Significance
Mesenchymal stem cells (MSCs) are thought to be derived from pericytes and exhibit a cellular, autonomous antimicrobial effector function that provides therapeutic potential against infectious diseases. However, the molecular mechanism remains unknown. Here, we demonstrate that human guanylate-binding protein 1 (hGBP1) is a key protective factor against Toxoplasma gondii infection in human MSCs (hMSCs). The recruitment of hGBP1 to the parasitophorous vacuole membrane in IFN-γ–stimulated hMSCs significantly inhibited T. gondii replication. Thus, our current study reveals an important function of hGBP1 in the defense against T. gondii and may shed new light on clarifying the mechanism of host defense properties of hMSCs.
Keywords: human stem cells, parasitic protozoan, innate immunity, in vitro cultivation
Abstract
Mesenchymal stromal cells (MSCs) have recently been shown to play important roles in mammalian host defenses against intracellular pathogens, but the molecular mechanism still needs to be clarified. We confirmed that human MSCs (hMSCs) prestimulated with IFN-γ showed a significant and dose-dependent ability to inhibit the growth of two types of Toxoplasma gondii [type I RH strain with green fluorescent proteins (RH/GFP) or type II PLK strain with red fluorescent proteins (PLK/RED)]. However, in contrast to previous reports, the anti-T. gondii activity of hMSCs was not mediated by indoleamine 2,3-dioxygenase (IDO). Genome-wide RNA sequencing (RNA-seq) analysis revealed that IFN-γ increased the expression of the p65 family of human guanylate-binding proteins (hGBPs) in hMSCs, especially hGBP1. To analyze the functional role of hGBPs, stable knockdowns of hGBP1, -2, and -5 in hMSCs were established using a lentiviral transfection system. hGBP1 knockdown in hMSCs resulted in a significant loss of the anti-T. gondii host defense property, compared with hMSCs infected with nontargeted control sequences. hGBP2 and -5 knockdowns had no effect. Moreover, the hGBP1 accumulation on the parasitophorous vacuole (PV) membranes of IFN-γ–stimulated hMSCs might protect against T. gondii infection. Taken together, our results suggest that hGBP1 plays a pivotal role in anti-T. gondii protection of hMSCs and may shed new light on clarifying the mechanism of host defense properties of hMSCs.
Mesenchymal stromal cells (MSCs) comprise a heterogeneous cell population endowed with multilineage differentiation potential and extensive immunomodulatory properties. MSCs have been successfully used to prevent and treat immune disorders, such as graft-versus-host disease, and emerging preclinical studies suggest that they might also protect against infectious challenges (1, 2). Recent studies showed that MSCs are located in the perivascular niche and constitute a subset of pericytes that are involved in both pathogen recognition and early inflammatory events (3). MSCs seem to impede pathogen growth and reduce the microbial burden by inhibiting growth through soluble factors or by enhancing the antimicrobial function of immune cells, as shown both in vitro and in vivo (2–5). For example, Nemeth et al. reported that mouse MSCs (mMSCs) prolonged the survival of septic mice and improved their organ (kidney, liver, and pancreas) functions (5). They achieved this result by enhancing IL-10 production from murine alveolar macrophages via MSC-secreted cyclooxygenase-2 (COX2) and prostaglandin E2 (PGE2) (5). Data from murine colitis models have shown that human adipose-derived MSCs protect against dextran-induced colitis by decreasing the secretion of proinflammatory cytokines and chemokines (6). However, the antimicrobial effector molecules in vertebrate MSCs are not universally the same (4–11). The antimicrobial effect of unstimulated hMSCs is mediated by the cathelicidin, LL-37 (4), as shown both in vitro and in vivo. In IFN-γ–stimulated hMSCs, by contrast, the antibacterial effect is mediated through the tryptophan-catabolizing enzyme, indoleamine 2,3-dioxygenase (IDO) (9). Conflicting results are also reported in mouse, in which the decision as to whether mMSCs increase the activity of phagocytes or not depends on the origin of these cells (11).
Toxoplasma gondii is an obligatory intracellular protozoan parasite that infects virtually all warm-blooded vertebrates, including humans. Clinical symptoms are rarely observed in most T. gondii-infected immunocompetent individuals. However, the parasite can cause severe disease and even death in immunosuppressed individuals, such as AIDS, cancer, and organ transplantation patients. T. gondii can actively invade host cells in vitro by dividing within a nonfusogenic parasitophorous vacuole (PV), a membrane structure formed during invasion that is maintained to surround the intracellular replicating parasites. However, this activity may not be completed in vivo due to the innate resistance mechanisms in host cells and, especially, in those that are naturally resistant to T. gondii (12).
During T. gondii infection, natural killer (NK) cells, neutrophils, CD4+ cells, and CD8+ T cells can all release IFN-γ, which is the central regulator of the immune response against T. gondii (12–14). In mouse cells, the most important IFN-γ–inducible effectors against T. gondii are likely to include inducible nitric oxide synthase (iNOS) (15), reactive oxygen species (ROS) (16), immunity-related p47 GTPases (IRGs) (17), and guanylate-binding proteins (GBPs) (18). Mice lacking a fragment of chromosome 3 that encodes GBP1, -2, -3, -5, -7, and -2ps were highly susceptible to T. gondii infection even after stimulation of IFN-γ (18), which indicates the importance of GBPs in immunity to T. gondii and provides insight into the antimicrobial effects of IFN-γ (18). It has been confirmed that members of the GBP family, namely GBP1, -6, -7, and -10, all play a key role in IFN-γ–mediated cell-autonomous immunity against bacterial infection and that GBP1, in particular, is essential for function in macrophage cell lines (19). However, IFN-γ–mediated immunity to intracellular pathogens seems to be cell type specific and occurs in a species-specific manner. IFN-γ–stimulated human monocytes and mouse macrophages are able to produce high levels of ROS to kill the parasite (15, 16). However, ROS production is not induced in T. gondii-infected human macrophages (20). Human fibroblasts can also display IFN-γ–dependent cell-autonomous immunity against T. gondii although the involvement of IDO remains controversial (21, 22). Thus, data from animal models may not directly apply to human toxoplasmosis, and the nature/relevance of innate immunity against T. gondii infection in humans is much less well understood. It is, therefore, useful to understand the fate of hMSCs (an important cell source for tissue/organ recovery) in T. gondii infection (23). In addition, the molecular mechanisms through which hMSCs augment anti-toxoplasmosis remain unclear.
To elucidate the functional contribution of human MSCs to host defense against T. gondii, we conducted a detailed analysis of gene expression in hMSCs before and after IFN-γ stimulation using genome-wide RNA sequencing (RNA-seq) and generated human GBP1 (hGBP1-), hGBP2-, and hGBP5-knockdown hMSC cell lines. hGBP1, but not other hGBPs or IDO knockdown, partially restored the IFN-γ–dependent anti-T. gondii response in hMSCs. Moreover, we observed a significant increase in colocalization of endogenous hGBP1 in the PV membrane of T. gondii-infected cells. These data clearly show that hGBP1 is associated with the replication of intracellular T. gondii upon IFN-γ stimulation, suggesting an important function for hGBP1 in the defense against T. gondii.
Results
The Growth of T. gondii in Human MSCs Is Dose Dependently Inhibited by IFN-γ Pretreatment.
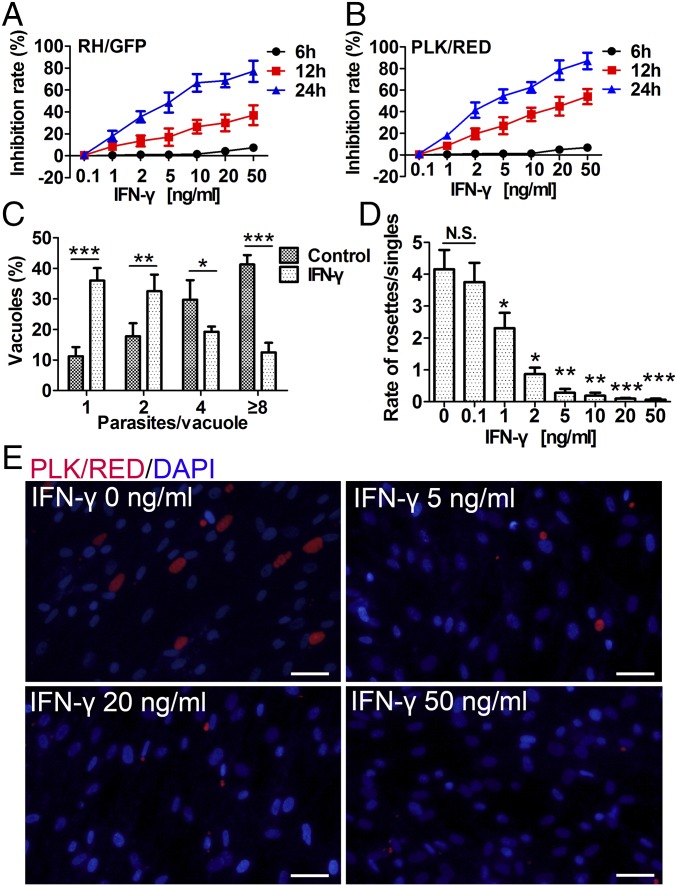
First, we tested the effects of IFN-γ–stimulated human MSCs on T. gondii growth. Human MSCs were pretreated with various concentrations of IFN-γ for 48 h and then infected with two T. gondii strains [type I strain RH with green fluorescent proteins (RH/GFP) and type II strain PLK with red fluorescent proteins (PLK/RED)], respectively. The fluorescence-labeled intracellular parasites were counted microscopically at multiple time points postinfection, and the inhibition rates of T. gondii growth were calculated. The growth of T. gondii was found to be dose-dependently inhibited by IFN-γ pretreatment. In addition, along with IFN-γ incubation time, the inhibition rate also significantly increased (Fig. 1A). Interestingly, the same concentrations of IFN-γ had stronger inhibitory effects on the PLK/RED strain than on the RH/GFP strain (Fig. 1B). Furthermore, the number of parasites per vacuole at 24 h postinfection (PLK/RED, Fig. 1C) and the ratio between parasite rosettes and single parasites at 48 h postinfection (PLK/RED) (Fig. 1D) were significantly lower in IFN-γ–treated hMSCs than in untreated hMSCs (RH/GFP) (Fig. S1 A and B). Representative fluorescence images of intracellular T. gondii growth at 48 h postinfection are shown in Fig. 1E (PLK/RED) and Fig. S1C (RH/GFP). To exclude the possibility that the presence of IFN-γ in the culture medium could have a direct effect on parasites, we assessed the effect of conditioned medium (CM), obtained from IFN-γ–stimulated MSCs (at 48 h postinfection), on T. gondii infection. The CM from pretreated cultures did not have any significant effect on the ability of T. gondii to invade hMSCs or to replicate, compared with CM from unstimulated hMSCs (RH/GFP, P = 0.34; PLK/RED, P = 0.45) (Fig. S2). These results strongly suggest that human MSCs act against T. gondii using some intracellular products that can be induced by IFN-γ.
Fig. 1.
IFN-γ–stimulated human MSCs dose-dependently inhibit the growth of T. gondii. Human MSCs were pretreated for 48 h with IFN-γ and then infected with T. gondii (RH/GFP or PLK/RED). (A and B) T. gondii growth inhibition rates were calculated at 6, 12, and 24 h postinfection (A, RH/GFP; B, PLK/RED). (C) At 24 h postinfection, the number of parasites per vacuole in IFN-γ–stimulated (20 ng/mL) hMSCs and unstimulated hMSCs (controls) were calculated (PLK/RED). (D) At 48 h postinfection, ratios of intracellular T. gondii rosettes and PVs containing a single parasite were presented (PLK/RED). (E) Representative fluorescence images of D. DNA was stained with DAPI. (Scale bars: 20 μm.) All presented values are means ± SD, n = 4. Statistical significance was indicated by comparison with controls. *P < 0.05; **P < 0.01; and ***P < 0.001; N.S., not significant.
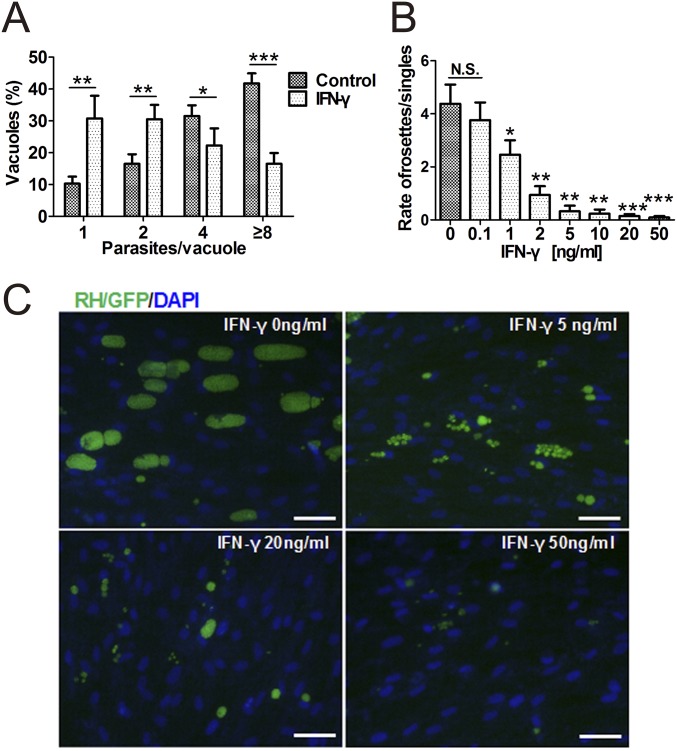
Fig. S1.
The growth of T. gondii in IFN-γ–stimulated hMSCs. Human MSCs were pretreated for 48 h with the indicated concentrations of IFN-γ and then infected with T. gondii strains (RH/GFP, moi = 0.5). (A) At 24 h postinfection, the number of parasites per vacuole in IFN-γ–stimulated hMSCs (IFN-γ, 20 ng/mL) and unstimulated hMSCs (controls) were calculated. Indicated values are means ± SD, n = 4. (B) At 48 h postinfection, the numbers of intracellular T. gondii rosettes and parasitophorous vacuoles (PVs) containing a single parasite were counted microscopically, and ratios were calculated. Indicated values are means ± SD, n = 4. *P < 0.05, **P < 0.01, ***P < 0.001, compared with the controls. N.S., not significant. (C) Representative fluorescence images of intracellular T. gondii growth were shown at 48 h postinfection. (Scale bars: 20 μm.) DAPI, 40,6-diamidino-2-phenylindole.
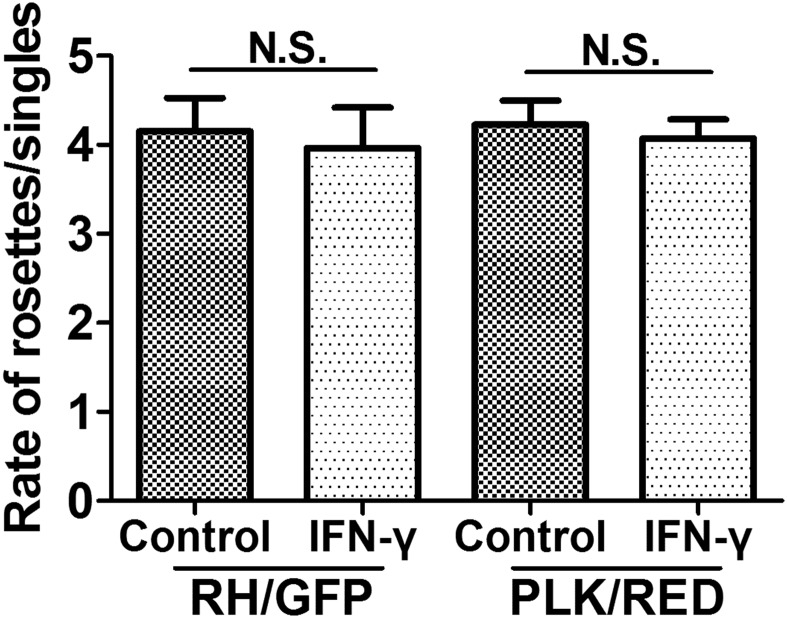
Fig. S2.
The effect of conditioned media (CM) from IFN-γ–treated MSCs on T. gondii growth of RH/GFP and PLK/RED strains. Toxoplasma gondii were incubated in the CM of IFN-γ–activated (20 ng/mL) MSCs for 30 min and then infected with untreated MSCs. At 48 h postinfection, the ratio between the quantity of intracellular T. gondii rosettes and the quantity of PVs containing a single parasite was calculated. The presented values are means ± SEM, n = 4.
The Anti-Toxoplasma Activity of Human MSCs Is Not Associated with IDO.
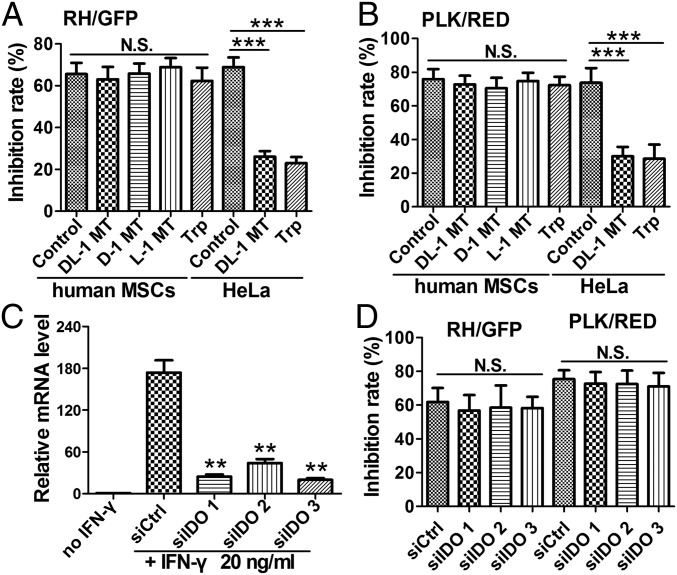
Previous studies showed that, in some human cell types (e.g., HeLa cells), IFN-γ stimulation inhibits T. gondii growth by depletion of tryptophan (Trp), via the activity of IDO (22, 24). To assess whether inhibiting the IDO activity could affect the proliferation of T. gondii in IFN-γ–stimulated MSCs, we tested the effects of three IDO-specific inhibitors (DL-1 MT, D-1 MT, and L-1 MT) or excess amounts of Trp, respectively. Our results revealed that both, inhibiting the IDO activity by 1-methyl-D/L-tryptophan and adding Trp, significantly decreased the antimicrobial effect against T. gondii in IFN-γ–stimulated HeLa cells (P < 0.001) (Fig. 2A), confirming that HeLa cells rely on IDO-mediated degradation of Trp for their ability to resist T. gondii infection. In human MSCs, in contrast, Trp and all three different IDO inhibitors failed to rescue both strains of the parasite from IFN-γ–stimulated growth inhibition (Fig. 2 A and B).
Fig. 2.
IFN-γ–mediated resistance in hMSCs does not require IDO. (A and B) Human MSCs or HeLa cells were stimulated with 20 ng/mL IFN-γ and treated with 1 mM Trp, or IDO inhibitor (DL-1 MT, D-1 MT, or L-1 MT), or the same volume of solvent (0.1 M NaOH, control) for 48 h. The cells were then infected with T. gondii (A, RH/GFP; B, PLK/RED) for 24 h, and growth inhibition was calculated. (C and D) Human MSCs were transfected with a control siRNA (siCtrl) or siRNAs targeting three different regions of IDO (siIDO1, -2, -3) for 24 h and treated with IFN-γ and infected with T. gondii as in A and B. The relative RNA levels of IDO in siIDO- and siCtrl-MSCs were examined by RT-PCR (C). T. gondii growth inhibition rates were calculated at 24 h postinfection (D). All results are expressed as the means ± SEM; **P < 0.01; ***P < 0.001; N.S., not significant; n = 4.
To further confirm these observations, we designed siRNAs that targeted three different regions of the IDO mRNA. Based on the RT-PCR results, we confirmed that they significantly decreased the expression of IDO stimulated by IFN-γ (by ∼90% in hMSCs treated with siIDO 1 and siIDO 3) (Fig. 2C). As shown in Fig. 2D, siRNA-mediated IDO knockdown also could not rescue the parasite from IFN-γ–mediated growth inhibition. These results suggest that the ability of human MSCs inhibiting the growth of T. gondii does not depend on Trp deprivation or IDO activity, which is in contrast to the results presented from a previously reported study (9).
IFN-γ–Inducible p65 Guanylate-Binding Proteins Are Significantly Up-Regulated in MSCs.
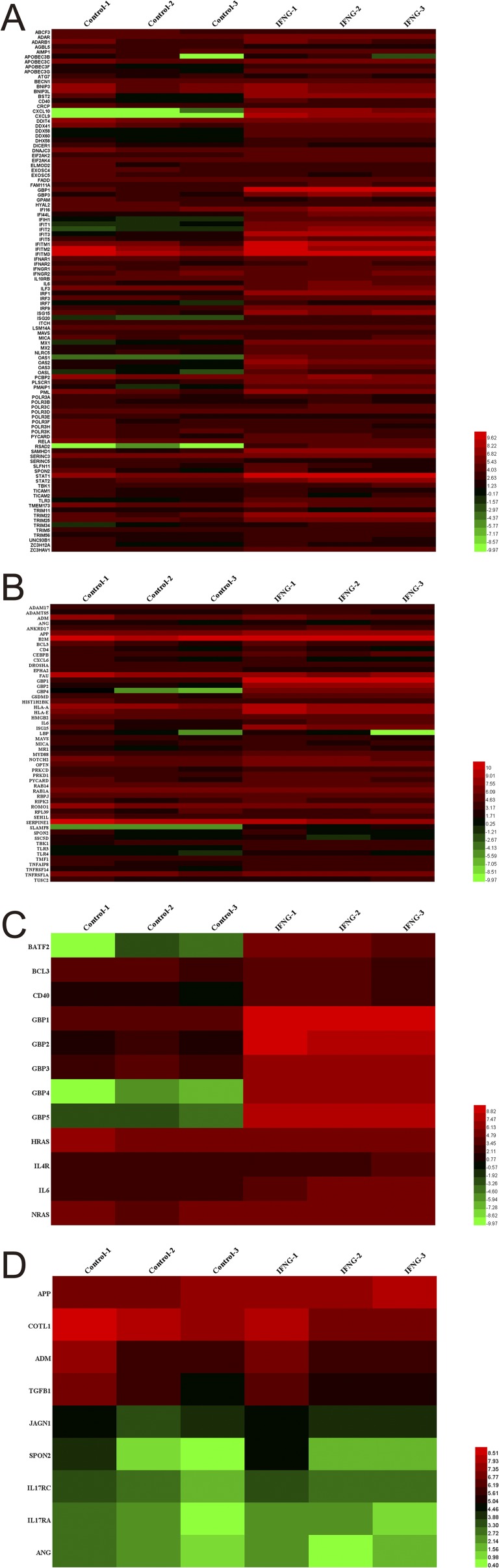
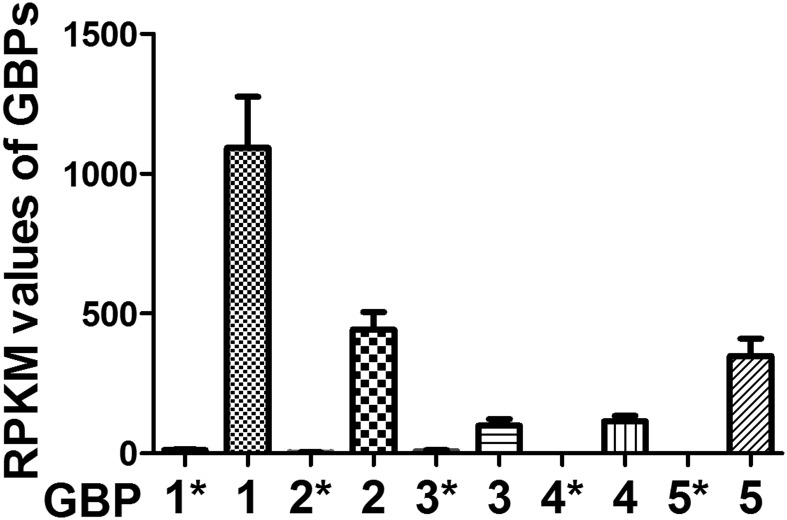
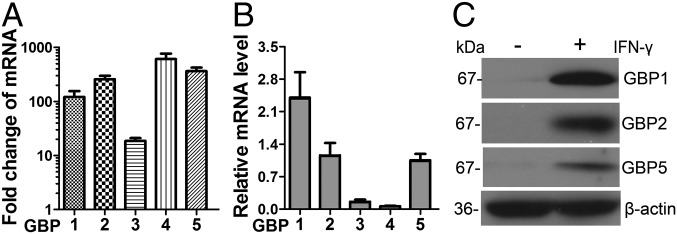
To examine the possible mechanism of activity of human MSCs against T. gondii infection, we performed genome-wide RNA-seq to assess the changes in the expression of host defense-related genes of MSCs treated with recombinant IFN-γ (20 ng/mL). Six sequencing libraries were constructed (three samples from each group). On average, more than 18 million 50-bp reads were obtained, of which more than 70% of the reads were aligned to unique locations in the genome, and included more than 17,000 coding genes from each library. The global transcriptional profiling data will be published elsewhere. MSCs from three different donors expressed a series of host defense-related factors. Through gene ontology (GO) analysis, we identified 106, 53, 12, and 9 expressed genes [reads per kilobase per million (RPKM) value > 5] that were associated with defense responses to viral, bacterial, protozoan, and fungal infections, respectively (Fig. S3). Previous reports have demonstrated that mouse GBPs are involved in T. gondii growth inhibition (18, 25, 26). Accordingly, we analyzed the expression of GBP family members in human MSCs before and after IFN-γ treatment. As shown in Fig. S4, the RPKM values for hGBP1, -2, -3, -4, and -5 significantly increased, and RT-PCR results further showed that the expression levels of hGBP1, -2, -4, and -5 were increased up to 100-fold after IFN-γ treatment (Fig. 3A). The relative abundances of the mRNAs for hGBP1, -2, and -5 compared with β-actin were significantly higher than that of hGBP3 and hGBP4 (Fig. 3B). Western blotting revealed that the protein expression levels of hGBP1, -2, and -5 were greatly increased after IFN-γ stimulation (Fig. 3C). These top three most highly expressed hGBPs (hGBP1, -2, and -5) were selected for further testing for their potential roles in human MSC activity against T. gondii infection.
Fig. S3.
RNA-seq data analysis. Human MSCs from three different donors were pretreated for 12 h with IFN-γ (20 ng/mL). Then, RNA library preparation and sequencing were performed as recommended by the manufacturer. Sequencing data were processed using Consensus Assessment of Sequence and Variation using the default settings (CASAVA, version 1.8.2; Illumina). Genes with an RPKM value more than five were enrolled in the functional analysis according to the Gene Ontology (GO) database. Heat map displays of genes related to host defense to virus (A), bacterium (B), protozoan (C), and fungus (D) are shown. The heatmaps of these genes were drawn with the RPKM value taking the logarithm of two.
Fig. S4.
RPKM values of hGBP1 to -5 in hMSCs before and after IFN-γ stimulation. Human MSCs were pretreated for 12 h with IFN-γ (20 ng/mL). Mean reads per kilobase per million (RPKM) values of hGBP1 to -5 in IFN-γ–stimulated MSCs, as assessed by RNA-seq. Indicated values are means ± SD, n = 3. *, data from nonstimulated hMSCs.
Fig. 3.
The expression levels of hGBPs are significantly up-regulated in IFN-γ–activated MSCs. Human MSCs were pretreated for 24 h with IFN-γ (20 ng/mL). (A) RT-PCR results show the magnitude of change of hGBP1 to -5 mRNA expression in IFN-γ–stimulated MSCs in comparison with prestimulated cells. (B) Relative mRNA levels of hGBP1 to -5 in IFN-γ–stimulated MSCs by RT-PCR with reference to beta-actin controls. (C) The protein levels hGBP1, -2, and -5 in IFN-γ–stimulated and nonstimulated MSCs, as assessed by Western blotting. All presented values are given as means ± SEM; n = 3.
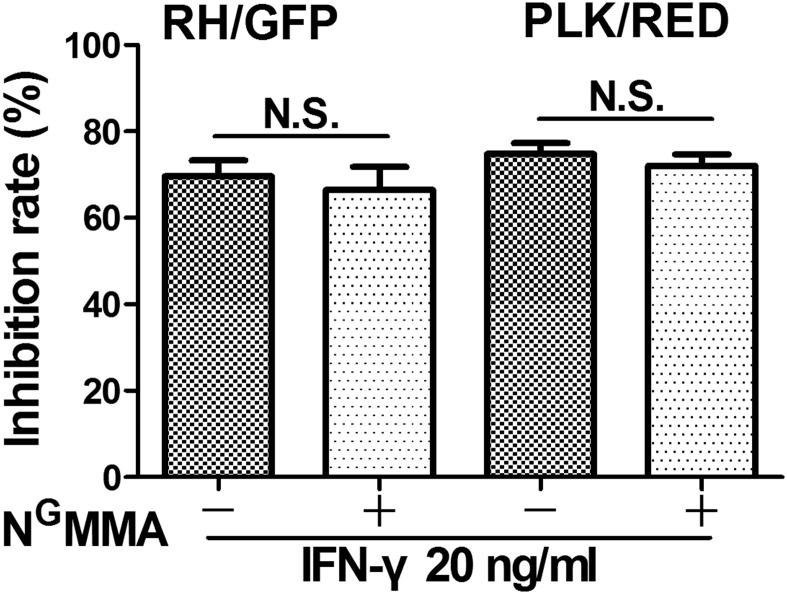
In mice, MSCs rely on nitric oxide (NO) produced by iNOS to protect themselves (9). Interestingly, we found that the expression of iNOS (NOS2) and human IRG genes was close to zero in MSCs both before and after stimulation with IFN-γ, which was also the case for the constitutive NOS (NOS1 and NOS3) genes, further supporting the notion that iNOS shows no increase in expression after stimulation. Using reverse transcriptase quantitative PCR and Western blotting, we also observed no iNOS expression in human MSCs. To further confirm these results, using the iNOS-specific inhibitor NG-monomethyl-l-arginine acetate (NGMMA), we revealed that inhibiting the iNOS activity did not influence the action of MSCs against T. gondii (Fig. S5). Taken together, our results indicate that the ability of human MSCs to inhibit the growth of T. gondii does not depend on iNOS.
Fig. S5.
iNOS activity on the effect of hMSCs against T. gondii. Human MSCs were stimulated with 20 ng/mL IFN-γ and treated with 100 μg/mL the iNOS-specific inhibitor NG-monomethyl-l-arginine acetate (NGMMA) for 48 h. The cells were then infected with the two strains of T. gondii (RH/GFP; PLK/RED) for 24 h (moi = 0.5), and growth inhibition rates were calculated for the parasites (Left, RH/GFP; Right, PLK/RED). The results are expressed as the means ± SEM (n = 4).
Generation of hGBP Knockdowns in Human MSCs.
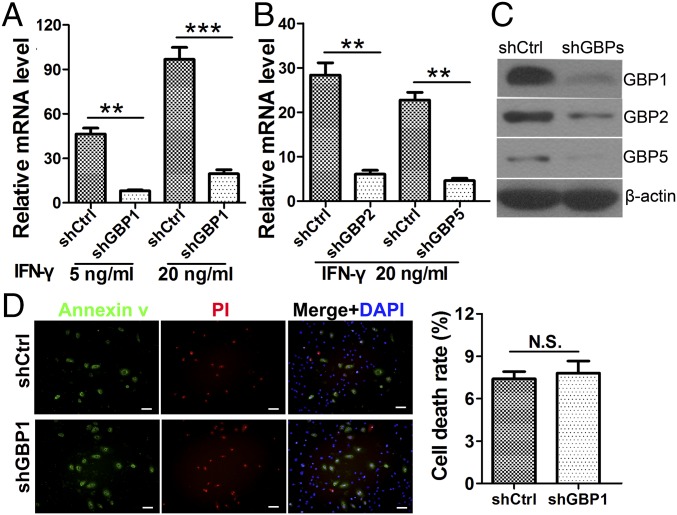
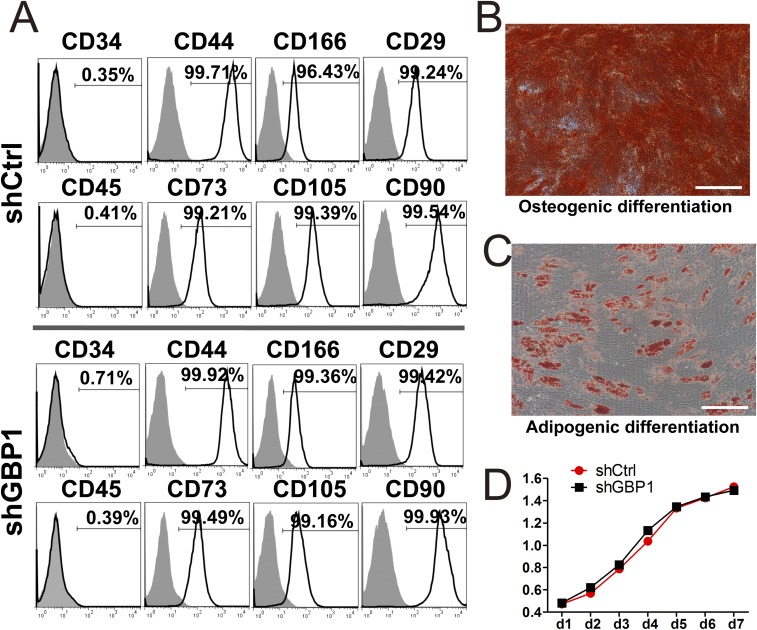
Stable hGBP1-, hGBP2-, and hGBP5-knockdown hMSC cell lines were generated using specific short hairpin RNA (shRNA) transcription cassettes and confirmed by RT-PCR (Fig. 4 A and B) using gene-specific primers (Table S1) and Western blotting (Fig. 4C). To test the characteristics of the three kinds of shGBP-MSCs, FACS was used to analyze hMSC surface markers. Compared with WT human MSCs, all transduced cells expressed the same panel of surface markers, exhibiting positive expression of CD29, CD44, CD73, CD90, CD166, and CD105, whereas detectable expression of the hematopoietic stem cell markers CD34 and CD45 was not found. Representative FACS results for shCtrl- and shGBP1-MSCs are shown in Fig. S6A. Our results clearly indicated that transduced cells maintained the cell-surface marker phenotype of human MSCs.
Fig. 4.
Characterization of shGBP-MSCs. Lentiviral shRNAs were used to knock down hGBP1, -2, and -5 (shGBPs), and pLKOpuro1 was used as a control (shCtrl). (A–C) Human MSCs transduced with shCtrl or shGBPs were stimulated with 20 ng/mL IFN-γ for 48 h, and the knockdown efficiencies of hGBP1 (A) and hGBP2 and -5 (B) were assessed by RT-PCR and Western blotting (C). n = 4. (D) MSCs transduced with shGBPs and shCtrl were cultured in serum-free medium for 48 h, and cell survival was tested using a LIVE/DEAD viability/cytotoxicity kit. n = 4. The results are expressed as the means ± SEM; **P < 0.01; ***P < 0.001; N.S., not significant; PI, propidium iodide staining. (Scale bars: 20 μm.)
Table S1.
Oligonucleotides used in this study
| Assay | Target gene | Sequence (5′–3′) |
| Quantitative real-time primers | β-actin | F: GATTACTGCTCTGGCTCCTAGC |
| R: GACTCATCGTACTCCTGCTTGC | ||
| IDO | F: AAGAAGTGGGCTTTGCTCTG | |
| R: CGCTGTGACTTGTGGTCTGT | ||
| hGBP1 | F: GTGGAACGTGTGAAAGCTGA | |
| R: CAACTGGACCCTGTCGTTCT | ||
| hGBP2 | F: GATTTCACCCTGGAACTGGA | |
| R: GGGTTCAGCTCTTCCTCCTT | ||
| hGBP3 | F: TTAATCTGCCCCGACTCTGT | |
| R: CATTGACCTTGATGCCTCCT | ||
| hGBP4 | F: GGCCCAAATGGAGAAGAAGT | |
| R: AGCCCCAGGTAGAGTGACAA | ||
| hGBP5 | F: AGGCCAAAGCAAGGTAGTGA | |
| R: ATGATGCCACCTGGAAGAGT | ||
| iNOS | F:TTTCCAAGACACACTTCACCA | |
| R: TCCTTTGTTACCGCTTCCAC | ||
| siRNA | siIDO1 | Sense: TGTTACAATGGGTAATGACAG |
| Antisense: GTCATTACCCATTGTAACAGA | ||
| siIDO2 | Sense: TATAATTACACATAATTACCT | |
| Antisense: GTAATTATGTGTAATTATACT | ||
| siIDO3 | Sense: TTAACTTCTCAACTCTTTCTC | |
| Antisense: GAAAGAGTTGAGAAGTTAAAC | ||
| Nontargeting control (siCtrl) | Sense: AACAAGATGAAGAGCACCAA | |
| Antisense: TTGGTGCTCTTCATCTTGTT | ||
| Short hairpin RNA primers | shGBP1 | AATTCAAAAACCAGATGAGTACCTGACATACCTCGAGGTATGTCAGGTACTCATCTGG |
| CCGGCCAGATGAGTACCTGACATACCTCGAGGTATGTCAGGTACTCATCTGGTTTTTG | ||
| shGBP2 | AATTCAAAAAGCAATTCAACTCATGCTTATTCTCGAGAATAAGCATGAGTTGAATTGC | |
| CCGGGCAATTCAACTCATGCTTATTCTCGAGAATAAGCATGAGTTGAATTGCTTTTTG | ||
| shGBP5 | AATTCAAAAACAAGGTAGTGATCAAAGAGTTCTCGAGAACTCTTTGATCACTACCTTG | |
| CCGGCAAGGTAGTGATCAAAGAGTTCTCGAGAACTCTTTGATCACTACCTTGTTTTTG |
Fig. S6.
The phenotype and differentiation capacity of shCtrl- and shGBP1-MSCs. (A) Representative flow-cytometric analysis of shCtrl- and shGBP1-MSCs. CD29, CD34, CD44, CD45, CD73, CD105, CD90, and CD166 expression in shCtrl- and shGBP1-MSCs was analyzed by fluorescence-activated cell sorting, which showed that transduced cells maintained the phenotype of human MSCs. (B) Alizarin Red S staining of osteogenic-differentiated shGBP1-MSCs. (C) Oil red O staining of adipogenic-differentiated shGBP1-MSCs. (Scale bar: 50 μm.) (D) Cell proliferation was analyzed by cell counting kit-8 (CCK8) assay.
To demonstrate that these cells still maintain their multipotency, they were cultured under conditions that could promote their differentiation into osteogenic or adipogenic lineages. After culture in osteogenic medium for 2 wk, the shGBP-MSCs were induced to differentiate into osteoblasts as confirmed by strong Alizarin Red S staining (Fig. S6B). Similarly, oil red O staining revealed the presence of lipid droplets in the cytoplasm of shGBP-MSCs on day 21 after adipogenic differentiation (Fig. S6C). The knockdown of hGBPs did not alter the proliferative properties of human MSCs, which were verified by CCK8 assays (P = 0.37) (Fig. S6D). Cell survival analysis showed that the cells cultured with serum-deprived conditions for 48 h produced only a mild, nonsignificant increase in the death rate that was similar to that found in shGBP1-MSCs (7.82 ± 0.94%) and shCtrl-MSCs (7.31 ± 0.73%; P = 0.21) (Fig. 4D). Similar features of sustained multipotency were also observed in shGBP2- and shGBP5-MSCs. These results demonstrated that the osteogenic and adipogenic differentiation, proliferation, and survival potential of shGBP-MSCs showed no observed differences from the control human MSCs.
hGBP1 Is a Key Factor for Inhibition of T. gondii Growth in Human MSCs Stimulated by IFN-γ.
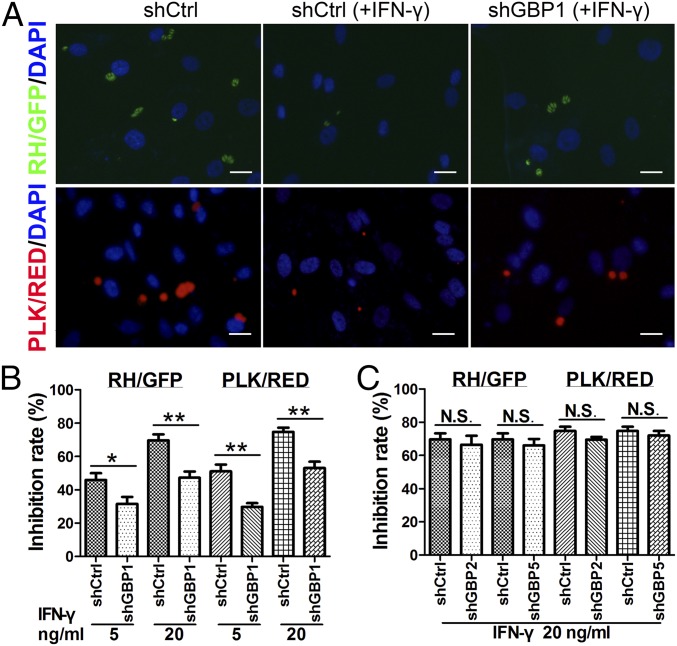
To investigate whether GBP family members contribute to the IFN-γ–induced antimicrobial effect of MSCs, we examined the effect of GBP knockdown in MSCs on the growth of T. gondii. Remarkably, compared with control cells, inhibition of T. gondii growth was rescued in shGBP1-MSCs [Fig. 5A, Upper (RH/GFP) and Lower (PLK/RED)] at 24 h postinfection. Specifically, using the RH/GFP and PLK/RED strains, we found the inhibition rates of T. gondii growth in MSCs at 5 and 20 ng/mL of IFN-γ were significantly reduced by shGBP1, compared with control MSCs (Fig. 5B). In contrast, there was no obvious difference between control cells and shGBP2- or shGBP5-MSCs at 24 h postinfection (Fig. 5C). Thus, our studies demonstrate that hGBP1 may play a major role in inhibiting T. gondii growth in IFN-γ–stimulated human MSCs, in contrast to hGBP2 and hGBP5, which do not.
Fig. 5.
IFN-γ–mediated resistance in MSCs requires hGBP1. Human MSCs transduced with shCtrl or shGBPs were stimulated with IFN-γ for 48 h and then infected with RH/GFP or PLK/RED. (A) Representative fluorescence images of intracellular T. gondii growth at 24 h postinfection. IFN-γ, 20 ng/mL. (Scale bars: 10 μm.) (B and C) The inhibition rates of T. gondii growth in the indicated knockdown and control cells were calculated at 24 h postinfection (RH/GFP; PLK/RED). The results are expressed as the means ± SEM n = 4. *P < 0.05; **P < 0.01; N.S., not significant.
Human GBP1 Is Recruited to the Parasitophorous Vacuole During T. gondii Infection.
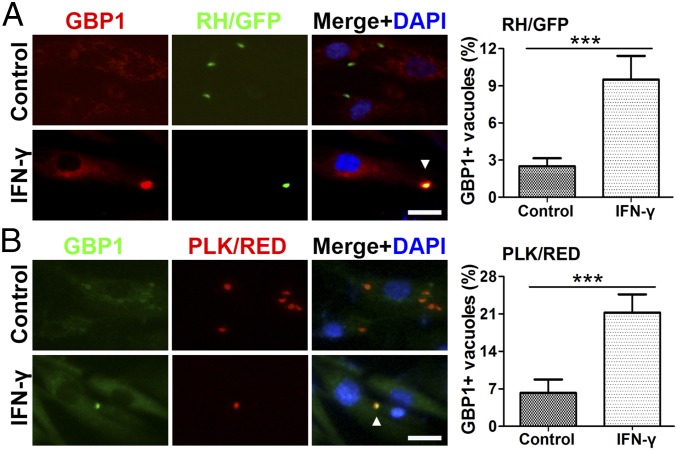
Recruitment of mouse GBPs to the PV was reported to be essential for the replication control of T. gondii (18, 25, 26). To explore the role of hGBP1 in human MSCs, we used immunofluorescence to assess its intracellular localization after T. gondii infection. Endogenous hGBP1 was observed in ∼10% of vacuoles belonging to IFN-γ–stimulated MSCs infected with RH/GFP strain at 3 h postinfection, but only 3% in the corresponding nonstimulated MSCs (Fig. 6A). A significantly higher percentage of PVs staining positive for hGBP1 was found in the cells infected with PLK/RED strain (Fig. 6B), which could potentially explain the stronger inhibitory effect of MSCs on PLK/RED versus RH/GFP strain. Collectively, these data indicated that the accumulation of hGBP1 on the PV membranes of IFN-γ–stimulated hMSCs could significantly inhibit the replication of T. gondii.
Fig. 6.
Localization of hGBP1 in T. gondii-containing parasitophorous vacuoles. The subcellular localization of hGBP1 in human MSCs was analyzed by immunostaining. (A and B) Cells were stimulated with IFN-γ (20 ng/mL) for 48 h, infected with T. gondii for 3 h, stained with anti-hGBP1 and DAPI (A, RH/GFP; B, PLK/RED). n = 4. The results are expressed as the means ± SEM ***P < 0.001. (Scale bars: 5 μm.)
Discussion
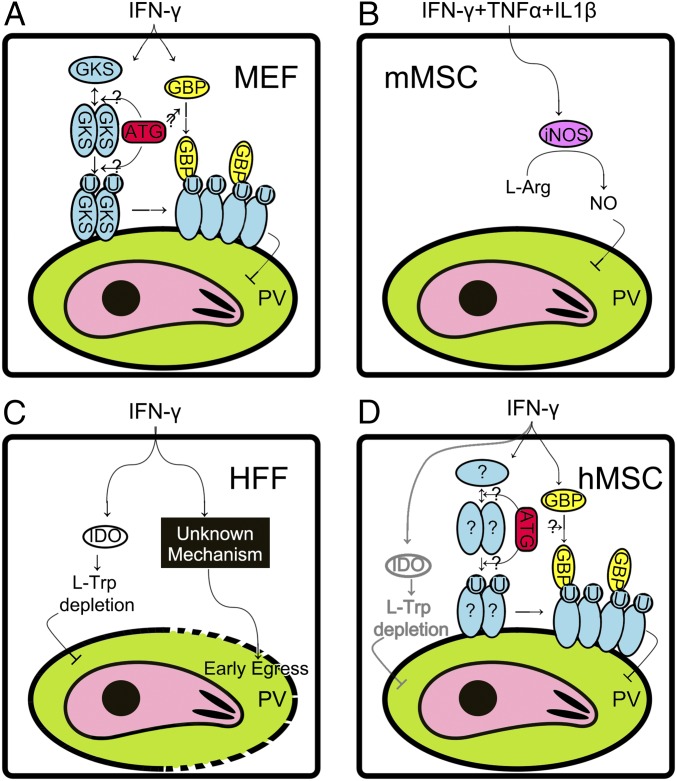
As an obligatory intracellular protozoan parasite, T. gondii is protected by the plasma membrane of the host cell and is thus able to escape from most antibody-based immune responses (12). Therefore, the host’s innate immune response is largely responsible for controlling T. gondii infection. Although numerous studies have demonstrated that T. gondii can grow in various somatic cells from warm-blooded animals and humans in vitro, the related disease condition is not commonly found in vivo. Previous studies have shown that an IFN-γ–mediated response against T. gondii infection seems to act in a cell type-specific and species-specific manner: e.g., nonlymphocytes shown in Fig. S7. In mice, astrocytes can use IRGs to restrict T. gondii infection; mMSCs use iNOS to produce NO; and skeletal muscle cells use both mechanisms (27). The mGBPs may also critically contribute to fighting T. gondii replication in vivo (18).
Fig. S7.
IFN-γ stimulates protection in nonlymphocytes against T. gondii infection. (A) GKS and GBP pathways in mouse embryo fibroblast cells (MEFs). IFN-γ stimulation up-regulates the expression of the glycine-lysine-serine motif containing immunity-related GTPase (GKS) proteins and guanylate-binding proteins (GBPs). GKS is activated via dimerization/oligomerization and translocated on the parasitophorous vacuole (PV) membrane (34), which induces ubiquitination (U) of the PV (44). Further, GKS recruits GBP on the PV membrane (26, 44), which inhibits growth of the parasite (18, 26, 42). Autophage proteins (ATG) are required for GKS and GBP recruitment although the mechanism is uncertain (45). (B) NO pathway in mouse mesenchymal stromal cells (mMSCs). IFN-γ, tumor necrosis factor α (TNFα), and interleukin 1β (IL1β) could induce inducible nitric oxide synthase (iNOS), but IFN-γ alone is not sufficient (9). iNOS consumes l-arginine and produces nitric oxide (NO), which inhibits the growth of parasites. (C) IDO pathway and an unknown pathway in human foreskin fibroblast cells (HEFs). IFN-γ up-regulates indoleamine 2,3-dioxygenase 1 (IDO), which results in depletion of l-tryptophan (Trp) and thus causes nutrition restriction of the parasites (21). Alternatively, an unknown pathway stimulates host cell death in response to the early egress of parasite (22, 53). (D) GBP pathway and IDO pathway in human mesenchymal stromal cells (hMSCs). The GBP pathway in hMSCs is functional in a similar manner to that in MEF cells, but GKS proteins were not identified in humans. An unknown factor (?) is yet to be revealed that may replace the GKS pathway. Alternatively, the IDO pathway may also be involved (9), but this involvement was not observed in our study.
However, many studies in animal models show significant differences from humans, and vast differences have been observed between immune and somatic cells of patients infected with T. gondii (14–22). High IDO activity, which could result in the depletion of Trp, is considered a key weapon against T. gondii growth in human fibroblasts treated with IFN-γ, but it does not seem to occur in mouse fibroblasts (20, 28, 29). Human MSCs treated with IFN-γ were found to exhibit an IDO-mediated antimicrobial effector function against various pathogens, suggesting a similar mechanism that might be used to curtail T. gondii (9). Our results, however, have indicated that IDO is not a main factor in the ability of human MSCs to inhibit the growth of T. gondii (Fig. 2D).
The iNOS-mediated production of NO is a well-known molecular effector against pathogens in the innate immune responses of various animals, including mMSCs (9) and skeletal muscle cells (27), and also in vivo (15, 30, 31). However, a previous study reported that iNOS protein expression was not detectable in human MSCs (9). Consistent with these results, our data also showed that MSCs derived from IFN-γ–stimulated human bone marrow neither expressed any detectable level of iNOS RNA nor displayed any inhibition of T. gondii's growth via iNOS activity. Therefore, it is highly possible that NO is not the main molecular effector against replication of T. gondii in human MSCs stimulated by IFN-γ.
It has been reported that an IRG protein-based resistance mechanism is essential to control T. gondii replication in mice (17, 27). Numerous IRGs were found to converge on vacuoles containing T. gondii in IFN-γ–activated mouse macrophages, fibroblasts, and astrocytes (32–34). IRG subclass m (IRGm) in mouse is a membrane marker for host cell self-recognition that is used to regulate the IRG pathway (35) and other IRGs such as the glycine-lysine-serine motif containing IRGs (also known as GKSs). Mouse IRGs were found to cooperate with mGBPs in acting against T. gondii infection (13, 14, 18). These findings suggested that mGBPs and mIRGs could play important roles in the IFN-γ–dependent, autonomous cellular control of toxoplasmosis. Furthermore, it can be predicted that these protein families could play broader roles in the host defense systems of murine cells. To our surprise, however, only one complete IRG (hIRGm) is present in the human genome, and it lacks the necessary IFN-γ induction element (33). In fact, based on our work, we also did not observe the up-regulation of any hIRGs in our RNA-seq experiments. Our data provide further evidence to suggest that hIRGm has little (if any) activity against T. gondii infection in humans, at least in the case of hMSCs. Without the cooperation of hIRGm, hGBPs, if present, may be sole contributors to antitoxoplasmosis activity.
In a similar manner to mIRGs, mGBPs belong to the IFN-inducible GTPase family. Kim et al. reported that mGBPs (at least mGBP1 and mGBP7) participated in antibacterial responses by recruiting host defense proteins, including phagocyte oxidase, antimicrobial peptides, and autophagic effectors within macrophages and mGBP1−/− mice (19). A recent report showed that mGBP2 and mGBP5 could promote bacteriolysis by controlling the activation of the inflammasome protein, AIM2, in mouse bone marrow-derived macrophages (36). In human cells, hGBP1 shows one of the highest induction levels in response to IFN-γ stimulation (37), which has been shown to be associated with viral infections, inflammatory bowel diseases, glioblastoma, and cutaneous lupus erythematosus (38–41).
In the case of toxoplasmosis, previous studies demonstrated that mGBP1, -2, -3, -6, -7, and -9 were recruited to T. gondii-containing PVs in IFN-γ–activated mouse embryonic fibroblasts or RAW 264.7 macrophages (42). Recently, an mGBP cluster on chromosome 3 (comprising the genes encoding mGBP1, -2, -3, -5, and -7) was found to cooperate with mIRGb6 and mIRGb10 during toxoplasmosis (18). Mice defective in p65 GTPases were highly susceptible to T. gondii infection, and GBPs deleted in chromosome 3 aneuploid macrophages were defective in IFN-γ–mediated inhibition of intracellular parasite growth (18). The recruitment of mGBP2 to T. gondii-containing PVs is essential for a cell’s ability to control T. gondii replication (25). Furthermore, recruitment of mGBP1 to PVs is associated with vesiculation and rupture and thus also protects against T. gondii infection (26). Both mGBP1 and mGBP2 have been shown to form tetramers and may also heterooligomerize to cooperatively activate GTPase activity (25).
The hGBP1, -2, and -3 proteins are paralogs of mGBP1 and mGBP2, and both hGBP1 and mGBP2 show isoprenylation, which targets proteins to intracellular membranes and/or facilitates protein/protein interactions (43). Such findings suggest that these GBPs may play similar roles in protecting human and mouse cells against T. gondii infection. Our present data clearly show that hGBP1 is recruited to T. gondii PVs (Fig. 6) and provides additional evidence that hGBP1 plays a role similar to that of mGBP1/2. Interestingly, our RNA-seq and RNAi data showed that hGBP1, not hGBP2, is the main responder in IFN-γ–stimulated human MSCs. Thus, hGBP1 is unlikely to interact with hGBP2 to execute this protective function. The third paralog, hGBP3, is also not likely to be a main effector, as its mRNA level was low and of little capability of up-regulation (Fig. 3). Thus, hGBP1 in human MSCs stimulated by IFN-γ could be a functional homodimer in the innate immune response.
Recently, Haldar et al. reported that the deposition of mGBPs on T. gondii-containing vacuoles needed IRG protein-dependent ubiquitination of PVs after priming their host cells with IFN-γ (44). The recruitment of hGBPs to the membranes of T. gondii-containing PVs is regulated by the autophagic protein, ATG16L1, in IFN-γ–stimulated human fibroblast-like (HAP1) cells (45). Here, using both the noncyst and cyst-forming strains (RH/GFP and PLK/RED) of T. gondii (46), we observed the recruitment of endogenous hGBP1 to T. gondii-containing PVs at 3 h postinfection in IFN-γ–stimulated human MSCs (Fig. 6). The signaling pathway that is responsible for recruiting hGBP1 to act against T. gondii is not yet fully understood. Future studies are needed to examine whether hGBPs contribute directly to membrane blebbing and the physical disruption of the PV membrane and/or whether they modulate the function of other effectors at this interface.
In summary, we herein define hGBP1 as an important effector molecule in the IFN-γ–triggered autonomous resistance of human MSCs against T. gondii infection. Our studies reveal that nonlymphocytes could apply various mechanisms of IFN-γ–triggered autonomous resistance against T. gondii infection in a cell type-specific and species-specific manner. Human MSCs offer an excellent example of a somatic cell type that plays a very important role in the host defense system by providing protection against intracellular pathogens such as T. gondii. The emerging involvement of MSCs in hematopoiesis and antimicrobial responses suggests that they are worthy of further development in the context of novel cellular antimicrobial therapies aimed at reducing infection-related morbidity and mortality, particularly in immunocompromised patients.
Materials and Methods
Cell Culture and Parasite Infection.
Human cells were isolated and/or cultured as described: MSCs (47), human foreskin fibroblasts (HFFs) (48), and HeLa cells (22) used in this study have been approved by the Institutional Review Board of Sun Yat-Sen University (2014064). Cell proliferation and cell survival assays were performed as described previously (49). Tachyzoites of T. gondii RH/GFP (50) and PLK/RED (51) were allowed to naturally egress from the host cells and were then quickly harvested using standard procedures (15). The effects of IFN-γ–treated human MSCs, nontreated human MSCs, or conditioned medium (CM) on the growth of T. gondii [multiplicity of infection (moi) = 0.5] were assessed by microscopic counting at different time points postinfection (18).
Small Interfering RNA and Short Hairpin RNA Knockdown.
In vitro silencing of IDO in hMSCs was performed as described (47), using three sets of IDO-siRNAs (Table S1). The short hairpin RNA (shRNA) expression vector pLKOpuro1 (Addgene) was used to express the control (shCtrl) and hGBP1, -2 and -5–specific shRNAs using specific oligos (Table S1). The helper plasmids psPAX2 and pMD2.G were used as lentiviral expression vectors (Addgene). The 293T host cells were used for packaging of the shRNAs. The MISSION Non-Target pLKOpuro1 control vector (Sigma-Aldrich) was used as a control (shCtrl). Pools of stably transduced cells were selected using puromycin (2 μg/mL) for 5 to 7 d. All experiments were performed in puromycin-free media, and each knockdown was validated by RT-PCR and Western blotting.
Other methods used in this paper can be found in SI Materials and Methods.
SI Materials and Methods
Chemicals and Reagents.
Recombinant human IFN-γ was purchased from R&D Systems. L-tryptophan (Trp), 1-methyl-DL-tryptophan (DL-1 MT), D-1 MT, and L-1 MT were purchased from Sigma-Aldrich. The primary antibodies against hGBP1, hGBP2, hGBP5, and β-actin, plus the relevant HRP-conjugated secondary antibodies, were all purchased from Santa Cruz. Monoclonal antibodies against CD29, CD34, CD44, CD45, CD73, CD90, CD105, and CD166 were purchased from BD Biosciences Pharmingen.
MSC Culture.
MSCs were derived from bone marrow aspirates (10 to 30 mL) of healthy voluntary donors after obtaining informed consent and according to guidelines approved by the Ethics Committee of Sun Yat-Sen University. Heparin-treated bone marrow was obtained by iliac crest aspiration from healthy donors who had provided informed consent. The MSCs were cultured and characterized as described previously and met all of the classification criteria for MSCs, as defined by the International Society of Cellular Therapy (52). Briefly, the unattached bone marrow cells were removed, and the cells that were positive for CD29, CD44, CD73, CD90, CD105, and CD166, but not CD34 or CD45, were collected by flow cytometry. These cells were confirmed as MSCs by validating that they could differentiate into osteoblasts and adipocytes. All of the MSCs used in this study were collected at passages 3 to 5. Each experiment was performed in triplicate using cells from at least three different donors.
Parasites and Infection.
Toxoplasma gondii culture supernatants were harvested 3 to 4 d after infection, host cell debris was removed by differential centrifugation (5 min, 50 × g; 15 min, 500 × g), and the obtained parasites were resuspended in medium, counted, and immediately used to inoculate host cells. All parasite strains and cell lines were routinely checked for Mycoplasma contamination, which was not detected.
Human MSCs stimulated with IFN-γ for 48 h were coincubated with freshly egressed T. gondii (moi = 0.5) for 2 h, and extracellular parasites were removed. Cells were counted in 28 viewing fields (40×) of tangential circles aligned horizontally across the center of each well, which represented 3.6% of the area of each well. This counting method yielded consistent results (variation < 8%) across duplicate wells and between different observers.
RT-PCR and RNA-seq.
RNA was isolated from human MSCs, using an RNeasy kit (Qiagen), and treated with DNase I to remove contaminating DNA. One microgram of total RNA was reverse transcribed to cDNA, and each target gene was amplified using the SYBR Green Master Mix (Bio-Rad) following the manufacturer’s protocol. The primer sequences are listed in Table S1. Quantitative real-time PCR (RT-PCR) was performed using a CFX96 Real-Time PCR System (Bio-Rad). Relative mRNA levels were calculated after normalization with respect to β-actin.
MSCs were treated with IFN-γ (20 ng/mL) for 12 h (n = 3). Then, RNA library preparation and sequencing were performed as recommended by the manufacturer (Genome Analyzer IIx; Illumina). Sequencing data were processed using Consensus Assessment of Sequence and Variation (Illumina) using the default settings. Genes with RPKM (reads per kilobase of exon per million mapped reads) values of more than five were enrolled in the functional analysis according to the Gene Ontology (GO) database. Genes related to host defense (GO:0051607 defense response to virus, GO:0042742 defense response to bacterium, GO:0042832 defense response to protozoan, GO:0050832 defense response to fungus) were selected to draw a heatmap with HemI (Heatmap Illustrator, version 1.0).
Western Blot Analyses.
Cell lysates were analyzed on a 12% SDS/PAGE gel, blotted, and probed with antibodies against hGBP1, hGBP2, and hGBP5 (all at 1:500) or β-actin (1:5,000). The bound primary antibodies were detected using peroxidase-coupled secondary antibodies and enhanced chemiluminescence (Cell Signaling Technologies).
Immunofluorescence Analyses.
Human MSCs (1 × 105) infected with T. gondii were fixed, permeabilized, blocked with 10% (vol/vol) goat serum, and incubated with anti-human GBP1 antibody (1:50) and then with Alexa Fluor 488/594-conjugated (green/red) IgG (Invitrogen). The samples were then washed three times with 0.01% Triton X-100 in PBS before the nuclei were stained with DAPI. The images were obtained using an IX71 fluorescence microscope (Olympus) and an LSM710 confocal microscope (Zeiss) and analyzed using ImageJ software (National Institutes of Health).
Statistical Analysis.
Statistical analysis was performed using GraphPad Prism software, and the data are given as the means ± SD or means ± SEM of at least three independent experiments. The data were compared using the Student’s paired t test, and Ps < 0.05 were considered to be statistically significant.
Acknowledgments
We thank the members of the authors’ laboratories who provided great help during the work. This work was supported by China Postdoctoral Science Foundation Grant 2015A030310428, National Basic Research Program of China 973 Program Grant 2010CB350000, National Natural Science Foundation of China Grants 81425016 and 31472058, and Key Scientific and Technological Projects of Guangdong Province Grant 2014B020226002.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. SRP095307).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1619665114/-/DCSupplemental.
References
- 1.Balan A, et al. Mesenchymal stromal cells in the antimicrobial host response of hematopoietic stem cell recipients with graft-versus-host disease--friends or foes? Leukemia. 2014;28(10):1941–1948. doi: 10.1038/leu.2014.127. [DOI] [PubMed] [Google Scholar]
- 2.Auletta JJ, Deans RJ, Bartholomew AM. Emerging roles for multipotent, bone marrow-derived stromal cells in host defense. Blood. 2012;119(8):1801–1809. doi: 10.1182/blood-2011-10-384354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paquet-Fifield S, et al. A role for pericytes as microenvironmental regulators of human skin tissue regeneration. J Clin Invest. 2009;119(9):2795–2806. doi: 10.1172/JCI38535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krasnodembskaya A, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28(12):2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Németh K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Rey E, et al. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58(7):929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 7.Wong JH, et al. Antifungal action of human cathelicidin fragment (LL13-37) on Candida albicans. Peptides. 2011;32(10):1996–2002. doi: 10.1016/j.peptides.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Gupta N, et al. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67(6):533–539. doi: 10.1136/thoraxjnl-2011-201176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meisel R, et al. Human but not murine multipotent mesenchymal stromal cells exhibit broad-spectrum antimicrobial effector function mediated by indoleamine 2,3-dioxygenase. Leukemia. 2011;25(4):648–654. doi: 10.1038/leu.2010.310. [DOI] [PubMed] [Google Scholar]
- 10.Hall SR, et al. Mesenchymal stromal cells improve survival during sepsis in the absence of heme oxygenase-1: The importance of neutrophils. Stem Cells. 2013;31(2):397–407. doi: 10.1002/stem.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mei SH, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182(8):1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 12.Yarovinsky F. Innate immunity to Toxoplasma gondii infection. Nat Rev Immunol. 2014;14(2):109–121. doi: 10.1038/nri3598. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: The major mediator of resistance against Toxoplasma gondii. Science. 1988;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 14.Sturge CR, Yarovinsky F. Complex immune cell interplay in the gamma interferon response during Toxoplasma gondii infection. Infect Immun. 2014;82(8):3090–3097. doi: 10.1128/IAI.01722-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, et al. Differences in iNOS and arginase expression and activity in the macrophages of rats are responsible for the resistance against T. gondii infection. PLoS One. 2012;7(4):e35834. doi: 10.1371/journal.pone.0035834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray HW, Juangbhanich CW, Nathan CF, Cohn ZA. Macrophage oxygen-dependent antimicrobial activity. II. The role of oxygen intermediates. J Exp Med. 1979;150(4):950–964. doi: 10.1084/jem.150.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard JC, Hunn JP, Steinfeldt T. The IRG protein-based resistance mechanism in mice and its relation to virulence in Toxoplasma gondii. Curr Opin Microbiol. 2011;14(4):414–421. doi: 10.1016/j.mib.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto M, et al. A cluster of interferon-γ-inducible p65 GTPases plays a critical role in host defense against Toxoplasma gondii. Immunity. 2012;37(2):302–313. doi: 10.1016/j.immuni.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Kim BH, et al. A family of IFN-γ-inducible 65-kD GTPases protects against bacterial infection. Science. 2011;332(6030):717–721. doi: 10.1126/science.1201711. [DOI] [PubMed] [Google Scholar]
- 20.Murray HW, Teitelbaum RF. L-arginine-dependent reactive nitrogen intermediates and the antimicrobial effect of activated human mononuclear phagocytes. J Infect Dis. 1992;165(3):513–517. doi: 10.1093/infdis/165.3.513. [DOI] [PubMed] [Google Scholar]
- 21.Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA. 1984;81(3):908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niedelman W, Sprokholt JK, Clough B, Frickel EM, Saeij JP. Cell death of gamma interferon-stimulated human fibroblasts upon Toxoplasma gondii infection induces early parasite egress and limits parasite replication. Infect Immun. 2013;81(12):4341–4349. doi: 10.1128/IAI.00416-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribitsch I, et al. Basic science and clinical application of stem cells in veterinary medicine. Adv Biochem Eng Biotechnol. 2010;123:219–263. doi: 10.1007/10_2010_66. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt SK, et al. Antimicrobial and immunoregulatory properties of human tryptophan 2,3-dioxygenase. Eur J Immunol. 2009;39(10):2755–2764. doi: 10.1002/eji.200939535. [DOI] [PubMed] [Google Scholar]
- 25.Degrandi D, et al. Murine guanylate binding protein 2 (mGBP2) controls Toxoplasma gondii replication. Proc Natl Acad Sci USA. 2013;110(1):294–299. doi: 10.1073/pnas.1205635110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selleck EM, et al. Guanylate-binding protein 1 (Gbp1) contributes to cell-autonomous immunity against Toxoplasma gondii. PLoS Pathog. 2013;9(4):e1003320. doi: 10.1371/journal.ppat.1003320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takács AC, Swierzy IJ, Lüder CG. Interferon-γ restricts Toxoplasma gondii development in murine skeletal muscle cells via nitric oxide production and immunity-related GTPases. PLoS One. 2012;7(9):e45440. doi: 10.1371/journal.pone.0045440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai W, Pan H, Kwok O, Dubey JP. Human indoleamine 2,3-dioxygenase inhibits Toxoplasma gondii growth in fibroblast cells. J Interferon Res. 1994;14(6):313–317. doi: 10.1089/jir.1994.14.313. [DOI] [PubMed] [Google Scholar]
- 29.Gazzinelli RT, Mendonça-Neto R, Lilue J, Howard J, Sher A. Innate resistance against Toxoplasma gondii: An evolutionary tale of mice, cats, and men. Cell Host Microbe. 2014;15(2):132–138. doi: 10.1016/j.chom.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao ZJ, et al. Lower expression of inducible nitric oxide synthase and higher expression of arginase in rat alveolar macrophages are linked to their susceptibility to Toxoplasma gondii infection. PLoS One. 2013;8(5):e63650. doi: 10.1371/journal.pone.0063650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao JM, et al. Investigation of infectivity of neonates and adults from different rat strains to Toxoplasma gondii Prugniaud shows both variation which correlates with iNOS and Arginase-1 activity and increased susceptibility of neonates to infection. Exp Parasitol. 2015;149:47–53. doi: 10.1016/j.exppara.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Martens S, et al. Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. PLoS Pathog. 2005;1(3):e24. doi: 10.1371/journal.ppat.0010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bekpen C, et al. The interferon-inducible p47 (IRG) GTPases in vertebrates: Loss of the cell autonomous resistance mechanism in the human lineage. Genome Biol. 2005;6(11):R92. doi: 10.1186/gb-2005-6-11-r92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khaminets A, et al. Coordinated loading of IRG resistance GTPases on to the Toxoplasma gondii parasitophorous vacuole. Cell Microbiol. 2010;12(7):939–961. doi: 10.1111/j.1462-5822.2010.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haldar AK, et al. IRG and GBP host resistance factors target aberrant, “non-self” vacuoles characterized by the missing of “self” IRGM proteins. PLoS Pathog. 2013;9(6):e1003414. doi: 10.1371/journal.ppat.1003414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meunier E, et al. Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat Immunol. 2015;16(5):476–484. doi: 10.1038/ni.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prakash B, Praefcke GJ, Renault L, Wittinghofer A, Herrmann C. Structure of human guanylate-binding protein 1 representing a unique class of GTP-binding proteins. Nature. 2000;403(6769):567–571. doi: 10.1038/35000617. [DOI] [PubMed] [Google Scholar]
- 38.Li M, et al. Guanylate binding protein 1 is a novel effector of EGFR-driven invasion in glioblastoma. J Exp Med. 2011;208(13):2657–2673. doi: 10.1084/jem.20111102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naschberger E, et al. Increased expression of guanylate binding protein-1 in lesional skin of patients with cutaneous lupus erythematosus. Exp Dermatol. 2011;20(2):102–106. doi: 10.1111/j.1600-0625.2010.01160.x. [DOI] [PubMed] [Google Scholar]
- 40.Britzen-Laurent N, et al. GBP-1 acts as a tumor suppressor in colorectal cancer cells. Carcinogenesis. 2013;34(1):153–162. doi: 10.1093/carcin/bgs310. [DOI] [PubMed] [Google Scholar]
- 41.Itsui Y, et al. Antiviral effects of the interferon-induced protein guanylate binding protein 1 and its interaction with the hepatitis C virus NS5B protein. Hepatology. 2009;50(6):1727–1737. doi: 10.1002/hep.23195. [DOI] [PubMed] [Google Scholar]
- 42.Degrandi D, et al. Extensive characterization of IFN-induced GTPases mGBP1 to mGBP10 involved in host defense. J Immunol. 2007;179(11):7729–7740. doi: 10.4049/jimmunol.179.11.7729. [DOI] [PubMed] [Google Scholar]
- 43.Nantais DE, Schwemmle M, Stickney JT, Vestal DJ, Buss JE. Prenylation of an interferon-gamma-induced GTP-binding protein: The human guanylate binding protein, huGBP1. J Leukoc Biol. 1996;60(3):423–431. doi: 10.1002/jlb.60.3.423. [DOI] [PubMed] [Google Scholar]
- 44.Haldar AK, et al. Ubiquitin systems mark pathogen-containing vacuoles as targets for host defense by guanylate binding proteins. Proc Natl Acad Sci USA. 2015;112(41):E5628–E5637. doi: 10.1073/pnas.1515966112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohshima J, et al. Role of mouse and human autophagy proteins in IFN-γ-induced cell-autonomous responses against Toxoplasma gondii. J Immunol. 2014;192(7):3328–3335. doi: 10.4049/jimmunol.1302822. [DOI] [PubMed] [Google Scholar]
- 46.Lun ZR, et al. Cancer in the parasitic protozoans Trypanosoma brucei and Toxoplasma gondii. Proc Natl Acad Sci U S A. 2015;112(29):8835–8842. doi: 10.1073/pnas.1502599112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng Y, et al. Mesenchymal stromal cells infusions improve refractory chronic graft versus host disease through an increase of CD5+ regulatory B cells producing interleukin 10. Leukemia. 2015;29(3):636–646. doi: 10.1038/leu.2014.225. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 49.Liu J, et al. Islet-1 overexpression in human mesenchymal stem cells promotes vascularization through monocyte chemoattractant protein-3. Stem Cells. 2014;32(7):1843–1854. doi: 10.1002/stem.1682. [DOI] [PubMed] [Google Scholar]
- 50.Nishikawa Y, et al. Characterisation of Toxoplasma gondii engineered to express mouse interferon-gamma. Int J Parasitol. 2003;33(13):1525–1535. doi: 10.1016/s0020-7519(03)00204-2. [DOI] [PubMed] [Google Scholar]
- 51.Takashima Y, et al. Detection of the initial site of Toxoplasma gondii reactivation in brain tissue. Int J Parasitol. 2008;38(5):601–607. doi: 10.1016/j.ijpara.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 52.Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 53.Yan X, Ji Y, Liu X, Suo X. Nitric oxide stimulates early egress of Toxoplasma gondii tachyzoites from Human foreskin fibroblast cells. Parasit Vectors. 2015;8(8):420. doi: 10.1186/s13071-015-1037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]