Significance
Cocaine-induced synaptic plasticity in the nucleus accumbens is implicated in neural adaptations that underlie addiction. Here we demonstrate that Wiskott-Aldrich syndrome protein (WASP) family verprolin homologous protein 1 (WAVE1) plays a selective role in medium spiny projection neurons that express D1 dopamine receptors (D1-MSNs) in the cellular and behavioral actions of cocaine. Our results suggest that chronic exposure to cocaine, followed by withdrawal, potentiates the signaling capacity of D1 dopamine and NMDA glutamate receptors, allowing WAVE1 activation in response to an acute cocaine challenge. WAVE1 activation is associated with morphological and functional decreases in glutamatergic synapses on D1-MSNs. Thus, we propose that WAVE1 is involved in a negative feedback mechanism of regulation of glutamatergic synapses as a part of the process of cocaine-induced synaptic plasticity.
Keywords: WAVE1, cocaine, striatum, medium spiny projection neurons, Cdk5
Abstract
Wiskott-Aldrich syndrome protein (WASP) family verprolin homologous protein 1 (WAVE1) regulates actin-related protein 2/3 (Arp2/3) complex-mediated actin polymerization. Our previous studies have found WAVE1 to be inhibited by Cdk5-mediated phosphorylation in brain and to play a role in the regulation of dendritic spine morphology. Here we report that mice in which WAVE1 was knocked out (KO) in neurons expressing the D1 dopamine receptor (D1-KO), but not mice where WAVE1 was knocked out in neurons expressing the D2 dopamine receptor (D2-KO), exhibited a significant decrease in place preference associated with cocaine. In contrast to wild-type (WT) and WAVE1 D2-KO mice, cocaine-induced sensitized locomotor behavior was not maintained in WAVE1 D1-KO mice. After chronic cocaine administration and following withdrawal, an acute cocaine challenge induced WAVE1 activation in striatum, which was assessed by dephosphorylation. The cocaine-induced WAVE1 dephosphorylation was attenuated by coadministration of either a D1 dopamine receptor or NMDA glutamate receptor antagonist. Upon an acute challenge of cocaine following chronic cocaine exposure and withdrawal, we also observed in WT, but not in WAVE1 D1-KO mice, a decrease in dendritic spine density and a decrease in the frequency of excitatory postsynaptic AMPA receptor currents in medium spiny projection neurons expressing the D1 dopamine receptor (D1-MSNs) in the nucleus accumbens. These results suggest that WAVE1 is involved selectively in D1-MSNs in cocaine-evoked neuronal activity-mediated feedback regulation of glutamatergic synapses.
Wiskott-Aldrich syndrome protein (WASP) family verprolin homologous protein (WAVE) initiates de novo actin polymerization through its ability to activate the actin-related protein 2/3 (Arp2/3) complex (1). Three members of the family (WAVE1–3) exist, with WAVE1 being highly expressed in brain (2). WAVE1 was purified from bovine or rat brain as part of a heteropentameric protein complex together with PIR121 [also called cytoplasmic FMR1-interacting protein 2, (Cyfip2)], Nck-associated protein 1 (Nckap1), Abl-interactor 2 (Abi2), and HSPC300 (3, 4). Whereas WAVE1 plays a key role in Arp2/3 complex binding and actin polymerization, other protein components in the complex are important for its subcellular localization and interaction with upstream ligands or activators (5, 6).
In our initial study in striatum, the WAVE1 complex was found to interact with p35, the regulatory subunit of cyclin-dependent kinase 5 (Cdk5) (4). Phosphorylation of WAVE1 at Ser310, Ser397, and Ser441 by Cdk5/p35, in the complex purified from rat brain, or with recombinant WAVE1, resulted in inhibition of actin polymerization in vitro (4). We also observed that the stoichiometry of WAVE1 phosphorylation by Cdk5 is high in brain, suggestive of the protein being largely inactive under basal conditions (4). However, studies using mouse striatal slices indicated that WAVE1 can be dephosphorylated at all three Cdk5 sites and thereby activated upon stimulation of D1 dopamine and of NMDA glutamate receptors, acting through cAMP- and Ca2+-mediated activation of protein phosphatase 2A and 2B, respectively (4, 7). These results indicate that WAVE1 is a neuronal activity-regulated phosphoprotein in the striatum.
We previously identified a pivotal role for Cdk5/p35 in cocaine-induced adaptive changes in brain (8). Cocaine is a highly addictive psychostimulant that exerts its effects on brain reward regions such as the nucleus accumbens (NAc), a ventral part of the striatum (9–11). The NAc receives dopaminergic input from the ventral tegmental area (VTA) and glutamatergic inputs from the medial prefrontal cortex (mPFC), hippocampus, and amygdala, among other regions (12). Cocaine elevates synaptic levels of dopamine by blocking reuptake of dopamine into presynaptic terminals (13, 14). However, chronic exposure to cocaine dampens dopamine signaling through increases in ΔFosB and Cdk5/p35 (8). Cdk5/p35 phosphorylates Thr75 of DARPP-32, a master regulator of dopamine signaling in striatal medium spiny projection neurons (MSNs), and thereby negatively regulates DARPP-32 function (15). Chronic exposure to cocaine also changes glutamatergic synapses in the NAc, including increasing dendritic spine density (9, 10, 16, 17). We showed that a Cdk5 inhibitor attenuated baseline dendritic spine density as well as cocaine-induced dendritic spine outgrowth in NAc core and shell, suggesting an involvement of Cdk5 in the regulation of dendritic spine density (18). WAVE1 is also known to regulate dendritic spine morphology (4). In the present study, we investigated the function of WAVE1 in the cellular and behavioral responses to cocaine. We found a critical role of WAVE1 expressed in a subpopulation of MSNs in cocaine-induced feedback regulation of dendritic spine density, maintenance of cocaine-induced locomotor sensitization, and cocaine reward.
Results
Expression of WAVE1 in D1- and D2-MSNs and Generation of Cell-Type–Specific WAVE1 KO Mice.
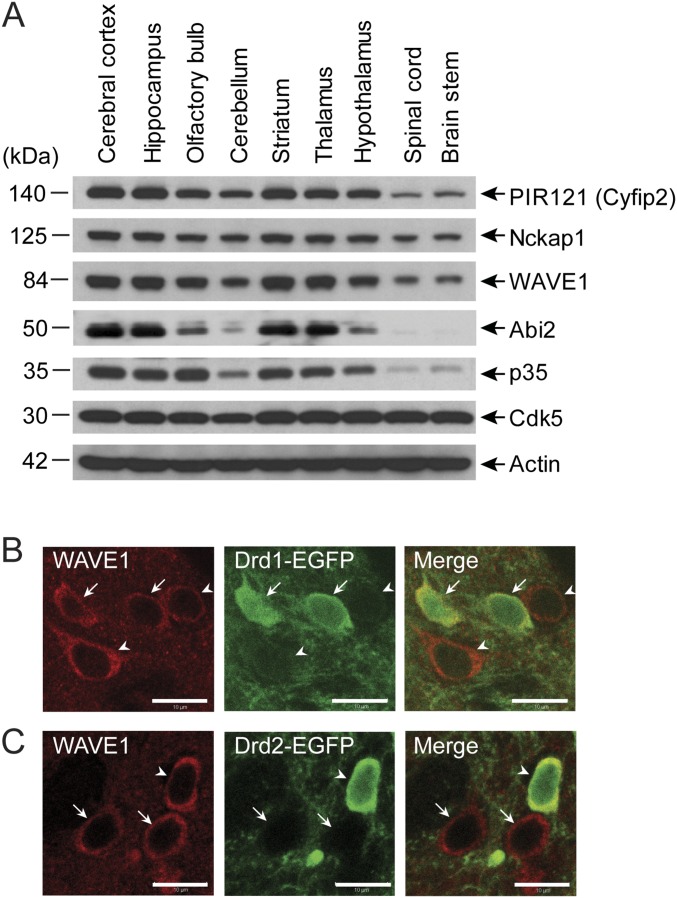
The WAVE1 complex is highly expressed in the forebrain, including cerebral cortex, hippocampus, striatum, and thalamus, and this expression pattern is well correlated with that of p35 (Fig. S1A) (2). MSNs, the major neuronal type in the striatum, are largely segregated into two distinct subpopulations expressing high levels of either dopamine D1 receptor (D1) or D2 receptor (D2) (19–21). We used bacterial artificial chromosome (BAC) transgenic mice expressing EGFP under the control of D1 (Drd1) or D2 (Drd2) promoters and examined the expression of WAVE1 and EGFP in D1- and D2-MSNs. Similar levels of WAVE1 immunostaining were observed in both D1- and D2-MSNs (Fig. S1 B and C). Anatomically, D1-MSNs and D2-MSNs in dorsal striatum project to distinct brain areas (22) but projections of D1-MSNs and D2-MSNs from the NAc are more overlapping and remain to be further defined (11, 23). Importantly, stimulation of MSNs by dopamine results in opposite regulation of cAMP and PKA via stimulatory G protein (Gs/Golf)-mediated signaling in D1-MSNs compared with inhibitory G protein (Gi/Go)-mediated signaling in D2-MSNs (24). Furthermore, molecular manipulations of D1-MSNs or D2-MSNs results in distinct behavioral effects of drugs of abuse (24). Thus, we decided to generate cell-type–selective KO lines to more specifically investigate WAVE1 function.
Fig. S1.
Expression of WAVE1 complex and Cdk5/p35 in the brain and expression of WAVE1 in D1- and D2-MSNs. (A) Immunoblotting of the components of the WAVE1 complex, Cdk5, p35, and actin in brain regions as indicated. (B and C) Immunohistochemically stained WAVE1 and EGFP in the striatum of Drd1a-EGFP mice (B) or Drd2-EGFP mice (C). Arrows and arrowheads indicate D1-MSNs and D2-MSNs, respectively. WAVE1 expression is observed in both D1-MSNs and D2-MSNs. (Scale bars, 10 µm.)
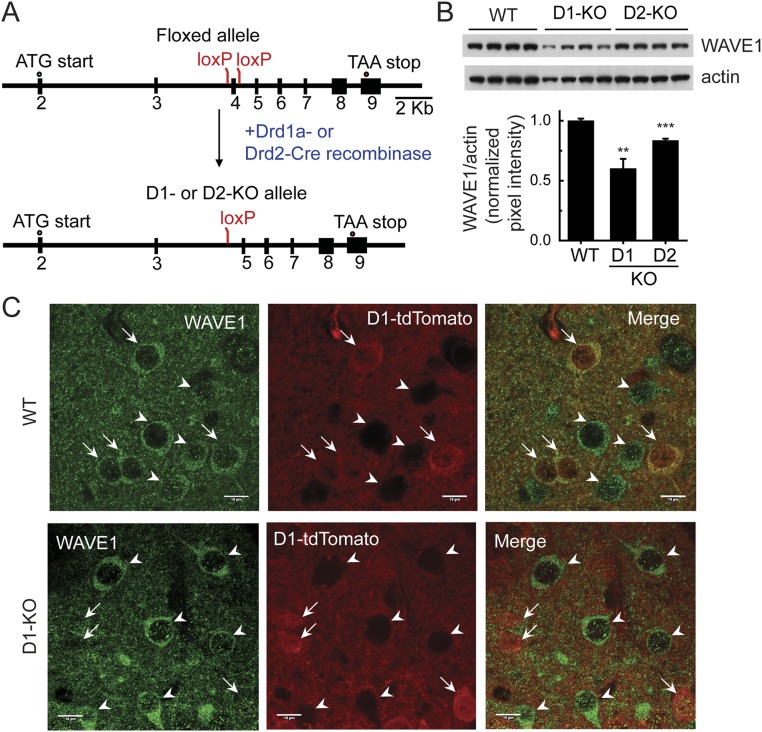
Previously we generated mice in which exon 4 of the WAVE1 gene was flanked by loxP sites (25). We bred floxed WAVE1 with either Drd1 or Drd2 promoter-driven Cre-recombinase lines (26) to delete WAVE1 in neurons expressing D1 (D1-KO) or D2 (D2-KO) receptors (Fig. S2A). WAVE1 protein in striatal lysates was reduced in both D1- and D2-KO mice, with a greater reduction being found in D1-KO mice (∼40% versus ∼17% of reduction) (Fig. S2B). Previous studies of Drd1-EGFP or Drd2-EGFP mice have indicated a comparable number of D1-MSNs and D2-MSNs (∼50% of neurons as Drd1-EGFP+ versus ∼40% of neurons as Drd2-EGFP+) (27). The reason for the greater reduction in total striatal WAVE1 in the D1-KO mice is therefore unclear, but could reflect higher expression of WAVE1 in D1-MSNs compared with that in D2-MSNs. We also used a Cre-recombinase–dependent tdTomato reporter line and confirmed that WAVE1 deletion was selectively dependent on Drd1-promoter–driven Cre-recombinase activity (Fig. S2C).
Fig. S2.
Generation of cell-type–specific WAVE1 KO mice. (A) WAVE1 D1-KO or D2-KO mice were generated by breeding floxed WAVE1 mice with Drd1a– or Drd2–Cre-recombinase mice. Exon 4 of the WAVE1 gene was flanked by loxP sites and deletion of exon 4 by Cre-recombinases created a frameshift in downstream exons, generating an early STOP codon. (B) Immunoblotting of total striatal lysates from WT, D1-KO, and D2-KO mice. Representative immunoblot images of WAVE1 and actin (Upper) and quantified WAVE1 levels normalized to actin levels are shown. Data represent means ± SEM compared with WT. n = 4 per group. **P < 0.01 and ***P < 0.001, t test. (C) Immunohistochemically stained WAVE1 in the striatum of WT mice harboring the Drd1a-Cre allele or WAVE1 D1-KO mice bred with a Cre-dependent tdTomato reporter line. Arrows and arrowheads indicate D1-MSNs and D2-MSNs, respectively. (Scale bars, 10 µm.)
Role for WAVE1 in D1-MSNs in Cocaine-Induced Behavior.
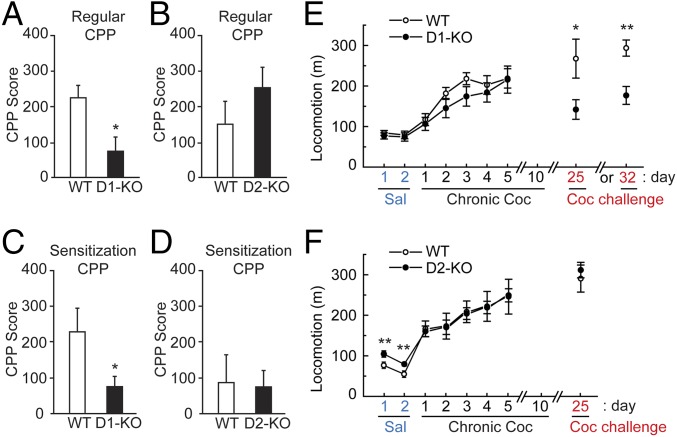
We used noncontingent cocaine administration (cocaine administration by experimenter) and assessed cocaine reward in a conditioned place preference (CPP) test. WAVE1 D1-KO mice showed a significantly reduced CPP score in both a standard CPP test (Fig. 1A) and a sensitization CPP test (Fig. 1C) compared with WT mice. In contrast, WAVE1 D2-KO mice did not show any significant difference compared with WT mice in either of the CPP tests (Fig. 1 B and D), suggesting a selective role for WAVE1 in D1-neurons in cocaine reward.
Fig. 1.
Cocaine-induced CPP and locomotor sensitization in WAVE1 D1- or D2-KO mice. (A and B) Cocaine-associated place preference in D1-KO mice (A) or D2-KO mice (B) and WT mice was assessed using a “regular” CPP test with cocaine (7.5 mg/kg, i.p.): WT versus D1-KO mice [t31 = 2.663, *P < 0.05, n = 17 (WT), 16 (D1-KO)] and WT versus D2-KO mice [n = 10 (WT), 10 (D2-KO)]. (C and D) “Sensitization” CPP was assessed in D1-KO (C) or D2-KO (D) and WT mice in which cocaine was administered (15 mg/kg) for 7 consecutive days followed by a 5-d withdrawal (WD). Preference for a low dose of cocaine (5 mg/kg) was then assessed: WT versus D1-KO mice [t15 = 2.271, *P < 0.05, n = 7 (WT), 10 (D1-KO)] and WT versus D2-KO mice [n = 8 (WT), 8 (D2-KO)]. (E and F) WT mice and WAVE1 D1-KO mice (E) or D2-KO mice (F) were treated daily with saline for 2 d. Cocaine (10 mg/kg, i.p.) was administered for 10 consecutive days. After chronic cocaine administration, WT and D1-KO mice were divided into two groups (n = 8 per group) and subjected to 2-wk or 3-wk WD (E), whereas WT and D2-KO mice were subjected to only a 2-wk WD (n = 11 per group) (F). Acute cocaine challenge (10 mg/kg) was administered following the 2-wk (day 25) or 3-wk WD (day 32). Locomotion was measured daily for 1 h after saline, cocaine (days 1–5), or acute cocaine challenge. Data represent means ± SEM. WT versus D1-KO mice were as follows: saline (days 1–2) and chronic cocaine (days 1–5) (n = 16 per group), cocaine challenge day 25 (t14 = 2.331, *P < 0.05, n = 8 per group) and cocaine challenge day 32 (t14 = 4.745, **P < 0.01, n = 8 per group). WT versus D2-KO mice were as follows: saline day 1 (t20 = 3.619, **P < 0.01, n = 11 per group) and saline day 2 (t20 = 3.611, **P < 0.01, n = 11 per group). t test was used.
We next investigated the effect of cocaine on locomotor sensitization using WAVE1 D1-KO or D2-KO mice and their corresponding WT controls. WAVE1 D1-KO mice and D2-KO mice both developed locomotor sensitization during chronic administration of cocaine (Fig. 1 E and F). However, after 2 or 3 wk of drug withdrawal, D1-KO mice showed a lower response to subsequent acute cocaine administration (Fig. 1E). D2-KO mice responded the same as WT mice to a cocaine challenge following withdrawal (Fig. 1F). These results suggest further that WAVE1 in neurons expressing the D1 receptor is required for the enduring effects of cocaine as measured by CPP and locomotion sensitization tests.
Regulation of WAVE1 Phosphorylation by Cocaine.
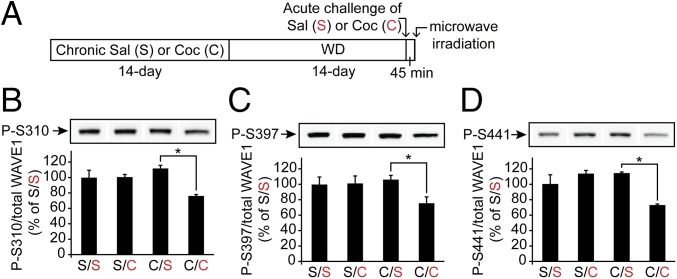
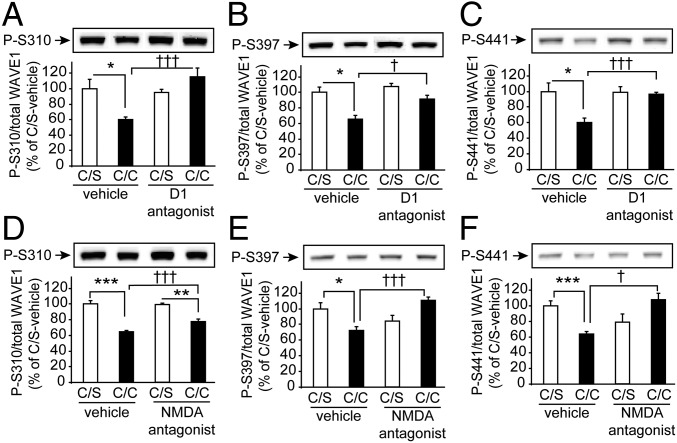
Previously, our studies showed that stimulation of striatal slices with a D1 agonist or NMDA led to dephosphorylation of WAVE1 at Ser310, Ser397, and Ser441, indicating neuronal activity-mediated stimulation of WAVE1 (4, 7). We anticipated that WAVE1 would be dephosphorylated in response to cocaine administration in vivo, because cocaine increases synaptic levels of dopamine, and activation of D1 is known to increase NMDA-receptor–mediated signaling (13, 28, 29). However, there was no significant change in phosphorylation, or of the total protein level, of WAVE1 in the striatum in response to acute injection of cocaine (45 min after 15 mg/kg) or chronic injection of cocaine (15 mg/kg daily for 14 d; 45 min after the last injection). Instead, we found that WAVE1 was significantly dephosphorylated at all three sites in response to an acute challenge with cocaine following chronic cocaine administration and a subsequent 14-d withdrawal period (Fig. 2). We did not see any significant change in total protein levels of WAVE1, p35, or Cdk5 (Fig. S3 A–C). Coinjection of a D1 antagonist (SCH23390) or an NMDA-receptor antagonist (MK801) with the acute cocaine challenge largely blocked the dephosphorylation of WAVE1 (Fig. 3 and Fig. S3 D and E), suggesting critical roles of D1- and NMDA-receptor signaling in WAVE1 dephosphorylation.
Fig. 2.
Dephosphorylation of WAVE1 by acute cocaine challenge following chronic administration of cocaine and withdrawal. (A) Cocaine treatment regimens. Saline (S) or cocaine (C, 15 mg/kg, i.p.) was administered for 14 consecutive days followed by 14-d withdrawal (WD). After acute cocaine (15 mg/kg) or saline administration, mice were killed 45 min later using focused microwave irradiation. (B–D) Phosphorylation of WAVE1 at S310 (P-S310) (B), S397 (P-S397) (C), and S441 (P-S441) (D) and total WAVE1 were measured in total striatum by immunoblotting. The levels were quantified and normalized to the levels of saline/saline (S/S). Data represent means ± SEM, n = 6 mice per group. *P < 0.05, C/S versus C/C, one-way ANOVA, Tukey’s post hoc test.
Fig. S3.
Protein levels of WAVE1, Cdk5, and p35 are not altered by acute cocaine challenge following chronic administration of cocaine and withdrawal. (A–C) Saline (S) or cocaine (C, 15 mg/kg) was administered (i.p.) for 14 consecutive days followed by a 14-d withdrawal. After acute cocaine (15 mg/kg) or saline administration, mice were then killed 45 min later using focused microwave irradiation. WAVE1 (A), Cdk5 (B), and p35 (the regulatory subunit of Cdk5, C) were measured by immunoblotting. The levels were quantified and normalized to the levels of S/S. Data represent means ± SEM, n = 6 mice per group. (D and E) After 14 d of withdrawal following 14 d of daily injection of cocaine (15 mg/kg), mice were coinjected with saline (C/S) or cocaine (15 mg/kg) (C/C) plus vehicle, a D1 antagonist, SCH23390 (0.25 mg/kg) (D), or an NMDA receptor antagonist, MK801 (0.25 mg/kg) (E). Mice were killed 45 min later using focused microwave irradiation. The levels of total WAVE1 were measured by immunoblotting. Quantified data were normalized to C/S vehicle. Data represent means ± SEM, n = 7 mice per group.
Fig. 3.
Effect of D1 or NMDA receptor antagonists on cocaine-induced WAVE1 dephosphorylation. After 14-d of withdrawal following 14-d of daily injection of cocaine (15 mg/kg), mice were coinjected with saline (C/S) or cocaine (15 mg/kg) (C/C) plus vehicle, a D1 antagonist, SCH23390 (0.25 mg/kg) (A–C), or an NMDA receptor antagonist, MK801 (0.25 mg/kg) (D–F). Mice were killed 45 min later using focused microwave irradiation. The levels of phospho- and total WAVE1 were measured by immunoblotting. Quantified data were normalized to C/S vehicle. Data represent means ± SEM, n = 7 mice per group. *P < 0.05, **P < 0.01, and ***P < 0.001 (C/S versus C/C). †P < 0.05 and †††P < 0.001 (C/C vehicle versus C/C antagonist as indicated). One-way ANOVA and Tukey’s post hoc test were used.
Role for WAVE1 in Cocaine-Induced Changes in Dendritic Spine Morphology.
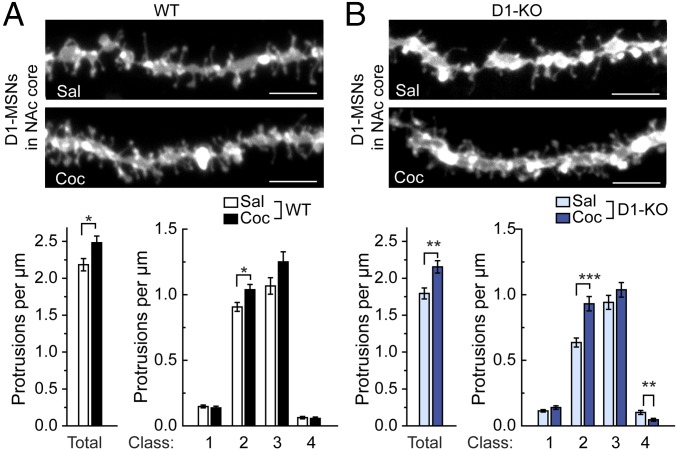
Plasticity of glutamatergic transmission in the NAc has emerged as a major contributing factor for addictive behavior (9, 10, 17). Previous studies showed that chronic administration of cocaine increases dendritic spine density in D1-MSNs (30–32). Dendritic spines are the postsynaptic part of glutamatergic synapses and actin polymerization is highly implicated in spine morphogenesis and stability (33, 34). Because WAVE1 plays a role in the regulation of dendritic spine morphology (4) and cocaine-induced behavior is altered in WAVE1 D1-KO mice (Fig. 1), we examined a possible role for WAVE1 in chronic cocaine-induced increase of spine density in D1-MSNs. We injected WT and WAVE1 D1-KO mice daily with saline or cocaine (15 mg/kg) for 14 d. Two days after the last injection, dendritic spine morphology of D1-MSNs was analyzed. We quantified total dendritic protrusions and also four classes of dendritic spines based on their length (35, 36). Class 1 represents stubby spines without a discernible neck, classes 2 and 3 represent short and long spines, respectively, and class 4 represents filopodial protrusions. In response to cocaine, we observed a comparable increase in the density of total spines and class 2 spines in the NAc core of WT or WAVE1 D1-KO mice (Fig. 4). The effect of chronic cocaine on dendritic spines in the NAc shell was relatively minimal in this experiment (Fig. S4). Our results suggest that WAVE1 is not required for the chronic cocaine-induced increase in dendritic spine density in D1-MSNs observed under these conditions.
Fig. 4.
Chronic cocaine-induced changes in dendritic spine density. WT mice (A) and WAVE1 D1-KO mice (B) were treated daily (i.p.) with saline (Sal) or cocaine (Coc) (15 mg/kg) for 14 d. Two days after the last injection, mice were perfused and dendritic spines were analyzed in DiI-labeled D1-MSNs (identified as enkephalin− cells) in the NAc core. (Upper panels) Representative images of dendritic segments. (Scale bars, 5 µm.) (Lower graphs) Quantification of total and four classes of dendritic protrusion density. Class 1 represents stubby spines without a discernible neck, classes 2 and 3 represent short and long spines, respectively, and class 4 represents filopodial protrusions. Data represent means ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001, Kolmogorov–Smirnov test. n = 14–16 mice per group. The total numbers of dendrites analyzed were 36 (WT-Sal), 30 (WT-Coc), 45 (KO-Sal), and 30 (KO-Coc).
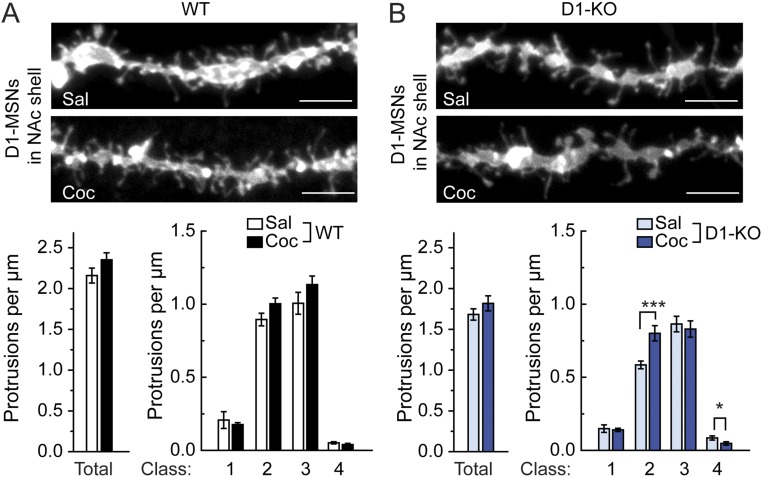
Fig. S4.
Chronic cocaine-induced changes in dendritic spine density in the NAc shell. WT mice (A) and WAVE1 D1-KO mice (B) were injected daily (i.p.) with saline or cocaine (15 mg/kg) for 14 d. Two days after the last injection, mice were perfused and dendritic spines were analyzed in DiI-labeled D1-MSNs (identified as enkephalin− cells) in the NAc shell. (Upper panels) Representative images of dendritic segments. (Scale bars, 5 µm.) (Lower graphs) Quantification of total and four classes of dendritic protrusion density. Data represent means ± SEM. *P < 0.05 and ***P < 0.001, Kolmogorov–Smirnov test. n = 15–16 mice per group. The total numbers of dendrites analyzed were 32 (WT-Sal), 30 (WT-Coc), 42 (KO-Sal), and 30 (KO-Coc).
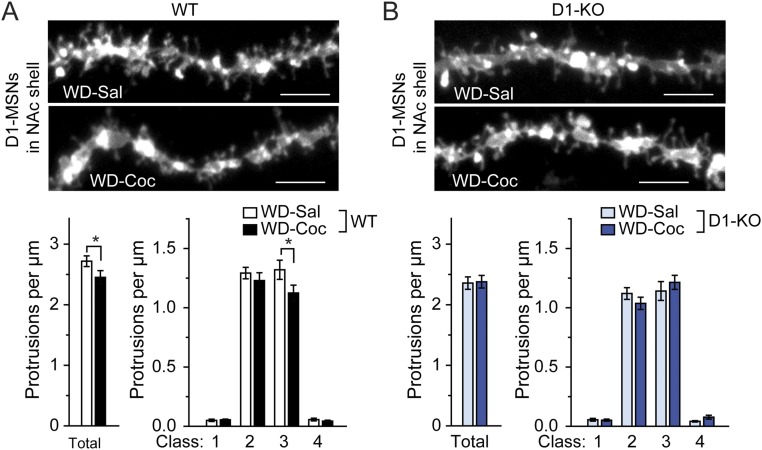
We next investigated possible changes in dendritic spines after an acute challenge with cocaine following withdrawal because WAVE1 dephosphorylation was observed under this condition (Fig. 2). Notably, an acute challenge with cocaine reduced total spine density in D1-MSNs in the WT NAc core but no decrease was found in D1-MSNs in WAVE1 KO NAc core (Fig. 5). The decrease in total spine density in WT mice was due to a decrease in spine density in classes 1–3 spines, whereas an opposing direction of spine changes in classes 1 and 2 was observed in WAVE1 D1-KO mice (Fig. 5). We also observed an acute cocaine-challenge–induced decrease in total and class 3 spine density in D1-MSNs in the NAc shell but the decrease was not observed in WAVE1 KO D1-MSNs in the NAc shell (Fig. S5).
Fig. 5.
Effect of acute cocaine challenge after withdrawal on dendritic spine density. WT mice (A) and WAVE1 D1-KO mice (B) were treated daily (i.p.) with cocaine (15 mg/kg) for 14 d. The mice were subsequently subjected to 14 d of withdrawal (WD) and then acutely challenged with saline or cocaine (15 mg/kg). One hour after the last injection, mice were perfused and dendritic spines were analyzed in DiI-labeled D1-MSNs in the NAc core as in Fig. 4. (Upper panels) Representative images of dendritic segments. (Scale bars, 5 µm.) (Lower graphs) Quantification of total and four classes of dendritic protrusion density. Data represent means ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001, Kolmogorov–Smirnov test. n = 15–16 mice per group. The total numbers of dendrites analyzed were 29 (WT-Sal), 30 (WT-Coc), 31 (KO-Sal), and 30 (KO-Coc).
Fig. S5.
Effect of acute challenge of cocaine after withdrawal on dendritic spine density in the NAc shell. WT mice (A) and WAVE1 D1-KO mice (B) were injected daily with cocaine (15 mg/kg) for 14 d. Mice were subsequently subjected to 14 d of withdrawal and then acutely challenged with saline or cocaine (15 mg/kg). One hour after the last injection, mice were perfused and dendritic spines were analyzed in DiI-labeled D1-MSNs in the NAc shell. D1-MSNs was identified as enkephalin−. (Upper panels) Representative images of dendritic segments. (Scale bars, 5 µm.) (Lower graphs) Quantification of total and four classes of dendritic protrusion density. Data represent means ± SEM. *P < 0.05, Kolmogorov–Smirnov test. n = 15–17 mice per group. The total numbers of dendrites analyzed were 30 (WT-Sal), 30 (WT-Coc), 32 (KO-Sal), and 34 (KO-Coc).
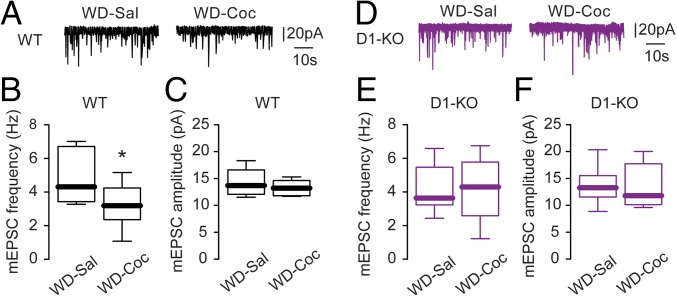
To examine the potential physiological consequences of a decrease in spine density in the NAc core, we examined AMPA glutamate receptor currents in this region. Consistently, acute cocaine challenge following withdrawal caused a significant reduction in frequency but not in amplitude of AMPA currents in WT mice (Fig. 6). This effect of cocaine on AMPA current frequency was abolished in WAVE1 D1-KO mice, supporting a critical role for WAVE1 in the decrease of functional glutamatergic synapses on D1-MSNs under these conditions (Fig. 6).
Fig. 6.
Effect of acute cocaine challenge after withdrawal on AMPA glutamate receptor-mediated synaptic currents in D1-MSNs in the NAc core. After 14 d of withdrawal (WD) from chronic exposure to cocaine (i.p., 15 mg/kg), WT (A–C) and WAVE1 D1-KO mice (D–F) were acutely challenged with saline or cocaine (5 mg/kg). Sample traces of mEPSC from WT (A) or D1-KO (D) D1-MSNs in the NAc core are shown with vertical and horizontal scale bars. mEPSC frequency (B) was reduced by an acute cocaine challenge without altering mEPSC amplitude (C) in WT mice (Sal challenge: n = 9 cells, 6 mice; Coc challenge: n = 6 cells, 3 mice). mEPSC frequency (E) and amplitude (F) were unchanged by an acute cocaine challenge after withdrawal in D1-KO mice (Sal challenge: n = 10 cells, 7 mice; Coc challenge: n = 8 cells, 6 mice). Data were analyzed by Mann–Whitney nonparametric analysis (*P < 0.05) and presented as box-and-whisker plots indicating the median, quartiles, and range.
Discussion
In this study, we identified a role for WAVE1 in cocaine reward and maintenance of locomotor sensitization. Using selective KO of WAVE1, we found decreased cocaine CPP and maintenance of locomotor sensitization in mice lacking WAVE1 in D1-expressing neurons. In contrast, mice lacking WAVE1 in D2-expressing neurons showed normal behavioral responses to cocaine. We also found a role for WAVE1 in the regulation of dendritic spine density in D1-MSNs in NAc in response to an acute challenge of cocaine following chronic cocaine exposure and 2 wk of withdrawal. This finding was associated with a decrease in the frequency of excitatory postsynaptic AMPA receptor currents in D1-MSNs. In further support of a selective role of WAVE1 in D1-mediated signaling, we found that following the acute challenge of cocaine in conjuction with chronic cocaine administration and withdrawal, cocaine-induced WAVE1 dephosphorylation was attenuated by coadministration of a D1 or an NMDA glutamate receptor antagonist.
Our previous studies indicated that WAVE1 is phosphorylated to a high level by Cdk5/p35 and as a result is largely inactive under basal conditions (4). Chronic cocaine exposure increases the levels of Cdk5 and p35 in the striatum (8). Increased levels of Cdk5/p35 phosphorylate DARPP-32 at Thr75 and reduce the efficacy of dopamine/cAMP/PKA/DARPP-32/protein phosphatase-1 signaling (8, 15). Cdk5/p35 also regulates the NR2B subunit of NMDA receptors by direct interaction and phosphorylation at Ser1116, resulting in facilitation of degradation of NR2B by calpain and inhibition of surface expression, respectively (37, 38). Thus, chronic cocaine-induced Cdk5/p35 expression would be expected to maintain WAVE1 in an inactive state through direct inhibitory phosphorylation as well as through inhibition of D1 and NMDA receptor signaling, which are the major pathways for WAVE1 activation (7). Consistent with this interpretation, WAVE1 was not required for the development of locomotor sensitization or for the increase of dendritic spine density observed following chronic exposure to cocaine. Instead, WAVE1 was dephosphorylated after an acute challenge of cocaine following chronic cocaine plus withdrawal, in a D1 and NMDA receptor signaling-dependent manner. Our previous studies showed that D1/cAMP/PKA signaling or NMDA receptor/calcium signaling leads to dephosphorylation and activation of WAVE1 (4, 7). Stimulation of D1 increases but stimulation of D2 decreases cAMP/PKA signaling (24). Furthermore, stimulation of D1 facilitates, whereas stimulation of D2 attenuates NMDA-mediated calcium responses in MSNs (39, 40). Thus, WAVE1 in D1-MSNs but not in D2-MSNs would be activated in response to cocaine. This biochemical activation mechanism likely underlies the selective role for WAVE1 in D1-MSNs in cellular and behavioral responses to cocaine observed in this study.
In this study, we used a noncontingent cocaine administration paradigm involving chronic cocaine administration, withdrawal, and acute challenge of cocaine. In this paradigm, we were able to observe preserved sensitization of cocaine reward (measured by CPP) and sensitization of locomotor activity. Following withdrawal from chronic cocaine exposure, morphological and functional plasticity of glutamatergic synapses in the NAc has been suggested as a critical mechanism underlying cocaine craving and relapse (10, 11). Strengthening of excitatory synapses in MSNs in the NAc involves the initial formation of silent synapses lacking AMPA receptors during chronic exposure to cocaine, followed by insertion of AMPA receptors into spines during withdrawal (9–11). Accumulating evidence indicates that adaptive cocaine action involves potentiation of glutamatergic afferents arising from mPFC, amygdala, and ventral hippocampus preferentially onto D1-MSNs (41–44), although D2-MSNs still contribute to certain behavioral features of cocaine action (45, 46). Previous studies showed that WAVE1 is highly localized to dendritic spines (47). WAVE1 KO neurons had decreased dendritic spine density, suggesting a role for WAVE1 in dendritic spine formation (4, 47). In the current study, we also observed lower baseline dendritic spine density in WAVE1 D1-KO MSNs compared with WT MSNs. However, WAVE1 was dispensable for chronic cocaine-induced spine formation. Instead, WAVE1 was required for a selective decrease in spine density in D1-MSNs in response to a cocaine challenge following withdrawal from chronic cocaine. We also observed that cocaine challenge decreased the frequency in AMPA currents in D1-MSNs in a WAVE1-dependent manner, supporting a role for WAVE1 in the pruning of glutamatergic synapses. Thus, our previous and current results together implicate WAVE1 in mediating distinct aspects of morphological plasticity that depend on the stage of spine development and the strength of input signals.
The dopamine system is intricately connected with the glutamatergic system in responses to acute and chronic exposure to cocaine. Dopaminergic neurons in the VTA provide dopaminergic input to NAc and mPFC and receive glutamatergic input from several corticolimbic structures such as mPFC, amygdala, and hippocampus (48–50). Glutamatergic input to the VTA increases the activity of dopaminergic neurons and enhances dopamine release in NAc (51–53). Dopamine also affects glutamatergic transmission by increasing the firing probability in pyramidal neurons in mPFC (54). The interactions of these two neurotransmitter systems in the VTA and mPFC might be involved in the increased glutamate release in the NAc observed after repeated cocaine administration (55, 56). Glutamate transmission in the NAc plays a key role in cocaine addiction, which is predominantly mediated by AMPA receptors in MSNs (17, 41, 57, 58). The synaptic strength of AMPA receptors in MSNs is also modulated by intracellular dopamine signaling. Cocaine-activated D1/cAMP/PKA signaling leads to phosphorylation of AMPA receptors and primes them for synaptic insertion (59, 60). The WAVE1-regulated decrease of glutamatergic synapses on D1-MSNs in NAc in response to cocaine challenge following withdrawal might be part of a homeostatic feedback mechanism to avoid excessive increases in excitatory input and to thereby maintain network stability (61, 62). It is also possible that WAVE1-mediated synaptic pruning is input specific. Recent studies indicate that cocaine-evoked plasticity in NAc specifically in glutamatergic afferents from mPFC onto D1-MSNs mediates correct action-outcome selection during cocaine seeking, whereas cocaine-evoked plasticity specifically in glutamatergic afferents from ventral hippocampus onto D1-MSNs mediates recognition of the correct context where cocaine can be obtained (41). It will be important to identify specific afferents regulated by WAVE1 and behavioral consequence of WAVE1-mediated synaptic plasticity.
Cyfip2 (PIR121) is a component of the WAVE1 complex, which we isolated as a Cdk5/p35 binding protein from the striatum (4). Recently, genetic screening of C57BL/6 substrains identified the Cyfip2 gene mutation S968F as a causative variant for different acute and sensitized locomotor responses to cocaine (63). Another genetic study of BXD mouse strains also identified Cyfip2 as a candidate gene underlying a different intake response in i.v. cocaine self-administration (64). As in the case of the mouse Cyfip2 S968F variant providing a differential cocaine response in mouse substrains (63), certain polymorphisms in WAVE1 complex proteins in humans might contribute to vulnerability in functional plasticity of glutamatergic synapses underlying addiction behavior such as cocaine craving and relapse. Characterization of WAVE1 mouse models using drug self-administration to measure clinically relevant drug craving and compulsive drug taking behavior (10, 65, 66), together with further understanding WAVE1-mediated synaptic plasticity in D1-MSNs, will be important topics for future research.
Methods
Animals and Treatments.
All procedures involving animals were approved by The Rockefeller University Institutional Animal Care and Use Committee and were performed in accordance with National Institutes of Health guidelines. BAC transgenic Drd1a-EGFP, Drd2-EGFP, Drd1a-Cre, and Drd2-Cre were from GENSAT (The Rockefeller University) (26, 67). A floxed WAVE1 line (25) was crossed with Drd1a-Cre (EY262) or Drd2-Cre (ER44) mouse lines. A double transgenic line harboring floxed WAVE1 and Drd1a-Cre alleles was crossed with a tdTomato reporter line (The Jackson Laboratory, 007909). Progeny were produced using in vitro fertilization and embryo-transfer techniques to generate the animals needed for the biochemical and behavioral tests. Cocaine (Sigma-Aldrich) was administered by i.p. injection.
Immunohistochemistry.
Mouse perfusion, preparation of brain slices, and immunohistochemistry were performed as described (30, 36). Anti-GFP (rabbit polyclonal, 1:1,000; Abcam), anti-WAVE1 (rabbit polyclonal, 1:1,000; V0101) (68) and anti-enkephalin (clone: NOC1, 1:250; Millipore) were used.
Locomotor Behavior.
WT, WAVE1 D1-KO, and D2-KO mice (12–16 wk of age) were habituated to saline injection (i.p.) for 2 consecutive days and then they received injections of cocaine (i.p. 10 mg/kg) for 10 consecutive days. WT and WAVE1 D1-KO mice were then divided into two groups: one group for 2-wk withdrawal and the other group for 3-wk withdrawal. After 2-wk or 3-wk withdrawal, mice received a challenge of cocaine (i.p. 10 mg/kg) to test the maintenance of sensitized locomotor activity. In the case of WAVE1 D2-KO mice and their WT mice, following a 2-wk withdrawal, mice received a challenge of cocaine (i.p. 10 mg/kg). Locomotor activity was measured daily in an open field chamber (42 × 42 × 30.5 cm) for 60 min after saline treatments, chronic cocaine treatments (first 5 d), and acute challenge with cocaine. Automated Accuscan software (Gerber Technology) was used to calculate total distance traveled.
Conditioned Place Preference.
CPP was carried out in a three-chambered box and a pretest, which preceded all training sessions, was performed as described previously (69). During 30-min training sessions in which partitions between end chambers were lowered, mice received two saline and two cocaine injections on 2 consecutive days (saline in the morning and cocaine in the afternoon) in opposite end chambers. One day after training, CPP was assessed during a training session in which all partitions between chambers were raised, mice were placed in the middle chamber, and time spent in individual chambers was monitored during a 20-min session. The testing session preference score was calculated as time spent in the cocaine-paired end chamber minus time spent in the saline-paired end chamber. For normal CPP tests (nonsensitization) a 7.5 mg/kg cocaine dose (i.p.) was used during the training session. For sensitization CPP experiments, mice were injected i.p. with a 15-mg/kg cocaine dose one time per day for 7 consecutive days in their home cage. This dosing was followed by a 5-d withdrawal period in which no cocaine was given. Mice were then injected with a low cocaine dose (5 mg/kg) for the sensitization CPP training sessions.
Phosphorylation Analysis and Immunoblotting.
Saline- or cocaine-injected mice were killed by focused microwave irradiation of the brain (model: TMW-6402C, Muromachi Kikai). The killing of rodents by microwave irradiation results in rapid protein denaturation, effectively protecting phosphorylated proteins from postmortem activity of protein phosphatases (70). Preparation of brain lysates, BCA protein assay, and immunoblotting using the Odyssey infrared imaging system (Licor) were done as described (7). Antibodies for total WAVE1, phosphorylation site-specific WAVE1, PIR121, Nckap1, Abi2, p35, and Cdk5 were used as described (4).
Dendritic Spine Analysis.
Perfusion of mouse brain, preparation of brain slices, and labeling brain slices with the lipophilic fluorescence dye 1,1′-diotadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) were performed as described (30, 36). Fluorescent dendritic images of MSNs were taken using a confocal microscope (Zeiss LSM 710) with an oil immersion lens (EC Plan-Neofluar 100×, 1.4 N.A.). DiI was excited using an Argon 514-nm laser line. A filterless scanhead was used for emission collection. Images of distal dendrites (second to fourth order dendrites) were examined in this study. For dendritic spines, a stack of images was acquired in the z dimension with an optical slice thickness of 0.4 µm (with oversampling). The pixel sizes along x and y dimensions were both 0.06 µm (with oversampling). To identify D1-MSNs, enkephalin was immnunostained and dendritic spine images were acquired from enkephalin− cells (D1-MSNs). All measurements were made manually with Metamorph image analysis software (Universal Imaging Corporation). Protrusions from dendrites were classified into four types based on their length as described (35, 36). Class 1 protrusions (also called stubby) were less than 0.5 µm, lacked a large spine head, and did not appear to have a neck; class 2, or shorter spines, were between 0.5 and 1.25 µm long; class 3, or longer spines, ranged between 1.25 and 3.0 µm; and class 4, or filopodial extensions, were filamentous protrusions longer than 3.0 µm.
Electrophysiological Analysis.
WT and WAVE1 D1-KO mice expressing Drd1-promoter–driven tdTomato were injected i.p. with a 15 mg/kg cocaine dose per day for 14 consecutive days. This dose was followed by a 14-d withdrawal and then an acute challenge of either saline or cocaine (5 mg/kg). Coronal slices were prepared from WT and WAVE1 D1-KO mice following procedures approved by the Northwestern University Animal Care and Use Committee, as described (62). Patch pipettes (3–4 MΩ) were filled with internal recording solution (in millimolar concentration): 120.0 CsMeSO3, 5.0 NaCl, 0.16 CaCl2, 10.0 TEA-Cl, 10.0 Hepes, 5.0 QX-314, 4.0 Mg-ATP, 0.3 Na-GTP. Recordings were performed on a submersion-style recording chamber with a fixed-stage Olympus BX51 upright microscope with continual perfusion of 95% O2/5% CO2 bubbled artificial cerebrospinal fluid (in millimolar concentration): 124 NaCl, 3 KCl, 2 CaCl2, 1 MgCl2, 26 NaHCO3, 1 NaH2PO4, and 16.66 glucose with (in micromolar concentration) 100.0 4-AP, 1.0 TTX, 10.0 gabazine, and 1.0 CGP 35348. tdTomato expression was used to verify D1-MSNs. Whole-cell patch clamp recordings were made using a Multiclamp 700B amplifier using custom-written freeware package WinFluor (John Dempster, University of Strathclyde, Glasgow, UK) as described (62, 71). Miniature excitatory postsynaptic currents (mEPSCs) were recorded in voltage clamp held at −70 mV. Data were analyzed using the MiniAnalysis program (Synaptosoft).
Acknowledgments
We thank Xiaozhu Zhang, Grant Martin, Hersh Trivedi, Caroline Holmes, Ryan Malpass, Cassandra Kooiker, Junghee Jin, and Jodi Gresack for technical assistance and discussion; Rada Norinsky and The Rockefeller University Transgenic Services Laboratory for in vitro fertilization and embro transfer services; and Elisabeth Griggs for graphics. This work was supported by Department of Defense US Army Medical Research Acquisition Activity Grants W81XWH-09-1-0392 (to Y.K.) and W81XWH-09-1-0402 (to P.G.); National Institutes of Health Grants DA010044 and MH090963 (to P.G.), R01DA014133 (to E.J.N.), and NS34696 (to D.J.S.); and grants from the JPB Foundation (to P.G. and D.J.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1621185114/-/DCSupplemental.
References
- 1.Rotty JD, Wu C, Bear JE. New insights into the regulation and cellular functions of the ARP2/3 complex. Nat Rev Mol Cell Biol. 2013;14(1):7–12. doi: 10.1038/nrm3492. [DOI] [PubMed] [Google Scholar]
- 2.Soderling SH, et al. Loss of WAVE-1 causes sensorimotor retardation and reduced learning and memory in mice. Proc Natl Acad Sci USA. 2003;100(4):1723–1728. doi: 10.1073/pnas.0438033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eden S, Rohatgi R, Podtelejnikov AV, Mann M, Kirschner MW. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature. 2002;418(6899):790–793. doi: 10.1038/nature00859. [DOI] [PubMed] [Google Scholar]
- 4.Kim Y, et al. Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature. 2006;442(7104):814–817. doi: 10.1038/nature04976. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z, et al. Structure and control of the actin regulatory WAVE complex. Nature. 2010;468(7323):533–538. doi: 10.1038/nature09623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen B, et al. The WAVE regulatory complex links diverse receptors to the actin cytoskeleton. Cell. 2014;156(1–2):195–207. doi: 10.1016/j.cell.2013.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceglia I, Kim Y, Nairn AC, Greengard P. Signaling pathways controlling the phosphorylation state of WAVE1, a regulator of actin polymerization. J Neurochem. 2010;114(1):182–190. doi: 10.1111/j.1471-4159.2010.06743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bibb JA, et al. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410(6826):376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- 9.Dong Y, Nestler EJ. The neural rejuvenation hypothesis of cocaine addiction. Trends Pharmacol Sci. 2014;35(8):374–383. doi: 10.1016/j.tips.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf ME. Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci. 2016;17(6):351–365. doi: 10.1038/nrn.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scofield MD, et al. The nucleus accumbens: Mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacol Rev. 2016;68(3):816–871. doi: 10.1124/pr.116.012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14(9):609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237(4819):1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 14.Amara SG, Sonders MS. Neurotransmitter transporters as molecular targets for addictive drugs. Drug Alcohol Depend. 1998;51(1–2):87–96. doi: 10.1016/s0376-8716(98)00068-4. [DOI] [PubMed] [Google Scholar]
- 15.Bibb JA, et al. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402(6762):669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- 16.Russo SJ, et al. The addicted synapse: Mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33(6):267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10(8):561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 18.Norrholm SD, et al. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;116(1):19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerfen CR, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250(4986):1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 20.Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86(2):353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 21.Gerfen CR. Molecular effects of dopamine on striatal-projection pathways. Trends Neurosci. 2000;23(10) Suppl:S64–S70. doi: 10.1016/s1471-1931(00)00019-7. [DOI] [PubMed] [Google Scholar]
- 22.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kupchik YM, et al. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 2015;18(9):1230–1232. doi: 10.1038/nn.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: Distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ceglia I, et al. APP intracellular domain-WAVE1 pathway reduces amyloid-β production. Nat Med. 2015;21(9):1054–1059. doi: 10.1038/nm.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong S, et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27(37):9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valjent E, Bertran-Gonzalez J, Hervé D, Fisone G, Girault JA. Looking BAC at striatal signaling: Cell-specific analysis in new transgenic mice. Trends Neurosci. 2009;32(10):538–547. doi: 10.1016/j.tins.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Dunah AW, Standaert DG. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J Neurosci. 2001;21(15):5546–5558. doi: 10.1523/JNEUROSCI.21-15-05546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ladepeche L, et al. Single-molecule imaging of the functional crosstalk between surface NMDA and dopamine D1 receptors. Proc Natl Acad Sci USA. 2013;110(44):18005–18010. doi: 10.1073/pnas.1310145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee KW, et al. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci USA. 2006;103(9):3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J, Park BH, Lee JH, Park SK, Kim JH. Cell type-specific alterations in the nucleus accumbens by repeated exposures to cocaine. Biol Psychiatry. 2011;69(11):1026–1034. doi: 10.1016/j.biopsych.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Graziane NM, et al. Opposing mechanisms mediate morphine- and cocaine-induced generation of silent synapses. Nat Neurosci. 2016;19(7):915–925. doi: 10.1038/nn.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hotulainen P, Hoogenraad CC. Actin in dendritic spines: Connecting dynamics to function. J Cell Biol. 2010;189(4):619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hlushchenko I, Koskinen M, Hotulainen P. Dendritic spine actin dynamics in neuronal maturation and synaptic plasticity. Cytoskeleton. 2016;73(9):435–441. doi: 10.1002/cm.21280. [DOI] [PubMed] [Google Scholar]
- 35.Vanderklish PW, Edelman GM. Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc Natl Acad Sci USA. 2002;99(3):1639–1644. doi: 10.1073/pnas.032681099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Y, et al. Methylphenidate-induced dendritic spine formation and DeltaFosB expression in nucleus accumbens. Proc Natl Acad Sci USA. 2009;106(8):2915–2920. doi: 10.1073/pnas.0813179106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawasli AH, et al. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci. 2007;10(7):880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plattner F, et al. Memory enhancement by targeting Cdk5 regulation of NR2B. Neuron. 2014;81(5):1070–1083. doi: 10.1016/j.neuron.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cahill E, et al. D1R/GluN1 complexes in the striatum integrate dopamine and glutamate signalling to control synaptic plasticity and cocaine-induced responses. Mol Psychiatry. 2014;19(12):1295–1304. doi: 10.1038/mp.2014.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu XY, et al. Modulation of D2R-NR2B interactions in response to cocaine. Neuron. 2006;52(5):897–909. doi: 10.1016/j.neuron.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Pascoli V, et al. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509(7501):459–464. doi: 10.1038/nature13257. [DOI] [PubMed] [Google Scholar]
- 42.Terrier J, Lüscher C, Pascoli V. Cell-type specific insertion of GluA2-lacking AMPARs with cocaine exposure leading to sensitization, cue-induced seeking, and incubation of craving. Neuropsychopharmacology. 2016;41(7):1779–1789. doi: 10.1038/npp.2015.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pascoli V, Turiault M, Lüscher C. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature. 2011;481(7379):71–75. doi: 10.1038/nature10709. [DOI] [PubMed] [Google Scholar]
- 44.MacAskill AF, Cassel JM, Carter AG. Cocaine exposure reorganizes cell type- and input-specific connectivity in the nucleus accumbens. Nat Neurosci. 2014;17(9):1198–1207. doi: 10.1038/nn.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bock R, et al. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci. 2013;16(5):632–638. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Creed M, Ntamati NR, Chandra R, Lobo MK, Lüscher C. Convergence of reinforcing and anhedonic cocaine effects in the ventral pallidum. Neuron. 2016;92(1):214–226. doi: 10.1016/j.neuron.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soderling SH, et al. A WAVE-1 and WRP signaling complex regulates spine density, synaptic plasticity, and memory. J Neurosci. 2007;27(2):355–365. doi: 10.1523/JNEUROSCI.3209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2(2):119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 49.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8(11):844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 50.Tzschentke TM, Schmidt WJ. Glutamatergic mechanisms in addiction. Mol Psychiatry. 2003;8(4):373–382. doi: 10.1038/sj.mp.4001269. [DOI] [PubMed] [Google Scholar]
- 51.Tzschentke TM, Schmidt WJ. Functional relationship among medial prefrontal cortex, nucleus accumbens, and ventral tegmental area in locomotion and reward. Crit Rev Neurobiol. 2000;14(2):131–142. [PubMed] [Google Scholar]
- 52.Blaha CD, Yang CR, Floresco SB, Barr AM, Phillips AG. Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur J Neurosci. 1997;9(5):902–911. doi: 10.1111/j.1460-9568.1997.tb01441.x. [DOI] [PubMed] [Google Scholar]
- 53.Floresco SB, Yang CR, Phillips AG, Blaha CD. Basolateral amygdala stimulation evokes glutamate receptor-dependent dopamine efflux in the nucleus accumbens of the anaesthetized rat. Eur J Neurosci. 1998;10(4):1241–1251. doi: 10.1046/j.1460-9568.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- 54.Lewis BL, O’Donnell P. Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential ‘up’ states in pyramidal neurons via D(1) dopamine receptors. Cereb Cortex. 2000;10(12):1168–1175. doi: 10.1093/cercor/10.12.1168. [DOI] [PubMed] [Google Scholar]
- 55.Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16(4):1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reid MS, Berger SP. Evidence for sensitization of cocaine-induced nucleus accumbens glutamate release. Neuroreport. 1996;7(7):1325–1329. doi: 10.1097/00001756-199605170-00022. [DOI] [PubMed] [Google Scholar]
- 57.Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93(4):1359–1367. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- 58.Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20(15):RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Snyder GL, et al. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J Neurosci. 2000;20(12):4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolf ME. Regulation of AMPA receptor trafficking in the nucleus accumbens by dopamine and cocaine. Neurotox Res. 2010;18(3–4):393–409. doi: 10.1007/s12640-010-9176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turrigiano G. Homeostatic synaptic plasticity: Local and global mechanisms for stabilizing neuronal function. Cold Spring Harb Perspect Biol. 2012;4(1):a005736. doi: 10.1101/cshperspect.a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fieblinger T, et al. Cell type-specific plasticity of striatal projection neurons in parkinsonism and L-DOPA-induced dyskinesia. Nat Commun. 2014;5:5316. doi: 10.1038/ncomms6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar V, et al. C57BL/6N mutation in cytoplasmic FMRP interacting protein 2 regulates cocaine response. Science. 2013;342(6165):1508–1512. doi: 10.1126/science.1245503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dickson PE, et al. Systems genetics of intravenous cocaine self-administration in the BXD recombinant inbred mouse panel. Psychopharmacology (Berl) 2016;233(4):701–714. doi: 10.1007/s00213-015-4147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: A review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 66.Pickens CL, et al. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34(8):411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gong S, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425(6961):917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 68.Soderling SH, et al. The WRP component of the WAVE-1 complex attenuates Rac-mediated signalling. Nat Cell Biol. 2002;4(12):970–975. doi: 10.1038/ncb886. [DOI] [PubMed] [Google Scholar]
- 69.Cahill ME, et al. Bidirectional synaptic structural plasticity after chronic cocaine administration occurs through Rap1 Small GTPase signaling. Neuron. 2016;89(3):566–582. doi: 10.1016/j.neuron.2016.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O’Callaghan JP, Sriram K. Focused microwave irradiation of the brain preserves in vivo protein phosphorylation: Comparison with other methods of sacrifice and analysis of multiple phosphoproteins. J Neurosci Methods. 2004;135(1–2):159–168. doi: 10.1016/j.jneumeth.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 71.Day M, Wokosin D, Plotkin JL, Tian X, Surmeier DJ. Differential excitability and modulation of striatal medium spiny neuron dendrites. J Neurosci. 2008;28(45):11603–11614. doi: 10.1523/JNEUROSCI.1840-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]