Significance
Antigen recognition by the immune system triggers rapid, specific, and protective responses, which are counterbalanced by inhibitory checkpoints to minimize potentially harmful immunity. The programmed death-1/ programmed death ligand-1 (PD-1/PD-L1) checkpoint is overreactive in cancer patients, curbing antitumor immunity. Whether a failing PD-1/PD-L1 checkpoint contributes to spontaneous autoimmune disease in humans is unknown. Here, we found that in patients with the autoimmune vasculitis giant cell arteritis, antigen-presenting cells provide insufficient negative signaling; unleashing highly activated T cells to infiltrate and damage the walls of large arteries. Thus, immunoinhibitory signals protect large arteries against inflammatory attack and checkpoint activation may be a suitable strategy to treat autoimmune vasculitis.
Keywords: vasculitis, T cells, immune checkpoint, autoimmunity, PD-1
Abstract
Giant cell arteritis (GCA) causes autoimmune inflammation of the aorta and its large branches, resulting in aortic arch syndrome, blindness, and stroke. CD4+ T cells and macrophages form organized granulomatous lesions in the walls of affected arteries, destroy the tunica media, and induce ischemic organ damage through rapid intimal hyperplasia and luminal occlusion. Pathogenic mechanisms remain insufficiently understood; specifically, it is unknown whether the unopposed activation of the immune system is because of deficiency of immunoinhibitory checkpoints. Transcriptome analysis of GCA-affected temporal arteries revealed low expression of the coinhibitory ligand programmed death ligand-1 (PD-L1) concurrent with enrichment of the programmed death-1 (PD-1) receptor. Tissue-residing and ex vivo-generated dendritic cells (DC) from GCA patients were PD-L1lo, whereas the majority of vasculitic T cells expressed PD-1, suggesting inefficiency of the immunoprotective PD-1/PD-L1 immune checkpoint. DC–PD-L1 expression correlated inversely with clinical disease activity. In human artery-SCID chimeras, PD-1 blockade exacerbated vascular inflammation, enriched for PD-1+ effector T cells, and amplified tissue production of multiple T-cell effector cytokines, including IFN-γ, IL-17, and IL-21. Arteries infiltrated by PD-1+ effector T cells developed microvascular neoangiogenesis as well as hyperplasia of the intimal layer, implicating T cells in the maladaptive behavior of vessel wall endogenous cells. Thus, in GCA, a breakdown of the tissue-protective PD1/PD-L1 checkpoint unleashes vasculitic immunity and regulates the pathogenic remodeling of the inflamed arterial wall.
Giant cell arteritis (GCA) is a granulomatous vasculitis with a stringent tissue tropism, named after the multinucleated giant cells that populate the inflammatory lesions in the arterial walls. Granulomatous infiltrates composed of CD4+ T cells and macrophages penetrate from the adventitia into the media and destroy the lamina elastica interna. T cells with identical T-cell receptor (TCR) sequences have been isolated from spatially distinct lesions (1, 2), highly suggestive for antigen-driven T-cell activation, yet no singular vasculitogenic antigen has been defined. Lesional T cells provide a spectrum of effector functions, suspiciously diverse, and ranging from the production of IFN-γ, IL-17, and IL-9 to IL-21 (3, 4). Similarly, multiple functional macrophage subsets participate in granuloma formation, spanning from macrophages committed to cytokine production (IL-6, IL-1β), to those releasing reactive oxygen species, to those providing growth factors (PDGF, FGF) and angiogenic factors (VEGF) (5–7).
The wall layers of large and medium vessels have features of an immunoprivileged niche (8) and the invasion of inflammatory cells in GCA essentially breaks this immune privilege. Under physiologic conditions endogenous dendritic cells (DC), so-called vascular DC (vasDC), populate the arterial wall in a vessel-specific distribution pattern and may protect against immune attack (9). Localized in the adventitial layer, such vasDC are close to the vasa vasorum, and are positioned between the vascular access to the wall and the immunoprivileged tissue site. Their role in controlling the influx of immune cells into the mural structures remains undefined. Similarly, understanding how antigen-nonspecific factors affect the intensity and the quality of the vasculitogenic immune response could redefine critical pathogenic processes with a major impact on immunosuppressive strategies (10).
T-cell–dependent immune responses are fine-tuned by a multitude of costimulatory and coinhibitory signals, provided by receptor–ligand interactions that modulate TCR-initiated signaling cascades (11). Such immune checkpoints are crucial for the maintenance of self-tolerance, prevent autoimmune disease, and protect against collateral tissue damage (12). Conversely, excessive expression of immune checkpoint proteins has been associated with immune resistance mechanisms, prominently used by tumor cells to escape from antitumor immunity (13). Recent successes in cancer immunotherapy have highlighted the importance of inhibitory immune checkpoints that stop antigen-reactive T cells. Specifically, monoclonal antibodies that block the programmed death-1/programmed death ligand-1 (PD-1/PD-L1) pathway have yielded unprecedented therapeutic benefit in patients with advanced solid tumors (14–16). PD-1 is expressed on activated T and B cells and its engagement by its ligands PD-L1 or PD-L2 disrupts kinase activity in the TCR-activation cascade through the phosphatase SHP2. Resulting immunosuppression involves several mechanisms, including T-cell apoptosis, T-cell exhaustion, T-cell anergy, T-cell IL-10 production, and Treg induction.
The contrasting scenario to immune resistance is exuberant immunity, leading to immune-mediated tissue injury and autoimmune diseases. PD-1 and PD-L1 deficiency have been associated with a lupus-like syndrome (17) and a dilated myocardiopathy (18), respectively. Lack of PD-L1 or PD-1 exacerbates murine diabetes and experimental autoimmune encephalitis (19, 20) and PD-L1 overexpression reduces spinal cord T-cell infiltrates (21). PD-L1−/− antigen-presenting cells (APCs) fail to convert naïve CD4+ T cells into Tregs (22), and PD-1−/− mice are prone to enrich for Th1 and Th17 cells (23).
Guided by a transcriptomic signature of GCA-affected arteries that lacked expression of the inhibitory ligand PD-L1, we have explored the role of the PD pathway in regulating the intensity and the functional orientation of vasculitogenic T-cell responses. GCA vascular lesions are occupied by PD-L1lo DCs and PD-1hi T cells. PD-L1lo DCs from GCA patients enhance T-cell activation and proliferative expansion. In a humanized mouse model of vasculitis, treatment with anti–PD-1 antibodies effectively accelerates the recruitment and retention of T cells and intensifies T-cell and macrophage responses in the inflamed artery. Vasculitogeneic T cells produce IFN-γ, IL-17, and IL-21, sustaining multifunctional effector functions. Accumulation of such multifunctional T-cell populations is associated with the rapid outgrowth of hyperplastic intima, activation of endothelial cells, and the induction of microvascular neoangiogenesis, connecting T cells to disease-relevant remodeling processes in the vascular wall. In essence, a dysfunctional inhibitory immune checkpoint exposes the vessel wall to inflammatory attack, formation of microvascular networks, and intimal hyperplasia, ultimately promoting ischemic organ damage. Reconstitution of a functional PD checkpoint could provide an entirely new strategy to treat medium- and large-vessel vasculitis.
Methods
Tissues, Cells, and Antibodies.
Temporal arteries were collected from diagnostic biopsies. A diagnosis of GCA was based on typical histological findings. Temporal arteries were considered negative for GCA if no inflammatory cells were identified on histology. Artery biopsies from patients with a diagnosis of polymyalgia rheumatic were excluded, as they can be negative by histology but have altered function of vasDC (24, 25). Normal human aorta, temporal, and axillary arteries were donated by organ donors. Patients were enrolled into the protocol if they had a diagnosis of biopsy-positive GCA or anti-cyclic citrullinated peptide-positive rheumatoid arthritis (RA) and had active disease. Patient demographic characteristics are listed in Table 1. Diagnostic biopsies of the lung and the skin of patients with granulomatosis with polyangiitis (GPA), a small-vessel vasculitis, served as controls. Age-matched healthy controls were recruited through the Stanford Blood Bank Research Program. A preexisting diagnosis of cancer, autoimmune disease, or chronic viral infection was considered an exclusion criterion.
Table 1.
Clinical characteristics of patients with GCA
| Parameters | Patients (n = 68) |
| Age (mean ± SD) | 72.7 ± 8.1 |
| Female | 76.5% |
| Ethnicity | |
| Caucasian | 86.8% |
| Hispanic | 8.8% |
| African American | 4.4% |
| Headaches | 72.1% |
| Eye involvement | 41.1% |
| Jaw claudication | 23.5% |
| Polymyalgia rheumatica | 61.8% |
| Disease duration (mean ± SD, mo) | 15.2 ± 24.0 |
| Disease activity | |
| High | 66.2% |
| Moderate | 11.8% |
| Low | 22.1% |
| ESR (mean ± SD, mm/h) | 44.6 ± 31.1 |
| CRP (mean ± SD, mg/dL) | 4.2 ± 4.5 |
| Untreated | 35.3% |
| Prednisone (mg/d, mean ± SD) | 13.4 ± 18.9 |
Peripheral blood mononuclear cells (PBMCs) were isolated from the peripheral blood of patients or healthy donors by density gradient centrifugation with Ficoll-Hypaque (Lymphoprep). Total and naïve CD4+ T cells were purified from PBMC by negative selection using the EasySep human total or naïve CD4+ T-Cell enrichment kits (Stemcell Technologies).
Antibodies used in the study are listed in Table S1. PCR primers are listed in Table S2.
Table S1.
Antibodies used in this study
| Antigens | Conjugated | Source |
| CD4 | FITC | BioLegend |
| CD14 | FITC | BioLegend |
| CD20 | APC | BioLegend |
| CD25 | APC | BD Pharmingen |
| CD80 | FITC | BioLegend |
| CD86 | FITC | BioLegend |
| CD273 (PD-L2) | PE | BioLegend |
| CD274 (PD-L1) | PE | BioLegend |
| PD-1 | APC | BioLegend/Bioxcell |
| CD3 | None | Dako |
| DC-SIGN | None | Abcam |
| PD-L1 | None | BioLegend |
| PD-1 | None | BioLegend |
| vWF | None | Abcam |
| α-SMA | None | Abcam |
| Rabbit IgG | Alexa Fluor 488 | Life Technologies |
| Mouse IgG | Alexa Fluor 546 | Life Technologies |
| Rabbit IgG | HRP | Dako |
| Mouse IgG | AP | Vector Laboratories |
Table S2.
Primers used this study
| Genes | Forward primers | Reverse primers |
| TCR | CCTTCAACAACAGCATTATTATTCCAG | CGAGGGAGCACAGGCTGTCTTA |
| T-bet | TGACCCAGATGATTGTGCTCCAGT | AATCTCGGCATTCTGGTAGGCAGT |
| RORC | GAGAGCTAGGTGCAGAGCTT | AACTTGACAGCATCTCGGGAC |
| GATA-3 | AGACCACCACAACCAAACT | GATGCCTTCCTTCTTCATAGTCA |
| Foxp3 | TTCAAGTTCCACAACATGCGACCC | GCACAAAGCACTTGTGCAGACTCA |
| IFN-γ | ACTAGGCAGCCAACCTAAGCAAGA | CATCAGGGTCACCTGACACATTCA |
| IL-17A | AACCGATCCACCTCACCTTGGAAT | TTCATGTGGTAGTCCACGTTCCCA |
| IL-4 | TGAACAGCCTCACAGAGCAGAAGA | CAGTTGTGTTCTTGGAGGCAGCAA |
| IL-10 | TCCTTGGTGGAGGACTTTAAGGGT | TGTCTGGGTCTTGGTTCTCAGCTT |
| IL-1 | AAGTACTGAGCTCGCCAGTGAAA | TTGCTGTAGTGGTGGTCGGAGATT |
| IL-6 | AGCCACTCACCTCTTCAGAACGAA | AGTGCCTCTTTGCTGCTTTCACAC |
| TNF-α | GGGACCTCTCTCTAATCAGCC | GTTATCTCTCAGCTCCACGCC |
| IL-7 | AGAGGAACCAGCTGCAGAGA | CAAGGATCAGGGGAGGAAGT |
| IL-15 | GAAGCCAACTGGGTGAATGT | TACTTGCATCTCCGGACTCA |
| IL-23p19 | ACTCAGCAGATTCCAAGCCTCAGT | TGGAGATCTGAGTGCCATCCTTGA |
| IL-27p28 | CCTGGTTCAAGCTGGTGTCT | CTCCTGGCAGGTGAGATTCC |
| IL-21 | TCCTGGCAACATGGAGAGGATTGT | AGCTGGCAGAAATTCAGGGACCAA |
| Bcl-6 | GTGACAAGGCCAGCAAAGAAG | GCTCTGTGGACTAACCAGACC |
| CXCR3 | GTCCTTGAGGTGAGTGACCA | CAGCAGAAAGAGGAGGCTGTA |
| CCR4 | CCTTGCCATCTCGGATCTGC | AGACCTAGCCCAAAAACCCAC |
| CXCR5 | CACGTTGCACCTTCTCCCAA | GGAATCCCGCCACATGGTAG |
| CCR5 | TCTGACCCTCACTTCCAACCCAAA | TGAGCATTTAGGGCAAGGAGACCA |
| CCR6 | TTCAGCGATGTTTTCGACTCC | GCAATCGGTACAAATAGCCTGG |
| CCR7 | ACACCAGACAGACAACACTGGGAA | GAGTCTGTGGGAGGCCAGAA |
| ICAM | AAGCCAAGAGGAAGGAGCAAGACT | ACACATGTCTATGGAGGGCCACTT |
| VCAM | TTGGAGTGCTTGGGCAGAAAGTTG | GTGTGCAACATGAAGGGAATGCCA |
| CD31 | TGCAGTGGTTATCATCGGAGTG | CGTTGTTGGAGTTCAGAAGTG |
| VE-Cadherin | CATTTGTCGTGCCTGAAGAC | ATGGTGAAAGCGTCCTGGTA |
| TWIST | ACTGGCCTGCAAAACCATAG | TGCATTTTACCATGGGTCCT |
| α-SMA | AGCACCATGATGCAAACCAAGACC | TCTGCGCCTTCATCAGCTCTTTCT |
| vWF | TTATTGTGGGCTCAGAAGGG | TGTACCATGAGGTTCTCAATGC |
| PD-L1 | GGTTGTGGATCCAGTCACCT | TTGGTGGTGGTGGTCTTACC |
| PD-1 | GTGCCTGTGGTTCTCTGTGGA | TCCGCTAGGAAAGACAATGG |
| PD-L2 | CAGTGCTATCTGAACCTGTGGT | CTGCAGGCCACCGAATTCTT |
| CD80 | ATGGTGGGCACAGAAGTAGC | AGGAAATCTGGGTTCTGGCG |
| CD86 | TGGTCAGGGAGGGGTTTTGG | GCCCCGGGTGATCTGTGTCT |
DC Generation and DC–T-Cell Cultures.
Freshly isolated PBMC were seeded onto tissue culture plates for 2 h and nonadherent cells were washed away. Adherent cell populations were >95% CD14+ by flow cytometry and were cultured in fresh complete medium supplemented with 50 ng/mL GM-CSF and 50 ng/mL IL-4 to generate monocyte-derived dendritic cells (MoDC), which were matured on day 6 with 100 ng/mL LPS or 100 U/mL IFN-γ. Gene-expression analysis was performed after 8 h, protein expression evaluated 24 h after stimulation. MoDC (8,000 cells per well) were cocultured with CD4+ T cells (24,000 cells per well) in 96-well round-bottom plates. CD4+CD25+ T cells were quantified by flow cytometry after 48 h. To assess T-cell proliferation, CD4+ T cells (24,000 cells per well) were labeled with carboxyfluorescein succinimidyl ester (CFSE) and cocultured with DCs (2,000 or 8,000 cells per well) for 5 d. Proliferation rates were analyzed by CFSE dilution, as previously described (26).
Human Artery-Severe Combined Immunodeficiency Mouse Chimeras.
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were purchased from The Jackson Laboratory and chimeras were generated as previously described (27). Normal human temporal or axillary arteries were engrafted subcutaneously into the back of the NSG mice. Six days after the surgery, mice received a single injection of 100 µg LPS subcutaneously. One to 2 d later, PBMC from GCA patients were adoptively transferred into the chimeras (1 × 107 cells per mouse). Chimeras were randomly assigned to two treatment arms. Arm A received 100 µg of anti-human PD-1 antibody intraperitoneally every other day over 1 wk. Arm B was treated with isotype control antibody. Grafts were harvested on days 21–25 and shock-frozen for RNA isolation or embedded into OCT for H&E or immunostaining.
Immunohistochemistry and Immunofluorescence.
Tissues were snap-frozen in OCT on dry ice and blocks were stored at –80 °C. Ten-micrometer sections were air-dried and fixed with acetone at 4 °C for 10 min. Endogenous peroxidases were inactivated with 0.3% H2O2 buffer for 15 min at room temperature and nonspecific binding sites were blocked with 1% normal goat serum for 30 min. Sections were incubated with primary rabbit anti-human CD3, anti-human DC-SIGN, and anti-human PD-L1 antibodies (1:100) at room temperature for 2 h, followed by HRP-conjugated goat anti-rabbit or AP-conjugated goat anti-mouse secondary antibodies for 1 h. Antibody binding was visualized by 3,3′-diaminobenzidine. Sections were counterstained with hematoxylin. CD3+ cells were enumerated in random visual fields distributed over the cross-sectional view of the artery. For immunofluorescence staining, frozen tissues were fixed with cold acetone for 10 min and covered with 1% normal goat serum at room temperature for 30 min. Slides were washed and incubated with anti-CD3 (1:100), anti-PD-1 (1:100), anti-von Willebrand factor (vWF; 1:100), and anti–α-smooth muscle actin (α-SMA; 1:200) antibodies at 37 °C for 60 min. Bound antibodies were visualized with secondary antibodies (Alexa Fluor 488 anti-rabbit Ab, Alexa Fluor 546 anti-mouse Ab) at 37 °C for 60 min and counterstained with DAPI. Images were taken with an Olympus fluorescence microscopy system.
Statistics.
All data are expressed as mean ± SEM. Statistical analysis was performed using GraphPad Prism 5.0 and differences were assessed by Student’s t test or paired Wilcoxon signed-rank test, as indicated. Two-tailed P < 0.05 was considered statistically significant. To adjust for multiple testing and control the false-discovery rate (at level 0.05), the Benjamini–Hochberg procedure (BH step-up procedure) was applied as appropriate.
Study Approval.
All procedures and biospecimen collections were approved by the Institutional Review Board at Stanford University and informed consent was obtained as appropriate. The animal protocol was approved by the Animal Care and Use Committee at Stanford University.
Additional data are available in SI Methods.
SI Methods
Flow cytometry.
PBMC or MoDC were stained with antibodies specific for CD4, CD14, CD20, CD25, PD-1, CD80, CD86, CD274 (PD-L1), and CD273 (PD-L2) at 4 °C for 30 min and analyzed with an LSR Fortessa cell analyzer (BD Biosciences). FlowJo (Tree Star) software was used for data analysis.
qPCR.
Total RNA was isolated and reverse-transcribed into cDNA as previously described (63). cDNA was amplified and SYBR green-based quantitative real-time PCR (RT-PCR) was performed in an Eppendorf PCR machine. Amplifications were performed as follows: 95 °C for 10 min and then 50 cycles at 95 °C for 15 s, 60 °C for 45 s. All transcripts were normalized to GAPDH transcripts. To control for interassay variability, gene expressions from the explanted artery grafts were normalized to the mean values of the control groups. PCR primers are listed in Table S2.
CD4+ T-Cell Lineage Differentiation.
Naïve CD4+ T cells were stimulated with anti-CD3/CD28 dynabeads in 96-well plates for 7 d. Anti–PD-1 antibody (1 µg/mL) was added to the culture media on day 0. Transcript expression of the lineage-determining transcription factors T-bet, RORC, Foxp3, and GATA-3, and the effector molecules IFN-γ, IL-17A, and IL-4 were analyzed by RT-PCR.
Results
Low Expression of the Inhibitory Ligand PD-L1 in GCA.
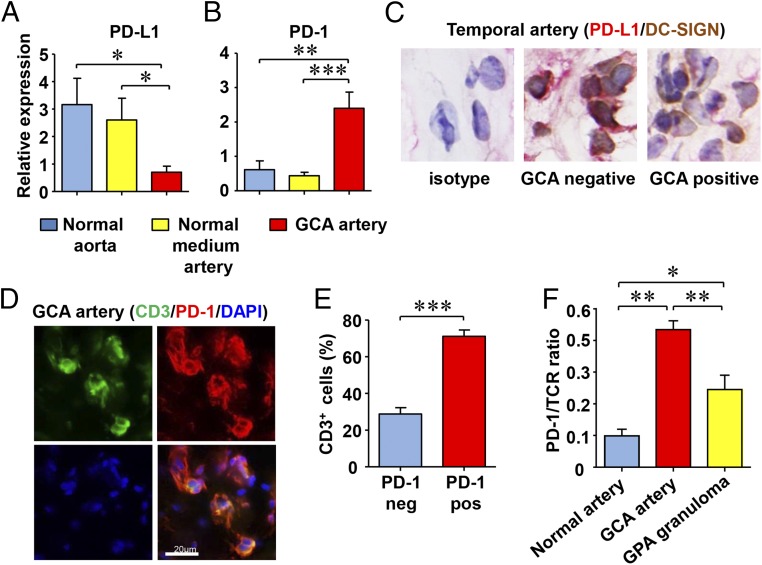
Vessel-wall invasive T cells in GCA are almost exclusively CD4+ memory cells that intermingle with highly activated macrophages and giant cells (28). T-cell–mediated immune responses are fine-tuned by counterbalancing stimulatory and inhibitory signals (e.g., costimulation through CD28-CD80/CD86 and coinhibition through PD-1/PD-L1). To assess whether such signals participate in GCA, we quantified PD-L1 and PD-1 transcripts in GCA-affected temporal artery specimens (Fig. 1) that derived from patients who had not yet started corticosteroid therapy. Noninflamed, normal aortic wall and noninflamed temporal and axillary arteries (medium artery targets of GCA) served as controls. PD-L1 transcripts were abundant in healthy arteries, but expressed at low levels in GCA-affected arteries (Fig. 1A). Conversely, PD-1 transcripts were essentially absent in healthy vessels, but present at high concentrations in arteries with GCA (Fig. 1B), reflective of the absence of T cells in normal arteries and dense T-cell infiltrates in the vasculitic lesions of GCA.
Fig. 1.
PD-L1lo DC and PD-1+ T cells in GCA. RNA was extracted from normal aortic wall, noninflamed medium-sized arteries, and from GCA-affected arteries (n = 10 each). In patients with GPA, granulomatous lesions in the lung and in the skin were examined (n = 10). (A) Expression of PD-L1 transcripts and (B) PD-1 transcripts was quantified by RT-PCR. (C) Tissue sections from temporal arteries were stained with anti–PD-L1 (red) and anti–DC-SIGN (brown) antibodies. (D) Tissue sections from GCA affected temporal arteries were stained with anti–PD-1 (red) and anti-CD3 (green) antibodies. Alexa Fluor 488 anti-rabbit (1:100) and Alexa Fluor 546 anti-mouse (1:100) secondary antibodies were used to visualize primary antibody binding. Representative images are shown. (E) Frequencies of CD3+PD-1+ T cells were quantified in vascular wall cross-sections. (F) Tissue expression of TCR and PD-1 was assessed in nonvasculitic and vasculitis-affected tissues (GCA or GPA). Ratios of PD-1/TCR are presented. Data are mean ± SEM from 10 different patient samples. *P < 0.05, **P < 0.01, ***P < 0.001. (Original magnification: 600×.)
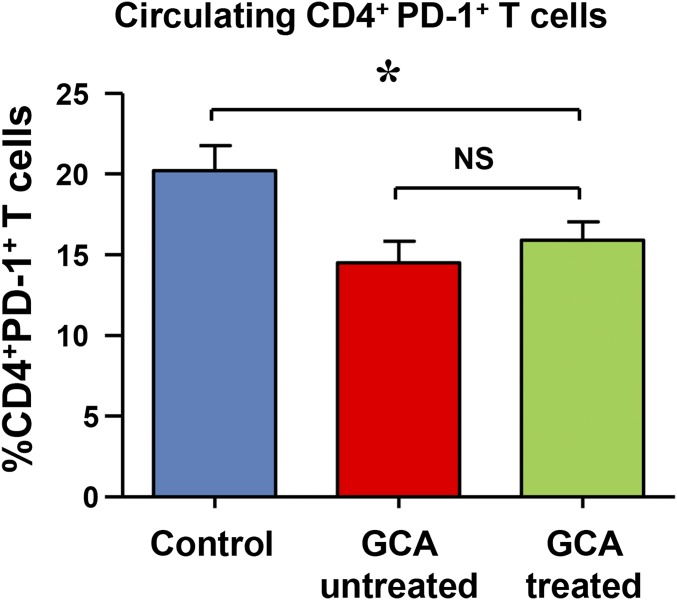
Immunohistochemical staining assigned PD-L1 expression in the noninflamed arteries to vascular DCs, endogenous cells typically localized at the media–adventitia border (9) (Fig. 1C). In case of granulomatous vasculitis, DC density increases greatly and lesional DCs up-regulate activation markers (e.g., CD83) (24, 29). Despite being highly activated, DCs participating in the vasculitic infiltrates consistently stained negative for PD-L1 (Fig. 1C). The majority of T cells in granulomatous lesions expressed the surface receptor PD-1 (Fig. 1 D and E), suggesting selective recruitment/retention of PD-1+ T cells. Frequencies of PD-1+ CD4+ T cells in the peripheral blood of glucocorticoid-treated or untreated GCA patients were 25% lower than in healthy controls (Fig. S1), compatible with trapping of such cells in the vasculitic lesions. To understand whether PD-1 high expression is a feature of all granulomatous vasculitides, we compared PD-1 mRNA levels in normal arteries, GCA-affected arteries, and in granulomatous tissue lesions from patients with GPA (Fig. 1F). As expected, the ratio of PD-1/TCR transcripts was low in normal. Accumulation of activated T cells in GPA granulomas resulted in higher PD-1/TCR ratios, but such ratios were markedly higher in GCA arteries, supporting the concept that vasculitic T cells in GCA arteries are preferentially PD-1+.
Fig. S1.
Frequencies of circulating CD4+ PD-1+ T cells. PBMCs from 21 GCA patients treated with glucocorticoids and 14 untreated GCA patients and 29 age-matched controls were stained with anti–PD-1 and anti-CD4 antibodies and analyzed by flow cytometry. Frequencies of CD4+PD-1+ cells are presented as mean ± SEM; *P < 0.05.
In essence, the tissue microenvironment of GCA lacks the inhibitory ligand PD-L1 and enriches for PD-1–expressing T cells.
Selective Defect of PD-L1 Expression in GCA DCs.
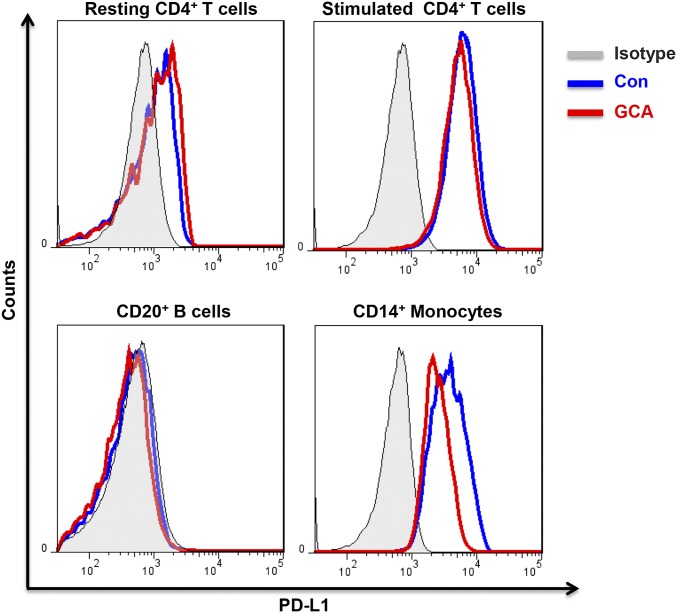
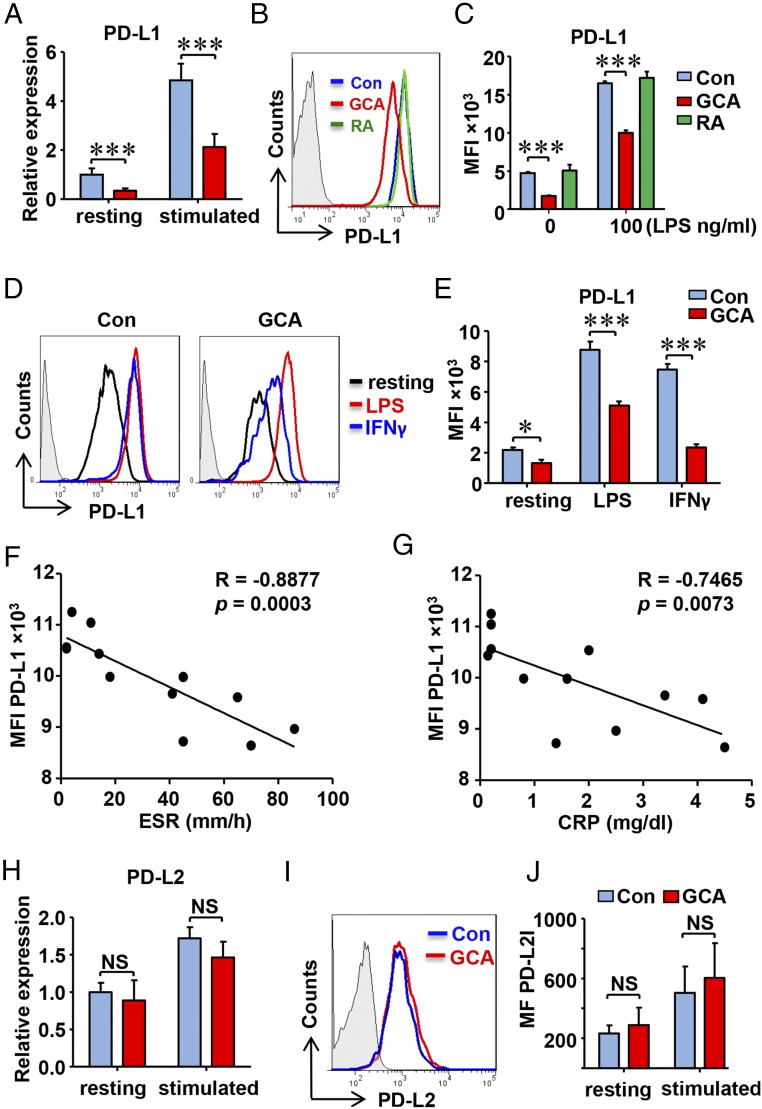
To examine whether GCA patients have a generalized defect in expressing PD-L1, we profiled PD-L1–expressing cells in the peripheral blood and generated MoDCs for functional studies. PD-L1 expression on resting and activated T cells, as well as on B cells, was indistinguishable between patients and age-matched controls (Fig. S2). In contrast, GCA CD14+ monocytes were PD-L1lo and this phenotype was maintained after differentiation into DCs (Fig. 2). In resting and LPS-activated GCA DCs, PD-L1 transcripts were markedly reduced (Fig. 2A). Flow cytometry confirmed PD-L1 protein reduction on resting and stimulated DCs (Fig. 2 B and C) to about 50% of expression levels in healthy counterparts. PD-L1 low-expression on GCA DCs was not solely a result of systemic inflammation; DCs generated from patients with active rheumatoid arthritis were identical to control DCs (Fig. 2 B and C).
Fig. S2.
PD-L1 expression on T cells, B cells, and monocytes in GCA patients. PBMC were collected from GCA patients with active vasculitis. Naïve CD4+ T cells were isolated and stimulated with anti-CD3/CD28 beads for 7 d. Cells were stained with anti-CD4, anti-CD20, anti-CD14, and anti–PD-L1 Ab. Data were acquired by flow cytometry. Representative flow charts are shown.
Fig. 2.
PD-L1lo DCs in GCA. DCs were generated from patients with GCA, patients with RA, and age-matched healthy controls (Con) and stimulated with LPS (100 ng/mL) for 8 h. (A) Relative expression of PD-L1 mRNA measured by quantitative PCR (qPCR). (B) Surface expression of the coinhibitory ligand PD-L1 in activated DC from healthy controls, RA patients and GCA patients quantified by flow cytometry. Representative histograms are shown. (C) Mean fluorescence intensities (MFI) of PD-L1 membrane expression from 12 control, GCA and RA samples. (D and E) DCs were stimulated with LPS (100 ng/mL) or IFN-γ (100 U/mL). Representative histograms (D) and MFIs from six experiments (E). (F and G) Correlation between PD-L1 expression on DC and serum ESR or CRP concentration in individual GCA patients. (H–J) PD-L2 expression was measured by qPCR and flow cytometry. Representative histograms are shown. Results are from six samples. All data are mean ± SEM; *P < 0.05, ***P < 0.001. NS, no significant difference.
To understand why GCA DCs lack PD-L1, they were stimulated with two distinct stimuli known to control PD-L1 expression (30, 31). Both LPS and IFN-γ induced strong up-regulation in the surface density of PD-L1 in healthy DCs. In GCA DCs, responses to both stimuli were dampened, particularly INF-γ–dependent induction (Fig. 2 D and E).
To test whether the PD-L1lo status was related to disease activity in GCA, we correlated DC PD-L1 protein levels with the erythrocyte sedimentation rate (ESR) and serum C-reactive protein concentrations (CRP), two biomarkers of the acute phase response in this vasculitis (Fig. 2 F and G). PD-L1 surface expression was particularly low in patients with the highest inflammatory activity.
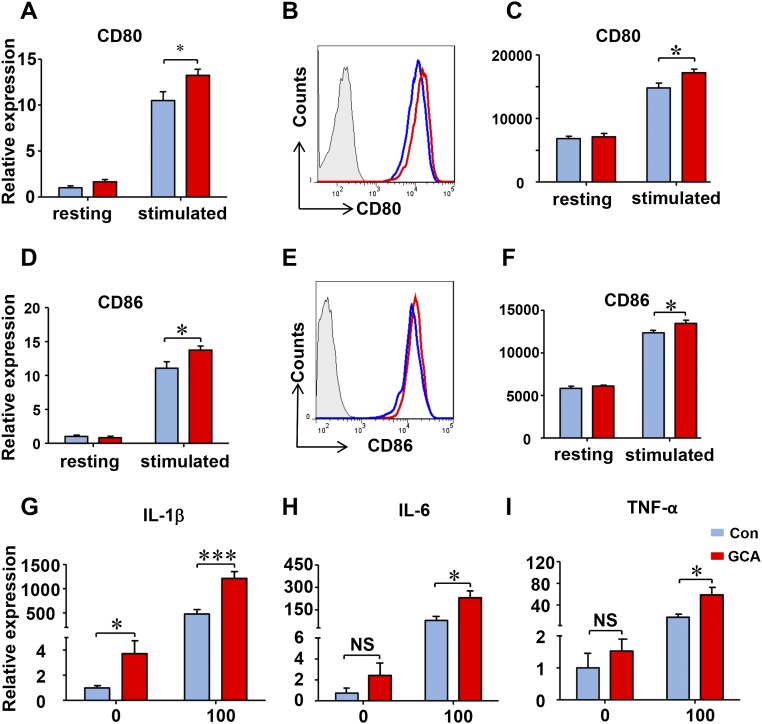
The defect was selective for PD-L1. Studies of transcript and protein expression for PD-L2, CD80, and CD86 demonstrated that the patient-derived DCs were perfectly capable to induce the costimulatory ligands as well as PD-L2 (Fig. 2 H–J and Fig. S3). Furthermore, the ability to produce proinflammatory cytokines (IL-1β, IL-6, TNF-α) was well maintained in patient-derived DC (Fig. S3).
Fig. S3.
Induction of cytokine genes in GCA DC. DCs were generated from GCA patients and healthy controls, stimulated with LPS (100 ng/mL) for 8 h for RT-PCR and 24 h for flow cytometry experiments. (A–F) Expression of CD80 and CD86 transcripts was measured by RT-PCR and membrane proteins were determined by flow cytometry in control and GCA DCs. (G–I) Cytokine transcripts (IL-1b, IL-6, and TNF-α) were measured by RT-PCR. Data from eight independent experiments are presented as mean ± SEM; *P < 0.05, ***P < 0.0001. NS, no significant difference.
These studies identified GCA DCs as PD-L1 low-expressing cells, enabling them to favor costimulatory over coinhibitory signals when functioning as APCs.
GCA DCs Are Hyperstimulatory.
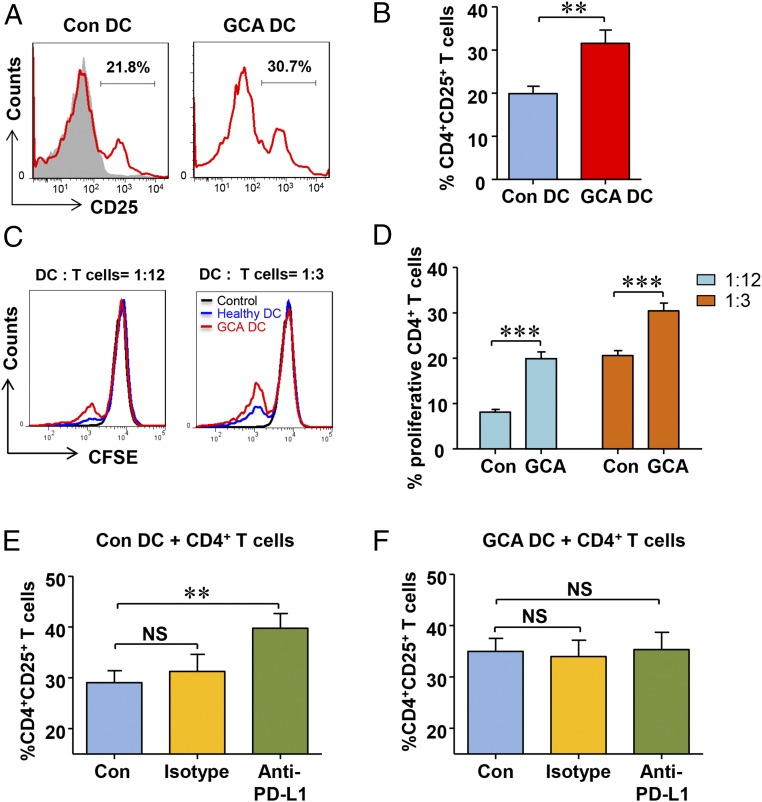
To examine how PD-L1lo DCs activate and instruct T cells, we measured DC-induced T-cell activation and expansion (Fig. 3). Lack of PD-L1 expression affected early steps of T-cell activation, measured by the frequency of CD4+ CD25+ T cells. As early as 48 h after stimulation, PD-L1lo DCs increased the frequency of activated T cells by about 50% (Fig. 3 A and B). To probe the impact of PD-L1lo DCs on T-cell proliferation, GCA DCs and control DCs were cultured with CFSE-labeled CD4 T cells, and 5 d later frequencies of dividing CD4+ T cells were measured. The effect of PD-L1 deficiency was particularly relevant under limiting conditions. In cultures containing 2,000 DCs (1 DC:12 T cells), the frequency of proliferating CD4+ T cells more than doubled in the presence of GCA DCs and remained significantly higher even at a higher DC:T-cell ratio (Fig. 3 C and D).
Fig. 3.
PD-L1lo DCs from GCA patients are hyperstimulatory and insensitive to PD-L1 blockade. DCs were generated from GCA patients and healthy controls (Con), stimulated with LPS for 24 h. Their stimulatory capacity was probed by coculturing them with CD4+ T cells purified from healthy donors. T-cell activation was quantified by the frequency of CD4+CD25+ T cells and T-cell proliferation was determined through CFSE dilution. (A) Activated CD4+CD25+ T cells quantified by flow cytometry after 48 h. Fluorescence minus one (FMO) controls superimposed as gray areas. (B) Percentage of activated CD4+ T cells in six GCA-control pairs. (C) Proliferation of CD4+ T cells was measured by flow cytometry after 5 d of coculture. Representative histograms of CSFE expression. The number of DCs per culture is indicated. (D) Frequencies of proliferating CD4+ T cells when cocultured with either control or patient-derived DCs. Results from six control-patient pairs. (E and F) Control and GCA DCs were cocultured with purified CD4+ T cells in the presence of anti–PD-L1 antibodies or isotype control. CD4+ T cells cocultured without DCs served as control. Frequencies of activated CD4+CD25+ T cells in six to eight independent experiments were measured after 48 h by flow cytometry. All data are mean ± SEM; **P < 0.01, ***P < 0.001.
To investigate whether the hyperactivation and hyperproliferation of T cells primed by GCA DCs was directly related to PD-L1 expression, anti–PD-L1 antibodies were included in the DC:T-cell cultures. Removing a negative signal by blocking the PD-L1/PD1 axis increased CD4+ T-cell responses by about 30% (Fig. 3E), confirming published data (32). Stimulatory effects of blocking access to PD-L1 were abolished in PD-L1lo GCA DCs (Fig. 3F). Frequencies of CD4+CD25+ T cells were similar between isotype control and anti–PD-L1 cultures, supporting the notion that PD-L1 on GCA DCs no longer participated in delivering negative signals.
Thus, PD-L1 deficiency on GCA DCs has functional implications, amplifying CD4+ T-cell responses.
Inhibiting PD-1/PD-L1 Interaction Intensifies Vascular Inflammation.
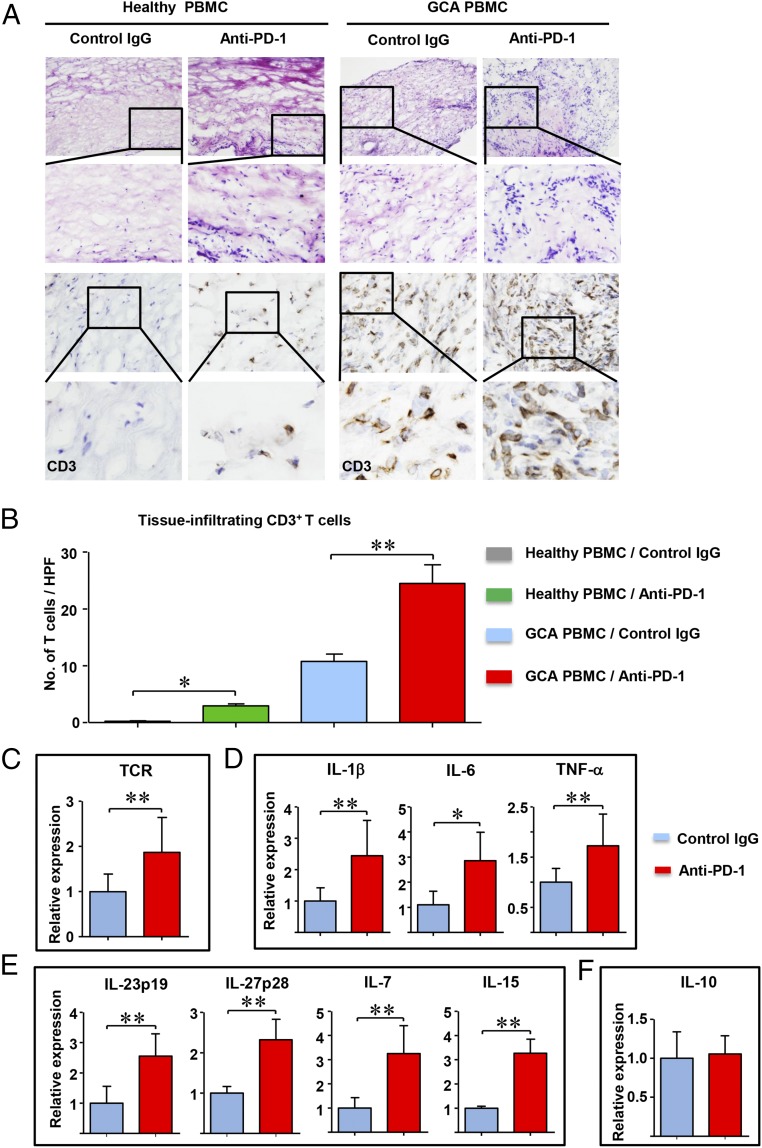
To explore whether a defect in PD-L1 expression has impact on pathogenic immune functions in vasculitis, we made use of a human artery–NSG mouse model (27). In this model system, human axillary arteries are engrafted into NSG mice and PBMC from GCA patients or healthy individuals are adoptively transferred into the chimeras. Patient-derived CD4+ T cells and monocytes infiltrate into the human vessel and form intramural infiltrates. If the chimeras are reconstituted with PBMC from non-GCA healthy donors, the engrafted human arteries remain free of inflammatory infiltrates. To test whether immune checkpoints are involved in the vasculitic response, chimeras were treated with a blocking anti–PD-1 antibody (Fig. 4). Histological and immunohistochemical examination of the explanted arteries confirmed that GCA PBMC, but not healthy PBMC, are able to induce vasculitis (Fig. 4 A and B). PD-1 checkpoint inhibition enabled very few healthy T cells to enter the vascular wall and no organized infiltrates were formed. PBMC from GCA patients induced vessel wall inflammation, with a significant increase in the density of the intramural T-cell infiltrate in anti–PD-1–treated chimeras. Tissue expression of TCR mRNA doubled after PD-1 blockade, in line with the histologic results (Fig. 4C).
Fig. 4.
Blocking of the PD-1/PD-L1 axis aggravates vascular inflammation. Sections of normal medium-sized arteries were engrafted into NSG mice and 7 d later the human-artery NSG chimeras were reconstituted with PBMC from GCA patients or age-matched healthy controls (Healthy PBMC). Mice were subsequently treated with anti–PD-1 antibodies or control IgG (100 µg, i.p.) given three times over 1 wk. Human arteries were explanted and processed for immunohistochemistry or RNA extraction. Relative gene expression was measured by RT-PCR. (A) Tissue sections from the explanted arteries were stained with H&E (Upper) or anti-CD3 antibodies (Lower) as in Fig. 1. (Original magnification: 200×.) (B) The density of the vessel wall infiltrate was evaluated by enumerating CD3+ T cells in 10 high-powered fields. Results from eight tissues are presented as mean ± SEM. (C) T-cell accumulation in the vessel wall was determined by the expression of TCR gene transcripts. (D–F) Transcriptome analysis for the indicated cytokines in control and anti–PD-1–treated arteries measured by RT-PCR. Gene expression data from 10 tissues are presented as mean ± SEM; *P < 0.05, **P < 0.01. After adjustment for multiple testing using the Benjamini–Hochberg method, the comparisons of TCR, IL-1β, IL-6, TNF-α, IL-23p19, ILP27p28, IL-7, and IL-15 are statistically significant with a false-discovery rate of less than 0.05.
Anti–PD-1–enhanced T-cell recruitment/retention had marked effects on the intensity of vessel wall inflammation. Gene-expression profiling revealed robust up-regulation of inflammatory cytokines—including IL-1β, IL-6, and TNF-α, which originate mostly in macrophages—and DC that participate in the granulomatous lesions (Fig. 4D). Notably, PD pathway blockade enhanced tissue transcript levels of four cytokines involved in T-cell activation and proliferation, including IL-7, IL-15, IL-23p19, and IL-27p28 (Fig. 4E). Thus, T cells were recruited into a T-cell tropic microenvironment, providing ideal conditions for unopposed T-cell expansion. PD-1 blockade had no effect on the antiinflammatory cytokine IL-10 (Fig. 4F).
In essence, inhibiting PD-1/PD-L1 interaction intensifies T-cell accumulation in the vessel wall and profoundly enhances tissue inflammation. Notably, PD-1 blockade is insufficient to convert healthy alloreactive T cells into vasculitogenic T cells.
PD-1 Checkpoint Inhibition Selects for Proinflammatory T Cells.
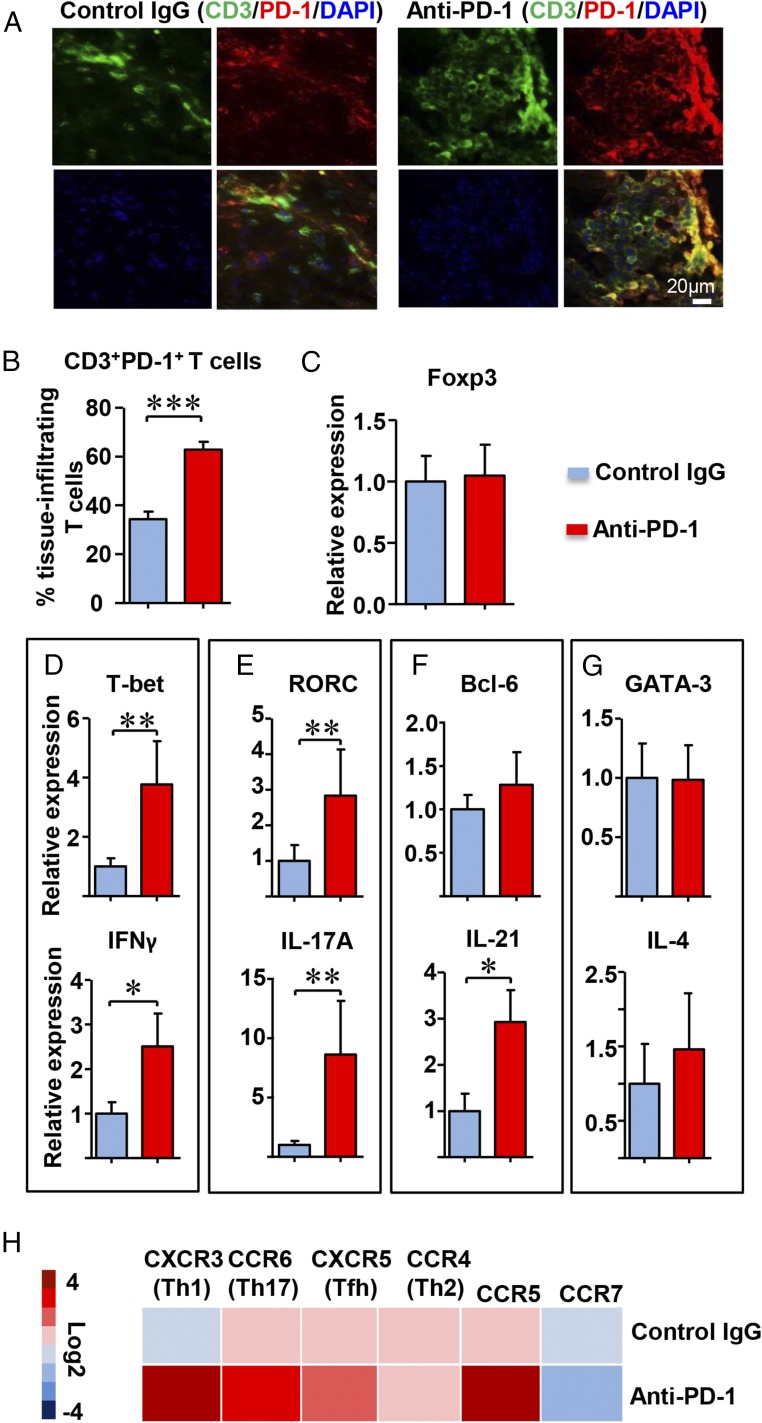
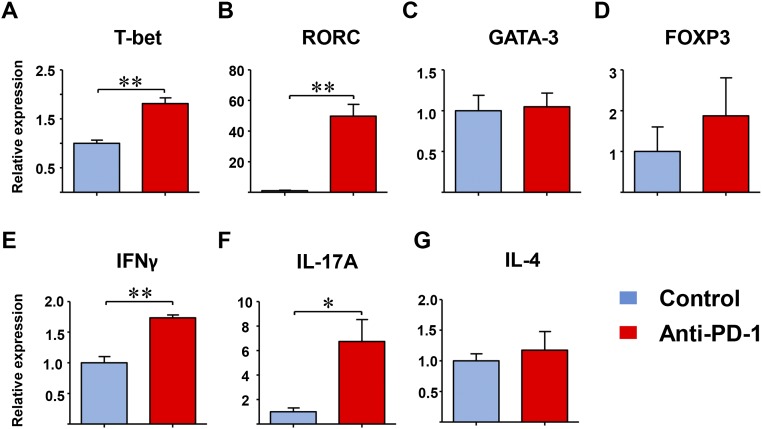
Inhibiting negative immune checkpoints should lead to unselected T-cell activation, restricted only by the preferential expression of the PD-1 receptor on memory and recently stimulated T cells. Alternatively, the absence of a PD-1–derived negative signal could lead to biased T-cell recruitment and retention. We first analyzed whether anti–PD-1 treatment enriched for PD-1+ T cells in the tissue infiltrates (Fig. 5A). Whereas only a small subset of lesional T cells expressed PD-1 in the vehicle-treated arteries, checkpoint inhibition clearly resulted in preferential recruitment of PD-1+ T cells (Fig. 5B). Eliminating inhibitory signaling shifted the T-cell population toward T-bet and RAR-related orphan receptor C (RORC)-expressing cells, whereas GATA3 expression, the lineage-determining transcription factors for the Th2 lineage, was similar in treated and untreated arteries (Fig. 5 C–G). In in vitro experiments, we confirmed that anti–PD-1 antibody was sufficient to shift the lineage commitment of T cells toward Th1 and Th17 differentiation (Fig. S4). In the inflamed arteries, gene expression for IFN-γ, IL-17A, and IL-21 were increased, clearly biasing the T-cell infiltrate toward proinflammatory effector functions (Fig. 5 D–F). Stable production of FoxP3, GATA3, and IL-4 transcripts supported the notion that removal of negative signaling enabled selected T cells to infiltrate into the tissue lesions (Fig. 5 C and G). Analysis of chemokine receptor expression demonstrated enrichment of CXCR3, CCR6, and CXCR5, predominantly found on Th1, Th17, and follicular helper T cells, respectively. Anti–PD-1 treatment resulted in preferential expression of the memory marker CCR5 and disadvantaged the naïve marker CCR7, suggestive for a shift in the balance between naïve and memory T cells (Fig. 5H).
Fig. 5.
PD-1 blockade selects for tissue-infiltrating T cells with proinflammatory functions. NSG mice carrying human arteries were reconstituted with GCA PBMC and treated with anti–PD-1 antibody or control Ig as in Fig. 4. Inflamed arteries were explanted and processed for gene-expression analysis by RT-PCR or embedded for immunohistochemically analysis. (A) Tissue sections were double-stained with anti-CD3 (green) and anti–PD-1 (red) antibodies. Merged pictures show PD-1+ T cells. (Original magnification: 600×.) (B) Percentages of CD3+PD-1+ cells were enumerated in cross-sections from seven isotype and anti–PD-1–treated arteries. (C–G) Tissue transcriptome analysis for lineage-determining transcription factors and T-cell effector molecules. Markers related to the same T-cell lineage are boxed. Data from 10 different tissues are presented as mean ± SEM. (H) Tissue transcriptome analysis for chemokine receptor genes. Results from 10 tissue grafts in each treatment arm are shown as a heat map. *P < 0.05, **P < 0.01, ***P < 0.001. After adjustment for multiple testing using the Benjamini–Hochberg method, the comparisons of T-bet, IFN-γ, RORC, IL-17A, and IL-21 are statistically significant with a false-discovery rate of less than 0.05.
Fig. S4.
Blocking PD-1 shifts T-cell differentiation toward Th1 and Th17 commitment. CD4+CD45RA+ T cells were purified and stimulated with anti-CD3/CD28 for 7 d in the absence and presence of anti–PD-1 antibodies (1 μg/mL). Gene expression of lineage-determining transcription factors and lineage-identifying cytokines was analyzed by RT-PCR. Data from three independent experiments are shown as mean ± SEM; *P < 0.05, **P < 0.01.
These experiments yielded unexpected results, demonstrating that the lack of inhibitory signaling lead to redistribution of lesional T cells, favoring IFN-γ–, IL-17–, and IL-21–producing effector T cells. Enrichment for CXCR3+, CCR6+, and CXCR5+ cells is compatible with a survival advantage for proinflammatory effector cells in the otherwise immunoprivileged tissue niche.
PD-1 Checkpoint Inhibition Aggravates the Maladaptive Remodeling of the Inflamed Arterial Wall.
In GCA, effector T cells contribute to a number of disease-relevant processes, most importantly, vessel wall restructuring (33). Inflamed arteries typically have thinning of the medial layer and intimal thickening, often leading to luminal occlusion. Several growth factors, including PDGF and FGF, have been implicated in driving myofibroblast migration and proliferation (34, 35). Intimal hyperplasia is consistently associated with marked neoangiogenesis (6, 36), creating microvascular networks to support recruitment of inflammatory cells and supplying oxygen and nutrients for outgrowing myofibroblasts. Ultimately, the remodeling of the arterial wall causes luminal compromise and gives rise to the ischemic complications of GCA, including blindness, stroke, and aortic arch syndrome (37). We explored whether the enrichment of PD-1+ T cells in the mural infiltrates has consequences for the outgrowth of new microvessels and for the expansion of the intimal layer. We measured the thickness of the tunica intima in explanted arteries from control- and anti–PD-1–treated chimeras (Fig. 6 A and B). Checkpoint inhibition led to rapid expansion of the intimal layer. In PD-1–blocked grafts, the intima was multilayered and densely packed (Fig. 6A), doubling in thickness (Fig. 6B). In control-treated arteries, the intima consisted of a single-cell layer of vWF+ endothelial cells and two to three layers of α-SMC+ myofibroblasts. In contrast, the hyperplastic intima in anti–PD-1–treated arteries was assembled from six to eight layers of α-SMC+ myofibroblasts, covered by large, partially vWF+α-SMC+ endothelial cells (Fig. 6C). Small vascular lumina appeared in the proximal media, and also in the vasa vasorum-containing adventitia (Fig. 6D). Anti–PD-1 treatment resulted in the brisk formation of new vWF+α-SMC+ microvessels. Enumeration in control and anti–PD-1–treated arterial grafts yielded a 100% increase in the number of microvessels after checkpoint inhibition (Fig. 6E). Removing negative signaling through the PD pathway was associated with endothelial activation, revealed by the robust induction of intercellular adhesion molecule (ICAM), vascular cell adhesion molecule (VCAM), CD31, vWF, and VE-cadherin (Fig. 6F). All markers increased by up to fourfold, when vehicle-treated and anti–PD-1–treated arteries were compared. Endothelial cells expanded in size and in the intensity of vWF expression (Fig. 6C), compatible with acute endothelial cell activation.
Fig. 6.
PD-1 blockade aggravates maladaptive remodeling of the inflamed arterial wall. NSG mice were engrafted with human arteries, reconstituted with GCA PBMC, and treated with anti–PD-1 or control Ig as in Fig. 4, and the thickness of the intimal layer was measured in cross-sections of explanted arteries. (A) H&E stains of cross-sections of explanted arteries. The intima-media border, identified through the lamina elastica interna, is indicated. (Original magnification: 200×.) (B) The thickness of the intimal layer was measured in anti–PD-1 and control-Ig–treated arteries. Results from four independent experiments are shown. (C) Tissue sections were double-stained with anti-vWF (green) and anti–α-SMA (red) antibodies. Representative images of the intima layer are shown. (D) Tissue sections were double-stained with anti-vWF (green) and anti–α-SMA (red) antibodies. Representative images of the adventitial layer are shown. (E) Numbers of microvessels were enumerated in 10 high-powered fields. Results from four independent experiments are shown. (F) Transcript levels for markers of endothelial activation and of myofibroblasts were measured in 10 tissues by RT-PCR and are shown as a heat map. Data are mean ± SEM; *P < 0.05, **P < 0.01.
In essence, the PD1/PD-L1–regulated inhibitory immune checkpoint has a major impact on the remodeling of the vessel wall in T-cell–induced vasculitis, affecting the process of neoangiogenesis, as well as intimal hyperplasia.
Discussion
Patients with the autoimmune vasculopathy GCA, a disease of the aorta and its major branches, have a spontaneous loss of the immunoinhibitory molecule PD-L1, which has devastating consequences for the course of their vasculitis. Ordinarily an immune privileged niche, the wall layers of GCA-affected arteries are occupied by PD-L1lo, CD80hi, CD86hi DCs, and CD4+ T cells arranged in granulomatous infiltrates. In the absence of negative signaling, PD-1+ T cells find a safe haven in an otherwise inaccessible tissue niche and sustain rampant yet selective inflammatory activity, affecting DCs, macrophages, endothelial cells, and vascular stromal cells. A functionally important outcome is the induction of intimal hyperplasia, the ultimate disease pathway causing blindness, stroke, and aortic arch syndrome. The deficiency of the immune-inhibitory checkpoint implicates antigen-nonspecific processes in GCA pathogenesis and opens the door to advanced immunomodulatory therapy and the attempt to reestablish the immunoprivilege of the arterial wall.
Physiologically, the coinhibitory ligand PD-L1 has limited expression in normal tissues, but is promptly up-regulated on APCs undergoing activation to jumpstart a negative feedback loop preventing T-cell overactivity and inflammatory tissue injury. Accordingly, PD-L1−/− mice are different from CTLA-4−/− mice, which develop spontaneous diffuse inflammatory infiltrates in normal organs (38). Mice lacking PD-L1 or PD-1 are highly susceptible to autoimmune disease, specifically when challenged with autoantigens (17, 20, 39). A unique feature of GCA is its stringent tissue tropism. The susceptible tissue niche is guarded by vasDC, strategically positioned outside of the media (33). Such vasDC display a vessel-specific Toll-like receptor (TLR) expression pattern, enabling them to respond in a vessel-specific way to danger signals (9). The closeness of vasDC to the vasa vasorum network allows them access to circulating pathogen- and damage-associated molecular patterns and monitoring of organism-wide danger signals. One possibility is that GCA’s breakdown of the arterial immunoprivilege is a direct consequence of PD-L1 loss, because vasDCs in healthy arteries are PD-L1+, even under resting conditions (Fig. 1).
PD-1+ T cells are highly enriched in vasculitic lesions (Fig. 1E). Notably, the frequency of PD-1+ T cells in the peripheral blood of GCA patients is reduced, compatible with the concept that inflamed arteries act as a sink for these powerful effector T cells. In line with recent observations that vasculitic lesions persist despite glucocorticoid therapy, frequencies of CD4+PD-1+ cells were similarly reduced in treated and untreated patients. A reduction of circulating PD-1–expressing cells contrasts with data reported in small-vessel vasculitis (40). Patients with granulomatosis with polyangiitis have higher frequencies of circulating PD1+ T cells, whereas renal lesions do not enrich PD-1+ T cells, suggesting fundamentally different roles of such T cells in different disease categories. In transcriptome studies, we confirmed that enrichment of PD-1 in the tissue lesions occurs to a much higher degree in GCA than in GPA (Fig. 1F). Much about the role of PD-1+ T cells in the immune system has been learned from the clinical application of PD pathway inhibitors in cancer patients (14). Immune checkpoint inhibitors have emerged as a powerful approach to activating therapeutic antitumor immunity and have provided valuable insights into the process of maintaining self-tolerance in humans. Durable antitumor responses have been induced in patients treated with anti–PD-1 antibodies, particularly in solid tumors with high immunogenicity (14). Removing a break in the immune system, however, comes with a price; many of the patients treated with checkpoint inhibitors develop immune-related adverse events that manifest with diffuse and tissue injurious inflammation of the gut, skin, endocrine glands, liver, and lung, but potentially of any organ system (16). Patients treated with antibodies blocking checkpoint signaling have been reported to develop GCA (41). Thus, patients treated with PD pathway blockers should be monitored for inflammatory disease in their large arteries.
Unblocked PD-1+ T cells in tumor patients elegantly destroy cancer cells, probably through a plethora of mechanisms (42). We interrogated effector functions of PD-1+ T cells in a humanized mouse model, in which GCA T cells, B cells, and monocytes induce vasculitis in engrafted human arteries. Blocking PD-1 access resulted in massive enrichment of PD-1+ T cells in the arterial wall. PD-1 is induced as a late marker of T-cell activation and the intense expression of PD-1 on tissue-residing T cells argues for a widespread and acute immune activation (43). A broad spectrum of inflammatory mediators was up-regulated in the treated chimeras, including markers related to DCs and macrophage function (IL-1β, IL-6, TNF-α, IL-23p19, IL-27p28), as well as stromal cell function (IL-7, IL-15). The process was selective, as expression of the antiinflammatory mediator IL-10 was unaffected. PD-1 blockade also imposed selectivity on T-cell effector functions, biasing the infiltrate toward T-bet and RORC expression and away from FoxP3 and GATA-3. Transcriptome analysis indicated multiplicity of T-cell effector molecules, ranging from IFN-γ to IL-17 to IL-21. All of these T-cell effector molecules have been detected in GCA vascular lesions (3, 44). Notably, PD-1 checkpoint inhibition in this model system was not able to simply convert healthy alloreactive T cells into wall-infiltrating T cells (Fig. 4), indicating that T cells from GCA patients are particularly susceptible to the unleashing effect of anti–PD-1 antibodies.
The PD-1 pathway has been implicated in two major mechanisms thwarting self-reactive T cells, inducing immunosuppressive Tregs, and directly inhibiting effector T cells (45). Data on Treg function in GCA are sparse, but a recent study has provided evidence that GCA patients are lacking a potent immunoregulatory CD8 Treg population (26), compatible with PD-L1 deficiency shaping a vasculitogenic T-cell repertoire. When analyzing disease-relevant effector T cells, PD-1 appeared to be expressed on multiple T-cell lineages (Fig. 5), with PD-1 blockade allowing entrance and survival of IFN-γ, IL-17, and IL-21 producers, but disfavoring IL-4+ and FoxP3+ cells. These findings suggest selectivity in how PD-1–mediated signaling interferes with the TCR-dependent signaling cascade. PD-1 ligation on T cells is believed to preferentially attenuate the PI3K/AKT pathway (46, 47), leading to suppression of the mammalian target of rapamycin-dependent signaling knot, which is critically involved in lineage commitment, regulation of bioenergetics, and T-cell expansion (48, 49). In the absence of sufficient PD-1 signaling, a scenario exemplified in the PD-L1–deficient GCA lesions, T cells gain proliferative potential, differentiate into metabolically hyperactive effector cells, and commit to proinflammatory cytokine production.
PD-1+ T cells in vasculitic lesions have profound impact on stromal and endothelial cells in the vessel wall. We observed three processes that were accelerated in the inflamed arteries after checkpoint inhibition (Fig. 6): (i) formation of microvessels, which are believed to sprout off the adventitial vasa vasorum network and invade into more proximal parts of the vessel wall; (ii) endothelial activation; and (iii) size expansion of the intimal layer. In temporal artery biopsies from GCA patients, neovessel formation and intimal thickening have been correlated with IFN-γ tissue levels (36), thus implicating T-cell effector functions with wall remodeling. Whether this is a direct or indirect consequence of T cells guiding the behavior of endothelial cells and myofibroblasts remains to be investigated. Conceivably, unopposed PD-1+ T cells could activate macrophages and DCs, which in turn could modify the functioning of nonimmune cells in the tissue microenvironment. The expansion of α-SMA+ cells forming a lumen-compromising neotissue was a fast process that occurred in only 1 wk. Although multiple mechanisms contribute to intimal hyperplasia, the chimeric mouse model allows eliminating some and exploring others. Chimeric mice have no access to human bone marrow, thus bone-marrow-derived stem cells cannot play a role in promoting intimal outgrowth. All precursor cells for the expanding myofibroblasts occupying the hyperplastic intima must be part of the human vessel wall that is engrafted into the animals. Gene-expression studies demonstrated up-regulation of the transcription factor TWIST in explants from anti–PD-1–treated chimeras. TWIST has been implicated in endothelial-mesenchymal transition (50, 51), but how T cells could achieve regulatory control of intimal hyperplasia remains unexplored.
Our data do not suggest that PD-1+ T cells have signs of immune exhaustion (52, 53); in contrast, they appear strongly activated and activate surrounding immune and stromal cells. In a recent study, the exhaustion marker PD-1 was associated with beneficial suppression of the “nonexhausted” T-cell state driven by costimulation and the balance between costimulation/exhaustion signatures was predictive of clinical outcomes in infectious versus autoimmune diseases (54). Indeed, inducing T-cell exhaustion was proposed as a therapeutic strategy in autoimmunity (55). In the current study, PD-1+ T cells were critical perpetrators of autoimmune functions and the lack of PD-L1 expression on APCs translated into a detriment for the patient, suggesting that different disease states may require a different balance of costimulatory and coinhibitory signals.
An important question raised by the present work is the mechanism leading to PD-L1 low-expression in GCA DC. Neither T cells nor B cells were affected by this defect, but monocytes shared the PD-L1lo phenotype. PD-L1 expression is considered to be regulated by the inflammatory milieu, with cytokines inducing PD-L1 and PD-L2 expression. Type I and type II IFNs and TNF-α have all been implicated in driving up PD-L1 (56–59). For human monocytes and DCs, TLR ligands are potent PD-L1 inducers. Patient-derived DC had low responses to the TLR4 ligand LPS, but were even less responsive to IFN-γ. IFN-γ is a key cytokines in the vasculitic lesions of GCA, literally exposing the DC to a IFN-γ–high environment (60). We considered the possibility that low-expression of PD-L1 is an age-dependent factor, reflective of vascular aging, but neither in healthy arteries nor in GCA patients was the donor age predictive for the level of PD-L1 expression. More detailed studies of PD-L1 transcription in patient-derived and control DC are needed to understand the molecular underpinnings of this defect.
Data presented here have considerable implications for the understanding and the management of medium- and large-vessel vasculitis. First, the granulomatous nature of the infiltrates has fostered models proposing specific antigens driving disease. No conclusive evidence for such antigens has been provided and data presented here favor a critical role for antigen-nonspecific immune regulation, and emphasize the protective nature of a balanced immune system. Second, guided by the observation that DC in healthy arteries—even in the resting state—express PD-L1, we propose that negative signaling through PD-1 plays a critical role in establishing and maintaining the immune privilege of the arterial wall. In that model, loss of immunoinhibitory signaling would make the tissue site susceptible to immune invasion. The strong enrichment of PD-1+ T cells in the vasculitic lesions of GCA supports a disease-critical role of these effector cells, which definitely have no signs of exhaustion but appear to be strongly activated. Granulomatous tissue lesion in GPA patients do not have a similar signature (Fig. 1F), arguing against the inflammatory environment present in every tissue infiltrate as the sole reason for PD-1 up-regulation. Expression of PD-1, rather than assignment to a single functional lineage, appears to be the common denominator of vasculitogenic T cells in GCA. Third, this report implicates T cells in the process of intimal hyperplasia, a critical event in patients developing blindness, stroke, or aortic arch syndrome. Taken together, these observations should encourage rethinking the therapeutic approaches in GCA. Chronic glucocorticoid therapy fails to remove the vasculitic infiltrates (61, 62), with Th1-committed effector T cells particularly resistant to treatment. Additionally, targeting individual inflammatory markers will unlikely provide a curative intervention for a multipronged immune attack, which involves a multitude of effector functions. Repairing the PD-1/PD-L1 pathway to reestablish immunoinhibitory signals and revive a protective “molecular shield” may allow reconstitution of the artery’s immune privilege. Eliminating/blocking the relatively small population of PD-1+ T cells may be an equally effective approach to protect the vessel wall. Finally, understanding the spontaneous deficiency of PD-L1 in GCA DC would have direct impact on the field of cancer immunotherapy, in which avoidance of PD-L1 up-regulation on cancer cells represents a major therapeutic strategy to undermine immune resistance. The present work unifies efforts in inflammatory vessel disease and in cancer immunotherapy to optimize immunostimulatory and immunoinhibitory signals for disease management.
Acknowledgments
This work was supported by the National Institutes of Health Grants R01 AR042527, R01 HL117913, R01 AI108906, and P01 HL129941 (to C.M.W.), and R01 AI108891, R01 AG045779, U19 AI057229, U19 AI057266, and I01 BX001669 (to J.J.G.). R.W. received fellowship support from the Govenar Discovery Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616848114/-/DCSupplemental.
References
- 1.Weyand CM, et al. Distinct vascular lesions in giant cell arteritis share identical T cell clonotypes. J Exp Med. 1994;179(3):951–960. doi: 10.1084/jem.179.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grunewald J, et al. CD4+ and CD8+ T cell expansions using selected TCR V and J gene segments at the onset of giant cell arteritis. Arthritis Rheum. 1994;37(8):1221–1227. doi: 10.1002/art.1780370817. [DOI] [PubMed] [Google Scholar]
- 3.Weyand CM, Younge BR, Goronzy JJ. IFN-γ and IL-17: The two faces of T-cell pathology in giant cell arteritis. Curr Opin Rheumatol. 2011;23(1):43–49. doi: 10.1097/BOR.0b013e32833ee946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciccia F, et al. Difference in the expression of IL-9 and IL-17 correlates with different histological pattern of vascular wall injury in giant cell arteritis. Rheumatology (Oxford) 2015;54(9):1596–1604. doi: 10.1093/rheumatology/kev102. [DOI] [PubMed] [Google Scholar]
- 5.Weyand CM, Wagner AD, Björnsson J, Goronzy JJ. Correlation of the topographical arrangement and the functional pattern of tissue-infiltrating macrophages in giant cell arteritis. J Clin Invest. 1996;98(7):1642–1649. doi: 10.1172/JCI118959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weyand CM, Goronzy JJ. Arterial wall injury in giant cell arteritis. Arthritis Rheum. 1999;42(5):844–853. doi: 10.1002/1529-0131(199905)42:5<844::AID-ANR2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 7.Rittner HL, et al. Tissue-destructive macrophages in giant cell arteritis. Circ Res. 1999;84(9):1050–1058. doi: 10.1161/01.res.84.9.1050. [DOI] [PubMed] [Google Scholar]
- 8.Dal Canto AJ, Swanson PE, O’Guin AK, Speck SH, Virgin HW. IFN-gamma action in the media of the great elastic arteries, a novel immunoprivileged site. J Clin Invest. 2001;107(2):R15–R22. doi: 10.1172/JCI11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pryshchep O, Ma-Krupa W, Younge BR, Goronzy JJ, Weyand CM. Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation. 2008;118(12):1276–1284. doi: 10.1161/CIRCULATIONAHA.108.789172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pober JS, Tellides G. Participation of blood vessel cells in human adaptive immune responses. Trends Immunol. 2012;33(1):49–57. doi: 10.1016/j.it.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4(5):336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura H, Honjo T. PD-1: An inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol. 2001;22(5):265–268. doi: 10.1016/s1471-4906(01)01888-9. [DOI] [PubMed] [Google Scholar]
- 13.Iwai Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99(19):12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamid O, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garon EB, et al. KEYNOTE-001 Investigators Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 16.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 18.Nishimura H, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 19.Keir ME, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203(4):883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latchman YE, et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci USA. 2004;101(29):10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirata S, et al. Prevention of experimental autoimmune encephalomyelitis by transfer of embryonic stem cell-derived dendritic cells expressing myelin oligodendrocyte glycoprotein peptide along with TRAIL or programmed death-1 ligand. J Immunol. 2005;174(4):1888–1897. doi: 10.4049/jimmunol.174.4.1888. [DOI] [PubMed] [Google Scholar]
- 22.Francisco LM, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAlees JW, et al. Differential control of CD4(+) T-cell subsets by the PD-1/PD-L1 axis in a mouse model of allergic asthma. Eur J Immunol. 2015;45(4):1019–1029. doi: 10.1002/eji.201444778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma-Krupa W, et al. Activation of arterial wall dendritic cells and breakdown of self-tolerance in giant cell arteritis. J Exp Med. 2004;199(2):173–183. doi: 10.1084/jem.20030850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weyand CM, et al. Vascular dendritic cells in giant cell arteritis. Ann N Y Acad Sci. 2005;1062:195–208. doi: 10.1196/annals.1358.023. [DOI] [PubMed] [Google Scholar]
- 26.Wen Z, et al. NADPH oxidase deficiency underlies dysfunction of aged CD8+ Tregs. J Clin Invest. 2016;126(5):1953–1967. doi: 10.1172/JCI84181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piggott K, et al. Blocking the NOTCH pathway inhibits vascular inflammation in large-vessel vasculitis. Circulation. 2011;123(3):309–318. doi: 10.1161/CIRCULATIONAHA.110.936203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner AD, Björnsson J, Bartley GB, Goronzy JJ, Weyand CM. Interferon-gamma-producing T cells in giant cell vasculitis represent a minority of tissue-infiltrating cells and are located distant from the site of pathology. Am J Pathol. 1996;148(6):1925–1933. [PMC free article] [PubMed] [Google Scholar]
- 29.Slobodin G, et al. Biopsy-negative giant cell arteritis: Does anti-CD83 immunohistochemistry advance the diagnosis? Eur J Intern Med. 2007;18(5):405–408. doi: 10.1016/j.ejim.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong H, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 32.Brown JA, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170(3):1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 33.Weyand CM, Goronzy JJ. Immune mechanisms in medium and large-vessel vasculitis. Nat Rev Rheumatol. 2013;9(12):731–740. doi: 10.1038/nrrheum.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaiser M, Weyand CM, Björnsson J, Goronzy JJ. Platelet-derived growth factor, intimal hyperplasia, and ischemic complications in giant cell arteritis. Arthritis Rheum. 1998;41(4):623–633. doi: 10.1002/1529-0131(199804)41:4<623::AID-ART9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 35.Lozano E, Segarra M, García-Martínez A, Hernández-Rodríguez J, Cid MC. Imatinib mesylate inhibits in vitro and ex vivo biological responses related to vascular occlusion in giant cell arteritis. Ann Rheum Dis. 2008;67(11):1581–1588. doi: 10.1136/ard.2007.070805. [DOI] [PubMed] [Google Scholar]
- 36.Kaiser M, Younge B, Björnsson J, Goronzy JJ, Weyand CM. Formation of new vasa vasorum in vasculitis. Production of angiogenic cytokines by multinucleated giant cells. Am J Pathol. 1999;155(3):765–774. doi: 10.1016/S0002-9440(10)65175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makkuni D, et al. Is intimal hyperplasia a marker of neuro-ophthalmic complications of giant cell arteritis? Rheumatology (Oxford) 2008;47(4):488–490. doi: 10.1093/rheumatology/ken012. [DOI] [PubMed] [Google Scholar]
- 38.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 39.Dong H, et al. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20(3):327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 40.Wilde B, et al. Aberrant expression of the negative costimulator PD-1 on T cells in granulomatosis with polyangiitis. Rheumatology (Oxford) 2012;51(7):1188–1197. doi: 10.1093/rheumatology/kes034. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein BL, Gedmintas L, Todd DJ. Drug-associated polymyalgia rheumatica/giant cell arteritis occurring in two patients after treatment with ipilimumab, an antagonist of ctla-4. Arthritis Rheumatol. 2014;66(3):768–769. doi: 10.1002/art.38282. [DOI] [PubMed] [Google Scholar]
- 42.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12(4):237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hokey DA, et al. Activation drives PD-1 expression during vaccine-specific proliferation and following lentiviral infection in macaques. Eur J Immunol. 2008;38(5):1435–1445. doi: 10.1002/eji.200737857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terrier B, et al. Interleukin-21 modulates Th1 and Th17 responses in giant cell arteritis. Arthritis Rheum. 2012;64(6):2001–2011. doi: 10.1002/art.34327. [DOI] [PubMed] [Google Scholar]
- 45.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parry RV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci USA. 2001;98(24):13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delgoffe GM, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30(6):832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12(5):325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cooley BC, et al. TGF-β signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci Transl Med. 2014;6(227):227ra34. doi: 10.1126/scitranslmed.3006927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cooke VG, et al. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell. 2012;21(1):66–81. doi: 10.1016/j.ccr.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 53.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 54.McKinney EF, Lee JC, Jayne DR, Lyons PA, Smith KG. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. 2015;523(7562):612–616. doi: 10.1038/nature14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 56.Mühlbauer M, et al. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol. 2006;45(4):520–528. doi: 10.1016/j.jhep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Schreiner B, et al. Interferon-beta enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: Relevance for the immune modulatory effect in multiple sclerosis. J Neuroimmunol. 2004;155(1-2):172–182. doi: 10.1016/j.jneuroim.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 58.Mazanet MM, Hughes CC. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J Immunol. 2002;169(7):3581–3588. doi: 10.4049/jimmunol.169.7.3581. [DOI] [PubMed] [Google Scholar]
- 59.Kinter AL, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181(10):6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 60.Weyand CM, et al. Disease patterns and tissue cytokine profiles in giant cell arteritis. Arthritis Rheum. 1997;40(1):19–26. doi: 10.1002/art.1780400105. [DOI] [PubMed] [Google Scholar]
- 61.Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med. 2003;349(2):160–169. doi: 10.1056/NEJMra022694. [DOI] [PubMed] [Google Scholar]
- 62.Deng J, Younge BR, Olshen RA, Goronzy JJ, Weyand CM. Th17 and Th1 T-cell responses in giant cell arteritis. Circulation. 2010;121(7):906–915. doi: 10.1161/CIRCULATIONAHA.109.872903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shirai T, et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med. 2016;213(3):337–354. doi: 10.1084/jem.20150900. [DOI] [PMC free article] [PubMed] [Google Scholar]