathway. Aging (Albany NY) 2010;2(6):344–352. doi: 10.18632/aging.100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 59.Schmitz M, et al. Loss of gene function as a consequence of human papillomavirus DNA integration. Int J Cancer. 2012;131(5):E593–E602. doi: 10.1002/ijc.27433. [DOI] [PubMed] [Google Scholar]
- 60.Tóth ZE, Mezey E. Simultaneous visualization of multiple antigens with tyramide signal amplification using antibodies from the same species. J Histochem Cytochem. 2007;55(6):545–554. doi: 10.1369/jhc.6A7134.2007. [DOI] [PubMed] [Google Scholar]
- 61.Hopman AH, Ramaekers FC, Speel EJ. Rapid synthesis of biotin-, digoxigenin-, trinitrophenyl-, and fluorochrome-labeled tyramides and their application for In situ hybridization using CARD amplification. J Histochem Cytochem. 1998;46(6):771–777. doi: 10.1177/002215549804600611. [DOI] [PubMed] [Google Scholar]