Significance
The role of thermodynamically driven processes in inherently active biological systems has daunted scientists for decades. One such conundrum emerges in the formation of membraneless organelles that, according to in vitro studies, assemble via thermodynamically driven phase separation but harbor numerous active processes within themselves. Disentangling the role of thermodynamic and active processes in their formation is, however, impossible in minimal in vitro systems of individual constituent components. In this work, we introduce a microfluidics-based temperature assay to address this question at its full complexity in vivo and use it to study the assembly of six nucleolar proteins in Drosophila embryos. Although four of these proteins follow the phase separation model, the recruitment of others to the nucleolus is active.
Keywords: liquid–liquid phase separation, intracellular phase transition, membrane-less organelle, RNA granule, Drosophilanucleologenesis
Abstract
Membraneless organelles play a central role in the organization of protoplasm by concentrating macromolecules, which allows efficient cellular processes. Recent studies have shown that, in vitro, certain components in such organelles can assemble through phase separation. Inside the cell, however, such organelles are multicomponent, with numerous intermolecular interactions that can potentially affect the demixing properties of individual components. In addition, the organelles themselves are inherently active, and it is not clear how the active, energy-consuming processes that occur constantly within such organelles affect the phase separation behavior of the constituent macromolecules. Here, we examine the phase separation model for the formation of membraneless organelles in vivo by assessing the two features that collectively distinguish it from active assembly, namely temperature dependence and reversibility. We use a microfluidic device that allows accurate and rapid manipulation of temperature and examine the quantitative dynamics by which six different nucleolar proteins assemble into the nucleoli of Drosophila melanogaster embryos. Our results indicate that, although phase separation is the main mode of recruitment for four of the studied proteins, the assembly of the other two is irreversible and enhanced at higher temperatures, behaviors indicative of active recruitment to the nucleolus. These two subsets of components differ in their requirements for ribosomal DNA; the two actively assembling components fail to assemble in the absence of ribosomal DNA, whereas the thermodynamically driven components assemble but lose temporal and spatial precision.
Membraneless organelles are highly concentrated assemblies of proteins and RNAs that provide specialized microenvironments for particular cellular functions (1). Recent studies suggest that such organelles may form via a liquid–liquid phase separation (LLPS) process, in which the constituent components spontaneously assemble on reaching a critical concentration at a given temperature (2–5). LLPS provides an attractive energy-efficient mechanism for cells to organize different biochemical reactions spatially, whereas the liquid nature of the emerging organelles, such as P granules and nucleoli (2, 6), allows for rapid exchange of molecules. The role of LLPS has been supported by studies in which the purified RNA binding proteins that localize to such subcellular bodies in vivo also self-assemble in vitro (3–11). However, because of the complexity of living cells, our current understanding of the role of LLPS in membraneless organelle assembly is by far limited to in vitro studies. Particularly, membraneless organelles are multicomponent, and the interactions between different components can enhance or diminish the ability of individual proteins to phase separate inside living cells (12). Therefore, the behavior of the individual components in the simplified in vitro systems are not necessarily predictive of their behavior in vivo with all native interacting partners. Such limitations of in vitro systems underline the necessity for a rigorous in vivo assessment of the LLPS model.
An alternative mechanism for the formation of membraneless organelles in vivo is active assembly. Based on this model, an enzymatic reaction couples an energy source, such as ATP, to a reaction, which results in the formation of membraneless organelles. Several studies suggest that active transport of components can drive the formation of high-concentration assemblies in vivo. For example, the formation of stress granules and the growth of P bodies in response to stress rely on motor proteins (13, 14). Similarly, transport of AMPA receptors to high-concentration synaptic puncta, which resemble membraneless organelles, is dependent on Kinesin-1 (15). Many active processes occur constantly in living cells and also, within membraneless organelles, which could also contribute to the formation of high-concentration assemblies. For example, several membraneless organelles, such as nucleoli and histone locus bodies, form at active sites of transcription that would provide high concentrations of nascent RNA. Any protein that can bind to the nascent RNA would thus be enriched at these sites of transcription without requiring any other mechanism.
Finally, the LLPS and active recruitment models for the formation of membraneless organelles are not mutually exclusive. For instance, the presence of actively transcribed RNA can modulate the demixing behavior of certain phase-separating proteins (7, 16). In addition, posttranslational modifications, such as phosphorylation of proteins, can regulate their localization to membraneless organelles, potentially by changing intermolecular interactions that govern LLPS (17–19). Nevertheless, in the absence of an in vivo assay for evaluation of the LLPS model, it is not quite clear to what extent the formation of membraneless organelles is through an LLPS and to what extent it is driven by active reactions.
Here, we introduce an in vivo temperature-based assay to test the LLPS model for the assembly of membraneless organelles and use it to study the mechanism by which six different nucleolar proteins (Fig. 1A and Table S1) localize to the nucleolus in Drosophila melanogaster cleavage-stage embryos. Development in Drosophila embryos starts without a nucleolus and proceeds through 13 rapid nuclear divisions followed by a pause at the interphase of nuclear cycle 14 (NC14). Transcription of ribosomal DNA (rDNA) begins at NC11 (16). To follow the formation of a visible nucleolus, we used six fluorescently tagged nucleolar proteins involved in different steps of ribosomal biogenesis and localized to different subcompartments of the nucleolus (16, 20) (Fig. 1A and Table S1). For comparisons, one of the proteins, Fibrillarin, was tagged with the red fluorescent protein, TagRFP (hereafter RFP-Fib) and always coexpressed with the other five EGFP-tagged proteins. At room temperature, all six proteins show a similar temporal pattern of accumulation into the nucleolus at 5–8 min into the interphase of NC13, with the median emergence times for assemblies being 5 0.5 min for nucleolar phosphoprotein 140 (Nopp140); 6 0.5 min for Fib, Modulo (Mod), and Rpl135 [also known as RNA polymerase I subunit 135 (RpI135) in Drosophila]; 7 0.5 min for Nucleostemin1 (Ns1); and 8 0.5 min for Pitchoune (Pit) (Methods has details of measurements; Fig. 1B). The timing of nucleolus formation is extremely reproducible and tightly constrained. At NC12, only 5 of 40 studied embryos show transient Fib and Mod and either Nopp140 or Ns1 foci; one embryo showed exclusively Mod assemblies, and two showed exclusively Fib assemblies. Visible nucleolar accumulation of all six proteins is always detectable during interphase of NC13 and stable after it is observed. The concentrations of these proteins in the nucleoplasm increase during syncytial NCs, with the maximum levels of all proteins coinciding with the first-time emergence of nucleolar assemblies at NC13 (Fig. S1) (16). Although the emergence of assemblies at high concentrations is consistent with the concentration dependence of phase separation processes, it is not sufficient to rule out the possibility of active assembly, because the latter would also lead to the formation of assemblies at higher (substrate) concentrations.
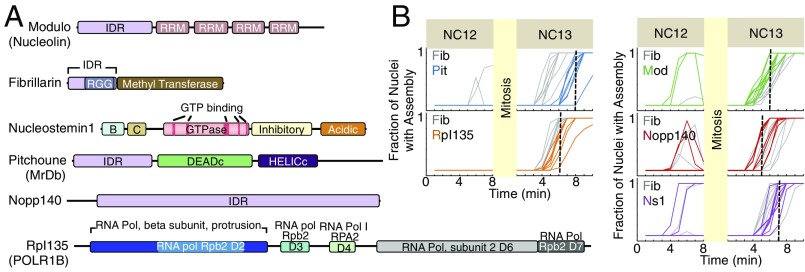
Fig. 1.
First-time assembly of six nucleolar proteins at 22 °C. Structural features of six nucleolar proteins studied are depicted in A. The structural elements of Ns1 were previously reported (21). For other proteins, the structural features were determined using National Center for Biotechnology Information conserved domain search (22) and InterPro (23) as well as IUPRED (24) for detection of disordered regions. Fib is a methyltransferase that associates with C/D box small nucleolar RNAs to form small nucleolar ribonucleoprotein complexes; the inhibitory domain of Ns1 prevents its nucleolar localization when GTP is not bound (25). The DEADc domain in Pitchoune, characterized by the conserved motif Asp-Glu-Ala-Asp (DEAD), contains an ATP binding region. B, basic region; C, coiled coil; D, domain; RGG, R/G-rich domain; RRM, RNA recognition motif. (B) The first-time emergence of assemblies for different nucleolar proteins at 22 °C at NC12–NC13 is depicted. Each line represents the fraction of nuclei in a single embryo that has detectable foci of each nucleolar protein, with the dashed lines marking the median for the first-time formation of each GFP-tagged protein (number of embryos in each panel = 8) (details are in Methods). Median for Fib is 6 min into interphase of NC13. Time 0 marks the beginning of interphase.
Table S1.
Proteins used in this study
| Protein | Function | Human homolog | Subcompartment |
| RpI135 | rDNA transcription | POLR1B | FC (39) |
| Nopp140 | A molecular chaperone for targeting snoRNP to the nucleolus (40, 41); directly interacts with a subunit of PolI and RpA194 and recruits it to rDNA genes (42) | hNopp140 | DFC (43) |
| Fib | In snoRNPs; has RGG domain for binding to RNA; is a 2′-O-methyltransferase (44) | Fib | DFC (45) |
| Mod | Has direct interaction with pre-rRNA and processing factors (i.e., U3 snoRNP) and recruits them to the early cleavage sites A1 and A2 (46, 47); anchors the centromere to the nucleolus; possibly formation of heterochromatin; gene expression (48, 49) | Nucleolin | Mainly DFC, some in GC (50) |
| Pit | DEAD box RNA helicase (51) | Myc-regulated DEAD box protein (MrDb) | Unknown |
| Ns1 | Has intrinsic GTPase activity (21); involved in processing 32S pre-rRNA into 28S rRNA (52), large ribosomal subunit biogenesis, and regulation of cell cycle (21, 53) | Nucleostemin | GC (21) |
Drosophila nucleoli lack FC (54), and the nucleolar subcompartment is mainly based on amniotes. DFC, dense fibrillar component; FC, fibrillar center; GC, granular component; RGG, R/G-rich domain; snoRNP, small nucleolar ribonucleoprotein complex.
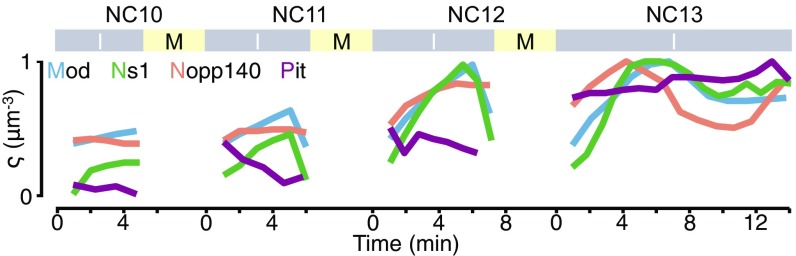
Fig. S1.
The nucleoplasmic concentrations of the nucleolar proteins increase by NC. The normalized nucleoplasmic intensities (arbitrary units) per unit volume, excluding the nucleoli, for EGFP-Mod, EGFP-Ns1, EGFP-Nopp140, and Pit-EGFP are shown as . Times 0 marks the beginning of interphase (I) and end of mitosis (M).
Temperature Dependence of First-Time Assembly Formation.
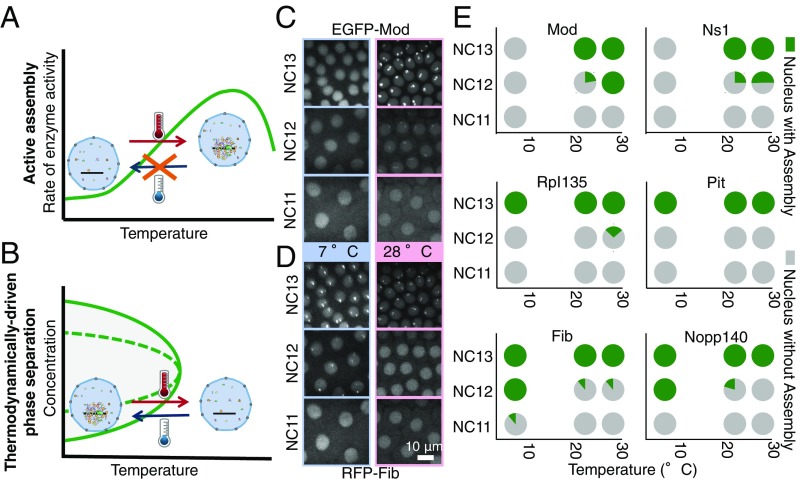
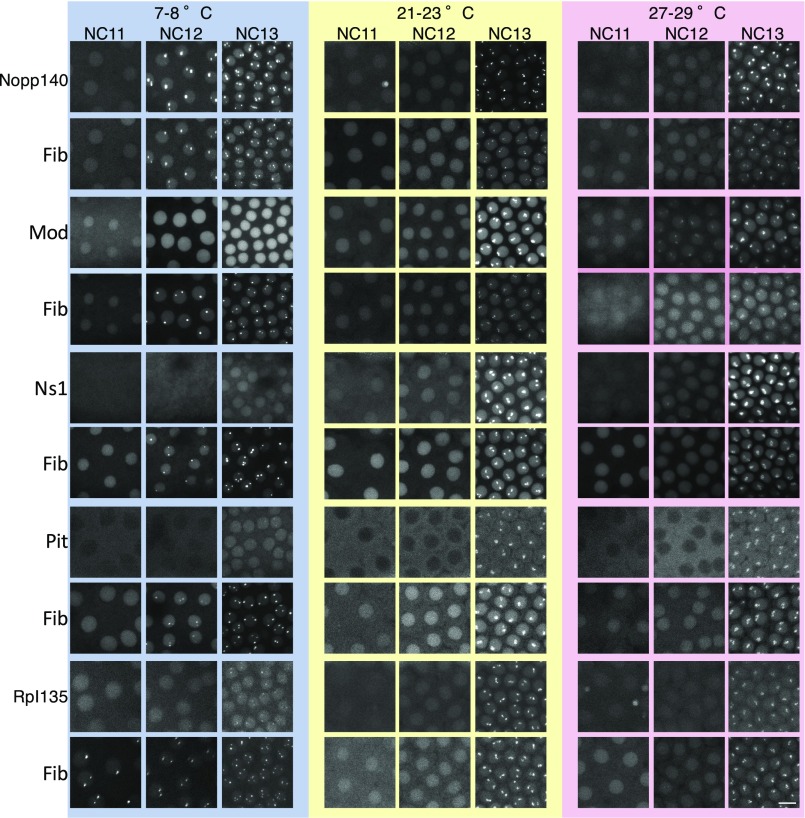
One feature that distinguishes thermodynamic LLPSs from active assembly processes is temperature dependence. The rate of an active assembly process is expected to decrease at lower temperatures, because the collisions that drive molecular interactions are less frequent at lower temperatures and the enzymes that couple the process to a high-energy source, such as ATP, typically operate more slowly (Fig. 2A) (26). Thermodynamic LLPS, in contrast, is generally enhanced at lower temperatures, because the entropic cost of demixing becomes smaller than its energetic advantage at lower temperatures (the exceptions are in Discussion; Fig. 2B). Therefore, allowing the embryos of the Drosophila to develop at lower temperatures will delay the processes dependent on active assembly, whereas it will enable thermodynamic LLPS to occur at earlier stages of development when the concentrations of the nucleolar proteins are too low to allow accumulation at room temperature (16) (Fig. S1). We used a microfluidic device (Fig. S2) (27) that allows a Drosophila embryo to develop at 7 °C to 29 °C. The temperature of the embryo in the device is controlled by the temperature of the water stream passing through the channels, and it is monitored by a built-in thermometer (details are in Methods). When the temperature at which development occurs is lowered to 7 °C to 8 °C, all studied embryos show Fib foci at NC12 [i.e., one cycle earlier than room temperature, with one embryo also showing assemblies as early as NC11 (n = 15)] (Fig. 2E and Fig. S3). Likewise, Nopp140 always localizes to Fib foci at NC12 (n = 3), suggesting that the assembly of these two proteins occurs via LLPS-dependent processes. Unlike Fib and Nopp140, the accumulation of two other nuclear proteins (Mod and Ns1) is delayed at 7 °C to 8 °C and does not occur at its normal time during NC13 (Fig. 2 C–E and Fig. S3). Higher temperatures (28 °C to 29 °C) have the opposite effect on Mod and Ns1, facilitating their accumulation (NC12 vs. NC13; n = 3), whereas the first emergence of Fib and Nopp140 remains at NC13, unchanged from that at room temperature. The premature assemblies of Mod and Ns1 that form without the enrichment of the phase-separating components seem morphologically similar to their assemblies in the normal nucleolus at NC13. The accumulation pattern of two other tested proteins, a subunit of RNA polymerase I (Rpl135) and Pit, seems to be insensitive to temperatures in the range that we have been able to produce with our microfluidic device. It is rather striking that the assembly of different proteins proceeds through mechanisms that scale differently with temperature, despite the fact that they all accumulate to the nucleolus simultaneously at ambient temperatures at which the embryo normally develops. In addition, these mechanisms are independent of one another, because the proteins in each group can assemble in the absence of the others. The observations presented above are consistent with Nopp140 and Fib following an LLPS mechanism, whereas Ns1 and Mod behave as actively separating proteins.
Fig. 2.
Temperature uncouples the first-time assembly of different nucleolar proteins. The temperature dependence of two possible mechanisms for the formation of assemblies is illustrated qualitatively in A and B. (A) The rate of active processes is determined by the rate of the enzyme that couples them to an energy source. Therefore, active assembly is faster at higher temperatures and slower at lower temperatures. (B) This behavior is the opposite of a thermodynamic LLPS, in which more condensation occurs at lower temperatures (exceptions are in Discussion). (C and D) An embryo coexpressing (C) EGFP-Mod and (D) RFP-Fib is imaged at 7 °C and 28 °C to determine the first-time emergence of assemblies. Images are maximum-projected. (E) The percentages of nuclei with or without assemblies at each temperature for each cell cycle are depicted in a pie chart for different nucleolar proteins. Number of embryos is three or more for each condition.
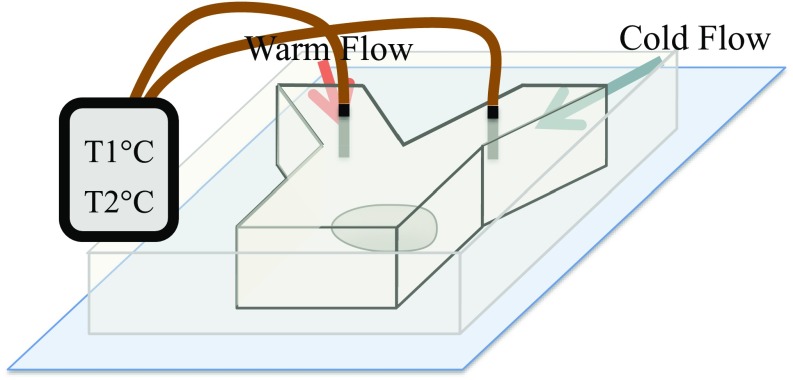
Fig. S2.
Schematic of temperature-control microfluidic device. Slight modifications have been applied to the previously reported microfluidic device (27) to allow for live confocal imaging with objective and monitoring of the temperature live. The embryo was placed on a polydimethylsiloxane (PDMS)-coated cover glass to accommodate the short working distance of the confocal objective, and a built-in thermometer was added to the designed for live monitoring of the temperature.
Fig. S3.
Differential temperature dependence of nucleolar proteins. Five different transgenic lines coexpressing RFP-Fib with one of the EGFP-tagged proteins Nopp140, Mod, Ns1, Pit, or Rpl135 were allowed to develop at different temperatures as discussed in Methods, and the maximum-projected images for NC11–NC13 for each temperature are shown. The brightness and contrast of the images have been modified for presentation purposes. (Scale bar: m.)
Reversibility of Assembly Formation.
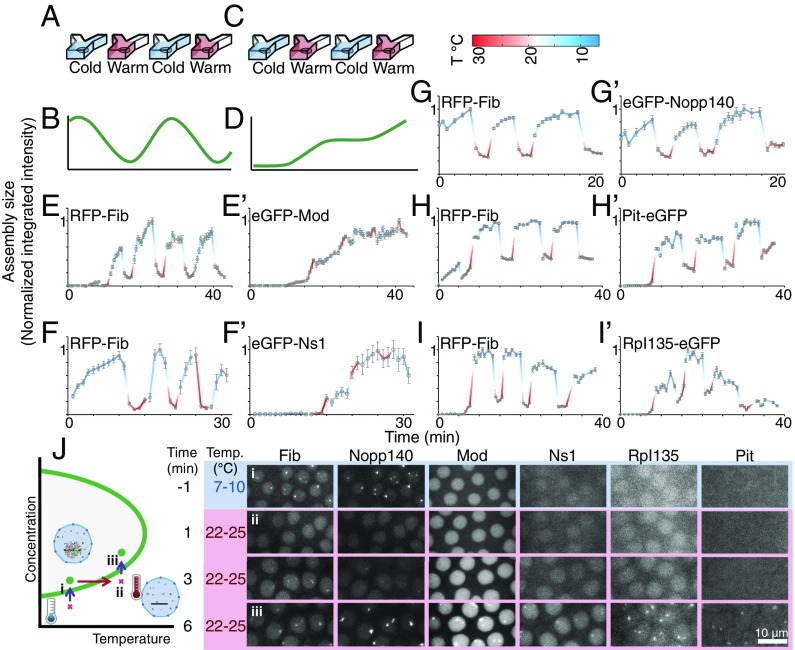
A second distinctive feature of thermodynamically driven LLPS is its reversibility. A small increase in temperature increases the entropic cost of demixing and makes the components of the two phases more miscible, leading to a reduction in the size and concentration of the nucleolar assemblies. If the change in temperature is large enough to cross the phase boundary in Fig. 2B, complete mixing of the two phases should occur. This reversibility in response to changes in the temperature has been observed in vitro for several protein solutions, including bovine lens protein II crystalline (28), hnRNPA1 (29), low-complexity domain of FUS (9), and the N terminus of Ddx4 (11). This behavior contrasts with that of an active process, which is facilitated at higher temperatures and expected to only decelerate and stall when the temperature is lowered but would not be expected to reverse (Fig. 3 A–D). To test this property further, we examined the reversibility of accumulation at the nucleolus in response to rapid changes in temperature. Such rapid changes should minimize the impact of the temperature shift on upstream factors, such as nuclear localization or protein synthesis. Accordingly, we allowed embryos to reach interphase of NC14 at 22 °C and then, reduced the temperature to 7 °C while performing confocal imaging. Next, the temperature was shifted up to 31 °C within a few minutes by manually changing the source of water stream from a cooling block to a heating block. The distributions of the nucleolar proteins were recorded again. To switch back to low temperature, the source of the water stream was changed back to a cooling block. This process was repeated three to six times, and changes in the nucleolar intensity of different proteins at various temperatures were measured from the resulting images. All studied embryos were coexpressing a GFP-tagged nucleolar protein with RFP-Fib for comparison. As depicted in Fig. 3 E–J and G′, Nopp140 and Fib show reversible changes in nucleolar localization in response to rapid oscillations in temperature. Higher levels accumulate at the nucleolus at lower temperatures, whereas the protein rapidly redistributes to the nucleoplasm at higher temperatures. These changes are fully reversible, because the same behavior is observed on multiple rounds of temperature oscillations. Interestingly, by shifting the temperature from 8 °C to 25 °C during early NC13, when the concentrations of these proteins are sufficiently low (16) (Fig. S1), we are able to cross the phase boundary in Fig. 2B and fully dissolve Nopp140 and Fib assemblies (Fig. 3J and Movie S1 ) as the concentration of the proteins increases. These results are consistent with a thermodynamically driven LLPS mechanism for the nucleolar accumulation of Nopp140 and Fib. We cannot rule out a special case with a combination of forward and reverse active processes, in which the disassembly process is more sensitive to the changes in the temperature. However, this special case seems unlikely, because it is an energetically expensive way of reaching the same behavior.
Fig. 3.
An in vivo assay to test the reversibility of assembly formation by nucleolar proteins. (A and C) A microfluidic device was used to switch between cold and warm temperatures in less than 1 min during NC14. (B and D) Schematics qualitatively illustrate predicted trends in assembly size for (B) a thermodynamic LLPS and (D) an active assembly. (B) A thermodynamic LLPS is expected to reversibly condense and dissolve by changing the temperature, whereas (D) an active assembly is expected to be slow at low temperatures, fast at high temperatures, and irreversible. (E–I and E′–I′) The integrated intensity at each temperature/time for high-concentration assemblies of different nucleolar proteins is determined, and mean SEM for n > 10 nuclei is normalized to its maximum level. Time 0 is when the experiment begins. The changes in the integrated intensity of the assemblies of (E′–I′) EGFP-tagged nucleolar proteins compared with those of (E–I) RFP-Fib are shown. (J) During early NC13, (i) Fib and Nopp140 assemblies are first to form at 8 °C to 10 °C. (ii) As soon as Fib assemblies appear, the embryos are shifted to 22 °C to 25 °C, in which the critical concentrations for Fib and Nopp140 are higher than the nucleoplasmic concentrations of these two proteins. This shift, therefore, causes the assemblies to dissolve. (iii) As the concentrations of Fib and Nopp140 increase by time, the assemblies reappear. The schematic in Left qualitatively illustrates the state of each step (i–iii) in the phase diagram. Images are maximum-projected. Time 0 marks the time when the temperature shift was applied.
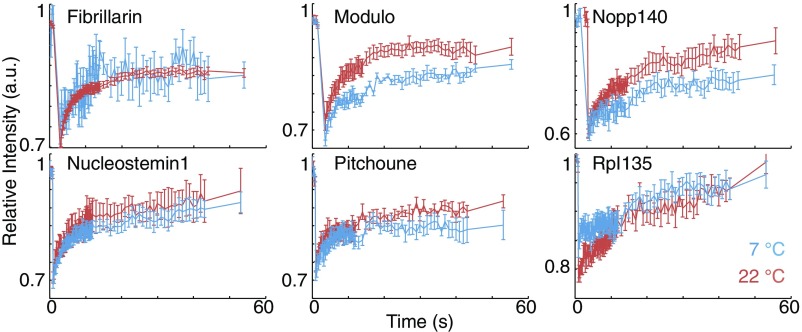
Unlike the first group of the nucleolar proteins discussed above, the formation of Mod and Ns1 assemblies is irreversible (Fig. 3E′ and F′). These two proteins are recruited to the nucleolus at higher temperatures, and their accumulation is slowed down at lower temperatures. Changing the temperature does not reverse any accumulation of these two proteins that has occurred previously in the nucleolus. This observed irreversibility is consistent with an active assembly. Alternatively, the assemblies could represent an LLPS with slow kinetics. To distinguish between these two possibilities, we performed fluorescent recovery after photobleaching (Fig. S4). Both Mod and Ns1 are highly dynamic, with for fluorescence recovery of less than 10 s, and therefore, the assemblies of these proteins are not kinetically arrested. The high motility of these proteins at 7 °C also argues against the involvement of motor proteins in their localization. Previous studies have shown that the GTPase, Ns1, requires binding to GTP for its localization to the nucleolus (25). Another possibility is a combination of active and LLPS processes, in which an initial active reaction, such as transcription of rDNA, phosphorylation, or binding to GTP, is required for the generation of key components, but a secondary thermodynamic LLPS drives the formation of assemblies. This hypothesis is consistent with the inhibition of assembly formation at low temperatures for Mod and Ns1 (Fig. 2). However, our reversibility results do not support the secondary LLPS mechanism. During NC14, the size of assemblies of these two proteins reaches a maximum level and saturates (equilibrates), suggesting that components capable of LLPS are no longer limiting. Under these conditions, LLPS-based movement in and out of the assemblies should depend on temperature—the size and magnitude of the assemblies should be reversible and change with rapid temperature shifts. We see such reversible behavior with other nucleolar components but not with Mod and Ns1, suggesting that, even in the saturated states characteristic of NC14, those two components do not follow characteristic LLPS behavior (Fig. 3E′ and F′).
Fig. S4.
Fluorescent recovery after photobleaching. The assembly of different nucleolar proteins have been photobleached, and the recovery of the fluorescent signal for the nucleolus has been quantified at 7 °C and 22 °C. Mean SEM is depicted for three or more nucleoli.
We also see a reversible behavior after rapid temperature shifts for the two proteins (Pit and Rpl135) that did not show obvious temperature dependence in the timing of their assembly during development. For Pit, the behavior was very similar to Nopp140 and Fib, suggesting that it may depend on LLPS (Fig. 3H′). Our previous failure to see earlier assembly of Pit at low temperatures may reflect the exclusion of Pit from nuclei before NC13 (Figs. S1 and S3). Rpl135 binds to the rDNA genes that are tandemly repeated hundreds of times, and this binding alone could give rise to Rpl135 foci. However, Rpl135 assemblies are present in mutant embryos lacking rDNA repeats (16) (Fig. 4A), arguing for the presence of alternative mechanism(s) for the recruitment of this protein. Consistent with that observation, during early NC14, successive alternations between low and high temperatures result in the reversible accumulation and redistribution of Rpl135 to the nucleolus, respectively (Fig. 3I′). Interestingly, unlike in the other phase-separating proteins, the nucleolar intensity of Rpl135 becomes almost insensitive to changes in temperature toward the end of NC14. This behavior suggests that the initial recruitment of Rpl135 to the nucleolus proceeds directly or indirectly through a thermodynamic LLPS but that secondary regulatory mechanisms later stabilize its nucleolar levels. Activation of transcription or association with the products of transcription could result in this observed behavior (30, 31).
Fig. 4.
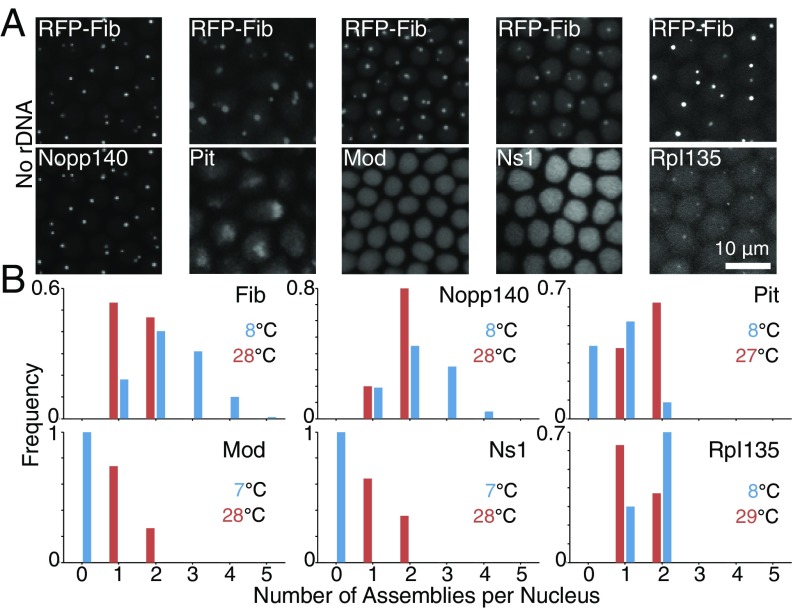
Transcription of rRNA coordinates the active and thermodynamic assemblies. (A) Each column depicts a mutant embryo lacking rDNA repeats at 22 °C and NC14 and coexpressing RFP-Fib with another EGFP-tagged nucleolar protein (Lower). Fib, Nopp140, and Rpl135 colocalize to variable numbers of assemblies in a nucleation-limited process, whereas Pit forms one diffuse assembly at the apical side of the nucleus. Mod and Ns1 do not form any assemblies in the absence of rDNA. Images are maximum-projected. (B) Distribution of the number of assemblies per nucleus in WT embryos for each of the nucleolar proteins at NC13 is depicted at high and low temperatures. At 22 °C, assemblies form at two rDNA repeats located on X and Y chromosomes. More than two foci observed for Fib and Nopp140 at 8 °C are indicative of unseeded assembly formation, whereas other proteins can only assemble at rDNA repeats in WT embryos.
Dependence of Assembly Formation on rDNA.
We have previously reported that the transcription of rRNA seeds the assembly of the phase-separating proteins, Fib and Rpl135, dictating the precision of an otherwise highly variable nucleation-limited process (16). In mutant embryos that lack rDNA, Fib and Rpl135 still assemble into nucleolar-like structures but with altered kinetics and greater variability. Because the temperature shift experiments presented above suggest that Fib’s incorporation and Rpl135’s initial recruitment into the nucleolus may be LLPS-dependent processes, we asked whether other nucleolar proteins show similar behaviors. Similar to Fib and Rpl135, other phase-separating nucleolar proteins, Nopp140 and Pit, are capable of forming high-concentration assemblies in the mutants lacking rDNA (Fig. 4A). In WT embryos at 22 °C, all six studied nucleolar proteins exclusively assemble at the two rDNA repeats of X and Y chromosomes (Fig. S3). However, more variability is observed in the number and position of Fib and Nopp140 foci in the absence of rDNA (Fig. 4A) (16). These morphological features were also observed in the premature Fib and Nopp140 assemblies that arise in WT embryos at 8 °C (Fig. 4B), suggesting that assembly at low temperature may not depend on seeding by RNA. The behavior of Pit differs from that of Fib and Nopp140, in that the assemblies that it forms in rDNA mutants accumulate to the apical side of the nucleus (Fig. 4A). These results suggest that, in the absence of rDNA, Pit may make use of an alternative nucleation site associated with heterochromatin. In contrast to the behavior of the LLPS-dependent proteins, rDNA is necessary for the two proteins that appear to accumulate through active recruitment (Mod and Ns1), because neither form assemblies in its absence (Fig. 4A). This behavior is similar to what was observed for these two proteins in WT embryos at low temperatures that are not permissive for active processes, such as transcription (Fig. 2). Consistent with the role of rDNA in active recruitment of Mod and Ns1, the maximum number of assemblies for these two proteins in WT embryos at different temperatures is two (Fig. 4B), suggesting that they can only assemble at rDNA repeats.
Discussion
The experiments presented here constitute an in vivo assessment of the phase separation model for the formation of membraneless organelles. This assessment is based on the two main features of LLPS, namely the temperature dependence and reversibility, which together can distinguish thermodynamically driven phase separations from active assemblies. Using our microfluidic-based temperature assay, we show that some nucleolar proteins are recruited to the nucleolus via a thermodynamic LLPS, whereas others are actively incorporated into the nucleolus. These findings refine our fundamental understanding of the mechanism and structural determinants for the formation of membraneless bodies in vivo in not only a developmental context but also, various diseases in which native structures are altered or pathological aggregates appear.
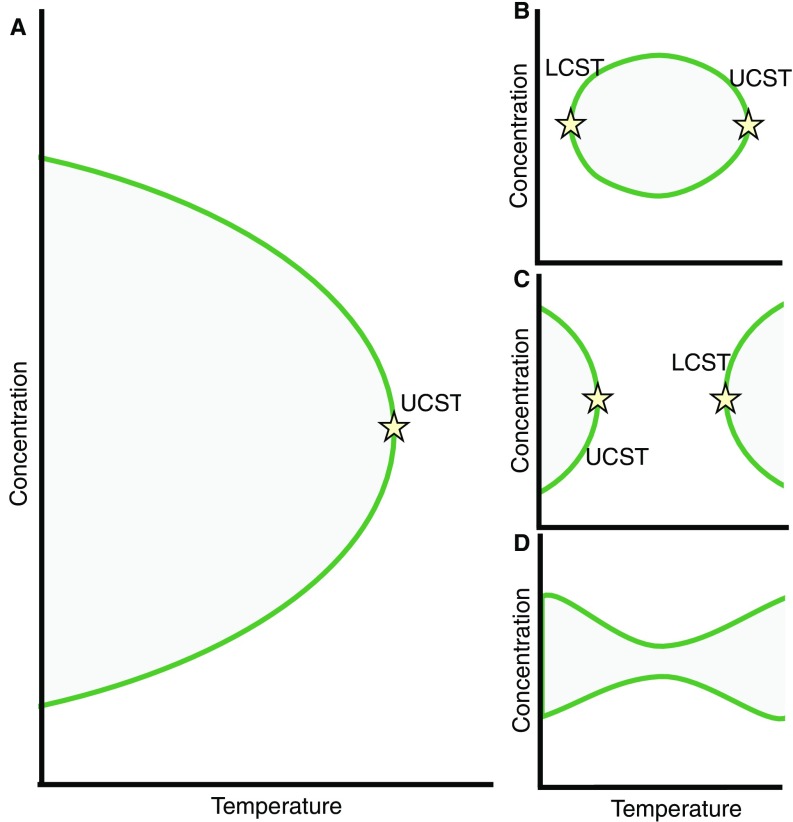
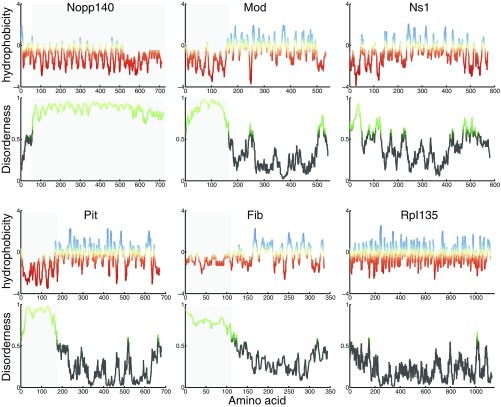
Several classes of LLPSs have been observed for polymer/solvent systems (Fig. S5) (32), and the assay presented in this work can distinguish between these different possibilities. Most LLPSs occur when cooling below a critical temperature because at higher temperatures, the entropic penalty of demixing increases and eventually overcomes the unfavorable energetic interactions between dissimilar entities. Such temperature dependence leads to an upper-critical solution temperature (UCST), above which the system is well-mixed at all compositions (Fig. S5A). Examples of UCST have been observed in vitro for the low-complexity domain of the RNA binding protein FUS (10), a disordered Nauge protein (11), and lipid bilayers (33). The phase-separating nucleolar proteins studied here (Fib, Nopp140, Pit, and Rpl135) all show this behavior, because their formation is reversibly enhanced at low temperatures. This behavior is consistent with the presence of polar residues at the disordered regions of these proteins (Fig. S6), which are predictive of LLPS with an UCST (34).
Fig. S5.
Schematic representation of different types of phase diagrams. A solution showing (A) phase separation with UCST, (B) closed loop where LCST is lower than UCST, (C) UCST lower than LCST, and (D) hourglass with no critical temperature (details are in Discussion).
Fig. S6.
IDR and hydrophobicity of six studied proteins. The hydrophobicity score (38) for each amino acid was determined using ExPASy, with red indicating hydrophilic residues and blue indicating hydrophobic residues. The disorderness of the proteins studied here was determined using IUPRED (24), with scores above 0.5 indicating disorder (light green). The shaded areas indicate the disordered regions.
There are, however, exceptions to this general behavior, which show a lower-critical solution temperature (LCST) (Fig. S5 B and C) or no critical temperature (Fig. S5D). Such anomalies are observed in two types of systems. The first anomaly is mostly observed in the polymer solutions at high temperatures and pressures, at which the compressibility of the solvent affects the mixing behavior (e.g., close to the vapor–liquid critical point of the solvent) and results in phase diagrams shown in Fig. S5 C and D (32). Obviously, such conditions are not physiologically relevant. The second type of anomaly is observed when highly directional specific interactions between dissimilar molecules prevent random mixing, leading to a “closed loop” in the phase diagram (Fig. S5B) (32). The presence of LCST has been observed for a spindle-associated protein, BuGZ (35). In this case, decreasing the temperature can prevent the formation of assemblies, similar to an active recruitment. However, unlike active processes, an LLPS with LCST is reversible, and therefore, after the formation of assemblies, lowering the temperature results in remixing of the two phases. Such remixing is not what we observe for Mod and Ns1, for which the assembly process that happens at high temperatures is irreversible (Fig. 3E′ and F′). This irreversibility is not caused by slower kinetics (Fig. S4) and is, thus, consistent with an active recruitment rather than an LLPS with LCST. In addition, these two proteins lack nonpolar residues at the disordered regions (Fig. S6) that are predictive of LLPS with LCST (34).
The large number of molecules studied here allows us to examine the structural features that govern in vivo phase separations. Previous studies suggest that the presence of intrinsically disordered regions (IDRs), repetitive modules, and RNA binding motifs in a protein enhance its phase separation propensity (3–10). The disorder tendency of the proteins studied here was assessed using IUPRED (24) (Fig. S6). Fib, Nopp140, and Pit all contain IDRs and assemble via an LLPS-dependent mechanism. In addition, Fib is a component of ribonucleoprotein complexes and therefore, has potential for multimerization through additional macromolecular constituents. Ns1 lacks IDRs and is shown to assemble actively (Fig. 3F′). An unexpected behavior, however, is observed for Mod, the Drosophila homologue of Nucleolin. Despite containing a disordered N terminus and four RNA binding motifs (Fig. 1 and Fig. S6), its recruitment to the nucleolus is through active assembly, suggesting that the presence of IDRs is not sufficient for an in vivo thermodynamic LLPS. Similarly, Rpl135 lacks any known structural feature that could lead to phase separation, and yet, its initial recruitment to the nucleolus is dependent on LLPS. This dependence could be direct via an unknown structural feature or indirect through binding to a phase-separating chaperon. For the three other proteins (Fib, Nopp140, and Pit), which contain IDRs and are recruited to the nucleolus through an LLPS-dependent mechanism, additional experiments will determine whether this dependence in vivo is direct or indirect. These observations underline the complexity of interactions that drive LLPS inside cells and the necessity for more in vivo studies that will allow us to refine the existing heuristics for identifying phase-separating proteins, an undertaking that could be the topic of future studies.
The observation of two independent mechanisms for the formation of a single organelle is surprising and suggests that the simultaneous accumulation observed during normal development may involve some global coordinator of the different mechanisms. Our observations confirm that the transcription of rDNA is responsible for the spatiotemporal regulation of LLPS as well as plays an essential role in recruiting nucleolar proteins, like Mod or Ns1, that depend on active assembly. Therefore, rDNA seems to function as the coordinator of the two independent mechanisms for the formation of the nucleolus.
Methods
Determination of the First-Time Emergence of Nucleolar Protein Assemblies.
The nucleoli were detected using an automated algorithm described before (16). The nuclei were detected independently for the two fluorescent proteins in each embryo. The first-time emergence ( of formation) is defined as the first time point at which at least one-half of the nuclei in the image has detectable assemblies. The average time for the first-time formation of assemblies for each protein is defined as the median for eight embryos studied.
Developing Embryos at Different Temperatures.
The embryos coexpressing RFP-Fib with a second EGFP-tagged nucleolar protein (Nopp140, Ns1, Mod, Pit, or Rpl135) were placed in the microfluidic device before NC10. For each temperature, the flow with the desired temperature would pass through one inlet, and the second inlet was blocked. For high-temperature experiments, the embryos were exposed to continuous flow of 28 °C to 29 °C from NC10 to NC13. For room temperature experiments, no flow was applied. Because low temperatures can disrupt microtubule assembly, the embryos were exposed to 7 °C to 8 °C flow only during interphases of NC11–NC13, and the mitosis was allowed to proceed at room temperature.
Reversibility Assay.
The switch between different temperatures was performed manually by alternating the inlet flow from the cooling block to the heating block. This process would be performed in about 30 s. After any change in the temperature, the objective lens and the cover glass contract or expand, shifting the embryo out of focus. The focus was, therefore, readjusted manually after each temperature shift for each image, and the temperature and time elapsed from the previous image were recorded.
SI Methods has details of materials and methods.
SI Methods
All image analyses were performed with ImageJ (Rasband WS; ImageJ; National Institutes of Health; 1997–2008) and MATLAB (MathWorks) routines.
Fabrication and Utilization of the Microfluidic Device.
A microfluidic device was fabricated as described previously (27) with slight modifications. The first modification was to allow for imaging with objective. Because of the short working distance of this objective, the embryos were directly mounted on a cover glass using heptane glue. The cover glass was coated with a thin layer of polydimethylsiloxane (PDMS) to reduce the heat transfer with the environment and also, ease the sealing of the channels. The microfluidic channel would then be placed on top of this cover glass (Fig. S2). A Fusion 200 Syringe Pump (Chemyx) was used to control the flow rate and pump the water through a cooling or heating block before reaching the microfluidics device. To achieve temperatures as low as 6 °C, the Tygon tubing containing the flow leaving the cooling block was placed in contact with dry ice on the confocal microscope stage before reaching the device. Finally, the temperature of the flow was measured with a built-in thermometer placed 5 mm upstream of the embryo in each inlet channel (Fig. S2). Confocal imaging was performed using a 63 HCX PL APO CS 1.4-N.A. oil-immersion objective on a Leica SP5 Laser-Scanning Confocal Microscope equipped with GaAsP HyD Detectors, z spacing of m, and zoom.
Transgenic Lines.
RpI135-EGFP and RFP-Fib transgenic lines were previously reported (16, 20). For EGFP-Fib, EGFP-Nopp140, EGFP-Mod, and EGFP-Ns1 transgenes, genomic fragments flanking each gene with lengths of 2.25, 5.6, 4.3, and 3.6 kb, respectively, were modified by replacing the start codon of each gene with a Drosophila codon-optimized EGFP and a linker inserted before the start of the genes. For Pit-EGFP, a 5.8-kb genomic segment flanking Drosophila melanogaster Pit was modified by replacing the stop codon with a linker and Drosophila codon-optimized EGFP. All five fragments were prepared by PCR and cloned into the pBabr vector. The resulting constructs were then inserted into the attP2 landing site in the Drosophila genome. Transgenic injections were performed by Genetic Services Inc. Primer and transgene sequences are available on request.
Fly Stocks and Genetics.
Fly stocks and crosses were maintained by standard methods at room temperature. Mutant embryos lacking rDNA have the genotype of C(1)DX/0 and were obtained by crossing C(1)DX/YBs;RFP-Fib;GFP-tagged nucleolar protein (EGFP-Nopp140, EGFP-Mod, EGFP-Ns1, or Pit-EGFP) virgins with C(1;Y)*,y1 wa/0 males. C(1)DX structurally lacks the nucleolus organizer regions (36, 37). Ten embryos from each cross are imaged using a HCX PL APO CS 1.4-N.A. oil-immersion objective on a Leica SP5 laser-Scanning Confocal Microscope equipped with GaAsP HyD Detectors, and genotypes are scored based on the number, position, size, and first-time emergence of high-concentration assemblies of Fib as described previously (16); 25% of embryos in these crosses are unambiguously scorable as C(1)DX/0.
Measuring the Integrated Intensities of Assemblies.
Confocal images were maximum intensity-projected, and the nuclei were detected using a Gaussian filter and thresholding and manually scanned to only include the nuclei that are in focus. A mask for high-concentration assemblies in each channel was prepared using a difference of Gaussian filter and a constant threshold for all images of the same channel. The mean integrated intensity of high-concentration assemblies per nucleus was then measured for each image and normalized to their maximum value at each experiment.
Measuring Nucleoplasmic Concentrations.
The nucleoplasmic concentration of EGFP-Nopp140, EGFP-Mod, EGFP-Ns1, and Pit-EGFP is reported as a function of intensity per unit volume () and has been measured from confocal images obtained using a HC PL APO Gly 0.7-N.A. objective on a Leica SP5 Laser-Scanning Confocal Microscope as described previously (20).
Determining Disorder Tendency and Hydrophobicity.
The D. melanogaster protein sequences were obtained from FlyBase, and IUPRED (iupred.enzim.hu) was used with the “long disorder” parameter to determine the disorder tendency (24). Hydrophobicity was determined using ExPASy’s ProtScale (web.expasy.org/protscale/) with the values of Kyte and Doolittle (38) and the following parameters: window size = 9, relative weight of the window edges compared with the window center = 100%, and no normalization of scale.
Fluorescent Recovery After Photobleaching.
To perform the fluorescent recovery after photobleaching experiment, a minimal area containing a whole nucleolus was photobleached, and the mean fluorescent intensity of that area was measured before and after photobleaching. The nucleoli that were photobleached were selected from nuclei that contain two distinct nucleoli to ensure a larger unbleached pool of fluorescent proteins. The mean intensity for each nucleolus at each time point was normalized to its average intensity of five consecutive time points preceding photobleaching.
Supplementary Material
Acknowledgments
We thank the Bloomington Drosophila Stock Center for fly stocks. We thank all members of the laboratory of E.W. and the Schupbach laboratory, especially S. Blythe for lively discussion. We also thank P. Debenedetti, S. Shvartsman, B. Machta, M. Levine, A. Haji-Akbari, and T. Schupbach for helpful comments. We thank S. Vyawahare and the Princeton Microfluidics Laboratory. This work was supported, in part, by National Institute of Child Health and Human Development Grant 5R37HD15587 (to E.W.). Additionally, E.W. is an Investigator with the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1615395114/-/DCSupplemental.
References
- 1.Matera AG, Izaguire-Sierra M, Praveen K, Rajendra TK. Nuclear bodies: Random aggregates of sticky proteins or crucibles of macromolecular assembly? Dev Cell. 2009;17(5):639–647. doi: 10.1016/j.devcel.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brangwynne CP, et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324(5935):1729–1732. doi: 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- 3.Li P, et al. Phase transitions in the assembly of multivalent signalling proteins. Nature. 2012;483(7389):336–340. doi: 10.1038/nature10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyman AA, Weber CA, Jülicher F. Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol. 2014;30:39–58. doi: 10.1146/annurev-cellbio-100913-013325. [DOI] [PubMed] [Google Scholar]
- 5.Courchaine EM, Lu A, Neugebauer KM. Droplet organelles? EMBO J. 2016;35(15):1603–1612. doi: 10.15252/embj.201593517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brangwynne CP, Mitchison TJ, Hyman AA. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc Natl Acad Sci USA. 2011;108(11):4334–4339. doi: 10.1073/pnas.1017150108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry J, Weber SC, Vaidya N, Haataja M, Brangwynne CP. RNA transcription modulates phase transition-driven nuclear body assembly. Proc Natl Acad Sci USA. 2015;112(38):E5237–E5245. doi: 10.1073/pnas.1509317112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elbaum-Garfinkle S, et al. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc Natl Acad Sci USA. 2015;112(23):7189–7194. doi: 10.1073/pnas.1504822112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murakami T, et al. ALS/FTD mutation-induced phase transition of FUS liquid droplets and reversible hydrogels into irreversible hydrogels impairs RNP granule function. Neuron. 2015;88(4):678–690. doi: 10.1016/j.neuron.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke KA, Janke AM, Rhine CL, Fawzi NL. Residue-by-residue view of in vitro FUF granules that bind the C-terminal domain of RNA polymerase II. Mol Cell. 2015;60(2):231–241. doi: 10.1016/j.molcel.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nott TJ, et al. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol Cell. 2015;57(5):936–947. doi: 10.1016/j.molcel.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sear RP, Cuesta JA. Instabilities in complex mixtures with a large number of components. Phys Rev Lett. 2003;91:245701. doi: 10.1103/PhysRevLett.91.245701. [DOI] [PubMed] [Google Scholar]
- 13.Loschi M, Leishman CC, Berardone N, Boccaccio GL. Dynein and kinesin regulate stress-granule and P-body dynamics. J Cell Sci. 2009;122(Pt 21):3973–3982. doi: 10.1242/jcs.051383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas MG, Loschi M, Desbats MA, Boccaccio GL. RNA granules: The good, the bad and the ugly. Cell Signal. 2011;23(2):324–334. doi: 10.1016/j.cellsig.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoerndli FJ, et al. Kinesin-1 regulates synaptic strength by mediating the delivery, removal, and redistribution of AMPA receptors. Neuron. 2013;80(6):1421–1437. doi: 10.1016/j.neuron.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falahati H, Pelham-Webb B, Blythe S, Wieschaus E. Nucleation by rRNA dictates the precision of nucleolus assembly. Curr Biol. 2016;26(3):277–285. doi: 10.1016/j.cub.2015.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dundr M, Misteli T. Biogenesis of nuclear bodies. Cold Spring Harbor Perspect Biol. 2010;2(12):a000711. doi: 10.1101/cshperspect.a000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang JT, et al. Regulation of RNA granule dynamics by phosphorylation of serine-rich, intrinsically disordered proteins in C. elegans. Elife. 2014;3:e04591. doi: 10.7554/eLife.04591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aumiller WM, Jr, Keating CD. Phosphorylation-mediated RNA/peptide complex coacervation as a model for intracellular liquid organelles. Nat Chem. 2016;8(2):129–137. doi: 10.1038/nchem.2414. [DOI] [PubMed] [Google Scholar]
- 20.Blythe SA, Wieschaus EF. Zygotic genome activation triggers the DNA replication checkpoint at the midblastula transition. Cell. 2015;160(6):1169–1181. doi: 10.1016/j.cell.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosby R, et al. Knockdown of the Drosophila GTPase nucleostemin 1 impairs large ribosomal subunit biogenesis, cell growth, and midgut precursor cell maintenance. Mol Biol Cell. 2009;20(20):4424–4434. doi: 10.1091/mbc.E08-06-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchler-Bauer A, et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015;43(Database issue):D222–D226. doi: 10.1093/nar/gku1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell A, et al. The InterPro protein families database: The classification resource after 15 years. Nucleic Acids Res. 2015;43(Database issue):D213–D221. doi: 10.1093/nar/gku1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dosztányi Z, Csizmok V, Tompa P, Simon I. IUPred: Web server for the prediction of intrinsically unstructured regions of proteins based on estimated energy content. Bioinformatics. 2005;21(16):3433–3434. doi: 10.1093/bioinformatics/bti541. [DOI] [PubMed] [Google Scholar]
- 25.Tsai RYL, McKay RDG. A multistep, GTP-driven mechanism controlling the dynamic cycling of nucleostemin. J Cell Biol. 2005;168(2):179–184. doi: 10.1083/jcb.200409053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark DS, Blanch HW. Biochemical Engineering. CRC Press; Boca Raton, FL: 1997. [Google Scholar]
- 27.Lucchetta EM, Lee JH, Fu LA, Patel NH, Ismagilov RF. Dynamics of Drosophila embryonic patterning network perturbed in space and time using microfluidics. Nature. 2005;434(7037):1134–1138. doi: 10.1038/nature03509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomson JA, Schurtenberger P, Thurston GM, Benedek GB. Binary liquid phase separation and critical phenomena in a protein/water solution. Proc Natl Acad Sci USA. 1987;84(20):7079–7083. doi: 10.1073/pnas.84.20.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molliex A, et al. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell. 2015;163(1):123–133. doi: 10.1016/j.cell.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller G, et al. hRRN3 is essential in the SL1-mediated recruitment of RNA Polymerase I to rRNA gene promoters. EMBO J. 2001;20(6):1373–1382. doi: 10.1093/emboj/20.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanij E, et al. UBF levels determine the number of active ribosomal RNA genes in mammals. J Cell Biol. 2008;183(7):1259–1274. doi: 10.1083/jcb.200805146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prausnitz JM, Lichtenthaler RN, de Azevedo EG. Molecular Thermodynamics of Fluid-Phase Equilibria. Prentice-Hall PTR; Upper Saddle River, NJ: 1998. [Google Scholar]
- 33.Veatch SL, Keller SL. Miscibility phase diagrams of giant vesicles containing sphingomyelin. Phys Rev Lett. 2005;94(14):148101. doi: 10.1103/PhysRevLett.94.148101. [DOI] [PubMed] [Google Scholar]
- 34.Quiroz FG, Chilkoti A. Sequence heuristics to encode phase behaviour in intrinsically disordered protein polymers. Nat Mater. 2015;14(11):1164–1171. doi: 10.1038/nmat4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang H, et al. Phase transition of spindle-associated protein regulate spindle apparatus assembly. Cell. 2015;163(1):108–122. doi: 10.1016/j.cell.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller HJ. A stable double X chromosome. Drosoph Inf Serv. 1943;17:61–62. [Google Scholar]
- 37.Ritossa FM, Spiegelman S. Localization of DNA complementary to ribosomal RNA in the nucleolus organizer region of Drosophila melanogaster. Proc Natl Acad Sci USA. 1965;53(4):737–745. doi: 10.1073/pnas.53.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 39.Scheer U, Rose KM. Localization of RNA polymerase I in interphase cells and mitotic chromosomes by light and electron microscopic immunocytochemistry. Proc Natl Acad Sci USA. 1984;81(5):1431–1435. doi: 10.1073/pnas.81.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Y, et al. Conserved composition of mammalian box H/ACA and box C/D small nucleolar ribonucleoprotein particles and their interaction with the common factor Nopp140. Mol Biol Cell. 2000;11(2):567–577. doi: 10.1091/mbc.11.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang C, Query CC, Meier UT. Immunopurified small nucleolar ribonucleoprotein particles pseudouridylate rRNA independently of their association with phosphorylated Nopp140. Mol Cell Biol. 2002;22(24):8457–8466. doi: 10.1128/MCB.22.24.8457-8466.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen H-K, Pai C-Y, Huang J-Y, Yeh N-H. Human Nopp140, which interacts with RNA polymerase I: Implications for rRNA gene transcription and nucleolar structural organization. Mol Cell Biol. 1999;19(12):8536–8546. doi: 10.1128/mcb.19.12.8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meier UT, Blobel G. Nopp140 shuttles on tracks between nucleolus and cytoplasm. Cell. 1992;70(1):127–138. doi: 10.1016/0092-8674(92)90539-o. [DOI] [PubMed] [Google Scholar]
- 44.Tollervey D, Lehtonen H, Jansen R, Kern H, Hurt EC. Temperature-sensitive mutations demonstrate roles for yeast fibrillarin in pre-rRNA processing, pre-rRNA methylation, and ribosome assembly. Cell. 1993;72(3):443–457. doi: 10.1016/0092-8674(93)90120-f. [DOI] [PubMed] [Google Scholar]
- 45.Ochs RL, Lischwe MA, Spohn WH, Busch H. Fibrillarin: A new protein of the nucleolus identified by autoimmune sera. Biol Cell. 1985;54(2):123–133. doi: 10.1111/j.1768-322x.1985.tb00387.x. [DOI] [PubMed] [Google Scholar]
- 46.Ginisty H, Sicard H, Roger B, Bouvet P. Structure and functions of nucleolin. J Cell Sci. 1999;112(Pt 6):761–772. doi: 10.1242/jcs.112.6.761. [DOI] [PubMed] [Google Scholar]
- 47.Ginisty H, Amalric F, Bouvet P. Nucleolin functions in the first step of ribosomal RNA processing. EMBO J. 1998;17(5):1476–1486. doi: 10.1093/emboj/17.5.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perrin L, et al. Dynamics of the sub-nuclear distribution of Modulo and the regulation of position-effect variegation by nucleolus in Drosophila. J Cell Sci. 1998;111(Pt 18):2753–2761. doi: 10.1242/jcs.111.18.2753. [DOI] [PubMed] [Google Scholar]
- 49.Perrin L, Benassayag C, Morello D, Pradel J, Montagne J. Modulo is a target of Myc selectively required for growth of proliferative cells in Drosophila. Mech Dev. 2003;120(6):645–655. doi: 10.1016/s0925-4773(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 50.Biggiogera M, et al. Nucleolar distribution of proteins B23 and nucleolin in mouse preimplantation embryos as visualized by immunoelectron microscopy. Development. 1990;110(4):1263–1270. doi: 10.1242/dev.110.4.1263. [DOI] [PubMed] [Google Scholar]
- 51.Zaffran S, et al. A Drosophila RNA helicase gene, pitchoune, is required for cell growth and proliferation and is a potential target of d-Myc. Development. 1998;125(18):3571–3584. doi: 10.1242/dev.125.18.3571. [DOI] [PubMed] [Google Scholar]
- 52.Romanova L, et al. Critical role of nucleostemin in pre-rRNA processing. J Biol Chem. 2009;284(8):4968–4977. doi: 10.1074/jbc.M804594200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma H, Pederson T. Depletion of the nucleolar protein nucleostemin causes G1 cell cycle arrest via the p53 pathway. Mol Biol Cell. 2007;18(7):2630–2635. doi: 10.1091/mbc.E07-03-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knibiehler B, Mirre C, Rosset R. Nucleolar organizer structure and activity in a nucleolus without fibrillar centres: The nucleolus in an established Drosophila cell line. J Cell Sci. 1982;57:351–364. doi: 10.1242/jcs.57.1.351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.