Significance
The carotid body (CB) is the major sensory organ responsible for monitoring arterial blood oxygen content. Glomus cells in the CB express heme oxygenase 2 (HO-2) and cystathionine-γ-lyase (CSE), enzymes that generate the gasotransmitters carbon monoxide (CO) and hydrogen sulfide (H2S), respectively. In normoxia, CO inhibits CSE from producing H2S. During hypoxia, HO-2 produces less CO, resulting in increased H2S production, which stimulates CB activity, leading to increases in respiratory rate, heart rate, and blood pressure. We report that decreased CO and increased H2S generation in the CB causes sleep apnea in HO-2 knockout mice and spontaneously hypertensive rats. An inhibitor of CSE eliminates sleep apnea when administered to these animals, suggesting that this approach may have therapeutic utility in patients with sleep apnea.
Keywords: central apnea, chemoreflex, hypertension, obstructive apnea, oxygen sensing
Abstract
Sleep apnea, which is the periodic cessation of breathing during sleep, is a major health problem affecting over 10 million people in the United States and is associated with several sequelae, including hypertension and stroke. Clinical studies suggest that abnormal carotid body (CB) activity may be a driver of sleep apnea. Because gaseous molecules are important determinants of CB activity, aberrations in their signaling could lead to sleep apnea. Here, we report that mice deficient in heme oxygenase-2 (HO-2), which generates the gaseous molecule carbon monoxide (CO), exhibit sleep apnea characterized by high apnea and hypopnea indices during rapid eye movement (REM) sleep. Similar high apnea and hypopnea indices were also noted in prehypertensive spontaneously hypertensive (SH) rats, which are known to exhibit CB hyperactivity. We identified the gaseous molecule hydrogen sulfide (H2S) as the major effector molecule driving apneas. Genetic ablation of the H2S-synthesizing enzyme cystathionine-γ-lyase (CSE) normalized breathing in HO-2−/− mice. Pharmacologic inhibition of CSE with l-propargyl glycine prevented apneas in both HO-2−/− mice and SH rats. These observations demonstrate that dysregulated CO and H2S signaling in the CB leads to apneas and suggest that CSE inhibition may be a useful therapeutic intervention for preventing CB-driven sleep apnea.
Gasotransmitters are a unique class of signaling molecules responsible for a diverse set of physiologic responses (1). Unlike other transmitters, they are not stored in vesicles. Instead, they are synthesized in response to a stimulus and released instantly. Their biological actions are mediated either by direct modification of target proteins or by activation of metallo-enzymes. Nitric oxide (NO) was the first gasotransmitter identified, whereas more recent studies have established roles for the gases carbon monoxide (CO) and hydrogen sulfide (H2S) (1).
Emerging evidence implicates CO and H2S in O2 sensing by the carotid body (CB), the principal sensory organ for monitoring O2 levels in the arterial blood (2, 3). Glomus cells, the O2-sensing cells in the CB, express heme oxygenase-2 (HO-2) and cystathionine-γ-lyase (CSE), which are enzymes that produce CO and H2S, respectively (3, 4). Under normoxic conditions, CO inhibits CSE from producing H2S through protein kinase G-dependent signaling (5). HO-2 produces less CO in hypoxia, thereby resulting in increased H2S production. H2S stimulates CB sensory nerve activity and initiates the CB chemoreflex, leading to increased heart rate, respiratory rate, and blood pressure, which are critical for maintaining cardiorespiratory homeostasis. Aberrant CO-H2S signaling in the CB has important physiological consequences. For instance, compared with Sprague–Dawley rats, Brown–Norway (BN) rats exhibit impaired O2 sensing by the CB due to increased CO and decreased H2S levels. As a consequence of the blunted CB chemoreflex, exposure of BN rats to hypobaric hypoxia does not stimulate breathing, leading to severe pulmonary edema (6).
Sleep apnea, characterized by periodic cessation of breathing during sleep, is a highly prevalent respiratory disorder affecting ∼10% of adults in the United States (7). Patients with sleep apnea exhibit several sequelae, including hypertension, stroke, and various neurocognitive and metabolic complications (8). Clinical studies suggest that a hyperactive CB chemoreflex is an important driver of pathological sequelae in sleep apnea patients (9–11). We hypothesized that an augmented CB chemoreflex stemming from disrupted CO-H2S signaling may lead to sleep apnea. This possibility was tested in HO-2–deficient (HO-2−/−) mice, which exhibit a hypersensitive CB chemoreflex due to a constitutive imbalance in CO-H2S signaling (5). We found that HO-2−/− mice exhibit a high incidence of apneas during sleep. Moreover, lowering the hyperactive CB chemoreflex by genetic ablation or pharmacologic inhibition of CSE activity in HO-2−/− mice normalized breathing and prevented apneas. Similar to HO-2−/− mice, spontaneously hypertensive (SH) rats also exhibited increased CB activity due to high levels of CSE-derived H2S (6). We found that SH rats also display a high incidence of apneas, which was corrected by treating SH rats with a CSE inhibitor.
Results
HO-2 −/− Mice Exhibit Irregular Breathing with Apneas and Hypopneas.
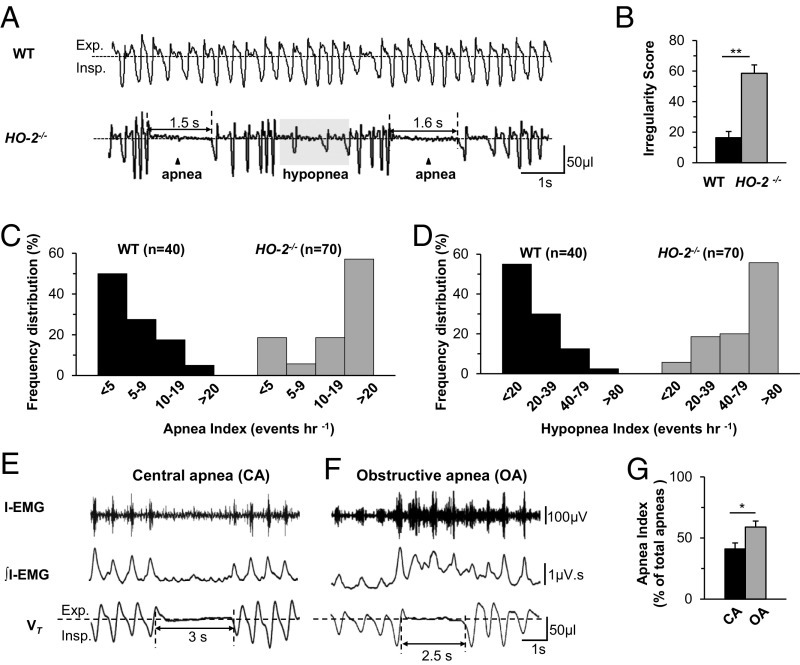
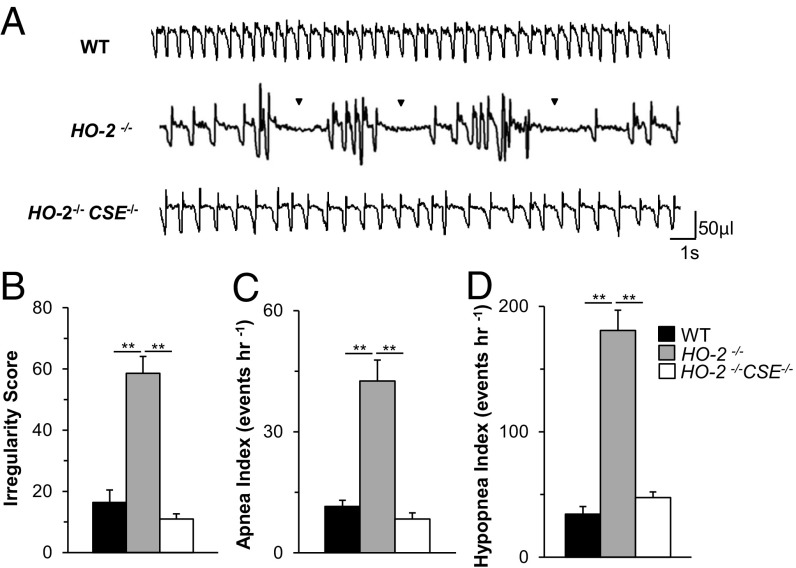
Basal breathing was monitored in unsedated 6- to 9-mo-old WT and HO-2−/− mice by plethysmography. Compared with WT mice, HO-2−/− mice exhibited irregular breathing with episodes of both apnea and hypopnea (Fig. 1A). The irregular breathing was quantified by analyzing the variations in the total duration of breaths (TTOT) as described (12, 13). HO-2−/− mice displayed higher irregularity scores than WT mice (Fig. 1B).
Fig. 1.
Irregular breathing with apnea and hypopnea in HO-2–null mice. Breathing was monitored continuously for 6 h by plethysmography in unsedated WT and HO-2−/− mice. (A) Examples of breathing in 6-mo-old, female WT and HO-2−/− mice. Exp., expiration; Insp., inspiration. Arrowhead indicates apnea, and shaded area represents hypopnea. The duration of apnea events (in seconds) is shown. (B) Irregularity score (mean ± SEM from 8 WT and HO-2−/− mice each). **P < 0.01. (C and D) Frequency distribution of apnea index (events per hour; C) and hypopnea index (events per hour, D) in age-matched WT and HO-2−/− mice, which were analyzed by the χ2 test. χ2 = 34.6 (C) and 48.3 (D), with 3 degrees of freedom and P < 0.001. (E–G) Obstructive and central apneas in HO-2−/− mice. Electrodes were chronically implanted in the inspiratory intercostal muscles of HO-2−/− mice to record electromyographic activity (I-EMG) and integrated I-EMG (∫I-EMG), along with breathing (VT, tidal volume), for 6 h in unsedated mice. (E and F) Example of central apnea (CA), with cessation of breathing and absence of I-EMG (E), and obstructive apnea (OA), characterized by cessation of breathing with increased I-EMG (F). The duration of apnea events (in seconds) is shown. (G) OA and CA indices in HO-2−/− mice (mean ± SEM, n = 12 each).
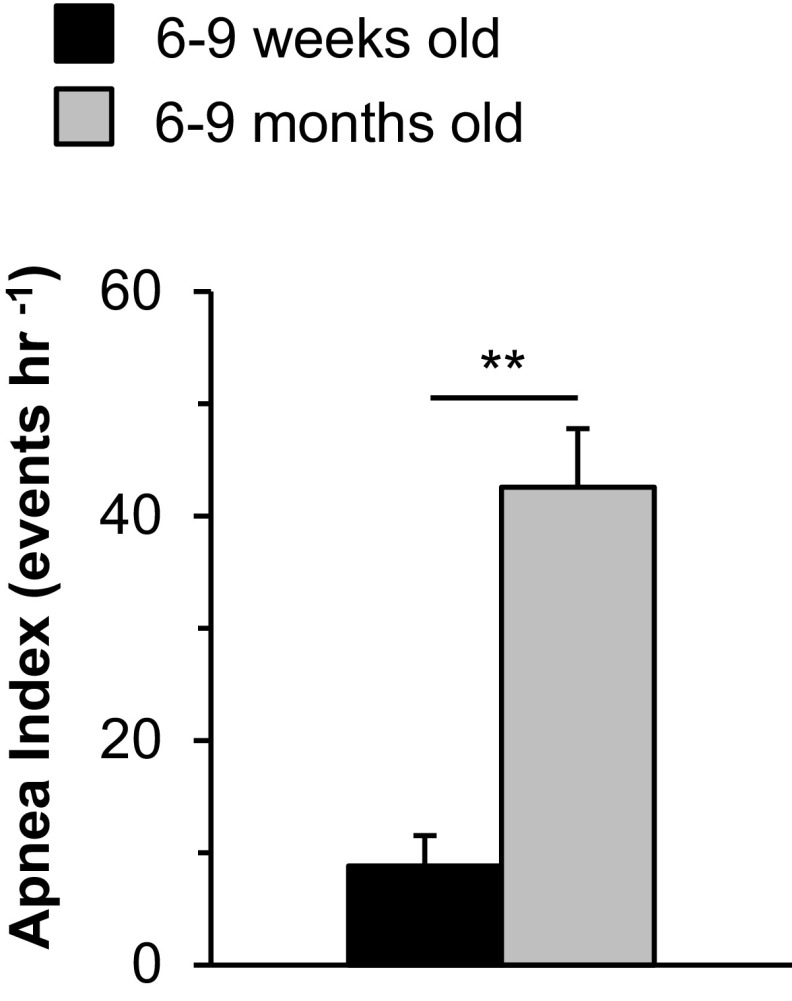
The number of apneas (defined as cessation of breathing for more than the duration of 2.5 breaths, excluding postsigh apneas, and sniffs) per hour was analyzed and presented as the apnea index. A majority of the HO-2−/− mice (40/70, 57%) had 20 or more apnea events per hour (Fig. 1C). In contrast, only 2/40 WT mice (5%) exhibited such frequent apneas (Fig. 1C). The apnea duration varied in individual mice, ranging from 1.3 to 4.0 s. Among HO-2−/− mice, the apnea index was higher at 6 to 9 mo compared with 6 to 9 wk (Fig. S1).
Fig. S1.
Comparison of the apnea index in 6- to 9-wk-old and 6- to 9-mo-old HO-2−/− mice. Data are shown as mean ± SEM; n = 17 mice in each group. **P < 0.01.
Hypopnea was defined as a breathing event with ≥30% reduction in tidal volume and is presented as the hypopnea index (hypopnea events per hour). Fifty-six percent of the HO-2−/− mice (39/70), but only 2.5% of the WT mice (1/40), had a hypopnea index of ≥80 (Fig. 1D). Analysis of arterial blood gases showed lower partial pressure of O2 (pO2), lower O2 saturation, and elevated pCO2 levels in HO-2−/− mice (Table S1). These results demonstrate that HO-2−/− mice exhibit irregular breathing with higher apnea and hypopnea indices than WT mice.
Table S1.
Arterial blood gas analysis in WT and HO-2−/− mice
| Blood gas variables | WT (n = 10) | HO-2−/− (n = 10) |
| PaO2, mmHg | 99 ± 4 | 83 ± 4** |
| PaCO2, mmHg | 36 ± 2.4 | 42 ± 2.3* |
| pH | 7.29 ± 0.02 | 7.23 ± 0.05 |
| SaO2, % | 95 ± 1.5 | 84 ± 3.9* |
P < 0.05; **P < 0.01.
Central and Obstructive Apneas in HO-2−/− Mice.
Apneas are of two types, central and obstructive. Central apneas are characterized by the absence of breathing and respiratory muscle activity, and obstructive apneas are associated with impeded airflow and increased respiratory muscle activity (11, 14). We sought to characterize the apneas exhibited by HO-2−/− mice. To this end, we recorded both breathing and inspiratory intercostal muscle electromyographic (I-EMG) activity with chronically implanted electrodes in unsedated HO-2−/− mice. Some apneas were associated with complete absence of I-EMG activity, indicative of central apnea (Fig. 1E). Other apneas were accompanied by an increase in I-EMG activity, indicative of obstructive apnea (Fig. 1F). On average, HO-2–null mice experienced obstructive apnea more frequently than central apnea (Fig. 1G).
Apneas and Hypopneas Occur During Sleep.
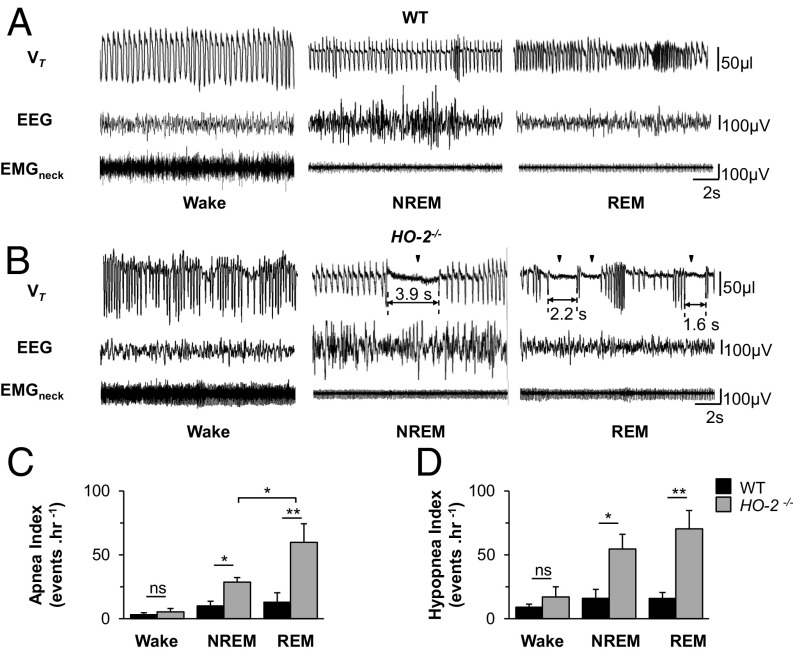
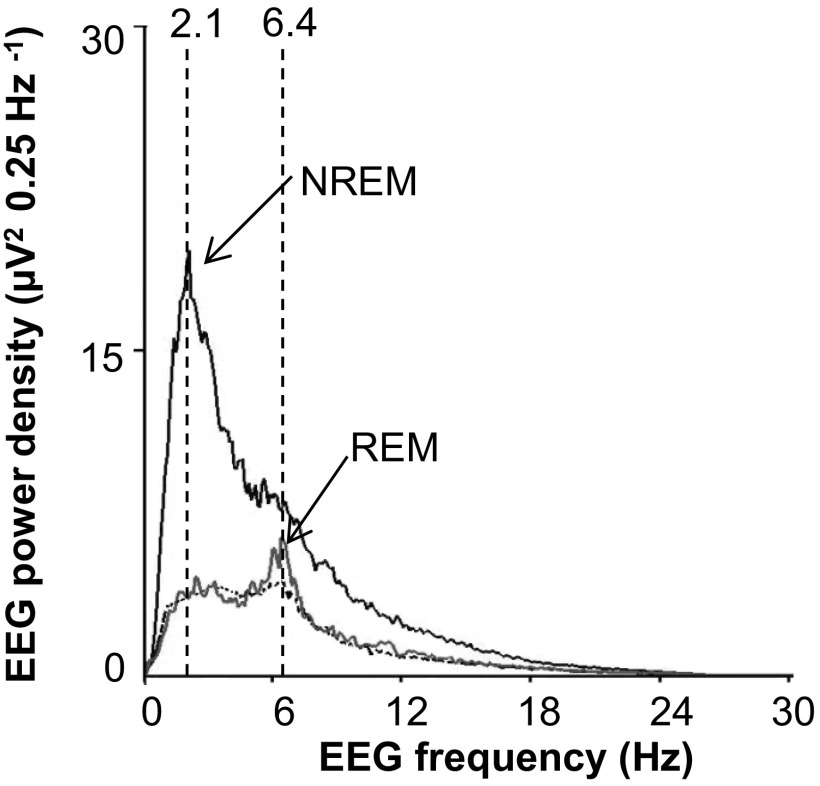
To determine whether sleep–wake state influences the apnea and hypopnea index, an electroencephalogram (EEG) and an electromyogram of neck extensor muscles (EMGneck) were recorded with chronically implanted electrodes, along with breathing, in unsedated WT and HO-2−/− mice. Wake state was characterized by EEG activity of mixed frequency, with low amplitude and high muscle tone. Rapid eye movement (REM) sleep was identified by both the frequency of theta waves (6 to 9 Hz) and muscle atonia. Non-REM (NREM) sleep was characterized by high-amplitude slow waves in the delta frequency range (1 to 4 Hz) and low muscle tone (15, 16) (Fig. 2 A and B and Fig. S2).
Fig. 2.
HO-2−/− mice exhibit sleep apnea. Electrodes were chronically implanted for monitoring electroencephalographic (EEG) and electromyographic activity of neck muscles (EMGneck) in WT and HO-2−/− mice. Tidal volume (VT), EEG, and EMGneck were monitored for 6 h. (A and B) Examples of VT, EEG, and EMGneck when awake or in NREM or REM sleep in a WT (A) and HO-2−/− (B) mouse are shown. (C and D) Apnea (C) and hypopnea (D) index (number of events per hour, mean ± SEM) in WT and HO-2−/− mice (n = 7 each). *P < 0.05; **P < 0.01; ns, not significant.
Fig. S2.
Power spectral analysis of the electroencephalogram (EEG) in an HO-2−/− mouse. Non-rapid eye movement (NREM) sleep showed a peak frequency of 2.1 Hz, and REM sleep displayed peak frequency at 6.4 Hz. The power spectral analysis was the basis for identifying NREM and REM sleep in the data presented in Fig. 3.
Consistent with earlier studies (17, 18), we found reduced tidal volume during NREM and REM sleep, and increased respiratory rate during REM sleep, in WT mice (Fig. 2A). The apnea and the hypopnea indices were lowest during the wake state, and were similar in WT and HO-2−/− mice (Fig. 2). NREM or REM sleep had little impact on the apnea or hypopnea index in WT mice (Fig. 2 A, C, and D). In contrast, HO-2–null mice showed increased apnea and hypopnea indices in NREM and REM sleep, with higher indices in REM compared with NREM sleep (Fig. 2 B–D).
The CB Chemoreflex Contributes to Apnea.
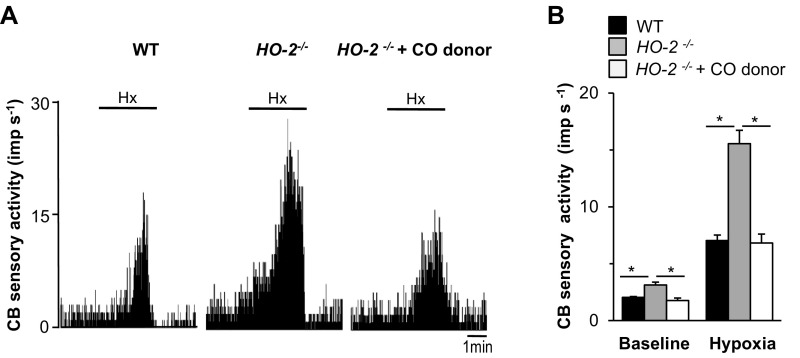
We next sought to determine the mechanisms causing apneas in HO-2−/− mice. An exaggerated CB chemoreflex has been implicated as a driver of apneas (19–21). Endogenous CO derived from HO-2 is a physiological inhibitor of CB sensory activity (4), and HO-2–null mice exhibit markedly diminished CO levels in the CB and heightened CB sensitivity to hypoxia (5). Administration of CORM-3, a CO donor, eliminated the enhanced hypoxic sensitivity of CBs from HO-2–null mice (Fig. S3). The heightened CB sensitivity to hypoxia was associated with augmented hypoxic ventilatory response (HVR), a hallmark of the CB chemoreflex, and i.p. administration of CORM-3 prevented the exaggerated HVR in HO-2–null mice (Fig. 3 A–C). We hypothesized that, if enhanced CB activity causes apneas, the CO donor should restore normal breathing in HO-2–null mice. This possibility was tested in HO-2–null mice by monitoring breathing before and after administration of CORM-3. Remarkably, CORM-3 completely prevented apneas and restored normal breathing in HO-2−/− mice (Fig. 3 C–E). The inhibitory effects of the CO donor were evident within 10 min after administration of CORM-3 and lasted for 2 h. These findings suggest that increased CB activity causes apneas in HO-2–null mice.
Fig. S3.
Effect of CORM-3, a CO donor, on sensory nerve response to hypoxia (Hx, PO2 = 40 ± 5 mmHg) in CBs from HO-2−/− mice monitored ex vivo. (A) Response to Hx of CBs from WT mice and HO-2−/− mice in the presence and absence of 15 µM CORM-3 in perfusate. Horizontal bar represents the duration of Hx challenge. (B) Sensory response to Hx presented as the change in impulses per second (Hx − baseline), mean ± SEM, from 6 WT and 7 HO-2−/− mice. *P < 0.05.
Fig. 3.
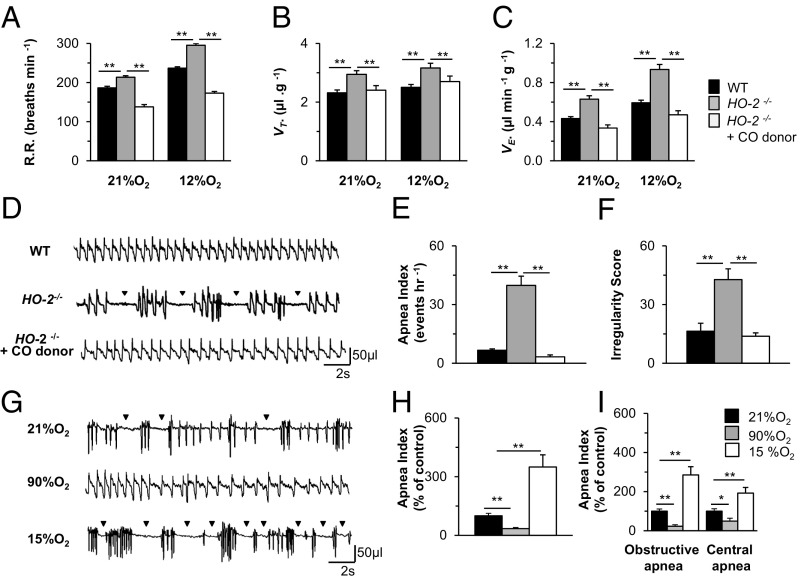
Carotid body (CB) chemoreflex contribution to apneas. Breathing response to 21% and 12% O2 in WT and HO-2−/− mice. Breathing responses in HO-2−/− mice were monitored before and after i.p. administration of 50 mg/kg CORM-3, a CO donor. (A) Respiratory rate (R.R., breaths per minute), (B) tidal volume (VT, microliters per gram of body weight), and (C) minute ventilation (R.R. x VT = VE, microliters per minute per gram of body weight). Data are mean ± SEM from WT and HO-2−/− mice with and without CORM-3 (n = 8 each). (D) Examples of breathing patterns in 6- to 9-mo-old WT and HO-2−/− mice, with and without CORM-3, are shown. (E and F) Apnea index (E) and irregularity score (F) are presented as mean ± SEM from WT and HO-2−/− mice with and without CORM-3 (n = 8 each). (G) Breathing patterns in an HO-2−/− mouse exposed to 21%, 90%, or 15% inspired O2. (H and I) Apnea index (H) and classification into obstructive and central apneas (I) during breathing 90% and 15% O2 expressed as percentage of control (21% O2). Number of mice: 21% O2, n = 21; 90% O2, n = 13; and 15% O2, n = 15 (E); and n = 6 HO-2−/− mice (F). *P < 0.05; **P < 0.01. Data from panels A–C were obtained from 6- to 9-wk-old WT and HO-2−/− mice and panels D–I from 6- to 9-mo-old WT and HO-2−/− mice.
To further establish a role for the CB chemoreflex in driving apneas, CBs were bilaterally denervated in HO-2–null mice. Remarkably, CB denervation proved lethal to all four HO-2−/− mice tested. We previously reported that HO-2–null CBs are capable of responding to changes in in O2 levels, due to a compensatory increase in the glomus cell expression of neuronal nitric oxide (NO) synthase, which catalyzes O2-dependent production of another gas messenger, NO (5). Therefore, as an alternative approach to modulating CB activity, HO-2–null mice were exposed to different concentrations of inspired O2. Exposure of the mice to hyperoxia (90% O2), which inhibits CB activity (22), markedly reduced the apnea index and restored stable breathing (Fig. 3 F and G). In contrast, exposure to hypoxia (15% O2), which stimulates CB activity, markedly increased the apnea index (Fig. 3 F and G). Hyperoxia decreased both obstructive and central apneas whereas hypoxia increased the number of obstructive and central apneas by three- and twofold, respectively (Fig. 3H).
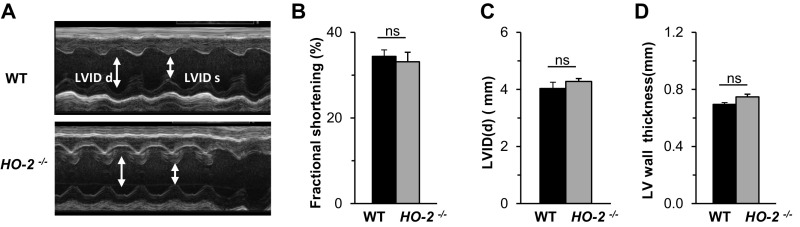
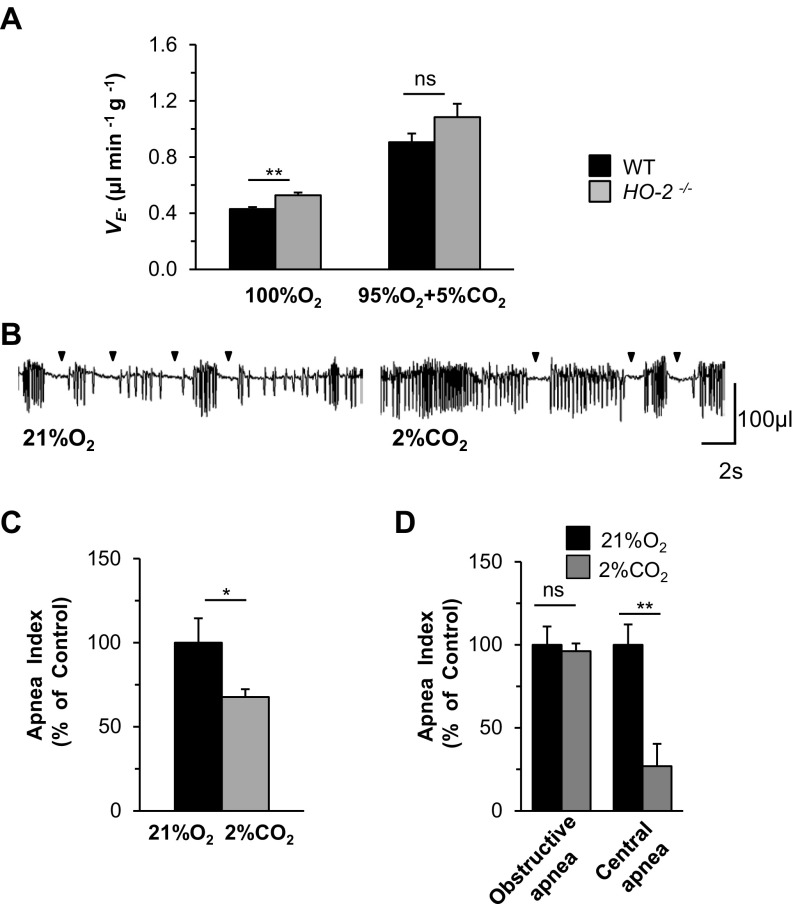
In addition to an augmented CB chemoreflex, cardiomyopathy (23) and reduced chemosensitivity to CO2 (24) can also result in apnea. Assessment of cardiac function by echocardiography revealed that fractional shortening, left ventricular diameter, and ventricular wall thickness were all comparable in WT and HO-2−/− mice (Fig. S4). The ventilatory response to CO2 was indistinguishable in HO-2−/− and WT mice (Fig. S5A). Exposure of HO-2−/− mice to 2% CO2 for 30 min, which stimulates central chemoreceptors, led to only a modest reduction (26%) in the apnea index, which was primarily due to reduced central apneas (Fig. S5 B–D). Together, these observations suggest that an enhanced CB chemoreflex, rather than cardiomyopathy or reduced chemosensitivity to CO2, is the major driver of apneas in HO-2–null mice.
Fig. S4.
Cardiac function in WT and HO-2−/− mice. (A) Representative transthoracic echocardiographic M-mode images of the left ventricle in 6-mo-old WT and HO-2−/− mice. Arrows indicate left ventricular internal dimensions in diastole (LVIDd) and systole (LVIDs). (B–D) Fractional shortening (FS) (B), LVIDd (C), and LV wall thickness (D) are shown as mean ± SEM from WT and HO-2−/− mice (n = 6 each).
Fig. S5.
(A) Breathing response to 100% O2 and 95% O2 + 5% CO2 in WT and HO-2−/− mice (n = 8 each). (B) Effect of breathing 2% CO2 in an HO-2−/− mouse. Arrowheads indicate apneas. (C and D) Apnea index (C, n = 14 mice) and obstructive and central apnea indices (D; n = 6 mice) while breathing 21% O2 or 21% O2 + 2% CO2 in HO-2–null mice. Data are presented as mean ± SEM; *P < 0.05; **P < 0.01; ns, not significant.
Absence of Apneas in HO-2/CSE Double-Null Mice.
The exaggerated CB activity in HO-2–null mice was shown to be due to increased CSE-derived H2S production in the CB, and HO-2/CSE double-null mice exhibit absence of CB hypersensitivity to hypoxia (5). We hypothesized that HO-2/CSE double-null mice with normal CB sensitivity to hypoxia should exhibit stable breathing compared with HO-2−/− mice. Indeed, HO-2−/−CSE−/− mice showed remarkably stable breathing without apneas or hypopneas (Fig. 4 A–D). These findings suggest that chronic activation of the CB chemoreflex by CSE-derived H2S contributes to apnea in HO-2−/− mice.
Fig. 4.
Absence of apneas in HO-2−/−CSE−/− mice. (A) Examples of breathing in unsedated and age- and gender-matched WT, HO-2−/−, and HO-2−/−CSE−/− mice. Arrowhead indicates apnea. Shown are (B) irregularity score, (C) apnea index, and (D) hypopnea index. Data are shown as mean ± SEM, n = 8 mice each. **P < 0.01.
Pharmacologic Blockade of CSE Prevents Apneas.
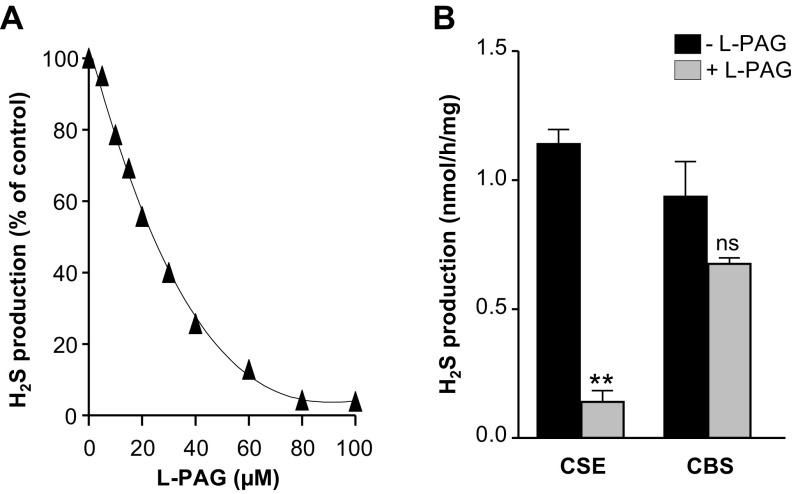
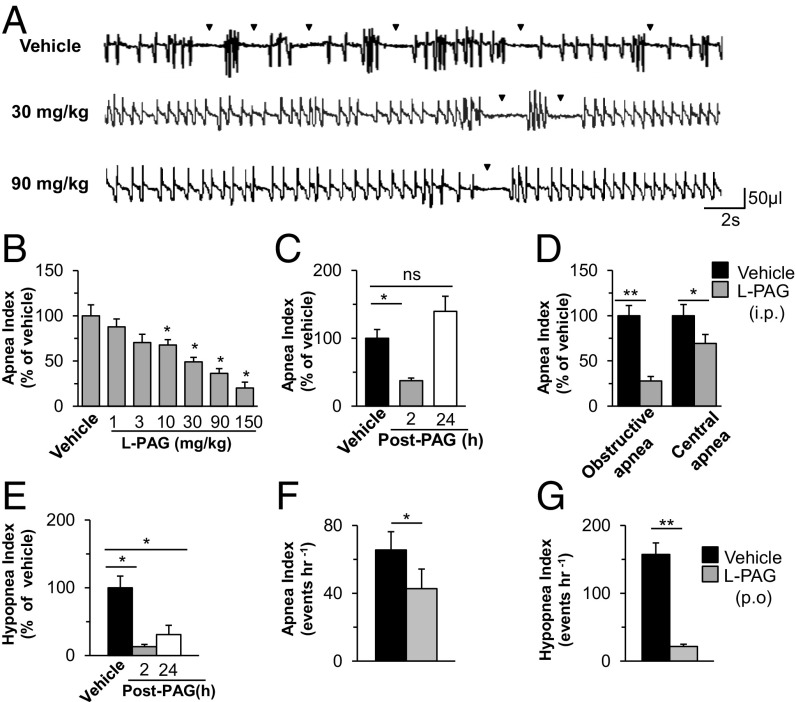
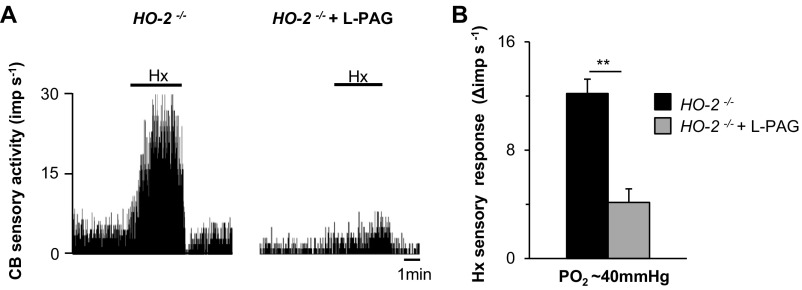
We next examined whether pharmacologic blockade of CSE stabilizes breathing in HO-2−/− mice. We first analyzed the efficacy and selectivity of l-propargylglycine (l-PAG), an inhibitor of CSE activity (25), using an in vitro assay. l-PAG inhibited CSE-derived H2S production in a dose-dependent manner whereas it had no effect on the activity of cystathionine β-synthase (CBS), another H2S-synthesizing enzyme (Fig. S6). We then examined the effects of systemic administration of l-PAG on breathing in HO-2−/− mice. The i.p. administration of l-PAG reduced the apnea index in a dose-dependent manner, with an median effective dose of 30 mg/kg (Fig. 5 A and B). The reduced apnea index by l-PAG was evident within 2 h after administration, and the apnea index returned to vehicle-treated levels after 24 h (Fig. 5C). l-PAG reduced the frequency of both obstructive and central apneas (Fig. 5D). l-PAG also lowered the hypopnea index, and, unlike the apnea index, it remained low 24 h after l-PAG administration (Fig. 5E). l-PAG (30 mg/kg i.p.) markedly reduced the CB sensory response to hypoxia in HO-2−/− mice (Fig. S7). Oral administration of l-PAG (30 mg/kg) was equally effective in reducing the apnea and hypopnea indices (Fig. 5 F and G). Oral or i.p. administration of l-PAG was well-tolerated, with no sign of overt toxicity.
Fig. S6.
(A) Effect of l-PAG, at concentrations from 0 to 100 µM, on H2S production in an in vitro assay using mouse liver homogenates. (B) Effect of l-PAG (30 µM) on H2S generation by CSE and CBS in mouse liver homogenates. Data are presented as mean ± SEM from four independent experiments. **P < 0.01; ns, not significant.
Fig. 5.
CSE inhibitor normalizes breathing in HO-2−/− mice. (A) Effects of i.p. administration of vehicle (saline) or l-PAG (30 or 90 mg/kg) on breathing in an HO-2−/− mouse. Arrowheads indicate apneas. (B) Dose–response of l-PAG on apnea index, presented as percentage of vehicle treatment, in HO-2–null mice (n = 15). (C) The onset and reversal of the effects of l-PAG (30 mg/kg i.p.) on apnea index (n = 16 mice each). (D) Effect of l-PAG (30 mg/kg i.p.) on obstructive and central apnea index in HO-2–null mice (n = 6). (E) The onset and reversal of the effects of l-PAG (30 mg/kg i.p.) on hypopnea index (n = 16). (F and G) Effect of l-PAG (30 mg/kg p.o.) on apnea (F) and hypopnea (G) index in HO-2−/− mice (n = 12). Data in B–G are presented as mean ± SEM; *P < 0.05; **P < 0.01; ns, not significant.
Fig. S7.
Effect of l-PAG on CB sensory nerve response to hypoxia (Hx). HO-2−/− mice received either vehicle or l-PAG (30 mg/kg) via i.p. route. CBs were harvested, and sensory nerve response to Hx (PO2 = 40 ± 5 mmHg) was monitored ex vivo. (A) CB response to hypoxia in an HO-2−/− mouse treated with vehicle (HO-2−/−) or l-PAG (HO-2−/− + l-PAG). Horizontal bar represents the duration of Hx challenge. (B) Hx sensory response presented as the change in impulses per second (Hx − baseline), mean ± SEM from 15 vehicle-treated and 13 l-PAG–treated HO-2 −/− mice. **P < 0.01.
CSE Inhibitor Prevents Apneas in SH Rats.
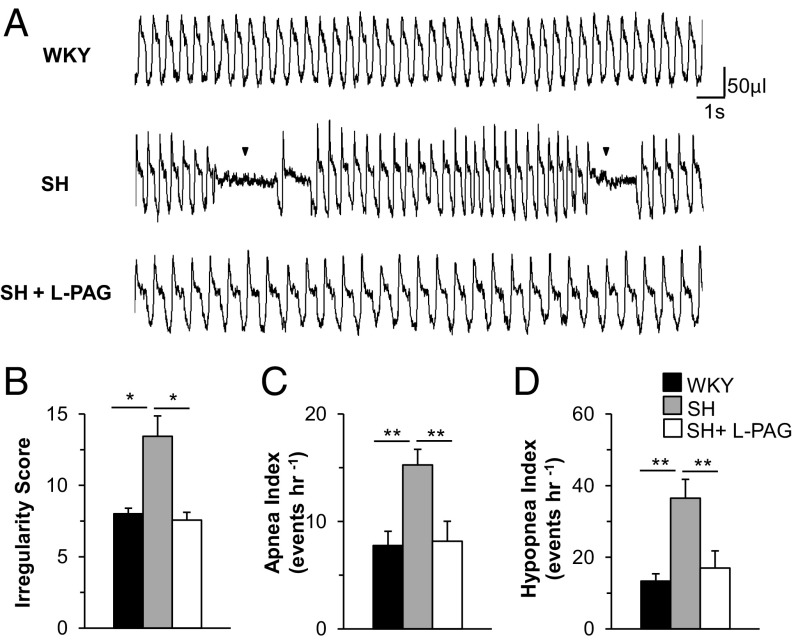
Even before the development of hypertension, SH rats exhibit a heightened CB response to hypoxia, which is associated with increased CSE-derived H2S levels and decreased CO levels, as well as reduced HO-2 activity (6), similar to what was observed in the HO-2−/− mice. Accordingly, we hypothesized that SH rats also display high apnea and hypopnea indices. To test this possibility, we monitored breathing in 4- to 5-wk-old unsedated SH and WKY rats (parental strain for SH rats). Although WKY rats displayed stable breathing, SH rats showed irregular breathing, with high apnea and hypopnea indices (Fig. 6 A–D). Because l-PAG normalized the CB hypoxic response in SH rats (6), we examined whether l-PAG affects the frequency of apneic episodes. Within 2 h of i.p. administration of l-PAG (30 mg/kg), the breathing of SH rats became stable, and the apnea and hypopnea indices were markedly decreased (Fig. 6 A–D).
Fig. 6.
l-PAG prevents apneas in prehypertensive spontaneously hypertensive (SH) rats. (A) Examples of breathing patterns in 4-wk-old male WKY, SH, and l-PAG–treated (30 mg/kg i.p.) SH rats. Arrowheads in the Middle panel indicate apneas. Shown are (B) irregularity score, (C) apnea index, and (D) hypopnea index. Data are mean ± SEM for WKY, SH, and l-PAG–treated SH rats (n = 8 each). *P < 0.05; **P < 0.01.
Discussion
The present study establishes that dysregulated signaling of the gasotransmitter CO can lead to sleep apnea. HO-2−/− mice, which have low CO levels, exhibited several key features of sleep apnea that have been reported in humans. Like patients with sleep apnea, HO-2–null mice exhibit high apnea and hypopnea indices during NREM and REM sleep but not when awake. Previously, rodents were thought to exhibit only central apneas (26) whereas sleep apnea patients most often exhibit both obstructive and central apneas (10, 11, 27). By simultaneously monitoring respiratory muscle activity and breathing, we demonstrated that HO-2−/− mice exhibit both central and obstructive apneas. Aged HO-2–null mice exhibited a greater apnea index than younger mice, mirroring the higher incidence of sleep apnea in middle-aged compared with younger adults (28). Besides apneas, HO-2–null mice also showed a higher hypopnea index, another common feature in patients with sleep apnea. The severity of apneas and hypopneas was reflected in deranged arterial blood gases manifested as mild hypoxemia and hypercapnia (Table S1). Because CB hypersensitivity to hypoxia is seen in HO-2–null mice even at a young age (5), it is likely that chronic activation of the CB chemoreflex leads to more frequent apneas with progressing age. However, further studies are needed to exclude the possibility that HO-2–null mice exhibit evidence of lung injury that might contribute to apneas by causing hypoxemia. Remarkably, systemic administration of CORM-3, a CO donor, completely prevented the apnea phenotype in HO-2–null mice, supporting the notion that the irregular breathing stems from reduced HO-2–derived CO levels. SH rats, which have decreased CO levels due to decreased HO-2 activity (6), also displayed increased apnea and hypopnea indices. Together, these findings demonstrate that low CO levels lead to an increased incidence of apnea in both rats and mice.
How might reduced CO signaling lead to more frequent apneas? Clinical studies suggest that an augmented CB chemoreflex can drive apneas in patients with sleep apnea (19–21). HO-2−/− mice displayed heightened CB sensitivity to hypoxia and augmented HVR, a hallmark of the CB chemoreflex, and these effects were prevented by administration of CORM-3. Remarkably, CORM-3 restored normal breathing in HO-2−/− mice, implicating the enhanced CB chemoreflex in causing apneas. Moreover, hyperoxia reduced, whereas hypoxia increased, the apnea index. SH rats also exhibit an augmented CB chemoreflex (29). Recent studies showed that increased H2S production from CSE is a major signaling mechanism mediating the exaggerated CB activity in HO-2−/− mice (5) and SH rats (6). Remarkably, genetic ablation of CSE normalized breathing in HO-2−/− mice, and pharmacologic blockade of CSE by l-PAG was equally effective in preventing apneas in both HO-2–null mice and SH rats. The same doses of l-PAG that normalized breathing also reduced CB activity, suggesting that the CB is a major site of l-PAG action. However, we cannot rule out the possibility that l-PAG also affects CSE activity in brainstem neurons (30).
In addition to the generation of H2S from cysteine, CSE also catalyzes the conversion of cystathionine to cysteine (31). However, CB activity is unaffected by cysteine at concentrations as high as 100 µM, whereas an H2S donor leads to robust CB activation at concentrations as low as 30 µM (3), indicating that CSE-derived H2S, rather than cysteine, is the reaction product that mediates CB activation. Taken together, these findings suggest that activation of the CB by CSE-derived H2S, and the resulting chemoreflex, drives apneas in rodents with reduced CO levels due to impaired HO-2 activity.
The CB has been proposed to drive central apneas (20, 21), but our results suggest that the CB chemoreflex may contribute to both central and obstructive apneas. How might an augmented CB reflex contribute to obstructive apnea, which occurs due to physical obstruction of the upper airway, often due to decreased tone of the tongue muscle? The enhanced CB chemoreflex may initially result in central apneas that then secondarily lead to obstruction of the upper airway due to reduced muscle tone of the tongue. Specifically, a recent study found that reactive oxygen species (ROS) disrupt transmission between neurons responsible for respiratory rhythm and the hypoglossal motor neurons controlling tongue muscle tone in mice that were exposed to chronic intermittent hypoxia to simulate central apneas (32). Because increased CB activity leads to increased ROS levels in the central nervous system (33), CB-stimulated ROS signaling may disrupt neural control of the tongue, leading to obstructive apnea. Aside from the CB chemoreflex, whether abnormal upper airway anatomy also contributes to the obstructive apnea in HO-2−/− mice remains to be investigated.
The current standards of care for patients with obstructive and central apnea are continuous positive airway pressure (CPAP) (34–36) and adaptive servo-ventilation (37, 38), respectively. However, a substantial number of obstructive apnea patients do not respond to CPAP therapy, and many find CPAP difficult to tolerate (27, 39, 40). Adaptive ventilation in heart failure patients was associated with a mortality rate of 30%, with no demonstrable benefits for central apnea (41). These findings suggest that current treatment options are suboptimal for normalizing breathing in sleep apnea patients. Although a CO donor prevented apneas in HO-2–null mice, its therapeutic potential is limited by a short half-life and likely increase in met-hemoglobin levels in arterial blood. In contrast to HO-2–null mice, HO-2/CSE double-null mice displayed a remarkable absence of apneas. Unlike WT mice, exposure of CSE-null mice to chronic intermittent hypoxia to simulate apneas did not cause enhanced CB activity and hypertension (42). It should be emphasized that CSE-null mice show normal CB responses to hypercapnia (3). Thus, genetic alteration resulting in long-term inhibition of CSE activity seems to be a well-tolerated and effective means of preventing apneas in mice.
Our results suggest that pharmacologic targeting of the CB with a CSE inhibitor, such as l-PAG, might prevent apneas. Notably, l-PAG reduced the number of obstructive and central apneas, as well as hypopneas, in a dose-dependent and reversible manner in HO-2−/− mice. Moreover, l-PAG exhibited efficacy after either oral and i.p. administration. The response to l-PAG was rapid and reversible and did not result in overt toxicity within the dose range tested. Although these observations provide proof-of-concept for the therapeutic potential of CSE inhibitors, the doses of l-PAG that were required to normalize breathing were relatively high. Consequently, future studies are needed to develop more potent CSE inhibitors. Nonetheless, pharmacologic modulation of the CB chemoreflex by an inhibitor of H2S synthesis, as shown in the present study, has the potential to significantly improve the clinical management of sleep apnea.
Sleep apnea is a multifactorial respiratory disease. Aside from an inherently hyperactive CB chemoreflex due to HO-2 loss of function, sleep apnea can occur as a result of abnormal upper airway anatomy or dysfunctional central CO2 chemoreceptors (24, 43) and secondary to a number of major medical conditions, including heart failure (44), renal failure (45), stroke (46), and diabetes/metabolic syndrome (47–49). It remains to be determined whether blockade of the CB chemoreflex with CSE inhibitors also normalizes breathing in patients with sleep apnea due to these other etiologies.
Materials and Methods
Preparation of Animals.
Experiments were approved by the Institutional Animal Care and Use Committee of the University of Chicago and were performed on age- and body-weight–matched male and female WT (C57/BL6; Charles River) and HO-2−/− mice (colony maintained by S. H. Snyder), as well as WKY and SH rats (Charles River) unless otherwise noted. HO-2 and CSE double-knockout mice were created by initially crossing HO-2−/− females with CSE−/− males (from R. Wang, Department of Biology, Laurentian University, Sudbury, ON, Canada).
Measurement of Breathing.
Breathing was continuously monitored under room air from 1000 to 1600 h by whole-body plethysmography, at ambient temperature maintained at 25 ± 1 °C as described (3). Apneas and hypopneas were scored by two individuals who were blinded to genotype. The breathing irregularity score was determined by the following formula: absolute (TTOTn − TTOTn-1)/(TTOTn+1) × 100%, where TTOT represents total duration of a breath as described (12, 13).
Echocardiography.
Transthoracic echocardiography was performed in mice anesthetized with 1.5% (vol/vol) isoflurane in oxygen using an ultrasound imaging system (VisualSonics 770). Fractional shortening (FS) was calculated from the equation: FS = (EDD − ESD)/EDD × 100%, where EDD is the end-disastolic dimension and ESD is the end-systolic dimension of the left ventricle.
EEG and Electromyogram.
Electrodes for monitoring EEG and nuchal electromyogram (EMG) were implanted in mice that were anesthetized by i.p. injection of ketamine (90 mg/kg) and xylazine (9 mg/kg). EEG/EMG signals along with breathing were recorded for 6 h. The data were collected using PowerLab 8/35 (AD Instruments) and scored off-line with commercial software (RemLogic) in a 10-s epoch.
Measurement of Respiratory Muscle Activity.
Stainless steel electrodes were placed in the inspiratory intercostal muscles of mice anesthetized with ketamine/xylazine. Five days after the surgery, breathing and respiratory muscle EMG activity were recorded for 6 h using PowerLab 8/35 (AD Instruments) and scored off-line with commercial software (RemLogic).
Ex Vivo CB Recording.
Sensory nerve activity was recorded from CBs that were harvested from anesthetized mice as previously described (3, 5). Sensory nerve responses to hypoxia were monitored for 3 min. The pO2 in the medium was determined by a blood gas analyzer (ABL 5). The hypoxic response was measured as the difference between the sensory nerve activity at baseline and during hypoxia (Δ impulses per second).
H2S Assay.
Tissue homogenates were prepared from freshly perfused mouse liver to measure H2S generation from CSE or CBS. H2S was measured using the methylene blue assay as described previously (3, 5). l-cysteine was used as the substrate for the CSE-derived H2S assay whereas l-cysteine and homocysteine were used as substrates to assess CBS-derived H2S, as described previously (50). H2S concentrations were calculated using a molar extinction coefficient of 71,089 M−1⋅cm−1 at 670 nm and expressed as nmol per h per mg of protein.
Data Analysis.
All data are reported as mean ± SEM unless otherwise stated in the figure legends. Statistical analysis was performed with either one-way or two-way analysis of variance (ANOVA) with repeated measures followed by post hoc Tukey’s test. The χ2 test was used for analysis of the distribution of apneas and hypopneas. The Mann–Whitney test was used to analyze normalized data. All P values of <0.05 were considered significant.
Acknowledgments
This research was supported by National Institutes of Health grants UH3-HL90554 (to N.R.P.), PO1-HL90554 (to N.R.P.), and MH018501 (to S.H.S.) and a Medical Scientist Training Program T32 grant (to C.V.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620717114/-/DCSupplemental.
References
- 1.Mustafa AK, Gadalla MM, Snyder SH. Signaling by gasotransmitters. Sci Signal. 2009;2(68):re2. doi: 10.1126/scisignal.268re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar P, Prabhakar NR. Peripheral chemoreceptors: Function and plasticity of the carotid body. Compr Physiol. 2012;2(1):141–219. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng Y-J, et al. H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci USA. 2010;107(23):10719–10724. doi: 10.1073/pnas.1005866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prabhakar NR, Dinerman JL, Agani FH, Snyder SH. Carbon monoxide: A role in carotid body chemoreception. Proc Natl Acad Sci USA. 1995;92(6):1994–1997. doi: 10.1073/pnas.92.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan G, et al. Protein kinase G-regulated production of H2S governs oxygen sensing. Sci Signal. 2015;8(373):ra37. doi: 10.1126/scisignal.2005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng YJ, et al. Inherent variations in CO-H2S-mediated carotid body O2 sensing mediate hypertension and pulmonary edema. Proc Natl Acad Sci USA. 2014;111(3):1174–1179. doi: 10.1073/pnas.1322172111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peppard PE, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St-Onge MP, et al. American Heart Association Obesity, Behavior Change, Diabetes, and Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council Sleep duration and quality: Impact on lifestyle behaviors and cardiometabolic health: A scientific statement from the American Heart Association. Circulation. 2016;134(18):e367–e386. doi: 10.1161/CIR.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orr JE, Malhotra A, Sands SA. Pathogenesis of central and complex sleep apnoea. Respirology. 2017;22(1):43–52. doi: 10.1111/resp.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea: Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckert DJ, Malhotra A, Jordan AS. Mechanisms of apnea. Prog Cardiovasc Dis. 2009;51(4):313–323. doi: 10.1016/j.pcad.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg SK, et al. Systemic delivery of MeCP2 rescues behavioral and cellular deficits in female mouse models of Rett syndrome. J Neurosci. 2013;33(34):13612–13620. doi: 10.1523/JNEUROSCI.1854-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derecki NC, et al. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484(7392):105–109. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol. 2013;3(1):141–163. doi: 10.1002/cphy.c110057. [DOI] [PubMed] [Google Scholar]
- 15.Mang GM, Franken P. Sleep and EEG phenotyping in mice. Curr Protoc Mouse Biol. 2012;2(1):55–74. doi: 10.1002/9780470942390.mo110126. [DOI] [PubMed] [Google Scholar]
- 16.Mochizuki T, Klerman EB, Sakurai T, Scammell TE. Elevated body temperature during sleep in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2006;291(3):R533–R540. doi: 10.1152/ajpregu.00887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman L, et al. Ventilatory behavior during sleep among A/J and C57BL/6J mouse strains. J Appl Physiol (1985) 2004;97(5):1787–1795. doi: 10.1152/japplphysiol.01394.2003. [DOI] [PubMed] [Google Scholar]
- 18.Sato S, et al. Rapid increase to double breathing rate appears during REM sleep in synchrony with REM: A higher CNS control of breathing? Adv Exp Med Biol. 2010;669:249–252. doi: 10.1007/978-1-4419-5692-7_50. [DOI] [PubMed] [Google Scholar]
- 19.Mansukhani MP, Wang S, Somers VK. Chemoreflex physiology and implications for sleep apnoea: Insights from studies in humans. Exp Physiol. 2015;100(2):130–135. doi: 10.1113/expphysiol.2014.082826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie A, Rutherford R, Rankin F, Wong B, Bradley TD. Hypocapnia and increased ventilatory responsiveness in patients with idiopathic central sleep apnea. Am J Respir Crit Care Med. 1995;152(6 Pt 1):1950–1955. doi: 10.1164/ajrccm.152.6.8520761. [DOI] [PubMed] [Google Scholar]
- 21.Xie A, Wong B, Phillipson EA, Slutsky AS, Bradley TD. Interaction of hyperventilation and arousal in the pathogenesis of idiopathic central sleep apnea. Am J Respir Crit Care Med. 1994;150(2):489–495. doi: 10.1164/ajrccm.150.2.8049835. [DOI] [PubMed] [Google Scholar]
- 22.Prabhakar NR. Sensing hypoxia: Physiology, genetics and epigenetics. J Physiol. 2013;591(9):2245–2257. doi: 10.1113/jphysiol.2012.247759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nerbass FB, et al. Obstructive sleep apnea and hypertrophic cardiomyopathy: A common and potential harmful combination. Sleep Med Rev. 2013;17(3):201–206. doi: 10.1016/j.smrv.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Kumar NN, et al. Regulation of breathing by CO2 requires the proton-activated receptor GPR4 in retrotrapezoid nucleus neurons. Science. 2015;348(6240):1255–1260. doi: 10.1126/science.aaa0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asimakopoulou A, et al. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE) Br J Pharmacol. 2013;169(4):922–932. doi: 10.1111/bph.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato T, Saito H, Seto K, Takatsuji H. Sleep apneas and cardiac arrhythmias in freely moving rats. Am J Physiol. 1990;259(2 Pt 2):R282–R287. doi: 10.1152/ajpregu.1990.259.2.R282. [DOI] [PubMed] [Google Scholar]
- 27.Gilmartin GS, Daly RW, Thomas RJ. Recognition and management of complex sleep-disordered breathing. Curr Opin Pulm Med. 2005;11(6):485–493. doi: 10.1097/01.mcp.0000183061.98665.b0. [DOI] [PubMed] [Google Scholar]
- 28.Okuro M, Morimoto S. Sleep apnea in the elderly. Curr Opin Psychiatry. 2014;27(6):472–477. doi: 10.1097/YCO.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 29.Tan ZY, et al. Chemoreceptor hypersensitivity, sympathetic excitation, and overexpression of ASIC and TASK channels before the onset of hypertension in SHR. Circ Res. 2010;106(3):536–545. doi: 10.1161/CIRCRESAHA.109.206946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul BD, et al. Cystathionine γ-lyase deficiency mediates neurodegeneration in Huntington’s disease. Nature. 2014;509(7498):96–100. doi: 10.1038/nature13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gadalla MM, Snyder SH. Hydrogen sulfide as a gasotransmitter. J Neurochem. 2010;113(1):14–26. doi: 10.1111/j.1471-4159.2010.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia AJ, 3rd, et al. Chronic intermittent hypoxia alters local respiratory circuit function at the level of the preBötzinger complex. Front Neurosci. 2016;10:4. doi: 10.3389/fnins.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng YJ, et al. Regulation of hypoxia-inducible factor-α isoforms and redox state by carotid body neural activity in rats. J Physiol. 2014;592(17):3841–3858. doi: 10.1113/jphysiol.2014.273789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ip S, et al. Auto-titrating versus fixed continuous positive airway pressure for the treatment of obstructive sleep apnea: A systematic review with meta-analyses. Syst Rev. 2012;1:20. doi: 10.1186/2046-4053-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veasey SC, et al. Medical therapy for obstructive sleep apnea: A review by the Medical Therapy for Obstructive Sleep Apnea Task Force of the Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep. 2006;29(8):1036–1044. doi: 10.1093/sleep/29.8.1036. [DOI] [PubMed] [Google Scholar]
- 36.Combs D, Shetty S, Parthasarathy S. Advances in positive airway pressure treatment modalities for hypoventilation syndromes. Sleep Med Clin. 2014;9(3):315–325. doi: 10.1016/j.jsmc.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Javaheri S, Germany R, Greer JJ. Novel therapies for the treatment of central sleep apnea. Sleep Med Clin. 2016;11(2):227–239. doi: 10.1016/j.jsmc.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Yamauchi M, Combs D, Parthasarathy S. Adaptive servo-ventilation for central sleep apnea in heart failure. N Engl J Med. 2016;374(7):689. doi: 10.1056/NEJMc1515007. [DOI] [PubMed] [Google Scholar]
- 39.Antic NA, et al. The sleep apnea cardiovascular endpoints (SAVE) trial: Rationale, ethics, design, and progress. Sleep. 2015;38(8):1247–1257. doi: 10.5665/sleep.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quan SF, Awad KM, Budhiraja R, Parthasarathy S. The quest to improve CPAP adherence: PAP potpourri is not the answer. J Clin Sleep Med. 2012;8(1):49–50. doi: 10.5664/jcsm.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cowie MR, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095–1105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan G, et al. H2S production by reactive oxygen species in the carotid body triggers hypertension in a rodent model of sleep apnea. Sci Signal. 2016;9(441):ra80. doi: 10.1126/scisignal.aaf3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramirez JM, et al. Central and peripheral factors contributing to obstructive sleep apneas. Respir Physiol Neurobiol. 2013;189(2):344–353. doi: 10.1016/j.resp.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sands SA, Owens RL. Congestive heart failure and central sleep apnea. Sleep Med Clin. 2016;11(1):127–142. doi: 10.1016/j.jsmc.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Dharia SM, Unruh ML, Brown LK. Central sleep apnea in kidney disease. Semin Nephrol. 2015;35(4):335–346. doi: 10.1016/j.semnephrol.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Nopmaneejumruslers C, Kaneko Y, Hajek V, Zivanovic V, Bradley TD. Cheyne-Stokes respiration in stroke: Relationship to hypocapnia and occult cardiac dysfunction. Am J Respir Crit Care Med. 2005;171(9):1048–1052. doi: 10.1164/rccm.200411-1591OC. [DOI] [PubMed] [Google Scholar]
- 47.Trombetta IC, et al. Obstructive sleep apnea is associated with increased chemoreflex sensitivity in patients with metabolic syndrome. Sleep. 2013;36(1):41–49. doi: 10.5665/sleep.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iturriaga R, Del Rio R, Idiaquez J, Somers VK. Carotid body chemoreceptors, sympathetic neural activation, and cardiometabolic disease. Biol Res. 2016;49:13. doi: 10.1186/s40659-016-0073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sacramento JF, et al. Functional abolition of carotid body activity restores insulin action and glucose homeostasis in rats: Key roles for visceral adipose tissue and the liver. Diabetologia. 2017;60(1):158–168. doi: 10.1007/s00125-016-4133-y. [DOI] [PubMed] [Google Scholar]
- 50.Chiku T, et al. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem. 2009;284(17):11601–11612. doi: 10.1074/jbc.M808026200. [DOI] [PMC free article] [PubMed] [Google Scholar]