Significance
Fish exhibit remarkable sexual plasticity. However, the underlying mechanism of heat-induced sex reversal is still unclear. Here we first established the conditions for heat-induced reprogramming of sexual phenotypes in zebrafish through sex ratio analysis and gonad transcriptomics. Sex ratio response to heat was family-specific and resulted in masculinization. We observed two heat-induced gonadal transcriptomic profiles per sex in adults, among them neomales and, strikingly, females with an ovary but a “male-like” transcriptome. The latter indicates major transcriptomic reprogramming with preserved organ structure, an interesting observation in vertebrates. In all heat-treated juveniles, we also observed a male-like transcriptome. Overall, this study reveals novel lasting thermal effects on fish gonads, with practical implications for studying the effects of global warming in natural populations.
Keywords: zebrafish, temperature, masculinization, polygenic sex determination, global warming
Abstract
Understanding environmental influences on sex ratios is important for the study of the evolution of sex-determining mechanisms and for evaluating the effects of global warming and chemical pollution. Fishes exhibit sexual plasticity, but the underlying mechanisms of environmental effects on their reproduction are unclear even in the well-established teleost research model, the zebrafish. Here we established the conditions to study the effects of elevated temperature on zebrafish sex. We showed that sex ratio response to elevated temperature is family-specific and typically leads to masculinization (female-to-male sex reversal), resulting in neomales. These results uncovered genotype-by-environment interactions that support a polygenic sex determination system in domesticated (laboratory) zebrafish. We found that some heat-treated fish had gene expression profiles similar to untreated controls of the same sex, indicating that they were resistant to thermal effects. Further, most neomales had gonadal transcriptomes similar to that of regular males. Strikingly, we discovered heat-treated females that displayed a normal ovarian phenotype but with a “male-like” gonadal transcriptome. Such major transcriptomic reprogramming with preserved organ structure has never been reported. Juveniles were also found to have a male-like transcriptome shortly after exposure to heat. These findings were validated by analyzing the expression of genes and signaling pathways associated with sex differentiation. Our results revealed a lasting thermal effect on zebrafish gonads, suggesting new avenues for detection of functional consequences of elevated temperature in natural fish populations in a global warming scenario.
A major paradox in developmental biology is the lack of conservation of sex-determining mechanisms even in closely related taxa (1). Thus, in mammals and birds, sex is genetically canalized by a chromosomal sex determination (CSD) system of male (XX/XY in mammals) or female (ZW/ZZ in birds) heterogamety, driven by the action of a master sex-determining gene: sry in mammals and dmrt1 in birds (1). In contrast, fish sex can be very plastic, with the combination of genetic and environmental influences (2). In addition to fish species with CSD, there are species with polygenic sex determination (PSD), in which sex depends on the combined action of several promale and profemale genetic factors, and species with temperature-dependent sex determination (TSD) (3). Understanding how the environment influences sex ratios in vertebrates is of fundamental importance in the study of the evolution of sex-determining mechanisms (4, 5). It is also of practical relevance in evaluating the effects of climate change and chemical pollution (6, 7).
The effects of temperature on gene expression during sex differentiation have been investigated in different teleost species, including the African catfish, Clarias gariepinus (8), European sea bass, Dicentrarchus labrax (9, 10), Japanese flounder, Paralichthys olivaceus (11), pejerrey, Odontesthes bonariensis (12), and Nile tilapia, Oreochromis niloticus (13, 14). Regardless of the actual underlying sex-determining mechanism, a shared characteristic of all fish species in which temperature can alter sex ratios is that exposure to heat during early development up-regulates the expression of genes related to testis differentiation with a concomitant down-regulation of genes related to ovarian differentiation, as assessed in several species (e.g., 9, 13, 15–17). This results in an increase in the proportion of males (18) and implies that some genetic females (XX or ZW females) in CSD species, or fish that otherwise would develop as females in a PSD system, end up developing as phenotypic males despite their genetic makeup. These masculinized or sex-reversed females are called “neomales,” and have testicular structure (19) and gene expression profiles similar or identical to those of males (20, 21) and can produce viable sperm. In fact, in several farmed species with male heterogametic sex determination, hormone- or heat-induced XX neomales are crossed with regular XX females and used for the production of all-female stocks (22). However, a complete picture of the underlying molecular mechanisms of heat-induced sex reversal in fish, including signaling pathways, is far from clear. In addition, interesting problems such as why some females resist heat-induced masculinization whereas others do not and whether there are morphological and/or transcriptomic differences between these two classes of females have never been investigated.
The zebrafish (Danio rerio) is a small tropical fish that has become an established model for many fields of research. Sex differentiation starts about 10 d post fertilization (dpf) with the development of a juvenile ovary in all individuals, regardless of the actual sexual genotype (23, 24). Then, during the 21- to 35-dpf period, in the future males the juvenile ovaries undergo apoptosis and are transformed into testes, with interindividual variation in the timing and duration to complete this process. In contrast, ovarian differentiation continues in the future females (25), with an increase in cytoplasm volume of oocytes at around 60 dpf. Transcriptomic analysis of zebrafish sex differentiation has shown that apoptosis (24), activated by the p53 signaling pathway (26), is required for testis differentiation, whereas up-regulation of the canonical Wnt and NF-κB signaling pathways is needed for ovarian differentiation (27, 28).
Zebrafish sex differentiation is, therefore, well-understood in its major aspects. In contrast, its primary sex-determining system was unclear until recently. Data from most cytogenetic studies (for a review, see ref. 29) and breeding experiments (30, 31) indicated the lack of sex chromosomes, pointing to PSD, whereas a hormonal sex reversal experiment (32) and restriction site-associated DNA (RAD) mapping data from crosses involving wild-type parents pointed toward a ZZ/ZW CSD system (33, 34). Eventually, comparative analysis of wild-caught and domesticated lines indicated the presence of sex chromosomes in the former and the apparent loss of the region harboring the sex-determining gene during the domestication process in the latter (34). This change in the mode of SD might be the cause for domesticated zebrafish being more susceptible to environmental changes and therefore well-suited for studying the effects of elevated temperature on its development.
In domesticated zebrafish, the effects of elevated temperature have been studied after exposure periods concerning embryos (35), juveniles (36, 37), or development until adults (38, 39). In agreement with the general pattern in fish (18), elevated temperatures result in a higher number of males, although, surprisingly, one study reported more females (40). However, most of the above experiments involved progeny derived from a single family, mixtures of several broods, low sample sizes, or offspring from gynogenetic parents, the latter raising the possibility of unaccounted effects of inbreeding. In addition, transcriptomic studies in zebrafish on the effect of exposure to heat are limited and were usually performed shortly after heat treatment (41). Therefore, a detailed study on how elevated temperature affects sex ratios in a family-dependent manner and the long-term transcriptomic effects in domesticated zebrafish with PSD is not available.
Thus, domesticated strains of zebrafish, by virtue of having lost the primary switch of their SD system, provide an excellent opportunity to investigate, in a well-established model rich in genomic resources, the influences of the environment on sex determination and gonad differentiation. In this study, we used a combination of approaches to characterize the sex ratio response to temperature in this model and, importantly, to study the underlying molecular mechanisms responsible for heat-induced masculinization.
Results
Defining Conditions for Studying Temperature Effects on Zebrafish Sex Ratios.
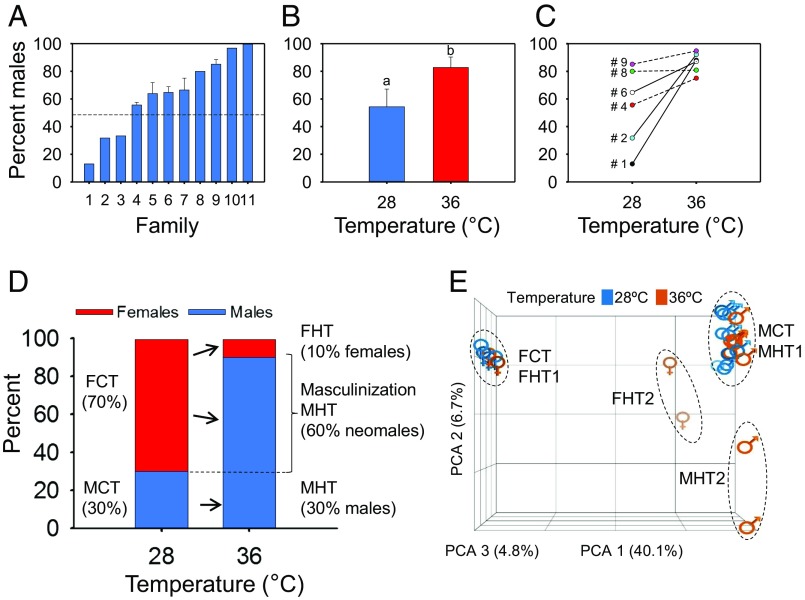
The sex ratio determined for 11 different pairs of the AB strain by growing their offspring to maturity at control temperature (28 °C) ranged from 13 to 100% males (Fig. 1A; for details, see SI Appendix, Materials and Methods). First, we performed experiments to determine whether temperature affected the adult sex ratio and, if so, to delineate the optimal conditions of heat treatment for this study. Thus, we subjected eight families (3, 4, 5, 6, 7, 8, 10, and 11) to four different temperatures: 22 °C, 28 °C, 34 °C, and 36 °C. Treatments were administered during three different developmental periods: 0 to 14, 7 to 21, and 18 to 32 dpf. The lowest temperature (22 °C; LT) did not cause a significant change in sex ratio compared with untreated controls in any of the three periods tested (SI Appendix, Fig. S1), and therefore this temperature was not further considered. Temperature treatment during the 0- to 14-dpf period resulted in survival rates below the acceptable standards recommended by the Organisation for Economic Cooperation and Development (OECD)’s Fish Sexual Developmental Test on zebrafish (42), and thus this period was excluded from the following experiments. The analysis of the 7- to 21- and 18- to 32-dpf periods gave similar results, so we chose the latter because fish were more robust after that treatment. Results obtained after applying 34 °C indicated that there was little or incomplete masculinization. At 36 °C (HT), a significant increase in the masculinization rate was observed compared with the control temperature (28 °C; CT) (P < 0.05; Fig. 1B). Although masculinization was still incomplete, the proportion of males obtained was higher than at 34 °C. Thus, we performed the subsequent heat treatments at 36 °C and during the exposure period of 18 to 32 dpf.
Fig. 1.
Masculinizing effect of early high-temperature treatment in zebrafish. (A) Sex ratio variability in zebrafish broods originating from 11 different families when reared at control temperature (28 °C). For five families (4 to 7 and 9), where two to six broods from repeated crosses were analyzed, the mean ± SEM is shown. A total of 1,202 fish were used. (B) Early treatment (18 to 32 dpf) with high temperature (36 °C) increased the proportion of males. The mean ± SEM of six independent families for control (28 °C; n = 421) and treated (36 °C; n = 316) groups is shown. Statistically significant differences were found between the two sex ratios (P < 0.05; χ2 test). (C) Genotype-dependent variation of the sex ratio response to high temperature (36 °C), as illustrated by the broods originating from six different families. Lines between data points represent significant (solid) and nonsignificant (dashed) differences between control (28 °C) and heat-treated (36 °C) groups. The number of offspring individuals sexed per family were as follows: family 1, n = 110; family 2, n = 92; family 4, n = 112; family 6, n = 168; family 8, n = 56; family 9, n = 199. # indicates family IDs from A. (D) Increase in male proportion at 90 dpf after early exposure to high temperature (36 °C; 7 to 32 dpf; n = 51) in a zebrafish brood originating from a family (#2) that produced a female-biased offspring (70% females) when reared at control temperature (28 °C; n = 41). FCT, females control temp. FHT, females high temp. MCT, males control temp. MHT, males high temp. (E) Clustering of untreated controls (28 °C; blue) and heat-treated (36 °C; orange) 90-dpf fish based on their gonadal transcriptome profiles by principal component analysis (PCA). Whereas the majority of males and females formed a tight group each, two males (MHT2) and two females (FHT2) with different profiles could be observed in both sexes among fish exposed to 36 °C.
Sex Ratio Response to High Temperature Is Family-Dependent in Zebrafish.
When the offspring from six families (1, 2, 4, 6, 8, and 9; Fig. 1A) were subjected to heat treatment (36 °C; 18 to 32 dpf) and were compared with their respective controls, three of them (1, 2, and 6) had a significant (P < 0.05) increase in the proportion of males, two of them (4 and 9) had a modest, nonsignificant (P = 0.07) increase, whereas for family 8 the sex ratio of the offspring remained unchanged (Fig. 1C). These results show that, although the extent of masculinization was family-specific, in most cases higher temperatures led to an increased proportion of males, denoting the existence of a clear genotype-by-environment (GxE) interaction during gonad differentiation. One of the families (2; 30% males; Fig. 1D) shows a female-biased family at control temperature that became male-biased (90% males) after heat treatment. Thus, it was estimated that a substantial proportion (60%) of the heat-treated fish underwent sex reversal to become phenotypic males (neomales; Fig. 1D). The remaining 10% in the population represent heat-resistant individuals that continued to develop as females despite the heat treatment. Whether there is an underlying genetic difference between these females and the neomales is not known.
The survival of control and heat-exposed fish was measured at three different time points: 6, 50, and 90 dpf. Survival was always within the acceptable limits defined by OECD-recommended standards (42). There was an overall significant (P < 0.01) reduction in the number of fish surviving at 50 dpf compared with 6 dpf for both control and heat-treated groups (SI Appendix, Fig. S2A), indicating that the mortality was not due to temperature. Thereafter, the number of surviving fish did not significantly change until they were sampled at the end of the experiment at 90 dpf. Furthermore, at each considered age there were no significant differences between the survivals of heat-treated fish compared with controls (SI Appendix, Fig. S2A). Therefore, observed sex ratio results are not due to differential survival of one sex with respect to the other.
At 90 dpf, there were no statistically significant differences between the body weight and standard length of males and females exposed to elevated temperature during the thermosensitive period compared with controls of the same sex (SI Appendix, Fig. S2B). These results indicated that effects observed in the gonadal transcriptome (see section below) of heat-exposed individuals were due to a direct temperature effect on the gonads and not to indirect temperature effects through possible differences in size.
When the maturation level of gonads was checked visually and histologically at 90 dpf, we found three types of gonads in both sexes: immature (type 1), maturing and differentiating (type 2), and mature and differentiated (type 3) (for a detailed description of these three gonadal types in both sexes, see SI Appendix, Materials and Methods, Fig. S3, and Table S1). We found differences in gonadal maturation depending on temperature treatment and sex. At control temperature, type 1 gonads were observed in more than half of the males examined, as opposed to only one-fifth of the females (SI Appendix, Fig. S2 C and D, respectively). Interestingly, at high temperature, a significantly higher percentage of type 3 gonads was observed in males (43.8% vs. 21.8%; P < 0.001) compared with controls (SI Appendix, Fig. S2C), whereas, in contrast, in females the proportion of type 3 gonads was reduced from ∼50% in the controls to ∼37% in the fish exposed to 36 °C, although differences were not statistically significant (SI Appendix, Fig. S2D).
Compared with 34 °C, treatment at 36 °C also increased the incidence of deformities, ranging from 0 to 42.6%, depending on the family, as observed in a subset of samples (n = 53 individuals). Deformities included a swollen belly, spinal cord malformation, and loss of pigmentation, regardless of the treatment period. However, all fish used for transcriptomic analysis had a normal morphology.
Transcriptomic Analysis of Adult Zebrafish Gonads Revealed an Unexpected Complexity in the Expression Profiles as a Result of Heat Treatment.
For microarray analysis, we used a custom-designed array (see SI Appendix, Materials and Methods for details) and individuals from one family (2; Fig. 1C) with a female-biased sex ratio (30% males) at 28 °C and with a strong masculinization effect (90% males) at 36 °C (Fig. 1D) to maximize the probability of finding neomales. We isolated the gonads from 4 adult females and 10 adult males at 90 dpf of each group (28 °C and 36 °C), and the whole gonad for each fish was hybridized individually. A higher number of males than females were analyzed to increase the chance for identifying potential differences between the transcriptomes of males and neomales.
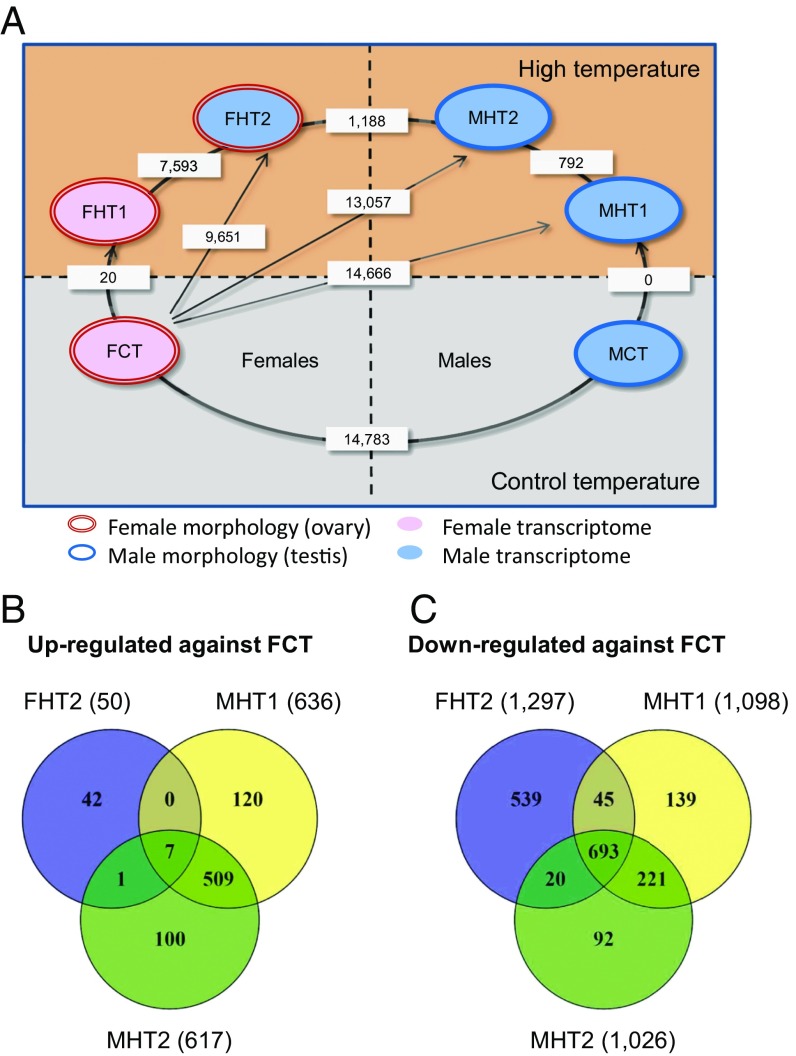
When the resulting expression profiles were subjected to principal component analysis (PCA; see Dataset S1 for PCA loadings), two distinct transcriptomic profiles were observed for the control group (28 °C; CT), corresponding to control females (FCT) and control males (MCT), as expected (Fig. 1E and SI Appendix, Table S2). A large number of transcripts (14,783) were differentially expressed between these two groups (Fig. 2A). These differences were not due to temperature but to sex, and represent a substantial proportion of protein-coding genes in zebrafish (43). This concurs with previous observations (44–46) showing large sex-related differences in the gonadal transcriptome of the adult zebrafish.
Fig. 2.
Gonadal transcriptome analysis shows differential expression between adults exposed to high temperature early in development. (A) Pairwise comparison of gonadal transcriptome profiles between control and heat-treated zebrafish grouped based on the results of principal component analysis. Only two different gonadal transcriptomes are observed at the control temperature (Lower), corresponding to control females (FCT) and control males (MCT). Exposure to high temperature resulted in two female transcriptomes, FHT1 and FHT2, as well as two male transcriptomes, MHT1 and MHT2 (Upper). The FHT2 and MHT2 transcription profiles differed not only from each other but also from those of control males (MCT) and females (FCT). Numbers of differentially expressed transcripts [fold change (FC), 1.5×; false discovery rate (FDR), P ≤ 0.01] between two phenotypes are indicated in the white rectangles. Sample sizes: FCT, n = 4; FHT1, n = 2; FHT2, n = 2; MCT, n = 10; MHT1, n = 8; MHT2, n = 2. (B and C) Venn diagram representation of biological process GO terms (B) up-regulated or (C) down-regulated in MHT1, MHT2, or FHT2 in pairwise comparison with FCT, as determined by microarray analysis (FC, 1.5×; FDR, P ≤ 0.01; see SI Appendix, Fig. S5 for similar Venn diagrams showing differentially expressed cellular component and molecular functions GO terms, as well as Dataset S4 for the complete list of all GO terms shown in this figure).
Among the 10 HT males randomly selected from this family for transcriptomic analysis, we observed two different profiles (Fig. 2A and SI Appendix, Fig. S4A): Eight of the 10 sampled HT males exhibited what we named the MHT1 profile (SI Appendix, Table S2), namely transcriptomically identical to MCT (a complete lack of differentially expressed transcripts; DETs; Fig. 1E). This resemblance was also reflected by comparing MHT1 and MCT males vs. FCT females, because a similar number of DETs were observed (14,666 and 14,783, respectively). The remaining two HT males had an MHT2 profile and formed a distinct cluster on the PCA plot (Fig. 1E). Based on the proportions of HT fish (Fig. 1D), we expected three or four normal males and six or seven neomales, which would have developed as females at control temperature (SI Appendix, Fig. S4B). This showed that some of the heat-induced neomales had gonadal expression profiles identical to those of regular males. Thus, both MHT2 males are likely to be neomales, with gonadal transcriptomes that differed from MHT1 males and FCT females by 792 and 13,057 DETs (Dataset S2), respectively. Likewise, half of the MHT1 males are also likely to be neomales.
For HT females, we found that (i) the PCA plot revealed two very distinct transcriptomic profiles, which we named FHT1 and FHT2 females (Fig. 1E); (ii) expression analysis showed that FHT2 females had a much higher number of DETs compared with FCT females (9,651) or even with FHT1 females (7,593) than with MHT2 males (1,188) (Fig. 2A); (iii) hierarchical clustering further supported the above observations by placing FHT2 females into the same clade with the males (SI Appendix, Fig. S4A). Together, these results showed that the gonadal transcriptome of FHT2 females was strikingly similar to that of males, despite having female gonadal morphology. On the other hand, the transcriptomic profile of the remaining high temperature-exposed females (FHT1) was very similar to that of control females, with only 20 DETs observed between the two groups (Fig. 2A and Dataset S2).
Thus, PCA-based classification of the gonadal transcriptome profiles, which explained more than half of the total observed variance (51.6%), allowed a clear arrangement of the different transcriptomic profiles in a Cartesian space (FCT→FHT1→FHT2→MHT2→MHT1→MCT) based on the magnitude of DETs among them (Fig. 2A and Dataset S2). In summary, we found a good match overall between the number of males and females, as determined by visual and histological gonadal analysis, and the results from the microarray-based transcriptomic analysis. Females appeared to show a more variable response to heat-induced masculinization than males, potentially indicating a threshold-based mechanism, and some of the gonadal/transcriptomic combinations produced in the former were unexpected.
Expression Analysis of Selected Genes Confirmed the Existence of Distinct Gonadal Transcriptomes in Both Sexes of Heat-Treated Zebrafish.
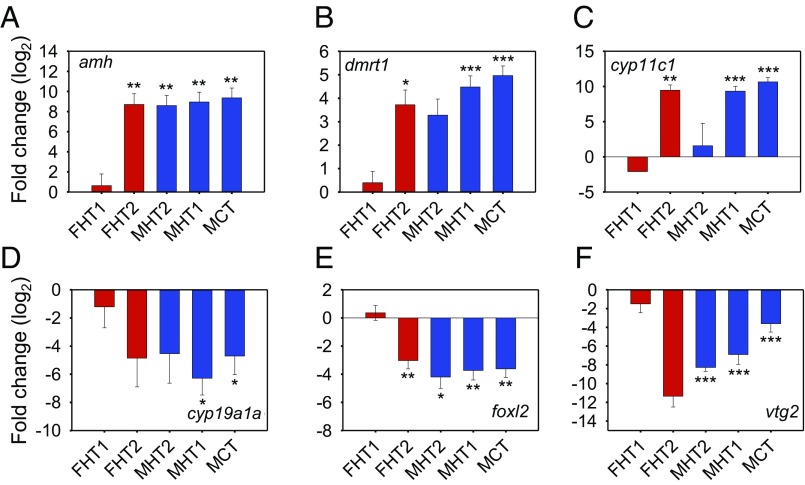
Next, we performed a pairwise comparison of the gonadal transcriptomic profiles of heat-treated fish that had the largest differences with those of control females (i.e., FHT2, MHT2, and MHT1 vs. FCT) and also comparisons among heated males and females (i.e., FHT1 vs. FHT2, FHT2 vs. MHT2, and MHT2 vs. MHT1) and searched for both up- and down-regulated DETs, creating separate lists per comparison (Dataset S2). Among these lists, we looked at the expression of 48 genes selected for their importance for reproduction and sex differentiation in fish (SI Appendix, Materials and Methods and Dataset S3). Among the comparisons, we found that some genes with promale function (e.g., dmrt1, amh, hsd11b2, cyp11c1) were up-regulated in control (MCT) and heated males (MHT1 and MHT2) compared with control females (FCT) but also in FHT2 heated females (FHT2 vs. FCT), whereas some genes with profemale function (e.g., cyp19a1a, foxl2, fancl, esr2a, figα) were down-regulated in all of the mentioned comparisons (full gene names and their abbreviations can be found in SI Appendix, Table S7, and in Dataset S2). None of these genes were differentially expressed between FHT1 and FCT, and only a few between MHT1 and MCT, MHT2 and MHT1, and MHT2 and FHT2, indicating transcriptomic similarity between the groups in these comparisons.
These lists were used for Gene Ontology (GO)-enrichment analysis (SI Appendix, Materials and Methods). In the biological process (BP) category, there were 700 GO terms found to be enriched between the three comparisons that are potentially involved in masculinization (Fig. 2 B and C). The differentially expressed cellular component (CC) and molecular functions (MF) GO terms are shown in SI Appendix, Fig. S5, and all of the above-mentioned GO terms are listed in Dataset S4. Some GO terms that were enriched in the up-regulated DET lists of FHT2, MHT2, and MHT1 were related to the male pathway, such as spermatid development (GO:0007286) and insulin-like growth factor binding (GO:0005520). On the other hand, the down-regulated DET lists were enriched with GO terms related to the female pathway, such as gonad development (GO:0008585), sex differentiation (GO:0046660), and ovarian follicle development (GO:0001541).
To validate the microarray results and obtain expression data from key reproduction-related genes, we performed quantitative (q)PCR analysis on a subset of 35 genes that showed significantly different expression levels in at least one of the following comparisons: MHT1 vs. FCT, MHT2 vs. FCT, FHT2 vs. FCT, and MCT vs. FCT. The direction and magnitude of change for over two-thirds of the genes examined (mean, 80.5%; range, 62.5 to 92.5%) showed an agreement between the two methods, thus validating our microarray results (SI Appendix, Tables S3 and S4).
A heat map was generated using the expression profiles of these 35 genes determined by qPCR (SI Appendix, Fig. S6). The dendrogram showed two major branches, one containing only females (FCT and FHT1 clades) and the other branch containing all of the males together with the two FHT2 females. This clustering showed that the transcriptome of FHT2 females was more similar to those of the males than of FCT females, confirming the previous PCA results (Fig. 1E). Furthermore, genes were grouped into two major branches, one containing genes involved in male sex differentiation (e.g., amh, dmrt1, cyp11c1, etc.) and the other containing genes involved in female sex differentiation (e.g., cyp19a1a, foxl2, vtg5, etc.) (Fig. 3 and SI Appendix, Fig. S6 and Table S3). The expression profiles of both gene subsets were in agreement with the expected changes in both sexes. Genes involved in male sex differentiation showed higher expression levels in the three male groups (MCT, MHT1, and MHT2) and, interestingly, in the FHT2 group as well (Fig. 3 A–C). The opposite pattern was observed for genes involved in female sex differentiation (Fig. 3 D–F).
Fig. 3.
Gene expression profiles of six candidate genes with sex-associated function support the expression profiles of FHT2 females and MHT2 males. These data were obtained by qPCR analysis of gonadal expression levels at 90 dpf. The expression level of three genes with “promale” function (A–C) and three with “profemale” function (D–F) are shown relative to those of control females (FCT). Females are marked in red (FHT1 and FHT2) and males are marked in blue (MCT, MHT1, and MHT2). Average values from 2 to 10 individuals per group are shown with standard error of the mean (SEM). Values that show a statistically significant difference from FCT are labeled as: *P < 0.05, **P < 0.01, or ***P < 0.001; all Student’s t test.
Then, we compared the expression profiles of these 35 validated genes across the six identified transcriptome types by performing pairwise comparison, with FCT as the reference group (SI Appendix, Table S3). Results showed that there were no significant differences between FHT1 and FCT, indicating a strong concordance in expression levels. Furthermore, 25 and 26 genes in MHT1 and MCT, respectively, showed significantly different expression levels (P < 0.01) compared with FCT. Compared with FCT, the FHT2 and MHT2 groups had 19 and 17 DE genes, respectively. These data provided yet another level of confirmation for, on one hand, the similarity between both female groups (FHT1 and FCT) and that of male groups MHT1 and MCT and, on the other hand, the Cartesian arrangement previously observed with the PCA.
Appearance of a Male-Like Transcriptome in Juveniles After Heat Exposure.
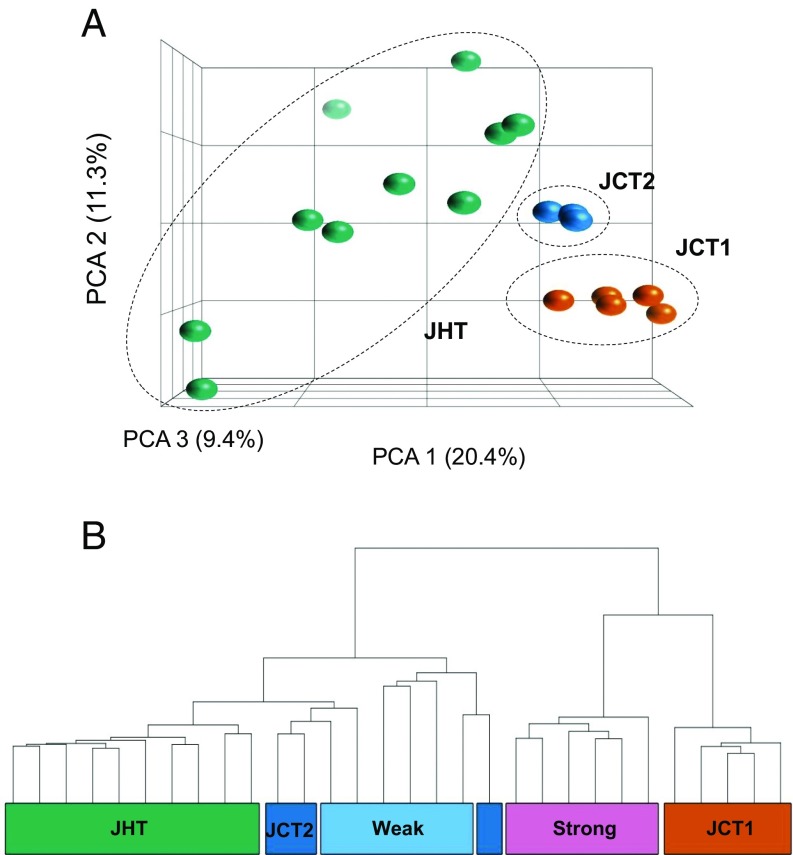
Results presented so far were obtained in adult zebrafish at 90 dpf, 2 mo after the end of the heat treatment, and thus represent long-lasting consequences of heat exposure on the gonadal transcriptome. We were also interested in assessing the immediate effects of heat exposure. Thus, we collected 10 control (JCT) and 10 heat-exposed (JHT) juveniles from another heat-responsive family (1; Fig. 1C; percent males at 90 dpf: controls, 13%; heat-exposed, 88%) by sampling them at 37 dpf, that is, 5 d after the end of the heat treatment, to avoid the period of heat shock protein induction. We performed microarray analysis on RNA samples obtained from body trunks (as at this stage the gonad was too small to isolate) and found two outliers from the JCT samples due to poor hybridization results, which were removed from further analysis. PCA of the microarray results revealed two groups in the control juveniles, named JCT1 (five individuals) and JCT2 (three individuals) (Fig. 4A). We found 425 DETs between these two groups (Dataset S2). Sexing of the juvenile zebrafish by examining external phenotypes at this age is not possible. Therefore, we included in the hierarchical clustering samples from the Tg(vasa:vasa-egfp) transgenic line (47) collected at similar age (35 and 42 dpf), because it allows for early sexing from 16 dpf onward (25). This way, we were able to infer the sex of the two juvenile groups at control temperature (JCT1 and JCT2). All JCT1 samples formed a clade with those transgenics that had strong gonadal fluorescence, indicating future females (Fig. 4B). In contrast, all JCT2 samples clustered with those that had very weak fluorescence intensity, denoting future males. Transcriptome-based molecular sexing for the whole group of JCT juveniles showed a female-biased sex ratio, in agreement with the adult sex ratio of this family. Then, we applied the same procedure to the JHT juveniles and found that all of them clustered with transgenic fish exhibiting weak gonadal fluorescence and JCT2 juveniles (Fig. 4B), indicating that all of them had a “male-like” transcriptomic profile after heat treatment even though they showed a diffuse profile on the PCA plot (Fig. 4A).
Fig. 4.
Genome-wide transcriptomic analysis of juveniles (control, JCT; heat-treated, JHT) analyzed at 37 dpf shows groups related to sex (JCT, n = 8; JHT, n = 10). (A) PCA plot produced by expression profiles showing JCT clustered into two groups, namely JCT1 (orange) with five individuals and JCT2 (dark blue) with three individuals. All 10 JHT individuals (green) were placed in a single group. (B) Hierarchical clustering of control and heat-exposed juveniles together with Tg(vasa:vasa-egfp) juveniles using the 425 differentially expressed transcripts between JCT1 (orange) and JCT2 (dark blue). Juveniles in the JCT1 group were most likely females, as they clustered with transgenic juveniles that showed strong fluorescence intensity (future females; pink). The JCT2 juveniles were most likely males, as they were in the same node as the transgenic juveniles with weak fluorescence intensity (future males; light blue). All of the 10 heat-treated juveniles (JHT; green) clustered with the weak fluorescence transgenic juveniles and JCT2, indicating a similar male-like transcriptomic profile regardless of their genetic sex.
We found 75 second-level GO terms (BP, CC, and MF) showing significant differences when comparing JCT2 vs. JCT1 (all of them down-regulated) and 295 GO terms in JHT vs. JCT1 (Dataset S4). There were 68 GO terms that were common between the two comparisons and therefore related to male differentiation (JCT2 and JHT groups). Some of those were involved in female sex differentiation and down-regulated, for example female gamete generation (GO:0007292) and oogenesis (GO:0046620). However, some of the GO terms related to the male pathway were also down-regulated compared with JCT1 (profemales), for example spermatid development (GO:0007286) and spermatogenesis (GO:0007283). Interestingly, and exclusively related to the heat response (only found in the JHT vs. JCT1 comparison), we found a down-regulation of some GO terms associated with female sex differentiation such as oocyte maturation (GO:0001556) and ovarian follicle development (GO:0001541), suggesting that reprogramming was taking place already at the time of heat exposure.
We also performed qPCR analysis on the juvenile samples using 26 of the 35 DE genes validated in adults. In the JCT2 vs. JCT1 and JHT vs. JCT1 comparisons, the expression of more than two-thirds (84.6% and 76.9%, respectively) of the genes examined showed a significant (P < 0.05 and P < 0.01, respectively) change in the same direction with both methods (SI Appendix, Table S4). Genes involved in testis differentiation, such as dkk3 or cyp11c1, were up-regulated in the JCT2 and JHT groups, whereas those related to ovarian development, such as cyp19a1a or zp2, were down-regulated, as previously found in adults. Overall, the qPCR results (SI Appendix, Fig. S7 and Table S4), together with the hierarchical clustering data (Fig. 4B), indicated that both JHT and JCT2 groups had a promale transcriptome, probably at different stages of gonadal development.
Different Effects of Heat from Juvenile to Adult.
Four different patterns of gene expression profiles from 50 genes were observed between heat-treated juveniles and adults in comparison with their respective controls (Table 1 and SI Appendix, Table S5). (i) Permanent effects involved genes that responded similarly both in juveniles and adults. For example, dnd expression was consistently and significantly down-regulated. (ii) Transient or inconsistent effects involved genes for which differences in expression were observed in juveniles but not in adults, or those that responded differently at the two stages. For example, sox9a was up-regulated in juveniles but not in adults, and foxl2 expression was up-regulated in juveniles but down-regulated in adults. (iii) Delayed effects included genes, such as amh or dnmt3b, for which differences were observed only in adults, long after the heat treatment had ceased. (iv) No effect involved genes, such as fshr, for which no significant differences were found between the treated and control groups in juveniles or adults.
Table 1.
Effects of heat on zebrafish gonad gene expression
| Juveniles | Adults | Type of heat effects | Number of genes | Examples |
| + | + | Permanent | 5 | ctnnbip1, ddx4, dkk3, dnd |
| + | − | Transient | 10 | piwil1, sox9a, foxl2, vtg5 |
| − | + | Delayed | 18 | amh, cyp19a1a, dmrt1, dnmt3b |
| − | − | None | 17 | fshr, nr3c1, sox3, tp53 |
Juveniles refer to juvenile high temperature (JHT) vs. juvenile control temperature (JCT1), whereas adults refer to female high temperature (FHT2) and male high temperature (MHT1 and MHT2) vs. FCT. A total of 50 genes with a cognate function in fish sex determination/differentiation in vertebrates were considered (see SI Appendix, Table S5 for the complete set of genes). Genes are not affected (−) or significantly (P < 0.01) affected (+) in the heat-treated groups compared with the controls.
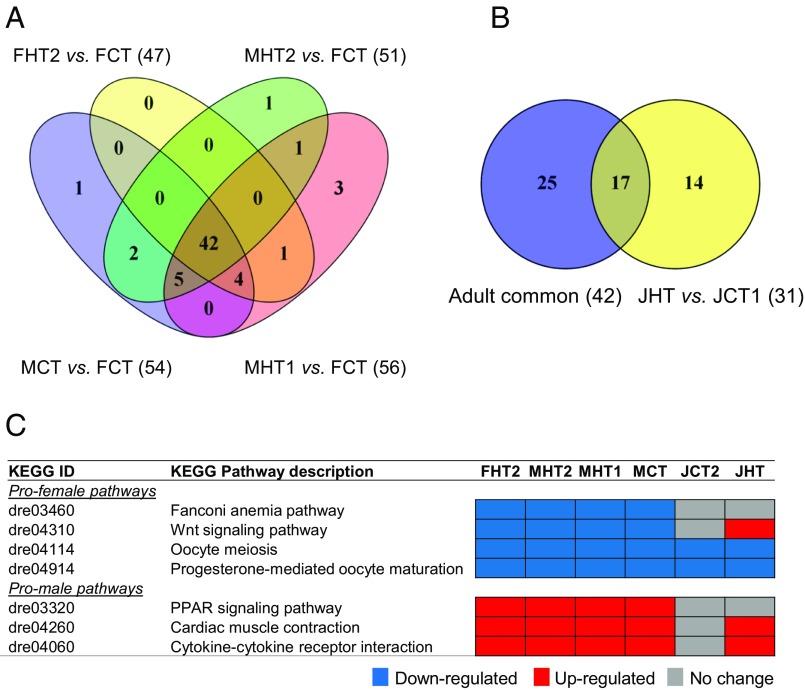
Then, we looked for cellular pathways involved in the heat response and sex differentiation in the gonads. A total of 256 Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were differentially expressed among juveniles and adults treated by heat (Dataset S5), 66 of which were unique and nonredundant pathways. Among the nonredundant pathways, 42 were enriched across the different adult groups analyzed (Fig. 5A) and included signaling pathways that were known to be involved in gonadal development. To further study the different effects of heat from juvenile to adult, we also analyzed the common pathways expressed in adults and juveniles, finding a total of 17 common pathways (Fig. 5B and Dataset S5). Four important pathways for ovarian development, namely Fanconi anemia, Wnt signaling, oocyte meiosis, and progesterone-mediated oocyte maturation, were down-regulated in FHT2 and in all of the adult male phenotype groups compared with FCT (Fig. 5C). On the other hand, three KEGG pathways that were involved in testis development were up-regulated in all of the adult male groups and FHT2 (Fig. 5C). These signaling pathways are the peroxisome proliferator-activated receptor (PPAR) signaling pathway, calcium signaling pathway in cardiac muscle contraction, and cytokine–cytokine receptor interaction.
Fig. 5.
Differentially expressed (DE) KEGG pathways found in juveniles and adult zebrafish exposed to heat during early stages of development (18 to 32 dpf) in comparison with controls. (A) Venn diagram of common DE KEGG pathways found in adults FHT2 vs. FCT, MHT2 vs. FCT, MCT vs. FCT, and MHT1 vs. FCT (FC, 1.5×; FDR, P ≤ 0.05). (B) Venn diagram of DE KEGG pathways found between the common KEGG pathways obtained in adults (total of 42) and the heat-treated juveniles (JHT vs. JCT1; FC, 1.5×; FDR, P ≤ 0.05). (C) List of pathways (four profemale and three promale) involved in the heat response and sex differentiation in the gonads of juvenile and adult fish showing early activation of promale pathways in the heat-treated juveniles. Up-regulated KEGG pathways are labeled in red, down-regulated are labeled in blue, and not differentially expressed are labeled in gray (FC, 1.5×; FDR, P ≤ 0.05).
The same pathways were also affected in the control male juveniles (JCT2) and heat-treated juveniles (JHT) compared with control female juveniles (JCT1). Two of the pathways with promale signaling elements (cardiac muscle contraction and cytokine–cytokine receptor interaction) were up-regulated in the JHT group but not differentially expressed in the JCT2 group (Fig. 5C). For the four profemale pathways, two of them (oocyte meiosis and progesterone-mediated oocyte maturation) were down-regulated in both JHT and JCT2 compared with JCT1, whereas the Wnt signaling pathway was up-regulated only in the JHT group (Fig. 5C).
Discussion
In this study, we first optimized the conditions to examine the effects of high temperature during early development on zebrafish sex ratios. Then, we focused on identifying the associated changes in the gonadal transcriptome at juvenile and adult stages.
Our results on sex ratio variation among, but not within, families of domesticated zebrafish corroborate previous observations from other PSD systems (48–50), namely that at normal temperatures offspring sex ratio has a genetic component with strong influence of parental genotypes (30). These data support the earlier claims that sex determination in domesticated zebrafish is polygenic (30, 31, 33, 51). Then, with the temperature experiments performed in this study, we added an additional level of complexity to this polygenic system by showing different family-by-temperature crossing reaction norms, hence demonstrating the existence of GxE interactions. Together, these results illustrate that domesticated zebrafish are more susceptible to external environmental cues, most likely as a consequence of having lost its sex-determining locus due to several generations of manipulations (34). Hence, it is an excellent model for investigating environmental influences on sex determination. Our results, therefore, emphasize the interplay between genetic and environmental influences and provide a potential explanation for the variable sex ratios observed in zebrafish facilities across the world, which are often unpredictable and biased.
The masculinizing effect of elevated temperature confirms previous results in zebrafish (36, 39) and correlates with results found in the literature in many other fish species (for reviews, see refs. 16 and 18). However, previous transcriptional studies in zebrafish exposed to temperature were restricted to the first 48 h postfertilization (41, 52). Our results, in contrast, provide a comprehensive analysis of the short- and long-term effects of heat on the gonadal transcriptome of both juvenile and adult zebrafish exposed to elevated temperatures during the period of sex differentiation.
We thus exploited temperature effects to study the gonadal expression profiles of heat-exposed fish, comparing individuals that persisted as males, persisted as females, or were neomales. During the course of this study, we examined the gonads of a total of 1,202 untreated fish, 454 fish exposed to low temperature, and 826 heat-treated fish. Not a single intersex individual with ovotestis was observed (i.e., all fish examined were either males or females). Nonetheless, the possibility of finding intersex individuals among all experimental fish cannot be completely discarded. A major finding is the discovery of two types of heat-exposed males and two types of heat-exposed females, with each type having a different transcriptomic profile. We found that most heat-treated males (MHT1) had a gonadal transcriptome identical to that of control males (MCT). The MHT1 males likely contain both genetic males and neomales, whereas the rest of heat-treated males (MHT2) were most likely neomales (described in detail below).
Heat-exposed females that remained phenotypic females were either unaffected (FHT1) or affected (FHT2). Both types of females maintained a normal ovarian morphology at the macroscopic level. The gonadal transcriptome of FHT1 females was essentially identical to that of control females (FCT); the underlying mechanism responsible for this resistance to heat is unknown and deserves further research. On the other hand, FHT2 females had gonad transcriptomes that were quite different from control ovaries and, surprisingly, differed by only ∼1,200 DETs from MHT2 males despite having different gonadal phenotypes. This difference represents just a fraction (8%) of the ∼15,000 DETs that were differentially expressed between untreated females (FCT) and males (MCT), which, in turn, account for a significant part of the estimated number of protein-coding genes (>25,000) in zebrafish.
In fish, masculinization by elevated temperature usually involves down-regulation of profemale genes, such as cyp19a1a and foxl2, and up-regulation of promale genes, such as dmrt1 or amh (8–14). In accordance with this general pattern, the FHT2 females with a male-like gonadal transcriptome exhibited down-regulation of both key profemale genes such as cyp19a1a and vtg5 as well as down-regulation of profemale pathways such as oocyte meiosis, progesterone-mediated oocyte maturation, canonical Wnt signaling (28, 53), and Fanconi anemia (26). Another profemale pathway, the NF-κB pathway, involved in blocking apoptosis through an inflammatory process during ovarian development (27), was not affected, although some of its genes (e.g., bcl2, nfkb, tradd) were down-regulated. On the other hand, genes related to testis differentiation such as amh and dmrt1 were up-regulated, together with male-related pathways such as the calcium signaling pathway in cardiac muscle contraction, which regulates Leydig cell steroidogenesis in mice (54). We speculate that these FHT2 females are “superfemales” that were able to resist heat-induced phenotypic masculinization, thus maintaining their ovarian morphology despite changing their gonadal transcriptome to a testis-like one. These FHT2 individuals did not likely have ovotestes, as neither the histological analysis of their siblings’ gonads nor extreme similarity of their gonadal transcriptome to that of control males provided such indications. Missexing the gonads based on their color and shape was unlikely, as adult zebrafish gonads are sufficiently different, allowing for their classification by trained eyes (SI Appendix, Tables S1 and S6). Thus, in our opinion, the most likely explanation for the apparent controversy between an ovarian gonadal phenotype and a testicular transcriptome is a major shift in the ovarian gene expression landscape to a testis-like one that was unable to force a complete gonadal transformation. Therefore, given their importance for reproduction, the potential resilience shown by gonads to the influence of external factors, particularly in an organism known for its plasticity, seems notable. An interesting open question for future studies concerns the number and type of additional genes that would need to be altered to cause not only transcriptional but also morphological sex reversal. Threshold hypothesis for heat-induced masculinization supports previous observations in the European sea bass in which heat-resistant fish were females that could not be masculinized by high temperature (55).
The discovery of heat-exposed females possessing ovaries with apparently normal macroscopic phenotype and a near-testicular expression profile potentially indicates major organ transcriptomic reprogramming in vivo during the course of gonadal development with preserved organ structure. It is known that, if conveniently directed, reprogramming can change cell identity (56). Further, epigenetic reprogramming is essential during vertebrate gonadogenesis and early development (57). Although targeting the stem cell microenvironment is believed to be important for proper tissue reprogramming (reviewed in ref. 58), earlier attempts at induced in vivo reprogramming in rodent models have resulted in the appearance of teratomas in multiple tissues (59). Our observation may be linked to the extraordinary plasticity of fish gonads compared with most other vertebrate tissues (60, 61), and constitutes an aspect that deserves further study.
We also explored the effects of heat on juvenile fish, allowing comparison of early vs. late changes in transcription profiles. Only 425 transcripts showed differential expression between the control juveniles of both sexes, thus representing a small fraction of DETs identified between adult testis and ovary. For the juvenile RNA extraction, a portion of the trunk containing the gonad was used, as it was technically challenging to isolate such a small gonad organ. Therefore, this low number of DETs could be due to the dilution effect from other tissues in the trunk. All heat-exposed juveniles exhibited a male-like gonadal transcriptome shortly after the end of the exposure period.
As with adults, female-related pathways such as oocyte meiosis and progesterone-mediated oocyte maturation were down-regulated. Interestingly, cardiac muscle contraction and cytokine–cytokine receptor interaction processes that contain promale pathways were up-regulated in the heat-exposed juveniles (JHT), as observed in the groups with adult males (MCT, MHT1, and MHT2) and heat-treated FHT2 females (Fig. 5C). However, these promale pathways were not differentially expressed in the juvenile control males (JCT2) compared with control females (JCT1). This suggests that heat exposure during the juvenile phase activates the promale pathways earlier than at the control temperature, resulting in the observed male-like transcriptomic profile.
Regarding the neomales referred to earlier, we found that the gonadal transcriptome of most neomales was very similar to that of regular control males. We cannot exclude the possibility that heat and other masculinizing factors such as stress (62) can produce neomales with characteristics very similar to males. In zebrafish facilities, these fish might be used as “males” in studies of genetics, development, and so forth. We do not know whether heat-induced neomales can produce male-biased offspring even in the absence of elevated temperature, resulting in an epigenetic transmission of altered states (63–65). In the European sea bass, the first link between environmental temperature and sex mediated by an epigenetic mechanism was described (10). Heat-exposed females exhibited increased DNA methylation of their cyp19a1a promoter, with a concomitant reduction in cyp19a1a mRNA levels, resulting in male-biased sex ratios (10). Epigenetic modifications of cyp19a1a were also reported during sex change in the rice field eel (66). In fish, transgenerational effects of heat exposure have been recently described (67). In the tongue sole, Cynoglossus semilaevis, which has a ZW/ZZ sex-determining system as in wild zebrafish, heat-induced neomales were responsible for the epigenetic transmission of DNA-altered states, resulting in the production of masculinized offspring even at normal temperatures (68). DNA methylation changes occur due to the activity of DNA methyltransferases (Dnmts). Dnmts have been identified in zebrafish, and there is evidence that their expression can change with temperature (69). In our study, we found that the expression of dnmt3b, together with other key reproduction genes (amh, cyp19a1a, dmrt1, etc.; Table 1), showed heat responses with delayed effects, as their expression was altered in adults but not in juveniles following termination of the heat treatment. These results indicate that heat-induced masculinization likely requires or involves epigenetic mechanisms and, based on the evidence from other fish species (70, 71), further studies should focus on transgenerational effects of heat and investigate whether these lasting effects could be detected in subsequent generations.
Zebrafish is considered to have a wide thermal tolerance zone (72) and lives in the 16.5 to 38.6 °C range of temperature in the wild (73, 74). Interestingly, the elevated temperature used in this study, 36 °C, falls within the above range. If the sensibility of natural populations were similar to that found in laboratory strains, even despite having an intact sex-determining mechanism, then the question is whether wild populations exposed to abnormally high temperature also contain sensitive families with a proportion of sex-reversed females (neomales) and females with ovaries expressing a male-like transcriptome, as found in our study. Chances of finding these fish would increase during particularly warm years, as a decline of wild-type zebrafish populations due to climate change has been described recently (38). In other species, such as Nile tilapia, which can also tolerate high temperatures, the presence of neomales has been strongly suggested in Kpandu and Koka natural populations (75). If they reach adulthood and reproduce, neomales can produce sex-biased (75% females) progeny in a ZZ/ZW system, if the WW genotype is viable (76), or 100% females in a XX/XY system (22). Severely biased sex ratios may have potentially detrimental consequences for population viability (77). Another important question is whether the FHT2 individuals with a masculinized gonadal transcriptome would be able to develop a fully mature gonad or not and, if so, whether they would produce viable germ cells. This question poses a puzzling technical difficulty, as it is impossible to identify an ovary with a male-like transcriptome without extracting the gonad for molecular analysis. Understanding the reproduction of these females with a male-like transcriptome would be a challenge for endocrinologists and reproductive physiologists alike. Finally, finding the equivalent of our FHT2 females in zebrafish and/or other species in the natural environment would mean that the potential effects of elevated temperature should be reconsidered.
Conclusions
In this study, we developed a protocol to learn the mechanisms of temperature-induced sex reversal in zebrafish. Our data demonstrated that in domesticated strains, the sex ratio response to elevated temperature is quite variable and family-specific, supporting a polygenic system of sex determination. We also show that elevated temperatures result in a variable number of neomales with a transcriptome similar to that of regular males but very different from that of unaffected females. Strikingly, we discovered that some individuals that have not been fully masculinized by the heat treatment had ovaries with a male-like transcriptome, potentially indicating transcriptomic reprogramming in vivo with preserved organ structure. To the best of our knowledge, this has never been reported, providing a sign of complex heat-induced masculinization effects in zebrafish that should serve to inspire further studies. These results reveal a previously unknown thermal effect on fish gonads, and suggest new avenues for the detection of functional consequences of elevated temperature in natural populations exposed to global warming.
Materials and Methods
SI Appendix provides a detailed description of the materials and methods used in this study.
Fish and Husbandry.
The AB zebrafish strain and the Tg(vasa:vasa-egfp) zebrafish line of AB strain (78) were used for the temperature treatment experiments. Breeding protocols and husbandry details can be found in SI Appendix, Materials and Methods.
Fish were housed at the experimental aquarium facilities of the Institute of Marine Sciences, CSIC according to approved institutional guidelines on the use of animals for research purposes and in agreement with European regulations of animal welfare (ETS N8 123,01/01/91). The Tg(vasa:vasa-egfp) zebrafish line was maintained at the Temasek Life Sciences Laboratory (TLL) Fish Facility according to the approved protocol of the TLL Institutional Animal Care and Use Committee [approval TLL (F)-10-001].
Temperature Treatments.
Temperature treatments initially involved 11 different families grown until adulthood plus additional families raised until they were juveniles. We first defined the conditions (intensity, duration, timing, and survival) to properly study the effects of elevated temperature on zebrafish sex ratios within OECD guidelines (42). The control and high temperatures were set at 28 and 36 °C, respectively, and the time of application was set at 18 to 32 d postfertilization, coinciding with the period of sex differentiation. Temperature treatments are described in detail in SI Appendix, Materials and Methods.
Sampling and Histological Analysis.
Gonads were collected at 37 (juveniles) and 90 (adults) dpf. To assess sex in adults, we used visual inspection of the gonad under a dissecting microscope. The dissection of Tg(vasa:vasa-egfp) juvenile zebrafish (35 and 42 dpf) was performed under a stereomicroscope equipped with a universal light source to ensure that the gonad (expressing vasa-egfp) was still intact in the trunk cavity after gutting. The fluorescence intensity observed is indicative of the future sex, with strong and weak gonadal fluorescence indicating future females and males, respectively. Fish sex was determined individually after dissection, and the sex ratio was calculated for each biological replicate. Both gonads were collected in the same tube and were stored in liquid nitrogen for subsequent gene expression analysis or fixed with PBS/paraformaldehyde and embedded in glycol methacrylate for histological analysis. Further details of sampling protocols and histological analysis are described in SI Appendix, Materials and Methods.
Microarray Analysis.
A previously validated NimbleGen (Roche) custom zebrafish expression microarray containing 31,477 unique sequences was used [National Center for Biotechnology Information Gene Expression Omnibus (GEO) Platform GPL17795]. Total RNA was extracted with TRIzol, and samples with mean RNA quality indicator (RQI) ≥9 were used. Raw fluorescence intensity data were retrieved from the scanned images by NimbleScan, and quantile normalization and background correction were applied. Microarray data were analyzed using Partek Genomics Suite according to MIAME guidelines (79), and DETs were identified by ANOVA using false discovery rate (FDR)-adjusted P ≤ 0.01 with at least a 1.5 fold change (FC) for multiple-hypothesis testing. Microarray results were deposited in the GEO database (accession no. GSE51434). Full details regarding the microarray analysis are described in SI Appendix, Materials and Methods.
Validation of Differential Gene Expression by qPCR Array.
A high-throughput real-time qPCR array was designed to quantify the expression level of 50 genes, including 35 genes used for validating the microarray results. The qPCR array was performed using the Biomark HD System (Fluidigm). All qPCR reactions were run in triplicate. The genes eef1a1l1 and rpl13a were used as reference. Quantification cycle (Cq) data were imported into GenEx Pro (MultiD Analyses AB) for analysis. The relative quantity was calculated using juveniles (JCT1) or adult females (FCT) at control temperature as reference. Complete details of the qPCRs and PCR data analysis can be found in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Francesc Maynou for assistance with statistical procedures, and Drs. Nigel Finn, Joan Cerdà, and Núria Montserrat for helpful comments. The laboratory of L.O. received financial support from the National Research Foundation, Prime Minister’s Office, Singapore under its Competitive Research Programme (NRF-CRP7-2010-01), Agri-Food and Veterinary Authority of Singapore, and Temasek Life Sciences Laboratory for this work. The F.P. laboratory was supported by Spanish Government Grants Aquagenomics (CDS2007-0002), Epigen-Aqua (AGL2010-15939), and Epifarm (AGL2013-41047-R). The Catalan Government supported fish facilities through a Xarxa de Recerca d’Aqüicultura (XRAq) grant. L.R. and N.D. were supported by Epigen-Aqua and Epifarm contracts, respectively. Travel between the two laboratories was supported by CSIC Grant i-LINK0195 (to F.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The microarray results reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database (accession no. GSE51434).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1609411114/-/DCSupplemental.
References
- 1.Cutting A, Chue J, Smith CA. Just how conserved is vertebrate sex determination? Dev Dyn. 2013;242(4):380–387. doi: 10.1002/dvdy.23944. [DOI] [PubMed] [Google Scholar]
- 2.Devlin RH, Nagahama Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture. 2002;208(3–4):191–364. [Google Scholar]
- 3.Penman DJ, Piferrer F. Fish gonadogenesis. Part I: Genetic and environmental mechanisms of sex determination. Rev Fish Sci. 2008;16(S1):16–34. [Google Scholar]
- 4.Heule C, Salzburger W, Böhne A. Genetics of sexual development: An evolutionary playground for fish. Genetics. 2014;196(3):579–591. doi: 10.1534/genetics.114.161158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uller T, Helanterä H. From the origin of sex-determining factors to the evolution of sex-determining systems. Q Rev Biol. 2011;86(3):163–180. doi: 10.1086/661118. [DOI] [PubMed] [Google Scholar]
- 6.Holleley CE, et al. Sex reversal triggers the rapid transition from genetic to temperature-dependent sex. Nature. 2015;523(7558):79–82. doi: 10.1038/nature14574. [DOI] [PubMed] [Google Scholar]
- 7.Söffker M, Tyler CR. Endocrine disrupting chemicals and sexual behaviors in fish—A critical review on effects and possible consequences. Crit Rev Toxicol. 2012;42(8):653–668. doi: 10.3109/10408444.2012.692114. [DOI] [PubMed] [Google Scholar]
- 8.Valenzuela N, Neuwald JL, Literman R. Transcriptional evolution underlying vertebrate sexual development. Dev Dyn. 2013;242(4):307–319. doi: 10.1002/dvdy.23897. [DOI] [PubMed] [Google Scholar]
- 9.Díaz N, Piferrer F. Lasting effects of early exposure to temperature on the gonadal transcriptome at the time of sex differentiation in the European sea bass, a fish with mixed genetic and environmental sex determination. BMC Genomics. 2015;16:679. doi: 10.1186/s12864-015-1862-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navarro-Martín L, et al. DNA methylation of the gonadal aromatase (cyp19a) promoter is involved in temperature-dependent sex ratio shifts in the European sea bass. PLoS Genet. 2011;7(12):e1002447. doi: 10.1371/journal.pgen.1002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaguchi T, Yamaguchi S, Hirai T, Kitano T. Follicle-stimulating hormone signaling and Foxl2 are involved in transcriptional regulation of aromatase gene during gonadal sex differentiation in Japanese flounder, Paralichthys olivaceus. Biochem Biophys Res Commun. 2007;359(4):935–940. doi: 10.1016/j.bbrc.2007.05.208. [DOI] [PubMed] [Google Scholar]
- 12.Fernandino JI, Hattori RS, Kimura H, Strüssmann CA, Somoza GM. Expression profile and estrogenic regulation of anti-Müllerian hormone during gonadal development in pejerrey Odontesthes bonariensis, a teleost fish with strong temperature-dependent sex determination. Dev Dyn. 2008;237(11):3192–3199. doi: 10.1002/dvdy.21731. [DOI] [PubMed] [Google Scholar]
- 13.D’Cotta H, Fostier A, Guiguen Y, Govoroun M, Baroiller JF. Search for genes involved in the temperature-induced gonadal sex differentiation in the tilapia, Oreochromis niloticus. J Exp Zool. 2001;290(6):574–585. doi: 10.1002/jez.1108. [DOI] [PubMed] [Google Scholar]
- 14.Poonlaphdecha S, et al. Temperature induced-masculinisation in the Nile tilapia causes rapid up-regulation of both dmrt1 and amh expressions. Gen Comp Endocrinol. 2013;193:234–242. doi: 10.1016/j.ygcen.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Fernandino JI, Hattori RS, Kishii A, Strüssmann CA, Somoza GM. The cortisol and androgen pathways cross talk in high temperature-induced masculinization: The 11β-hydroxysteroid dehydrogenase as a key enzyme. Endocrinology. 2012;153(12):6003–6011. doi: 10.1210/en.2012-1517. [DOI] [PubMed] [Google Scholar]
- 16.Guiguen Y, Fostier A, Piferrer F, Chang CF. Ovarian aromatase and estrogens: A pivotal role for gonadal sex differentiation and sex change in fish. Gen Comp Endocrinol. 2010;165(3):352–366. doi: 10.1016/j.ygcen.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Li CG, et al. Differential expression analysis of genes involved in high-temperature induced sex differentiation in Nile tilapia. Comp Biochem Physiol B Biochem Mol Biol. 2014;177–178:36–45. doi: 10.1016/j.cbpb.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Ospina-Álvarez N, Piferrer F. Temperature-dependent sex determination in fish revisited: Prevalence, a single sex ratio response pattern, and possible effects of climate change. PLoS One. 2008;3(7):e2837. doi: 10.1371/journal.pone.0002837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hliwa P, Bah M, Kuzminski H, Dobosz S, Ciereszko A. Ultrasound evaluation of the gonadal structure in sex-reversed rainbow trout females. Aquacult Int. 2014;22(1):89–96. [Google Scholar]
- 20.Kocmarek AL, Ferguson MM, Danzmann RG. Comparison of growth-related traits and gene expression profiles between the offspring of neomale (XX) and normal male (XY) rainbow trout. Mar Biotechnol (NY) 2015;17(2):229–243. doi: 10.1007/s10126-015-9612-5. [DOI] [PubMed] [Google Scholar]
- 21.Rougeot C, et al. Comparative study of the reproductive characteristics of XY male and hormonally sex-reversed XX male Eurasian perch, Perca fluviatilis. Theriogenology. 2004;62(5):790–800. doi: 10.1016/j.theriogenology.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Piferrer F. Endocrine sex control strategies for the feminization of teleost fish. Aquaculture. 2001;197(1–4):229–281. [Google Scholar]
- 23.Orban L, Sreenivasan R, Olsson PE. Long and winding roads: Testis differentiation in zebrafish. Mol Cell Endocrinol. 2009;312(1–2):35–41. doi: 10.1016/j.mce.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Uchida D, Yamashita M, Kitano T, Iguchi T. Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J Exp Biol. 2002;205(Pt 6):711–718. doi: 10.1242/jeb.205.6.711. [DOI] [PubMed] [Google Scholar]
- 25.Wang XG, Bartfai R, Sleptsova-Freidrich I, Orban L. The timing and extent of ‘juvenile ovary’ phase are highly variable during zebrafish testis differentiation. J Fish Biol. 2007;70:33–44. [Google Scholar]
- 26.Rodríguez-Marí A, Postlethwait JH. The role of Fanconi anemia/BRCA genes in zebrafish sex determination. Methods Cell Biol. 2011;105:461–490. doi: 10.1016/B978-0-12-381320-6.00020-5. [DOI] [PubMed] [Google Scholar]
- 27.Pradhan A, et al. Activation of NF-κB protein prevents the transition from juvenile ovary to testis and promotes ovarian development in zebrafish. J Biol Chem. 2012;287(45):37926–37938. doi: 10.1074/jbc.M112.386284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sreenivasan R, et al. Gonad differentiation in zebrafish is regulated by the canonical Wnt signaling pathway. Biol Reprod. 2014;90(2):45–56. doi: 10.1095/biolreprod.113.110874. [DOI] [PubMed] [Google Scholar]
- 29.Amores A, Postlethwait JH. Banded chromosomes and the zebrafish karyotype. Methods Cell Biol. 1999;60:323–338. doi: 10.1016/s0091-679x(08)61908-1. [DOI] [PubMed] [Google Scholar]
- 30.Liew WC, et al. Polygenic sex determination system in zebrafish. PLoS One. 2012;7(4):e34397. doi: 10.1371/journal.pone.0034397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liew WC, Orbán L. Zebrafish sex: A complicated affair. Brief Funct Genomics. 2014;13(2):172–187. doi: 10.1093/bfgp/elt041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tong SK, Hsu HJ, Chung BC. Zebrafish monosex population reveals female dominance in sex determination and earliest events of gonad differentiation. Dev Biol. 2010;344(2):849–856. doi: 10.1016/j.ydbio.2010.05.515. [DOI] [PubMed] [Google Scholar]
- 33.Anderson JL, et al. Multiple sex-associated regions and a putative sex chromosome in zebrafish revealed by RAD mapping and population genomics. PLoS One. 2012;7(7):e40701. doi: 10.1371/journal.pone.0040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson CA, et al. Wild sex in zebrafish: Loss of the natural sex determinant in domesticated strains. Genetics. 2014;198(3):1291–1308. doi: 10.1534/genetics.114.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abozaid H, Wessels S, Hörstgen-Schwark G. Effect of rearing temperatures during embryonic development on the phenotypic sex in zebrafish (Danio rerio) Sex Dev. 2011;5(5):259–265. doi: 10.1159/000330120. [DOI] [PubMed] [Google Scholar]
- 36.Abozaid H, Wessels S, Hörstgen-Schwark G. Elevated temperature applied during gonadal transformation leads to male bias in zebrafish (Danio rerio) Sex Dev. 2012;6(4):201–209. doi: 10.1159/000336297. [DOI] [PubMed] [Google Scholar]
- 37.Uchida D, Yamashita M, Kitano T, Iguchi T. An aromatase inhibitor or high water temperature induce oocyte apoptosis and depletion of P450 aromatase activity in the gonads of genetic female zebrafish during sex-reversal. Comp Biochem Physiol A Mol Integr Physiol. 2004;137(1):11–20. doi: 10.1016/s1095-6433(03)00178-8. [DOI] [PubMed] [Google Scholar]
- 38.Brown AR, et al. Climate change and pollution speed declines in zebrafish populations. Proc Natl Acad Sci USA. 2015;112(11):E1237–E1246. doi: 10.1073/pnas.1416269112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luzio A, Santos D, Fontaínhas-Fernandes AA, Monteiro SM, Coimbra AM. Effects of 17α-ethinylestradiol at different water temperatures on zebrafish sex differentiation and gonad development. Aquat Toxicol. 2016;174:22–35. doi: 10.1016/j.aquatox.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Sfakianakis DG, Leris I, Mylonas CC, Kentouri M. Temperature during early life determines sex in zebrafish, Danio rerio (Hamilton, 1822) J Biol Res (Thessalon) 2012;17:68–73. [Google Scholar]
- 41.Long Y, Li L, Li Q, He X, Cui Z. Transcriptomic characterization of temperature stress responses in larval zebrafish. PLoS One. 2012;7(5):e37209. doi: 10.1371/journal.pone.0037209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Organisation for Economic Cooperation and Development . Test No. 234: Fish Sexual Development Test. OECD; Paris: 2011. [Google Scholar]
- 43.Howe K, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496(7446):498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santos EM, et al. Gonadal transcriptome responses and physiological consequences of exposure to oestrogen in breeding zebrafish (Danio rerio) Aquat Toxicol. 2007;83(2):134–142. doi: 10.1016/j.aquatox.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 45.Small CM, Carney GE, Mo Q, Vannucci M, Jones AG. A microarray analysis of sex- and gonad-biased gene expression in the zebrafish: Evidence for masculinization of the transcriptome. BMC Genomics. 2009;10:579. doi: 10.1186/1471-2164-10-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sreenivasan R, et al. Transcriptomic analyses reveal novel genes with sexually dimorphic expression in the zebrafish gonad and brain. PLoS One. 2008;3(3):e1791. doi: 10.1371/journal.pone.0001791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krøvel AV, Olsen LC. Expression of a vas:EGFP transgene in primordial germ cells of the zebrafish. Mech Dev. 2002;116(1–2):141–150. doi: 10.1016/s0925-4773(02)00154-5. [DOI] [PubMed] [Google Scholar]
- 48.Bull JJ. Evolution of Sex Determining Mechanisms. Benjamin/Cummings; Menlo Park, CA: 1983. [Google Scholar]
- 49.Kosswig C. Polygenic sex determination. Experientia. 1964;20(4):190–199. doi: 10.1007/BF02135395. [DOI] [PubMed] [Google Scholar]
- 50.Moore EC, Roberts RB. Polygenic sex determination. Curr Biol. 2013;23(12):R510–R512. doi: 10.1016/j.cub.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Bradley KM, et al. An SNP-based linkage map for zebrafish reveals sex determination loci. G3 (Bethesda) 2011;1(1):3–9. doi: 10.1534/g3.111.000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Long Y, et al. Transcriptomic characterization of cold acclimation in larval zebrafish. BMC Genomics. 2013;14:612. doi: 10.1186/1471-2164-14-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nicol B, Guiguen Y. Expression profiling of Wnt signaling genes during gonadal differentiation and gametogenesis in rainbow trout. Sex Dev. 2011;5(6):318–329. doi: 10.1159/000334515. [DOI] [PubMed] [Google Scholar]
- 54.Abdou HS, Villeneuve G, Tremblay JJ. The calcium signaling pathway regulates Leydig cell steroidogenesis through a transcriptional cascade involving the nuclear receptor NR4A1 and the steroidogenic acute regulatory protein. Endocrinology. 2013;154(1):511–520. doi: 10.1210/en.2012-1767. [DOI] [PubMed] [Google Scholar]
- 55.Navarro-Martín L, Blázquez M, Viñas J, Joly S, Piferrer F. Balancing the effects of rearing at low temperature during early development on sex ratios, growth and maturation in the European sea bass (Dicentrarchus labrax). Limitations and opportunities for the production of highly female-biased stocks. Aquaculture. 2009;296(3–4):347–358. [Google Scholar]
- 56.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 57.Zhou LQ, Dean J. Reprogramming the genome to totipotency in mouse embryos. Trends Cell Biol. 2015;25(2):82–91. doi: 10.1016/j.tcb.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lane SW, Williams DA, Watt FM. Modulating the stem cell niche for tissue regeneration. Nat Biotechnol. 2014;32(8):795–803. doi: 10.1038/nbt.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abad M, et al. Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature. 2013;502(7471):340–345. doi: 10.1038/nature12586. [DOI] [PubMed] [Google Scholar]
- 60.Herpin A, Schartl M. Plasticity of gene-regulatory networks controlling sex determination: Of masters, slaves, usual suspects, newcomers, and usurpators. EMBO Rep. 2015;16(10):1260–1274. doi: 10.15252/embr.201540667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piferrer F, Ribas L, Díaz N. Genomic approaches to study genetic and environmental influences on fish sex determination and differentiation. Mar Biotechnol (NY) 2012;14(5):591–604. doi: 10.1007/s10126-012-9445-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fernandino JI, Hattori RS, Moreno Acosta OD, Strüssmann CA, Somoza GM. Environmental stress-induced testis differentiation: Androgen as a by-product of cortisol inactivation. Gen Comp Endocrinol. 2013;192:36–44. doi: 10.1016/j.ygcen.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 63.Jablonka E, Raz G. Transgenerational epigenetic inheritance: Prevalence, mechanisms, and implications for the study of heredity and evolution. Q Rev Biol. 2009;84(2):131–176. doi: 10.1086/598822. [DOI] [PubMed] [Google Scholar]
- 64.Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. 2010;21(4):214–222. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Youngson NA, Whitelaw E. Transgenerational epigenetic effects. Annu Rev Genomics Hum Genet. 2008;9:233–257. doi: 10.1146/annurev.genom.9.081307.164445. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Zhang S, Liu Z, Zhang L, Zhang W. Epigenetic modifications during sex change repress gonadotropin stimulation of cyp19a1a in a teleost ricefield eel (Monopterus albus) Endocrinology. 2013;154(8):2881–2890. doi: 10.1210/en.2012-2220. [DOI] [PubMed] [Google Scholar]
- 67.Salinas S, Munch SB. Thermal legacies: Transgenerational effects of temperature on growth in a vertebrate. Ecol Lett. 2012;15(2):159–163. doi: 10.1111/j.1461-0248.2011.01721.x. [DOI] [PubMed] [Google Scholar]
- 68.Chen S, et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat Genet. 2014;46(3):253–260. doi: 10.1038/ng.2890. [DOI] [PubMed] [Google Scholar]
- 69.Campos C, Valente LMP, Fernandes JMO. Molecular evolution of zebrafish dnmt3 genes and thermal plasticity of their expression during embryonic development. Gene. 2012;500(1):93–100. doi: 10.1016/j.gene.2012.03.041. [DOI] [PubMed] [Google Scholar]
- 70.Shao C, et al. Epigenetic modification and inheritance in sexual reversal of fish. Genome Res. 2014;24(4):604–615. doi: 10.1101/gr.162172.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun L-X, et al. Global DNA methylation changes in Nile tilapia gonads during high temperature-induced masculinization. PLoS One. 2016;11(8):e0158483. doi: 10.1371/journal.pone.0158483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spence R, Fatema MK, Ellis S, Ahmed ZF, Smith C. Diet, growth and recruitment of wild zebrafish in Bangladesh. J Fish Biol. 2007;71(1):304–309. [Google Scholar]
- 73.López-Olmeda JF, Sánchez-Vázquez FJ. Thermal biology of zebrafish (Danio rerio) J Therm Biol. 2011;36(2):91–104. [Google Scholar]
- 74.Spence R, Gerlach G, Lawrence C, Smith C. The behaviour and ecology of the zebrafish, Danio rerio. Biol Rev Camb Philos Soc. 2008;83(1):13–34. doi: 10.1111/j.1469-185X.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- 75.Bezault E, Clota F, Derivaz M, Chevassus B, Baroiller JF. Sex determination and temperature-induced sex differentiation in three natural populations of Nile tilapia (Oreochromis niloticus) adapted to extreme temperature conditions. Aquaculture. 2007;272:S3–S16. [Google Scholar]
- 76.Martínez P, et al. Genetic architecture of sex determination in fish: Applications to sex ratio control in aquaculture. Front Genet. 2014;5:340. doi: 10.3389/fgene.2014.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cotton S, Wedekind C. Population consequences of environmental sex reversal. Conserv Biol. 2009;23(1):196–206. doi: 10.1111/j.1523-1739.2008.01053.x. [DOI] [PubMed] [Google Scholar]
- 78.Krøvel AV, Olsen LC. Sexual dimorphic expression pattern of a splice variant of zebrafish vasa during gonadal development. Dev Biol. 2004;271(1):190–197. doi: 10.1016/j.ydbio.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 79.Brazma A, et al. Minimum information about a microarray experiment (MIAME)—Toward standards for microarray data. Nat Genet. 2001;29(4):365–371. doi: 10.1038/ng1201-365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.