Significance
Secreted pore-forming toxins are a common feature of bacterial virulence. Mycobacterium tuberculosis, the agent of human tuberculosis, has been reported to possess a pore-forming toxin called 6 kDa early secretory antigenic target (ESAT-6) that is secreted through a specialized secretion system called ESX-1 (ESAT-6 secretion system 1). We report here experiments showing that ESAT-6 does not lyse cells; the lytic activity previously attributed to this secreted protein is due to contaminating detergent in the recombinant protein preparations. Whereas the ESX-1 secretion system does lyse host cell membranes, we find this lysis is dependent on bacterial cell contact with the host membrane and results in tears in the membrane without any pore formation. Understanding the mechanism of this lysis may provide clues to how mycobacteria cause disease.
Keywords: Mycobacterium tuberculosis, Mycobacterium marinum, ESAT-6, ESX-1 secretion system, cell membrane lysis
Abstract
Mycobacterium tuberculosis and Mycobacterium marinum are thought to exert virulence, in part, through their ability to lyse host cell membranes. The type VII secretion system ESX-1 [6-kDa early secretory antigenic target (ESAT-6) secretion system 1] is required for both virulence and host cell membrane lysis. Both activities are attributed to the pore-forming activity of the ESX-1–secreted substrate ESAT-6 because multiple studies have reported that recombinant ESAT-6 lyses eukaryotic membranes. We too find ESX-1 of M. tuberculosis and M. marinum lyses host cell membranes. However, we find that recombinant ESAT-6 does not lyse cell membranes. The lytic activity previously attributed to ESAT-6 is due to residual detergent in the preparations. We report here that ESX-1–dependent cell membrane lysis is contact dependent and accompanied by gross membrane disruptions rather than discrete pores. ESX-1–mediated lysis is also morphologically distinct from the contact-dependent lysis of other bacterial secretion systems. Our findings suggest redirection of research to understand the mechanism of ESX-1–mediated lysis.
Tuberculosis is an ancient human disease caused by Mycobacterium tuberculosis (Mtb) that continues to be a leading infectious human killer despite the availability of effective chemotherapeutic regimens (1). There has been a decades-long search to better understand and identify the precise mechanisms by which Mtb causes disease. One virulence determinant that has been identified is a highly immunogenic secreted protein called ESAT-6 (6-kDa early secretory antigenic target) (2, 3). ESAT-6 was first identified as a secreted antigen that stimulated T cells (4). Humans and multiple laboratory animal species infected with Mtb uniformly show ESAT-6 reactivity (4–6). ESAT-6’s role in virulence received further support when its deletion in Mycobacterium bovis reduced virulence in guinea pigs (7).
At the same time as ESAT-6 was being implicated in mycobacterial virulence, parallel work discovered and ascribed a role in virulence to a specialized secretion system now called ESX-1. Comparisons of the genomes of Mtb and of the attenuated vaccine strain M. bovis bacillus Calmette–Guérin (BCG) revealed a chromosomal region called region of difference 1 (RD1) that was missing in BCG (8, 9). RD1 was confirmed to play a role in virulence by complementary genetic experiments that showed increased virulence when the region was expressed in BCG and attenuated virulence when it was removed from Mtb (10–12). In silico analysis revealed RD1 to encode ESAT-6 and also part of a previously unidentified secretion system (8, 13). The presence of this secretion system solved the conundrum of how ESAT-6 is secreted despite lacking a canonical signal sequence (13). This newly discovered secretion system was found to contain several other potential secretion substrates, yet it was named ESAT-6 secretion system 1 (ESX-1) (13, 14), highlighting the primary role ascribed to ESAT-6 in mycobacterial pathogenesis (2).
ESAT-6 was first hypothesized to exert virulence by lysing host cell membranes when a transposon insertion mutant in ESAT-6’s cotranscribed ESX-1 substrate 10 kDa culture filtrate antigen (CFP-10) was found to have lost cytolytic activity for a cultured pneumocyte cell line (15). When purified ESAT-6 and CFP-10 were examined for disruption of artificial lipid bilayers, it was found that that ESAT-6 but not CFP-10 caused membrane lysis (15). These results were confirmed in a series of reports, all implicating eukaryotic cell membrane lytic activity as ESAT-6’s virulence mechanism (16–18). When transposon mutants in the closely related species, Mycobacterium marinum (Mm), were screened to identify those defective for hemolysis, all attenuated mutants were within the ESX-1 locus and were defective for ESAT-6 secretion (16). Moreover, the mutants were avirulent and failed to lyse infected macrophages, implicating secreted ESAT-6 in mediating virulence through host cell cytolysis (16). Meanwhile, purified recombinant ESAT-6 (rESAT-6) was reported to be capable of disrupting liposome membranes (19), lysing red blood cells (RBCs) (18), and killing cultured macrophages (17, 18). Thus, the experimental evidence from both genetic and biochemical approaches firmly supported the idea that ESAT-6 was responsible for ESX-1–mediated bacterial pathogenicity and was due to its cytolytic activity.
The consensus of contemporary research ascribes a critical role for ESX-1–mediated membrane lysis during TB pathogenesis via permeabilization of the mycobacterial phagosome following bacterial entry into host macrophages (20–22). Phagosomal permeabilization has been found to activate host cytosolic sensing pathways that are exploited by the bacteria for growth and virulence (23, 24). By extension, ESAT-6 is held to be directly responsible for phagosomal permeabilization and its downstream pathogenic effects (25, 26).
In this report, we confirm that a functional ESX-1 secretion system is required for host cell membrane lytic activity and virulence. However, we find that ESAT-6 is not sufficient for ESX-1’s lysis of host cell membranes. Rather, we find that the various lytic activities attributed to ESAT-6 at neutral pH are the result of a widely used protocol to isolate ESAT-6, which leaves residual detergent in the preparations. We find that detergent-free ESAT-6 preparations can disrupt liposomes under acidic conditions, as previously observed (25, 27, 28). However, this intrinsic low pH-dependent activity of ESAT-6 is not responsible for mycobacterial phagosomal permeabilization in host macrophages.
Results
Mtb ESX-1 Secretion Is Linked to Virulence and Hemolysis.
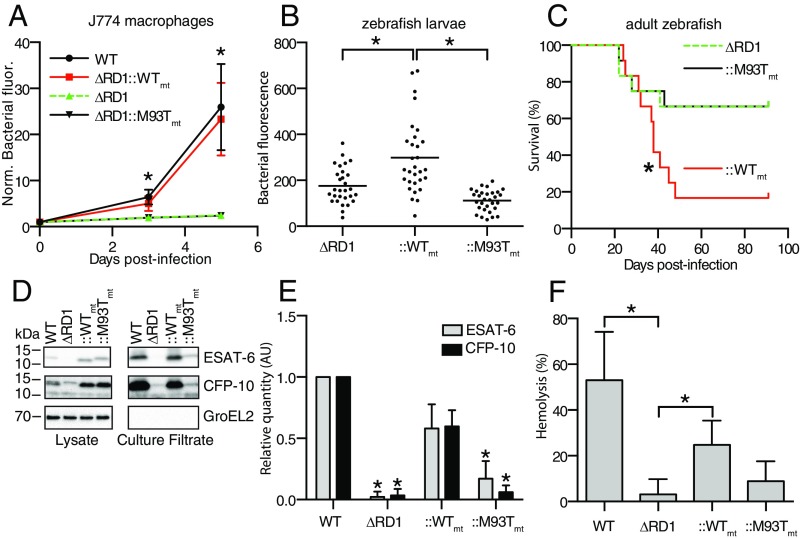
We previously showed that deleting the Mm RD1 orthologous region (MMAR_5446-MMAR_5455) results in similar attenuation phenotypes to those reported for Mtb. This strain, Mm−ΔRD1, is attenuated for growth in cultured mammalian macrophages as well as adult and larval zebrafish. The reduced bacterial burdens in the zebrafish are accompanied by increased survival (29, 30). To test whether the Mm and Mtb ESX-1 systems were functionally equivalent, we complemented Mm−ΔRD1 with a cosmid containing either the Mtb ESX-1 locus (Mm−ΔRD1::rv3861-rv3885mt, referred to as Mm−ΔRD1::WTmt) (11) or the Mtb ESX-1 locus bearing a point mutation in the gene encoding ESAT-6 (Mm−ΔRD1::M93Tmt), which fails to restore virulence in the mouse (31). Similar to the Mtb results, we found that Mm−ΔRD1::WTmt but not Mm−ΔRD1::M93Tmt rescued virulence (Fig. 1 A–C). We observed that Mm−ΔRD1::M93Tmt had diminished secretion not only of ESAT-6, but also of CFP-10 (Fig. 1 D and E), which has been shown to be dependent on ESAT-6 for its secretion in Mtb (32).
Fig. 1.
Mtb ESX-1 secretion is linked to virulence and hemolysis. (A) Bacterial growth in the J774 macrophage cell line as assessed by intracellular bacterial fluorescence. n = 12 fields per condition. *P < 0.05, two-way ANOVA with Dunnett’s test. (B) Bacterial burdens in 4-day-postinfection (dpi) zebrafish larvae as assessed by bacterial fluorescence. n = 30 larvae per condition. *P < 0.05, one-way ANOVA with Dunnett’s test. (C) Survival of adult zebrafish infected with 100 colony-forming units (CFU) of Mm. n = 12 fish per condition. *P < 0.05, log-rank (Mantel–Cox) test. (D) Immunoblot of Mm lysates and culture filtrates, representative of four experimental replicates. (E) Protein quantification from D by image densitometry, relative to WT culture filtrates. *P < 0.05, one-way ANOVA with Dunnett’s test relative to ::WT. (F) Contact-dependent sheep RBC lysis by 3.0 × 108 CFU Mm. n = 4 experimental replicates. (E and F) *P < 0.05, one-way ANOVA with Dunnett’s test relative to ∆RD1. Error bars, SD.
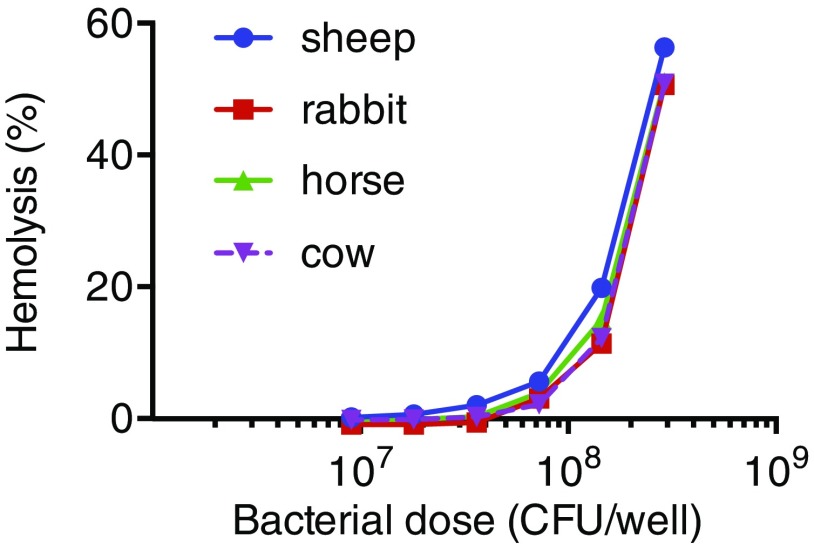
ESX-1 function in both Mtb and Mm has been associated with RBC lysis (16, 33). After confirming that wild-type (WT) Mm exhibited similar dose-dependent hemolytic activity for sheep, rabbit, horse, and cow RBCs (Fig. S1), we used sheep RBCs for subsequent experiments. We found that Mm−∆RD1 was defective for hemolysis (Fig. 1F) and furthermore that Mm−∆RD1::WTmt but not Mm−ΔRD1::M93Tmt rescued hemolytic activity (Fig. 1F). These findings show the functional equivalence of Mtb and Mm ESX-1 and confirm the requirement of ESX-1 secretion for both virulence and cytolytic activity.
Fig. S1.
No specific difference in mycobacterial lytic activity was observed between different species of RBCs. A total of 2 × 107 RBCs of the indicated species were mixed with described concentrations of WT Mm, and contact-dependent hemolysis was measured as described in SI Materials and Methods, Hemolysis Assays.
ESX-1–Mediated Hemolysis Is Contact Dependent.
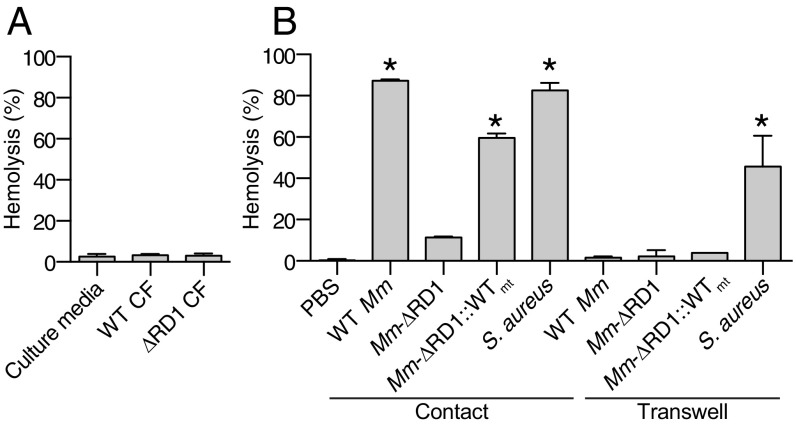
Prior work had suggested that ESAT-6 mediated ESX-1 membrane lytic activity through pore-forming activity (15, 18, 28, 34). If so, mycobacterial culture supernatants should produce hemolysis. However, even concentrated culture supernatants from WT bacteria are not hemolytic (Fig. 2A). This finding raised the possibility that ESX-1–mediated hemolysis is contact dependent as reported by King et al. (35). Moreover, they showed that only the virulent Mtb strain H37Rv conferred hemolysis; both its avirulent counterpart Mtb H37Ra and BCG were nonhemolytic. At the time of the King et al. (35) publication, it was not known that BCG has the RD1 deletion, which ablates ESX-1 function (8, 9), or that Mtb H37Ra is defective for ESX-1 secretion (36, 37). Reinterpreting the King et al. (35) paper in this new light, our finding that mycobacterial supernatants did not confer lysis strongly suggested to us that ESX-1 mediates exclusively contact-dependent hemolysis without any contact-independent lysis. We tested our hypothesis of contact-dependent hemolysis by placing Mm in direct contact with RBCs or by separating bacteria from RBCs, using the 0.4-μm cell barrier present in commercially available Transwell. Both WT Mm and Mm−∆RD1::WTmt were capable of hemolysis only when in direct contact with RBCs (Fig. 2B). In contrast, Staphylococcus aureus retained substantial hemolytic activity when separated by the Transwell barrier, consistent with its known ability to secrete hemolytic pore-forming toxins (38) (Fig. 2B). These results demonstrated that the hemolytic activity mediated by both Mtb and Mm ESX-1 requires direct bacterial cell contact with host membranes and accounts for essentially all mycobacterial hemolytic activity (Fig. 2B).
Fig. 2.
ESX-1–dependent hemolysis requires direct contact. (A) Hemolysis following addition of culture filtrate from WT Mm, Mm–ΔRD1, or uninoculated media. n = 6 experimental replicates. (B) Hemolysis following addition of the indicated bacterial strains, either in direct contact with RBCs or separated by a Transwell. n = 3 experimental replicates. *P < 0.05, one-way ANOVA with Dunnett’s test relative to ∆RD1 (contact). Error bars, SD.
ESX-1–Dependent Hemolysis Is Accompanied by Membrane Disruptions at Points of Bacterial Contact Without Apparent Pore Formation or Host Membrane Penetrating Bacterial Structures.
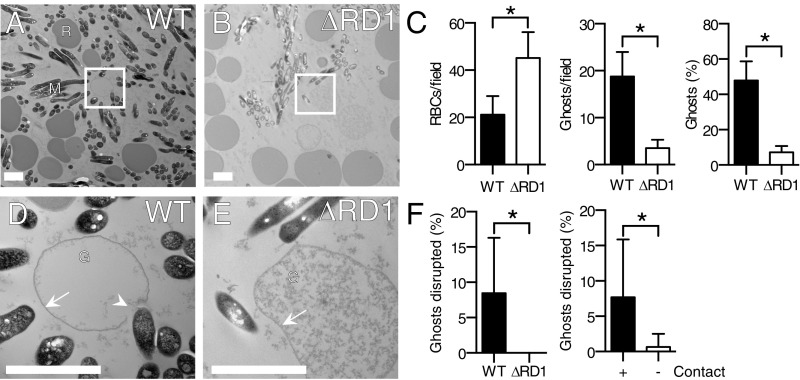
We assessed the morphology of RBC lysis by transmission electron microscopy (TEM). Consistent with our findings in the hemolysis assay, the total number of RBCs was reduced and RBC ghosts were more abundant following incubation with WT Mm (Fig. 3 A–C). Higher-magnification views revealed gross RBC membrane disruptions only in WT Mm samples; the few RBC ghosts seen in the Mm−ΔRD1 condition did not have any membrane disruptions (Fig. 3 D–F). WT membrane disruptions were predominantly at regions of bacterial contact with RBC membranes (11/12 or 91%) (Fig. 3D). Significantly more disruptions were observed at the point of contact with WT Mm (Fig. 3F). Mm did not induce discrete pores in the RBCs in contrast to what has been observed for hemolysis caused by classical pore-forming toxins, like S. aureus ⍺-hemolysin (Hla) (39) and Clostridium perfringens necrotic enteritis B-like toxin (NetB) (40) (Fig. S2).
Fig. 3.
ESX-1–dependent hemolysis is accompanied by membrane disruptions at points of bacterial contact without apparent pore formation. (A and B) Transmission electron micrographs of RBC pellets undergoing contact-dependent hemolysis by WT Mm and Mm–ΔRD1, respectively. M, Mm; R, RBCs. Boxes in A and B indicate the areas magnified in D and E, respectively. (C) Total RBCs, lysed ghosts, and lysed ghosts as percentage of total RBCs per field. n = 10 fields. (D and E) Membrane-disrupted ghost in contact with WT Mm (D) and intact ghost in contact with Mm–ΔRD1 (E). G, ghost; arrow, intact membrane; arrowhead, disrupted membrane. (F) Percentage of WT and Mm–ΔRD1 ghosts disrupted per field (Left) and percentage of contact-dependent or -independent WT ghost disruptions per field (Right). (Scale bar, 2 μm.) Error bars, SD. *P < 0.05, Student’s t test.
Fig. S2.
No pores were detected at point of contact-dependent hemolysis by Mm. (A) High-resolution electron micrograph (EM) of an RBC ghost (labeled “G”) and WT Mm (labeled “M”). Arrowhead indicates beginning of membrane disruption, whereas arrow indicates RBC plasma membrane. Box in A indicates area of increased magnification depicted in B. No pores were observed at highest magnification. (C) Example of S. aureus Hla pores forming on a RBC membrane as observed by negative stain EM. Reproduced with permission from ref. 39.
These findings corroborate the hypothesis that ESX-1 mediates contact-dependent membrane disruption and show that host cell lysis is accompanied by gross cell membrane disruptions at points of bacterial contact rather than by pore formation. Accordingly, we looked for morphological similarities to contact-dependent cell lysis mediated by specialized bacterial secretion systems of other bacteria. We did not observe any structures mediating contact between Mm and host cells such as the needle structures or pili that have been observed for the type III secretion-mediated contact-dependent hemolytic activity of Yersinia enterocolitica (41) or Escherichia coli (42). Moreover, the gross membrane disruptions we observed are in stark contrast to the normal morphology observed for RBC lysis upon contact with Shigella (43). Thus, the mycobacterial ESX-1 secretion system appears to mediate lysis through another distinct mechanism.
Most Membrane Lytic Activities Attributed to ESAT-6 Are Due to Residual Detergent in the Preparations.
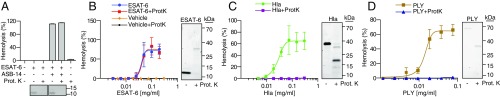
Our findings were contradicted by prior work suggesting that ESAT-6 alone directly lyses membranes by functioning as a secreted pore-forming protein (15, 18, 28, 34). We were puzzled by this discrepancy particularly because we too had previously found that the widely used, commercially prepared recombinant ESAT-6 [from Biodefense and Emerging Infections (BEI) Research Resources Repository] mediated hemolysis and so did the rESAT-6 we prepared using the published protocol (44). Further analysis of the purification protocol revealed that rESAT-6 showed hemolytic activity only when we included the endotoxin removal step—washing of the column with the zwitterionic detergent, ASB-14. When we omitted this wash step, we found the preparation had no hemolytic activity. This result made us wonder whether the lytic activity ascribed to ESAT-6 could be due to residual detergent. We compared the hemolytic activity of rESAT-6 prepared side by side with and without the detergent wash step and found that omitting the detergent wash step consistently yielded nonhemolytic rESAT-6 protein preparations (Fig. 4A). While our experiments were in progress, a paper was published that also found that rESAT-6 lysed RBCs only if the preparations were treated with ASB-14 (34). These authors interpreted the data to mean that detergent modifies the structure of ESAT-6, activating its lytic activity. If true, the hemolytic activity should be dependent on both ESAT-6 and detergent and should be abrogated by proteolyzing detergent-treated ESAT-6. However, we found no decrease in hemolytic activity of our rESAT-6 preparations following Proteinase K treatment (Fig. 4A). Similarly, Proteinase K treatment also failed to decrease the lytic activity of rESAT-6 obtained from BEI Resources (Fig. 4B). However, Proteinase K treatment completely abrogated the lytic activity of the classic secreted pore-forming toxins Hla and pneumolysin (PLY) (Fig. 4 C and D). In light of these results, we reviewed the ESAT-6 literature and found that publications reporting ESAT-6 cytolytic activity included detergent in the purification as determined from their described methods (Table 1). In contrast, those that did not use the detergent step reported ESAT-6 to be devoid of cytolytic activity (Table 1). Of note, both we and others (34) have found that recombinant CFP-10 preparations (including from BEI Resources) fail to lyse cells despite being subjected to the identical detergent wash step to that for ESAT-6. This result suggests that any residual detergent was removed during the subsequent wash and dialysis steps in the case of CFP-10 but not ESAT-6 because of tighter binding of detergent to the latter protein. In summary, we conclude that the cytolytic activity attributed to rESAT-6 is due to residual bound detergent.
Fig. 4.
Recombinant ESAT-6 lyses host membranes via residual contaminating detergent. (A) Lysis of RBCs treated with 0.06 mg/mL rESAT-6 prepared with or without ASB-14 and treated with Proteinase K (Prot. K.). n = 3 replicates (Top) and Coomassie Blue-stained gel of 0.6 µg of corresponding sample (Bottom). (B) RBC lysis following addition of serially diluted rESAT-6 (BEI) or vehicle ± Prot. K. n = 3 experimental replicates (Left) and Coomassie Blue-stained gel of 1.2 µg protein sample from each condition (Right). (C and D) RBC lysis following addition of serially diluted S. aureus α-hemolysin (Hla, ∼38 kDa) (C, Left) or Streptococcus pneumoniae pneumolysin (PLY, ∼70 kDa) (D, Left), with Coomassie Blue-stained gels of 1.2 µg protein on Right for each panel.
Table 1.
Literature reports of recombinant ESAT-6–mediated lysis parsed by detergent wash step use in the preparation
| Detergent used | Detergent not used | |||
| Phenomenon tested | Effect | No effect | Effect | No effect |
| Lipid bilayer disruption | 1* | 0 | 0 | 0 |
| Host cell lysis | 6† | 2‡ | 0 | 3§ |
| Liposome disruption | 0 | 0 | 4¶ | 0 |
Papers that reported recombinant ESAT-6–mediated lysis or membrane disruption were found through a PubMed Search. Whether detergent was used in the ESAT-6 preparation was determined from the Materials and Methods section in the case of ESAT-6 prepared in house or from the manufacturer’s website if it was purchased.
Ref. 15.
ESAT-6 Can Disrupt Liposomes at Acidic pH.
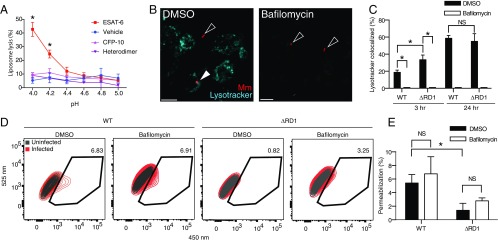
Whereas we had ruled out that ESAT-6 functions as a lytic protein at neutral pH, detergent-free rESAT-6 is reported to disrupt liposome membranes at pH ≤5 (Table 1) (25). We too found that our detergent-free ESAT-6 preparations lysed liposomes at acidic pH <4.5 (Fig. 5A). Also consistent with the prior report (25), rCFP-10 and an rESAT-6/CFP-10 heterodimer prepared from a bicistronic construct did not lyse liposomes (Fig. 5A). These findings confirmed that detergent-free ESAT-6 has intrinsic liposome-disrupting activity at acidic pH (25).
Fig. 5.
Recombinant ESAT-6 lyses membranes at acidic pH, whereas mycobacterial ESX-1–mediated lysis proceeds at neutral pH. (A) Quantification of pH-dependent liposome lysis by recombinant ESAT-6, but not CFP-10 or ESAT-6/CFP-10 heterodimer as measured by fluorescent ANTS release from DOPC liposomes. *P < 0.05, two-way ANOVA with Dunnett’s multiple comparisons. (B) Representative images of THP-1 macrophages infected with WT Mm 6 h after treatment. (Scale bar, 20 μm.) (C) Quantification of colocalization of WT Mm and Mm–ΔRD1 with acidified compartments. n = 3 technical replicates. *P < 0.05, Mann–Whitney U test. (D) Flow cytometry of WT- or ΔRD1-infected THP-1 macrophages. Gate highlights permeabilization events in live, infected macrophages. (E) Quantification of permeabilization events. n = 3 experimental replicates. Error bars, SD. *P < 0.05, one-way ANOVA with Tukey’s multiple-comparisons test.
ESAT-6’s pH-Dependent Membrane Lytic Activity Is Not Required for ESX-1–Mediated Macrophage Phagosomal Permeabilization.
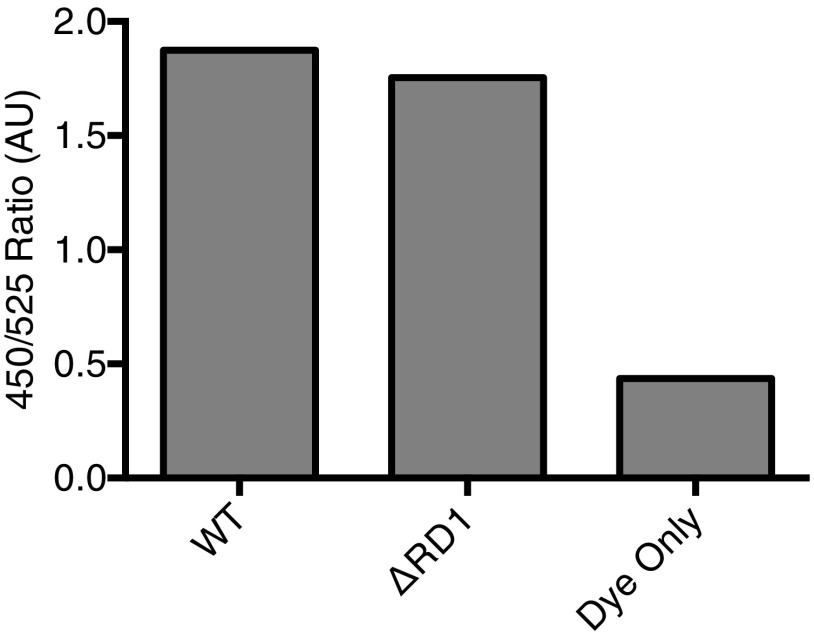
ESAT-6’s lytic activity at acidic pH is thought to be responsible for ESX-1’s ability to permeabilize macrophage phagosomes and allow mycobacteria to access the cytosol (25, 26). If so, then blocking phagosomal acidification with the vacuolar ATPase (vATPase) inhibitor, Bafilomycin (45), should decrease ESX-1–mediated phagosomal permeabilization. To test this, we first confirmed that Bafilomycin inhibited acidification of both WT Mm and Mm−∆RD1 containing phagosomes in the human macrophage cell line THP1 by staining with LysoTracker, an acidophilic dye that labels lysosomal compartments (46)(Fig. 5 B and C). Next, we tested the effect of Bafilomycin on mycobacterial phagosomal membrane permeabilization with an assay that uses the fluorescence resonance energy transfer (FRET)-based dye, CCF4-AM (22). CCF4-AM is absorbed into the host cytosol and produces a green fluorescent signal (525 nm) (22). If the mycobacterial phagosome is permeabilized, then the dye is cleaved by the endogenous Mm cell-surface–associated β-lactamase BlaC, which is otherwise inaccessible to the dye. Cleavage causes a loss of FRET and an increase in blue fluorescence (450 nm). We observed similar loss of FRET for WT Mm and Mm−∆RD1, showing they had similar BlaC activity (Fig. S3). Thus, phagosomal permeabilization can be measured by the shift from green to blue fluorescence within an infected cell (22). We used this dye to measure the phagosome-permeabilizing ability of WT Mm and Mm−∆RD1 following treatment with Bafilomycin or vehicle. We confirmed prior findings that permeabilization was increased in WT infection compared with Mm−∆RD1 (22) (Fig. 5 D and E and Fig. S4). However, we found no reduction in permeabilization in Bafilomycin-treated macrophages (Fig. 5E). Thus, acidification is not a prerequisite for permeabilization of the mycobacterial phagosome, suggesting that ESX-1–mediated phagosomal permeabilization does not occur through the acidic pH-dependent membrane lytic activity of ESAT-6. Rather, it appears that ESX-1–mediated phagosomal permeabilization proceeds through the same mechanism as RBC lysis that either is independent of ESAT-6 or requires additional mycobacterial determinants.
Fig. S3.
WT and RD1 Mm strains show similar in vitro lactamase activity as measured by CCF4-AM cleavage. The ratios depicted are values of the 450-nM emission peak divided by the 525-nm peak obtained from a 405-nm emission scan. Experiment depicts technical triplicate.
Fig. S4.
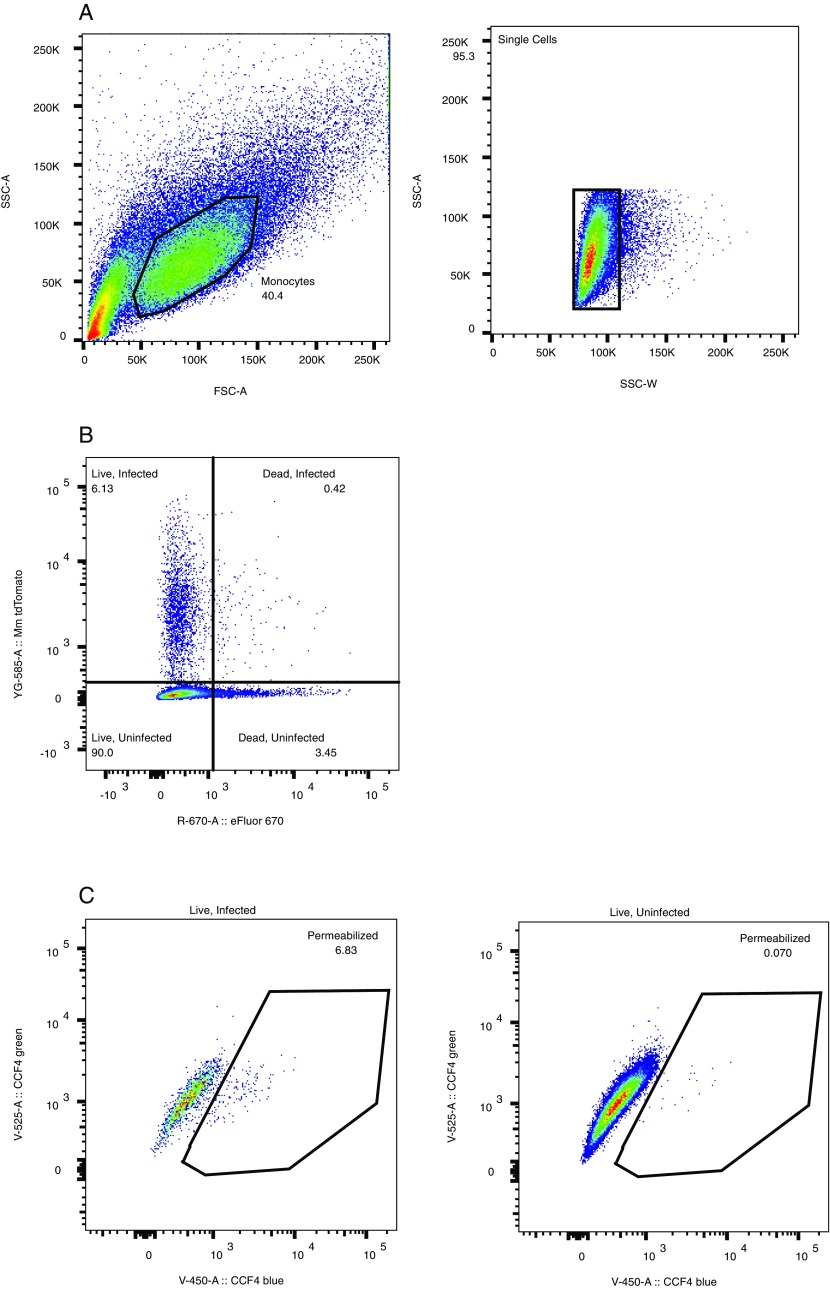
Representative flow cytometry gating scheme. (A) FSC-A, forward scatter A; SSC-A, side scatter A; SSC-W side scatter W. Black polygons indicate gated cell subsets. (B) Dot plot showing gating for infected/uninfected cells and live/dead cells based on tdTomato and Fixable Viability Dye eFluor 660 fluorescence. Numbers indicate the percentage of events in respective quadrants. (C) Black polygons indicate region containing permeabilization events. Dot plots are representative of three independent experiments.
Discussion
We show that ESAT-6 does not function as a pore-forming protein or possess intrinsic membrane lytic activity under physiological conditions. We find, however, that ESX-1–mediated cell lysis occurs through a contact-dependent mechanism that causes gross disruptions at points of bacterial contact. Other specialized bacterial secretion systems are known to mediate contact-dependent cytolysis (41, 42). It is tempting to speculate that this might be the case for mycobacteria as well, given a very recent study suggesting that the ESX-1 substrate EspC may form a surface-exposed filamentous structure spanning the mycobacterial cell envelope (47, 48). The ESX-1–mediated membrane disruptions we observe in RBCs look similar to the ESX-1–mediated phagosomal membrane degradation of infected myeloid cells observed by cryoelectron microscopy (20, 21), suggesting that it is relevant to mycobacterial pathogenesis. The intracellular pathogen Rickettsia also disrupts phagosomal membranes, and it does so via phospholipase A2 (49) in a manner that appears similar to these ESX-1–mediated membrane disruptions. Whereas mycobacterial phospholipases are not involved in Mtb’s phagosomal permeabilization (50), some hemolytic activity is attributed to a mycobacterial sphingomyelinase (33). In addition, a host phospholipase A2 may be involved in phagosomal permeabilization (51). Such findings leave open the possibility that ESX-1 regulates the activities of bacterial and host lipolytic enzymes to disrupt membranes. Alternatively, ESX-1–mediated lysis may occur through a secreted component (including ESAT-6) that needs to be activated by a mycobacterial (or even host) membrane component. Such a scenario would make lysis through a secreted lysin contact dependent and is exemplified in the case of Serratia marcescens hemolysin ShlA (52). ShlA is secreted and activated by its partner ShlB only in the presence of phosphatidylethanolamine, a lipid commonly found in biological membranes (52).
Our experiments raise the question of what precise role ESAT-6 plays in mycobacterial virulence. Prior reports that ESAT-6 secretion-defective mutants are defective for host cell lysis (15, 16, 53) are confounded by the finding that several ESX-1 substrates are mutually codependent for secretion (54, 55). However, because it is an immunodominant antigen, it is likely to have a role in virulence. Perhaps it acts downstream of ESX-1–mediated membrane permeabilization to directly influence pathogenesis by regulating immune determinants (56–58). The finding that ESAT-6 binds to liposomal membranes may reflect its ability to bind lipids and thereby eukaryotic cell membranes (19, 59) to initiate or subvert the requisite cell signaling events. In such a scenario, ESX-1–mediated phagosomal permeabilization would enable ESAT-6 to gain contact with the cytosol of the infected macrophage or even neighboring cells to influence their immune program.
Our quest to confirm and expand the model of ESAT-6 as a cytolytic, pore-forming toxin took an unexpected turn when a serendipitous omission in a well-defined and widely used purification protocol led us to reexamine the biological function that had been widely attributed to this mycobacterial virulence determinant. Our experiments led us to reinterpret ESX-1’s membrane lytic activity. We hope our findings will lead to a better understanding of this and of ESAT-6’s biological function.
Materials and Methods
The materials and methods used are detailed at length in SI Materials and Methods. Mycobacterial strains, plasmids, hemolysis and liposome lysis assays, zebrafish and macrophage infections, electron microscopy, production of recombinant proteins and culture filtrates, and the phagosomal permeabilization assay are described therein. Plasmids and mycobacterial strains used are listed in Table S1 and S2.
Table S1.
Plasmids used in this paper
| No. | Plasmid | Description | Source |
| 1 | pTEC31 | Mycobacterial plasmid containing the gene for the fluorescent protein tdTomato under the constitutive mycobacterial promoter msp12. Hygromycin plasmid in pTec27 swapped with kanamycin resistance. | (71) |
| 2 | pYUB412 | Empty vector for integration into attP site, conferring hygromycin resistance. | (15, 31) |
| 3 | 2F9-EsxA-WT | pYUB412 integrating cosmid containing M. tuberculosis RD1 region (base pairs 4,336,809–4,368,613) between PacI sites. This includes the gene encoding ESAT-6 (esxA). | (15, 31) |
| 4 | 2F9-EsxA-M93T | 2F9-EsxA integrating cosmid where EsxA codon 93 encoding methionine is mutated to threonine (ATG→ACC) | (31) |
| 5 | pMRLB.7–EsxA-His | Plasmid for expression and production of recombinant M. tuberculosis ESAT-6-6xHis | BEI |
| pMRLB.7::EsxA::His | |||
| 6 | pMRLB.46–EsxB-His | Plasmid for expression and production of recombinant M. tuberculosis CFP-10-6xHis | BEI |
| 7 | pET29–EsxB-EsxA-His | Bicistronically expressed ESAT-6-6x-His and CFP-10. For production of recombinant M. tuberculosis ESAT-6/CFP-10 heterodimer. | This work |
| 8 | pHla-His | Plasmid for expression and production of recombinant S. aureus Hla. | (76) |
| 9 | pPLY | Plasmid for expression and production of recombinant S. pneumoniae PLY. | (61) |
Table S2.
M. marinum strains used in this paper
| No. | Strain | Description | Antibiotic resistance | Fig. | Source |
| 1 | M strain | WT | None | Figs. 2 and 3 | (62) |
| 2 | ΔRD1 | M strain with a deleted RD1 locus. | None | Figs. 2 and 3 | (29) |
| 3 | ΔRD1::2F9-EsxA-WT (aka ΔRD1::WTmt) | Strain 2 containing plasmid 3. M strain harboring a deleted RD1 locus heterologously expressing the Mtb RD1 locus on an integrating plasmid. | Hygromycin | Fig. 2 | This work |
| 4 | WT::pYub412+tdtomato | M strain containing the empty vector pYUB412 and the fluorescent protein expressing psmp12::tdtomato vector. Contains plasmids 1 and 2. | Hygromycin, kanamycin | Figs. 1, 2, and 5 | This work |
| 5 | ΔRD1::pYub412+tdtomato | ΔRD1 strain containing the empty vector pYUB412 and the fluorescent protein expressing psmp12::tdtomato vector. Contains plasmids 1 and 2. | Hygromycin, kanamycin | Figs. 1, 2, and 5 | This work |
| 6 | ΔRD1::WTmt+tdtomato (ΔRD1::WTmt) | ΔRD1 strain containing the vector pYUB412 harboring the RD1 region of M. tuberculosis and the fluorescent protein expressing psmp12::tdtomato vector. Contains plasmids 1 and 3. | Hygromycin, kanamycin | Figs. 1 and 2 | This work |
| 7 | ΔRD1:: 2F9-ESAT-6M93T+tdtomato (ΔRD1::M93Tmt) | Same as strain 5, except that it harbors plasmids 1 and 4, expressing EsxA-M93T. | Hygromycin, kanamycin | Fig. 1 | This work |
Zebrafish husbandry and experiments were in compliance with guidelines from the UK Home Office and the US National Institutes of Health and approved by the University of Washington Institutional Animal Care and Use Committee.
SI Materials and Methods
Bacterial Strains.
All strains were derived from WT Mm purchased from American Type Culture Collection (ATCC) (strain M, ATCC no. BAA-535). RD1-deleted Mm (ΔRD1) was generated as described previously (29).
Production of Recombinant ESAT-6, CFP-10, and Pore-Forming Toxins.
Recombinant ESAT-6, CFP-10, and ESAT-6/CFP-10 heterodimer were prepared according to the protocol published by BEI Research Resources Repository (44). E. coli strain BL21 was transformed with plasmid pMRLB.7, plasmid pMRLB.46 [obtained through BEI Resources, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH)], or plasmid pET29-EsxB-EsxA-6xHis. Ten-milliliter starter cultures were grown to stationary phase overnight with shaking in Luria Bertani broth (Becton Dickinson no. 244610) with 100 μg/mL ampicillin (Sigma A0166-5G). These were used to inoculate 1 L of LB media to grow in shaking Fernbach flasks at 37 °C. Once cultures reached an OD600 between 0.5 and 0.8, recombinant protein production was induced using 250 μM IPTG (Sigma I5502-1G). Cultures were grown overnight, with shaking at 20 °C. Bacteria were then pelleted at 4,680 × g for 30 min and resuspended in 10 mL lysis buffer containing 20 mM Tris⋅HCl (pH 7.9), 500 mM NaCl, and 5 mM imidazole. A total of 1 mM phenylmethylsulfonyl fluoride (PMSF) (Sigma P7626-1G) was added as a protease inhibitor immediately before lysis. Bacteria were sonicated on ice, using a VC505 sonicator (Vibra Cell) with 4 × 30-s pulses at 30% power. Crude lysates were clarified by centrifugation at 38,724 × g for 30 min at 4 °C. For rESAT-6 and rCFP-10 1-mL Ni-NTA (Qiagen no. 30210) columns were equilibrated using lysis buffer. Columns were then washed with 20 column volumes (CVs) of lysis buffer. Next, columns were washed with 10 CVs of Burdick and Jackson water. One column of rESAT-6 received 10 CVs of 0.5% ASB14 (Sigma A1346-1G) in 10 mM Tris⋅HCl (pH 7.9); the other column received 10 CVs of 10 mM Tris⋅HCl (pH 7.9). Following detergent or control wash, another 10 CVs of 10 mM Tris⋅HCl (pH 7.9) were added. Recombinant protein was subsequently eluted using 1 M imidazole in 10 mM Tris⋅HCl (pH 7.6). Recombinant ESAT-6/CFP-10 heterodimer was purified using a HisTrap HP column (GE Healthcare) equilibrated with buffer A [20 mM Tris⋅HCl, 150 mM NaCl (pH 7.9)] and then eluted with a linear imidazole gradient from 0 mM to 500 mM in buffer A. Fractions containing heterodimer as observed by SDS/PAGE were then pooled and loaded onto an S200 size exclusion column equilibrated with buffer A (GE Healthcare). Protein-containing fractions, as assessed by A280, were collected for dialysis. Recombinant proteins were subsequently dialyzed in two 4-L stages, using 3,500-MWCO dialysis tubing in 10 mM ammonium bicarbonate (Sigma). Fractions were collected and monitored for recombinant protein elution and purity by SDS/PAGE followed by Coomassie staining. Protein concentrations from highly purified eluted fractions were quantified by A280, using a nanodrop 2000 spectrophotometer. Samples were aliquoted and frozen for subsequent use.
Recombinant S. aureus Hla and S. pneumoniae PLY were purified according to methods graciously provided by Julie Bubeck Wardenburg (personal communication). Briefly, plasmids 8 and 9 were used to transform BL21 E. coli. Isolated colonies were used to inoculate 100 mL LB broth under antibiotic selection and grown overnight at 37 °C with shaking at 120 rpm. A total of 50 mL of culture was used to inoculate 1 L of LB containing selective antibiotic. Samples were then grown on a 37 °C shaking incubator until cultures reached OD600 = 0.5–0.7. Toxin expression was induced by adding IPTG to a final concentration of 1 mM, and induction was allowed to proceed for 6 h. Bacteria were then pelleted at 4,680 × g for 30 min. Bacterial cultures were resuspended in 20 mL TGN buffer [50 mM Tris, 150 mM NaCl, 10% (vol/vol) glycerol, pH 7.5] containing 1 mM PMSF. Samples were then lysed by a French pressure cell. Cell supernatants were clarified by centrifugation at 38,724 × g for 30 min at 4 °C. Two-milliliter Ni-NTA columns were equilibrated in 10 CVs of TGN buffer containing 10 mM imidazole. Samples were then loaded onto Ni-NTA columns by gravity. Samples were then washed with 10 CVs of TGN containing 20 mM imidazole and 50 mM imidazole. Toxin was then eluted using 5 mL TGN containing 250 mM imidazole. Samples were then dialyzed into PBS containing 10% (vol/vol) glycerol. Fractions were collected and monitored for recombinant protein elution and purity by SDS/PAGE followed by Coomassie Blue stain. Protein concentrations from highly purified eluted fractions were quantified by A280, using a nanodrop 2000 spectrophotometer. Samples were aliquoted and frozen for subsequent use.
Hemolysis Assays.
Hemolysis was performed as described previously (18, 35). Defibrinated sheep RBCs (Fisher Scientific) were diluted to a concentration of 1% (vol/vol) and washed twice with PBS (125 mM NaCl, 16.6 mM Na2HPO4, 8.4 mM NaH2PO4, pH 7.4) and centrifuged at 3,200 × g for 5 min. Mycobacteria were cultured in 7H9 complete media [middlebrook 7H9 media (Becton Dickinson no. 271310) containing 0.05% tween 80, 0.2% (vol/vol) glycerol, 5 mg/mL BSA (fraction V; Sigma no. A3912), 0.005% oleic acid in 0.02 N NaOH, 2 mg/mL dextrose, 0.85 mg/mL NaCl] in a humidified CO2 incubator set to 33 °C and 5% CO2. For hemolysis assays, mycobacteria were inoculated at an OD600 = 0.01 from refrigerator stocks and grown until late-log phase (OD600 >1). A total of 5 mL of cultures was centrifuged at 3,200 × g for 10 min and subsequently washed twice with PBS. Assuming 1 OD600 unit of bacteria = 3 × 108 CFU/mL (68, 69), mycobacteria were concentrated to 9 × 108 mycobacteria in 100 μL. The mycobacteria were then mixed with 100 μL 1% RBCs in a 1.5-mL microcentrifuge tube and centrifuged at 3,200 × g for 5 min. Samples were incubated for 2 h at 33 °C. Following incubation, samples were resuspended and repelleted at 3,200 × g for 5 min. A total of 100 μL supernatant was collected and A405 and A540 were measured on a BMG CLARIOstar 96-well microplate reader. Hemolytic activity of PBS was used as a negative control, and hemolytic activity of 0.1% Triton X-100 (Sigma) was used as a positive control. Absorbance measurements were converted to percentage of hemolysis by the following formula:
To measure hemolytic activity, recombinant proteins were serially diluted in 100 μL PBS and mixed with 100 μL 1% RBCs in a round-bottom 96-well plate. Recombinant proteins were prepared as described above. rESAT6, Hla, and PLY were added to RBCs at a final concentration of 0.2 mg/mL, 0.3 mg/mL, and 0.075 mg/mL. Additional rESAT-6 was obtained through BEI Resources, NIAID, NIH: ESAT-6 Recombinant Protein Reference Standard, NR14868. Following a 30-min incubation at room temperature, samples were pelleted by centrifugation at 3200 × g for 10 min. A total of 100 μL supernatant was transferred to a flat-bottom 96-well plate and hemolytic activity was compared with hemolytic activity of PBS and 0.1% Triton X-100 as described above.
To measure hemolytic activity of culture filtrates, 10 µL of culture filtrates (described in SI Materials and Methods, Short-Term Culture Filtrate Production) was added to 90 µL PBS and 100 µL 1% RBCs and incubated for 2 h at 33 °C. Samples were then pelleted and supernatant was collected and assessed for hemoglobin as above.
To measure contact-dependent hemolysis, 100 μL of 1% RBCs was added to the bottom chamber of a Corning HTS Transwell with a 0.4-μm membrane (Sigma no. CLS3391) or to a round-bottom 96-well cluster plate. A total of 100 μL containing 9 × 108 CFU of PBS-washed WT Mm, Mm–ΔRD1, or S. aureus strain LAC (kindly provided by Juliane Bubeck Wardenburg, Department of Microbiology, University of Chicago) was added either to the upper chamber of the Transwell or to the round-bottom plate. The 96-well plate was centrifuged at 3,220 × g for 5 min, and both trays were incubated at 33 °C with 5% CO2. Contact lysis proceeded for 2 h, whereas Transwell lysis was allowed to proceed for 24 h. Following incubation, samples in the lower chamber of the Transwell were transferred to the 96-well cluster plate. All samples were resuspended and pelleted again. Supernatants were collected and assayed for hemolysis as before.
Measuring Liposome Lysis.
Liposomes were prepared as previously described (70). Briefly, cholesterol (Sigma) was dissolved in 99.8% (vol/vol) ethanol (Sigma) to 10 mg/mL. Phosphatidylcholine (Egg Yolk Phosphatidylcholine; Sigma) was dissolved in 99.8% (vol/vol) ethanol (Sigma) to 100 mg/mL. A total of 86 μL phosphatidylcholine solution was mixed with 80 μL cholesterol solution and ethanol evaporated using N2 gas and under vacuum. Lipids were resuspended in 400 μL 125 mM ANTS (Biotium) in PBS, gently vortexed, and then sonicated in a water bath (55 kHz) for 3 min. Suspensions were incubated at 4 °C overnight and then washed in PBS by centrifugation at 24,000 × g for 30 min at 10 °C. Liposomes were then resuspended in 400 μL PBS (∼1.5 × 109/mL) for use in assays.
For pH buffers 4.0–5.0, varied ratios of sodium phosphate and citric acid were combined in 150 mM NaCl. The resulting buffers were verified to have the correct pH and then diluted 20-fold in 150 mM NaCl to achieve final buffer concentrations. pH was validated again, before and after each experiment.
To measure liposome lytic activity of recombinant proteins, 3 µM rESAT-6, rCFP-10, heterodimer, or equivalent volume of vehicle was added to 100 µL pH buffer. For each condition, 5 µL liposomes were washed twice with the indicated pH buffer and resuspended in 100 µL buffer. Protein and liposome samples were combined in a microcentrifuge tube and incubated with shaking at 20 °C for 20 min. Intact liposomes were pelleted by centrifugation; supernatants were measured for ANTS fluorescence [excitation/emission (nm): 350 ± 15/520 ± 15]. Raw fluorescence data were converted to percentage of lysis, using the formula above with buffer as a negative control and buffer containing 0.1% Triton X-100 as a positive control for each pH.
Zebrafish Survival Assays.
Adult zebrafish survival following mycobacterial infection was assayed according to Swaim et al. (30). Adult zebrafish were reared in a recirculating aquarium (Aquatic Habitats). Weeks before infection, adult zebrafish were then moved to a biosafety level 2 flow-through aquarium (Aquatic Habitats) and allowed to acclimate. For infection, adult AB zebrafish were anesthetized in a bath of tank water containing 0.1% 3-aminobenzoic acid methyl ester (tricaine). Zebrafish were then infected by i.p. injection of a 10-μL PBS solution containing 100 CFU M. marinum, diluted from previously quantified and frozen single-cell suspensions. Twelve fish per condition were infected. One tank was kept per condition. Survival was recorded daily. Tanks were maintained under conditions compliant with laboratory standards outlined by the Institutional Animal Care and Use Committee (water temperature of ∼28 °C, pH of ∼7.4, and conductivity of ∼1,500 µS).
Intramacrophage Growth Assays.
J774A.1 macrophages (ATCC) were maintained in DMEM (Gibco) supplemented with 10% (wt/vol) heat-inactivated FBS (Labtech), 1% penicillin–streptomycin (Gibco), and 2 mM l-glutamine (Gibco). Twenty-four hours before infection 4.8 × 104 cells were plated per well in a flat-bottomed 96-well plate. J774A.1 cells were infected with 0.5–0.75 multiplicity of infection (MOI) of tdTomato-fluorescent WT, ΔRD1, ΔRD1::WT, and ΔRD1::M93T strains of Mm in antibiotic-free DMEM. Media were replaced every 48 h and course of infection was recorded by imaging regularly using an inverted fluorescence microscope for a total magnification of 100× (Nikon; Eclipse Ti-E). Fluorescence quantification of bacterial burden was assessed as previously described (71).
Measuring Phagosomal Permeabilization.
Phagosomal permeabilization was assessed as previously described (72). To measure in vitro lactamase activity of mycobacteria, 1 × 106 washed mycobacteria were added to 100 nM CCF4-AM (Invitrogen), 50 μg/mL porcine esterase liver extracts (Sigma) in 100 μL of PBS. This mix was incubated in the dark for 1 h at 37 °C, and a 405-nm emission scan was read on a BMG CLARIOstar 96-well microplate reader. To assay phagosomal permeabilization, THP-1 macrophages (ATCC) were maintained in RPMI-1640 (Sigma) supplemented with 10% (wt/vol) heat-inactivated FBS (Labtech), 1% penicillin–streptomycin, and 2 mM l-glutamine. Seventy-two hours before infection, cells were activated with 33 nM phorbol 12-myristate 13-acetate (Sigma) and plated at 6 × 105 cells/mL. Activated THP-1s were infected with single-cell suspensions of tdTomato-labeled WT Mm and Mm–ΔRD1 at an MOI of 1 for 6 h at 33 °C in EM medium (120 mM NaCl, 7 mM KCl, 1.8 mM CaCl2, 0.8 mM MgCl2, 5 mM glucose, 25 mM Hepes, pH 7.3). Forty-eight hours after infection cells were stained with Fixable Viability Dye eFluor660 (eBioscience). Cells were then harvested and stained for 1 h at room temperature with 8 µM CCF4-AM (Invitrogen) in EM medium supplemented with 2.5 µM probenecid. Finally, cells were fixed overnight at 4 °C in 4% (wt/vol) paraformaldehyde. Cells were analyzed in an LSRFortessa II cytometer, using FACSDiva software (BD Biosciences). At least 50,000 events per sample were collected. Data were analyzed using FlowJo (Treestar). Infected monocytes were identified using the gating scheme described in Fig. S4A and B. Permeabilization percentage among infected monocytes was calculated using a region defined by an increased 450-nm signal (indicating CCF4 dye cleavage and, therefore, phagosomal permeabilization) relative to that in uninfected samples. Events in this region were designated “permeabilized”. Percent permeabilization was then calculated as a ratio of events in the permeabilized field to total live and infected cells (exemplified in Fig. S4B and C).
For inhibition of phagosomal acidification, cells were incubated with 25 nM Bafilomycin A1 (Cambridge Bioscience) and phagosomal permeabilization assay was performed as above. Acidic lysosomes were identified by staining with 100 nM LysoTracker Far Red DND-99 (Molecular Probes) for 30 min before imaging. Confocal microscopy was performed using a Nikon A1 confocal microscope with a 20× Plan Apo 0.75-N.A. objective and galvano scanner to generate 10-µm z-stacks consisting of 1-µm optical sections. Data were acquired using NIS Elements version 4.4.
Measuring Bacterial Burden in Larvae.
Zebrafish larvae were prepared, infected, and measured for bacterial burden as previously described (71), with the following modifications.
WT 2 d postfertilization zebrafish larvae were infected with single-cell suspensions containing ∼150 CFU, 100 CFU, and 50 CFU of indicated strains of tdTomato-expressing M. marinum. In this experiment, ΔRD1, ΔRD1::WT, and ΔRD1::M93T were infected with 72 CFU, 72 CFU, and 69 CFU, respectively, as quantified by CFU enumeration of injected inoculum. Infected larvae were imaged by fluorescence microscopy at 4 d postinfection, and resulting images were analyzed by fluorescence pixel counting (FPC) to determine bacterial burden. For each larva, total number of fluorescent pixels was quantified using ImageJ, as described previously (71).
Fluorescence microscopy was performed using a Nikon Eclipse Ti-E equipped with a Ti-S-E Motor XY Stage, a C-HGFIE 130-W mercury light source, a 23/0.10 Plan Apochromat objective, and a Chroma ET-CY3 (49004) filter cube. Fluorescence images were captured with a Photometrics CoolSNAP HQ2 Monochrome Camera, using NIS-Elements (version 3.22). Fluorescence microscopy was performed as previously described (71, 73).
Visualization of Membrane Disruption by Electron Microscopy.
A total of 9 × 109 CFU of WT or Mm–ΔRD1 in 200 µL PBS were mixed with 125 µL prewashed 20% (vol/vol) RBCs in PBS. Samples were mixed and pelleted for 5 min at 1,000 × g on a bed of 5% (wt/vol) agarose/PBS and subsequently incubated at 33 °C for 1 h. A total of 160 µL of supernatant was replaced with 160 µL of 1/2 Karnovsky’s fixative [2% (wt/vol) paraformaldehyde, 2.5% vol/vol) glutaraldehyde, 0.2 M Cacodylate; Electron Microscopy Sciences] and moved to 4 °C. Following overnight incubation, 160 µL supernatant was replaced with 160 µL 1/2 Karnovsky’s fixative three times Samples were then transferred to the Fred Hutchinson Electron Microscopy Center for postfixation in 2% (wt/vol) OsO4, dehydration, EPON 812 embedding, ultrathin sectioning, transfer to 200-mesh grids, and poststaining with uranyl acetate/lead citrate as described by the Fred Hutchinson Electron Microscopy Procedures Manual (available at https://sharedresources.fredhutch.org/training/electron-microscopy-procedures-manual). Samples were imaged on a JEOL 1400 transmission electron microscope and imaged using a Gatan digital imaging camera and software. For analysis of membrane disruption, the observer was blinded as to the condition and then imaged 10 randomly selected fields at a magnification of 600×. The numbers of ghosts and red blood cells were quantified in each field. Each ghost was examined for membrane disruption. Membrane disruption was scored contact dependent if the disruption occurred within 100 nm of a Mycobacterium and as contact independent if disruption occurred at a distance greater than 100 nm from a Mycobacterium.
Short-Term Culture Filtrate Production.
Short-term culture filtrates were prepared as previously described (74). In brief, Mm were grown in 7H9 media to OD600 >3. The bacteria were then washed and transferred to modified Sauton’s medium (0.2 g/L KH2PO4, 0.5 g/L MgSO4.7H2O, 2 g/L citric acid, 0.05 g/L ferric ammonium citrate, 60 mL/L glycerol, 4.0 g/L asparagine, pH 7.4), normalized to OD600 = 1, and cultured for 48 h. Bacteria were pelleted at 3,220 × g for 20 min. Supernatants were collected, filtered through a 0.2-µm filter, supplemented with 1 mM PMSF, and concentrated ∼200-fold with an Amicon 3-kDa centrifugal filter (Merck Millipore). Bacterial pellets were resuspended with 1 mL ice-cold PBS in 1 mM PMSF and lysed with 500 μL of 0.1-mm glass beads (VWR) and then lysed by 3 × 30-s pulses in a bead-beater apparatus. Bacterial debris was separated by centrifugation at 20,000 × g for 10 min at 4 °C and supernatant was collected. The total protein in the culture filtrate and bacterial pellet was quantified using the Pierce BCA protein assay kit (ThermoFisher Scientific). A total of 1 µg protein from culture filtrates and supernatants was separated by SDS/PAGE. Presence of ESAT-6, CFP-10, and GroEL2 was assayed by Western blot, using the following antibodies: mouse anti-ESAT-6 clone 11G4 (1:1,000; Enzo life Sciences, BPD-HYB-076-08-02), rabbit anti-CFP-10 (1:500; BEI, product NR13801), and mouse anti-GroEL2 clone IT-56 (1:1,000; BEI, product NR-13655). ESAT-6 and CFP-10 abundance was quantified by blot densitometry relative to WT culture filtrates, using Bio-Rad Image Lab Software.
Preparing Single-Cell Suspensions.
Single-cell suspensions of mycobacteria were prepared as described by Takaki et al. (71) with minor modifications. Briefly, 35 mL mycobacterial cultures were inoculated from glycerol stocks and cultured under kanamycin selection as described above, in Cellstar 250-mL vented tissue culture flasks (Greiner Bio-One; GMBH no. 658170). Once cultures reached OD600 = 0.3–0.6, cultures were pelleted at 3,220 × g for 20 min at 20 °C. Cultures were resuspended in 5 mL middlebrook 7H9 complete media without supplemental tween 80 (7H9 OAD). Samples were passed 10 times through a 27-gauge needle connected to a 10-mL syringe. Passaged cultures were then pelleted at 100 × g for 1 min. A total of 4 mL supernatant was collected. The bacterial pellet was resuspended in 5 mL of 7H9 OAD and again passed 10 times through a syringe. This process was repeated until 15 mL supernatant was collected. A total of 7.5 mL supernatant was then passed through a 32-mm 5-μm acrodisc syringe filter (Pall no. 4650) and the process was repeated with the remaining supernatant, using a new syringe filter. Filtered supernatant was then centrifuged at 3,220 × g for 30 min at room temperature. A single-cell pellet was then resuspended in 200 μL 7H9 OAD and divided into 5-μL aliquots and stored at −80 °C. Bacterial concentrations (CFU/mL) were determined using the Miles and Misra method (75).
Acknowledgments
We thank Stanley Falkow for critical review of the manuscript and David Sherman, Richard James, and Mark Troll for advice and discussion. This work was supported in part by a Wellcome Trust Principal Research Fellowship, the National Institutes of Health (NIH) (Grant R37AI054503), and the National Institute of Health Research Cambridge Biomedical Research Centre (L.R.); National Science Foundation Graduate Research Fellowship Grant DGE-1256082 (to M.M.O.); NIH Training Grant Award T32 AI55396 (to F.C.); and Agence National de Recherche Grants ANR-14-JAMR-001-02 and ANR-10-LABX-62-IBEID (to R.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620133114/-/DCSupplemental.
References
- 1.Cambier CJ, Falkow S, Ramakrishnan L. Host evasion and exploitation schemes of Mycobacterium tuberculosis. Cell. 2014;159(7):1497–1509. doi: 10.1016/j.cell.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 2.Brodin P, Rosenkrands I, Andersen P, Cole ST, Brosch R. ESAT-6 proteins: Protective antigens and virulence factors? Trends Microbiol. 2004;12(11):500–508. doi: 10.1016/j.tim.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Gröschel MI, Sayes F, Simeone R, Majlessi L, Brosch R. ESX secretion systems: Mycobacterial evolution to counter host immunity. Nat Rev Microbiol. 2016;14(11):677–691. doi: 10.1038/nrmicro.2016.131. [DOI] [PubMed] [Google Scholar]
- 4.Andersen P, Andersen AB, Sørensen AL, Nagai S. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J Immunol. 1995;154(7):3359–3372. [PubMed] [Google Scholar]
- 5.Elhay MJ, Oettinger T, Andersen P. Delayed-type hypersensitivity responses to ESAT-6 and MPT64 from Mycobacterium tuberculosis in the guinea pig. Infect Immun. 1998;66(7):3454–3456. doi: 10.1128/iai.66.7.3454-3456.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravn P, et al. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J Infect Dis. 1999;179(3):637–645. doi: 10.1086/314640. [DOI] [PubMed] [Google Scholar]
- 7.Wards BJ, de Lisle GW, Collins DM. An esat6 knockout mutant of Mycobacterium bovis produced by homologous recombination will contribute to the development of a live tuberculosis vaccine. Tuber Lung Dis. 2000;80(4-5):185–189. doi: 10.1054/tuld.2000.0244. [DOI] [PubMed] [Google Scholar]
- 8.Mahairas GG, Sabo PJ, Hickey MJ, Singh DC, Stover CK. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol. 1996;178(5):1274–1282. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behr MA, et al. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284(5419):1520–1523. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 10.Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol. 2002;46(3):709–717. doi: 10.1046/j.1365-2958.2002.03237.x. [DOI] [PubMed] [Google Scholar]
- 11.Pym AS, et al. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med. 2003;9(5):533–539. doi: 10.1038/nm859. [DOI] [PubMed] [Google Scholar]
- 12.Lewis KN, et al. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guérin attenuation. J Infect Dis. 2003;187(1):117–123. doi: 10.1086/345862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tekaia F, et al. Analysis of the proteome of Mycobacterium tuberculosis in silico. Tuber Lung Dis. 1999;79(6):329–342. doi: 10.1054/tuld.1999.0220. [DOI] [PubMed] [Google Scholar]
- 14.Okkels LM, Andersen P. Protein-protein interactions of proteins from the ESAT-6 family of Mycobacterium tuberculosis. J Bacteriol. 2004;186(8):2487–2491. doi: 10.1128/JB.186.8.2487-2491.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu T, et al. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci USA. 2003;100(21):12420–12425. doi: 10.1073/pnas.1635213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao L-Y, et al. A mycobacterial virulence gene cluster extending RD1 is required for cytolysis, bacterial spreading and ESAT-6 secretion. Mol Microbiol. 2004;53(6):1677–1693. doi: 10.1111/j.1365-2958.2004.04261.x. [DOI] [PubMed] [Google Scholar]
- 17.Derrick SC, Morris SL. The ESAT6 protein of Mycobacterium tuberculosis induces apoptosis of macrophages by activating caspase expression. Cell Microbiol. 2007;9(6):1547–1555. doi: 10.1111/j.1462-5822.2007.00892.x. [DOI] [PubMed] [Google Scholar]
- 18.Smith J, et al. Evidence for pore formation in host cell membranes by ESX-1-secreted ESAT-6 and its role in Mycobacterium marinum escape from the vacuole. Infect Immun. 2008;76(12):5478–5487. doi: 10.1128/IAI.00614-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jonge MI, et al. ESAT-6 from Mycobacterium tuberculosis dissociates from its putative chaperone CFP-10 under acidic conditions and exhibits membrane-lysing activity. J Bacteriol. 2007;189(16):6028–6034. doi: 10.1128/JB.00469-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Wel N, et al. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129(7):1287–1298. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 21.Houben D, et al. ESX-1-mediated translocation to the cytosol controls virulence of mycobacteria. Cell Microbiol. 2012;14(8):1287–1298. doi: 10.1111/j.1462-5822.2012.01799.x. [DOI] [PubMed] [Google Scholar]
- 22.Simeone R, et al. Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog. 2012;8(2):e1002507. doi: 10.1371/journal.ppat.1002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wassermann R, et al. Mycobacterium tuberculosis differentially activates cGAS- and inflammasome-dependent intracellular immune responses through ESX-1. Cell Host Microbe. 2015;17(6):799–810. doi: 10.1016/j.chom.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Dey B, et al. A bacterial cyclic dinucleotide activates the cytosolic surveillance pathway and mediates innate resistance to tuberculosis. Nat Med. 2015;21(4):401–406. doi: 10.1038/nm.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Leon J, et al. Mycobacterium tuberculosis ESAT-6 exhibits a unique membrane-interacting activity that is not found in its ortholog from non-pathogenic Mycobacterium smegmatis. J Biol Chem. 2012;287(53):44184–44191. doi: 10.1074/jbc.M112.420869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng X, Sun J. Mechanism of ESAT-6 membrane interaction and its roles in pathogenesis of Mycobacterium tuberculosis. Toxicon. 2016;116:29–34. doi: 10.1016/j.toxicon.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Y, Keil V, Sun J. Characterization of Mycobacterium tuberculosis EsxA membrane insertion: Roles of N- and C-terminal flexible arms and central helix-turn-helix motif. J Biol Chem. 2015;290(11):7314–7322. doi: 10.1074/jbc.M114.622076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng X, et al. Characterization of differential pore-forming activities of ESAT-6 proteins from Mycobacterium tuberculosis and Mycobacterium smegmatis. FEBS Lett. 2016;590(4):509–519. doi: 10.1002/1873-3468.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volkman HE, et al. Tuberculous granuloma formation is enhanced by a mycobacterium virulence determinant. PLoS Biol. 2004;2(11):e367. doi: 10.1371/journal.pbio.0020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swaim LE, et al. Mycobacterium marinum infection of adult zebrafish causes caseating granulomatous tuberculosis and is moderated by adaptive immunity. Infect Immun. 2006;74(11):6108–6117. doi: 10.1128/IAI.00887-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brodin P, et al. Functional analysis of early secreted antigenic target-6, the dominant T-cell antigen of Mycobacterium tuberculosis, reveals key residues involved in secretion, complex formation, virulence, and immunogenicity. J Biol Chem. 2005;280(40):33953–33959. doi: 10.1074/jbc.M503515200. [DOI] [PubMed] [Google Scholar]
- 32.Berthet FX, Rasmussen PB, Rosenkrands I, Andersen P, Gicquel B. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10) Microbiology. 1998;144(Pt 11):3195–3203. doi: 10.1099/00221287-144-11-3195. [DOI] [PubMed] [Google Scholar]
- 33.Speer A, et al. Surface hydrolysis of sphingomyelin by the outer membrane protein Rv0888 supports replication of Mycobacterium tuberculosis in macrophages. Mol Microbiol. 2015;97(5):881–897. doi: 10.1111/mmi.13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Refai A, et al. Two distinct conformational states of Mycobacterium tuberculosis virulent factor early secreted antigenic target 6 kDa are behind the discrepancy around its biological functions. FEBS J. 2015;282(21):4114–4129. doi: 10.1111/febs.13408. [DOI] [PubMed] [Google Scholar]
- 35.King CH, Mundayoor S, Crawford JT, Shinnick TM. Expression of contact-dependent cytolytic activity by Mycobacterium tuberculosis and isolation of the genomic locus that encodes the activity. Infect Immun. 1993;61(6):2708–2712. doi: 10.1128/iai.61.6.2708-2712.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mostowy S, Cleto C, Sherman DR, Behr MA. The Mycobacterium tuberculosis complex transcriptome of attenuation. Tuberculosis. 2004;84(3-4):197–204. doi: 10.1016/j.tube.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Frigui W, et al. Control of M. tuberculosis ESAT-6 secretion and specific T cell recognition by PhoP. PLoS Pathog. 2008;4(2):e33. doi: 10.1371/journal.ppat.0040033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandenesch F, Lina G, Henry T. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: A redundant arsenal of membrane-damaging virulence factors? Front Cell Infect Microbiol. 2012;2:12. doi: 10.3389/fcimb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freer JH, Arbuthnott JP, Bernheimer AW. Interaction of staphylococcal α-toxin with artificial and natural membranes. J Bacteriol. 1968;95(3):1153–1168. doi: 10.1128/jb.95.3.1153-1168.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandes da Costa SP, et al. Identification of a key residue for oligomerisation and pore-formation of Clostridium perfringens NetB. Toxins. 2014;6(3):1049–1061. doi: 10.3390/toxins6031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoiczyk E, Blobel G. Polymerization of a single protein of the pathogen Yersinia enterocolitica into needles punctures eukaryotic cells. Proc Natl Acad Sci USA. 2001;98(8):4669–4674. doi: 10.1073/pnas.071065798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaw RK, Daniell S, Ebel F, Frankel G, Knutton S. EspA filament-mediated protein translocation into red blood cells. Cell Microbiol. 2001;3(4):213–222. doi: 10.1046/j.1462-5822.2001.00105.x. [DOI] [PubMed] [Google Scholar]
- 43.Clerc P, Baudry B, Sansonetti PJ. Plasmid-mediated contact haemolytic activity in Shigella species: Correlation with penetration into HeLa cells. Ann Inst Pasteur Microbiol. 1986;137A(3):267–278. doi: 10.1016/s0769-2609(86)80033-3. [DOI] [PubMed] [Google Scholar]
- 44. BEI Resources (2016) Production of Recombinant ESat-6 Under Non-Denaturing Conditions SOP (BEI Resources, Colorado State University, Fort Collins, CO). Available at csu-cvmbs.colostate.edu/Documents/dobos-rp004.pdf. Accessed October 28, 2016.
- 45.Ghigo E, et al. Coxiella burnetii survival in THP-1 monocytes involves the impairment of phagosome maturation: IFN-gamma mediates its restoration and bacterial killing. J Immunol. 2002;169(8):4488–4495. doi: 10.4049/jimmunol.169.8.4488. [DOI] [PubMed] [Google Scholar]
- 46.Levitte S, et al. Mycobacterial acid tolerance enables phagolysosomal survival and establishment of tuberculous infection in vivo. Cell Host Microbe. 2016;20(2):250–258. doi: 10.1016/j.chom.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lou Y, Rybniker J, Sala C, Cole ST. EspC forms a filamentous structure in the cell envelope of Mycobacterium tuberculosis and impacts ESX-1 secretion. Mol Microbiol. 2017;103(1):26–38. doi: 10.1111/mmi.13575. [DOI] [PubMed] [Google Scholar]
- 48.Ates LS, Brosch R. Discovery of the type VII ESX-1 secretion needle? Mol Microbiol. 2017;103(1):7–12. doi: 10.1111/mmi.13579. [DOI] [PubMed] [Google Scholar]
- 49.Walker DH, Feng HM, Popov VL. Rickettsial phospholipase A2 as a pathogenic mechanism in a model of cell injury by typhus and spotted fever group rickettsiae. Am J Trop Med Hyg. 2001;65(6):936–942. doi: 10.4269/ajtmh.2001.65.936. [DOI] [PubMed] [Google Scholar]
- 50.Le Chevalier F, et al. Revisiting the role of phospholipases C in virulence and the lifecycle of Mycobacterium tuberculosis. Sci Rep. 2015;5:16918. doi: 10.1038/srep16918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jamwal SV, et al. Mycobacterial escape from macrophage phagosomes to the cytoplasm represents an alternate adaptation mechanism. Sci Rep. 2016;6:23089. doi: 10.1038/srep23089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hertle R, et al. Specific phosphatidylethanolamine dependence of Serratia marcescens cytotoxin activity. Mol Microbiol. 1997;26(5):853–865. doi: 10.1046/j.1365-2958.1997.6031978.x. [DOI] [PubMed] [Google Scholar]
- 53.Brodin P, et al. Dissection of ESAT-6 system 1 of Mycobacterium tuberculosis and impact on immunogenicity and virulence. Infect Immun. 2006;74(1):88–98. doi: 10.1128/IAI.74.1.88-98.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fortune SM, et al. Mutually dependent secretion of proteins required for mycobacterial virulence. Proc Natl Acad Sci USA. 2005;102(30):10676–10681. doi: 10.1073/pnas.0504922102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Champion MM, Williams EA, Pinapati RS, Champion PA. Correlation of phenotypic profiles using targeted proteomics identifies mycobacterial esx-1 substrates. J Proteome Res. 2014;13(11):5151–5164. doi: 10.1021/pr500484w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Volkman HE, et al. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science. 2010;327(5964):466–469. doi: 10.1126/science.1179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chatterjee S, et al. Early secreted antigen ESAT-6 of Mycobacterium tuberculosis promotes protective T helper 17 cell responses in a toll-like receptor-2-dependent manner. PLoS Pathog. 2011;7(11):e1002378. doi: 10.1371/journal.ppat.1002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brilha S, et al. ESAT-6 drives MMP-10 gene expression and secretion in tuberculosis. Am J Respir Cell Mol Biol. September 21, 2016 doi: 10.1165/rcmb.2016-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Renshaw PS, et al. Structure and function of the complex formed by the tuberculosis virulence factors CFP-10 and ESAT-6. EMBO J. 2005;24(14):2491–2498. doi: 10.1038/sj.emboj.7600732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Macdonald SH, et al. Networked T cell death following macrophage infection by Mycobacterium tuberculosis. PLoS One. 2012;7(6):e38488. doi: 10.1371/journal.pone.0038488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lawrence SL, et al. Crystal structure of Streptococcus pneumoniae pneumolysin provides key insights into early steps of pore formation. Sci Rep. 2015;5:14352. doi: 10.1038/srep14352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramakrishnan L, Falkow S. Mycobacterium marinum persists in cultured mammalian cells in a temperature-restricted fashion. Infect Immun. 1994;62(8):3222–3229. doi: 10.1128/iai.62.8.3222-3229.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kinhikar AG, et al. Potential role for ESAT6 in dissemination of M. tuberculosis via human lung epithelial cells. Mol Microbiol. 2010;75(1):92–106. doi: 10.1111/j.1365-2958.2009.06959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Francis RJ, Butler RE, Stewart GR. Mycobacterium tuberculosis ESAT-6 is a leukocidin causing Ca2+ influx, necrosis and neutrophil extracellular trap formation. Cell Death Dis. 2014;5:e1474. doi: 10.1038/cddis.2014.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang X, et al. ESAT-6 inhibits production of IFN-gamma by Mycobacterium tuberculosis-responsive human T cells. J Immunol. 2009;182(6):3668–3677. doi: 10.4049/jimmunol.0803579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hemmati M, et al. Additive effect of recombinant Mycobacterium tuberculosis ESAT-6 protein and ESAT-6/CFP-10 fusion protein in adhesion of macrophages through fibronectin receptors. J Microbiol Immunol Infect. 2016;49(2):249–256. doi: 10.1016/j.jmii.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 67.Ganguly N, et al. Mycobacterium tuberculosis secretory proteins CFP-10, ESAT-6 and the CFP10:ESAT6 complex inhibit lipopolysaccharide-induced NF-kappaB transactivation by downregulation of reactive oxidative species (ROS) production. Immunol Cell Biol. 2008;86(1):98–106. doi: 10.1038/sj.icb.7100117. [DOI] [PubMed] [Google Scholar]
- 68.Peñuelas-Urquides K, et al. Measuring of Mycobacterium tuberculosis growth. A correlation of the optical measurements with colony forming units. Braz J Microbiol. 2013;44(1):287–289. doi: 10.1590/S1517-83822013000100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Veneman WJ, et al. Establishment and optimization of a high throughput setup to study Staphylococcus epidermidis and Mycobacterium marinum infection as a model for drug discovery. J Vis Exp. 2014;•••(88):e51649. doi: 10.3791/51649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Rooijen N, Hendrikx E. 2010. Liposomes for specific depletion of macrophages from organs and tissues. Liposomes, Methods in Molecular Biology, ed Weissig V (Humana, Totowa, NJ), pp 189–203. [DOI] [PubMed]
- 71.Takaki K, Davis JM, Winglee K, Ramakrishnan L. Evaluation of the pathogenesis and treatment of Mycobacterium marinum infection in zebrafish. Nat Protoc. 2013;8(6):1114–1124. doi: 10.1038/nprot.2013.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keller C, et al. Single cell measurements of vacuolar rupture caused by intracellular pathogens. J Vis Exp. 2013;•••(76):e50116. doi: 10.3791/50116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takaki K, Cosma CL, Troll MA, Ramakrishnan L. An in vivo platform for rapid high-throughput antitubercular drug discovery. Cell Rep. 2012;2(1):175–184. doi: 10.1016/j.celrep.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andersen P, Askgaard D, Ljungqvist L, Bennedsen J, Heron I. Proteins released from Mycobacterium tuberculosis during growth. Infect Immun. 1991;59(6):1905–1910. doi: 10.1128/iai.59.6.1905-1910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miles AA, Misra SS, Irwin JO. The estimation of the bactericidal power of the blood. J Hyg. 1938;38(6):732–749. doi: 10.1017/s002217240001158x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ragle BE, Bubeck Wardenburg J. Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect Immun. 2009;77(7):2712–2718. doi: 10.1128/IAI.00115-09. [DOI] [PMC free article] [PubMed] [Google Scholar]