Abstract
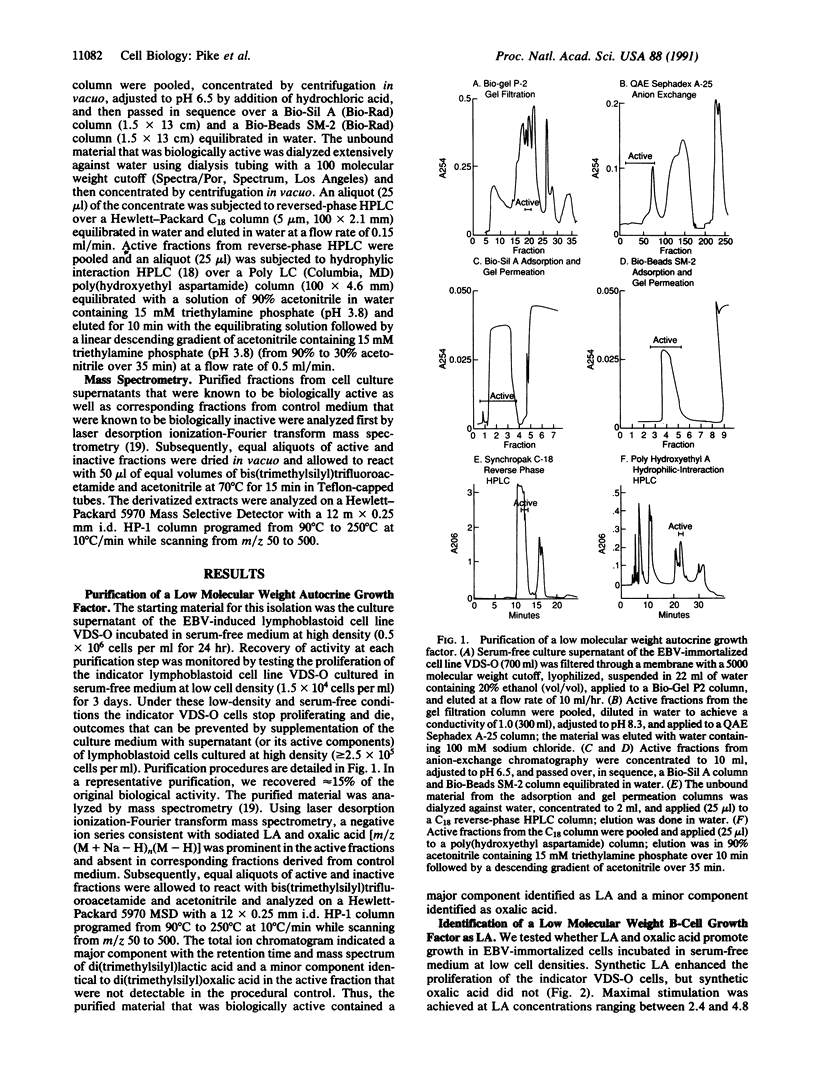
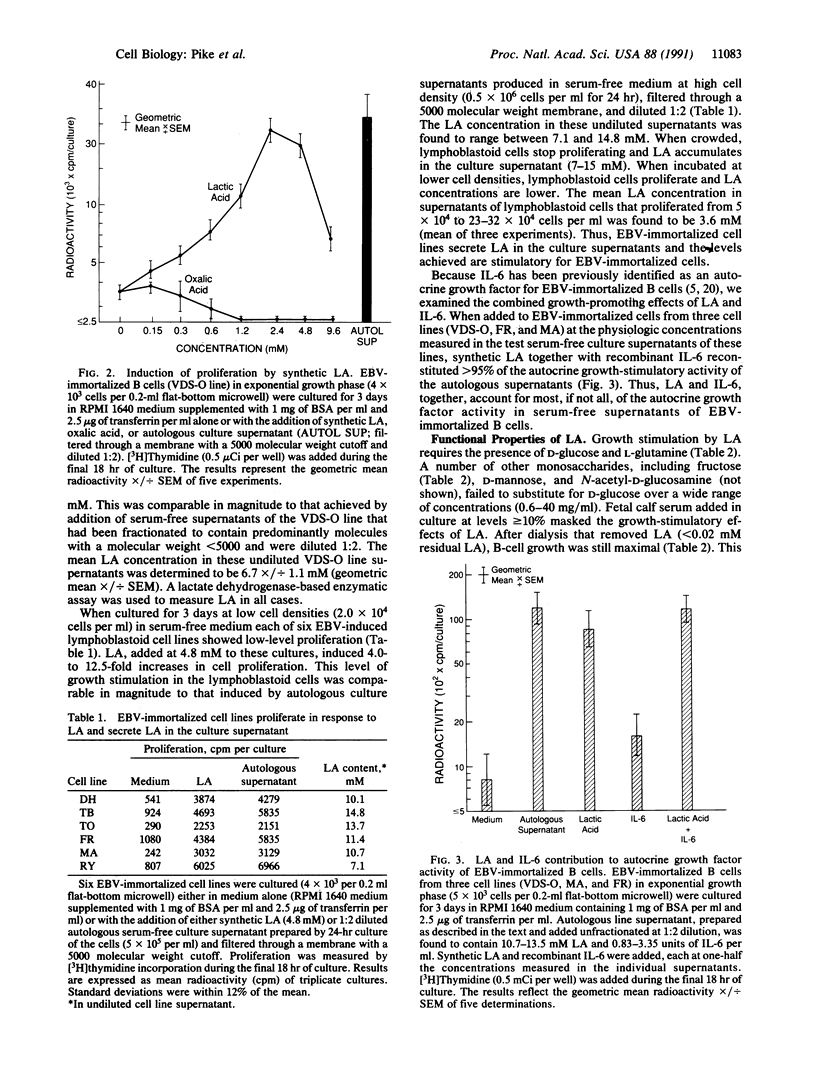
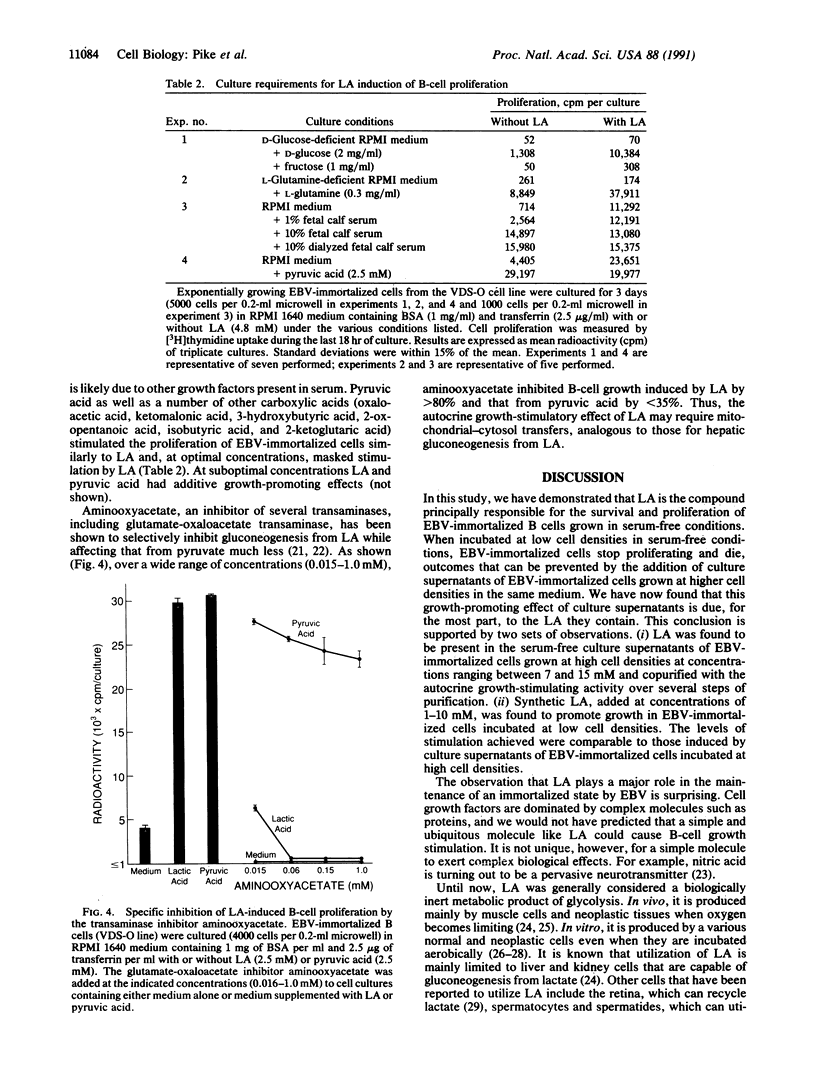
Growth and survival of Epstein-Barr virus (EBV)-immortalized B lymphocytes cultured at low cell densities require autocrine soluble factors. In this study, we have purified a low molecular weight autocrine soluble factor that promotes growth of EBV-immortalized B cells in serum-free conditions and identified it as lactic acid (LA). Synthetic LA stimulated growth in EBV-immortalized B cells at 1-10 mM, a concentration of LA measured in the culture supernatant of EBV-immortalized cell lines. LA alone was found to account for greater than 70% of the autocrine growth factor activity in serum-free supernatants of EBV-immortalized B cells. Aminooxyacetate, a glutamate-oxaloacetate transaminase inhibitor, specifically inhibited B-cell growth induced by LA, suggesting that this process requires mitochondrial-cytosol transfers. Thus, LA is an autocrine stimulatory molecule that in serum-free conditions is essential for the continuous proliferation of EBV-immortalized B cells. This represents an unexpected function for LA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert A. J. Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J Chromatogr. 1990 Jan 19;499:177–196. doi: 10.1016/s0021-9673(00)96972-3. [DOI] [PubMed] [Google Scholar]
- Best L., Yates A. P., Meats J. E., Tomlinson S. Effects of lactate on pancreatic islets. Lactate efflux as a possible determinant of islet-cell depolarization by glucose. Biochem J. 1989 Apr 15;259(2):507–511. doi: 10.1042/bj2590507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazar B. A., Sutton L. M., Strome M. Self-stimulating growth factor production by B-cell lines derived from Burkitt's lymphomas and other lines transformed in vitro by Epstein-Barr virus. Cancer Res. 1983 Oct;43(10):4562–4568. [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Burgos H. Angiogenic factor from human term placenta. Purification and partial characterization. Eur J Clin Invest. 1986 Dec;16(6):486–493. doi: 10.1111/j.1365-2362.1986.tb02166.x. [DOI] [PubMed] [Google Scholar]
- Dröge W., Roth S., Altmann A., Mihm S. Regulation of T-cell functions by L-lactate. Cell Immunol. 1987 Sep;108(2):405–416. doi: 10.1016/0008-8749(87)90223-1. [DOI] [PubMed] [Google Scholar]
- Elstow S. F., Schor A. M., Weiss J. B. Bovine retinal angiogenesis factor is a small molecule (molecular mass less than 600). Invest Ophthalmol Vis Sci. 1985 Jan;26(1):74–79. [PubMed] [Google Scholar]
- Finlay C. A., Cristofalo V. J. Autocrine stimulation of WI38 cell proliferation in the presence of glucocorticoids. Characteristics of the stimulatory factor(s) involved in this response. Exp Cell Res. 1987 Jan;168(1):191–202. doi: 10.1016/0014-4827(87)90428-9. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Mueckler M. M., Usher P., Lodish H. F. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987 Mar 20;235(4795):1492–1495. doi: 10.1126/science.3103217. [DOI] [PubMed] [Google Scholar]
- Goldman S. S. Gluconeogenesis in the amphibian retina. Lactate is preferred to glutamate as the gluconeogenic precursor. Biochem J. 1988 Sep 1;254(2):359–365. doi: 10.1042/bj2540359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J., Ley S. C., Melamed M. D., Aman P., Hughes-Jones N. C. Soluble factor requirements for the autostimulatory growth of B lymphoblasts immortalized by Epstein-Barr virus. J Exp Med. 1984 May 1;159(5):1554–1559. doi: 10.1084/jem.159.5.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERZENBERG L. A., ROOSA R. A. Nutritional requirements for growth of a mouse lymphoma in cell culture. Exp Cell Res. 1960 Nov;21:430–438. doi: 10.1016/0014-4827(60)90275-5. [DOI] [PubMed] [Google Scholar]
- Henle W., Diehl V., Kohn G., Zur Hausen H., Henle G. Herpes-type virus and chromosome marker in normal leukocytes after growth with irradiated Burkitt cells. Science. 1967 Sep 1;157(3792):1064–1065. doi: 10.1126/science.157.3792.1064. [DOI] [PubMed] [Google Scholar]
- Hiraki Y., Rosen O. M., Birnbaum M. J. Growth factors rapidly induce expression of the glucose transporter gene. J Biol Chem. 1988 Sep 25;263(27):13655–13662. [PubMed] [Google Scholar]
- Hotta S. S. Oxidative metabolism of isolated brain mitochondria: changes caused by aminooxyacetate. Arch Biochem Biophys. 1968 Sep 20;127(1):132–139. doi: 10.1016/0003-9861(68)90209-9. [DOI] [PubMed] [Google Scholar]
- Jennings M. L., Adams-Lackey M. A rabbit erythrocyte membrane protein associated with L-lactate transport. J Biol Chem. 1982 Nov 10;257(21):12866–12871. [PubMed] [Google Scholar]
- Kallinowski F., Vaupel P., Runkel S., Berg G., Fortmeyer H. P., Baessler K. H., Wagner K., Mueller-Klieser W., Walenta S. Glucose uptake, lactate release, ketone body turnover, metabolic micromilieu, and pH distributions in human breast cancer xenografts in nude rats. Cancer Res. 1988 Dec 15;48(24 Pt 1):7264–7272. [PubMed] [Google Scholar]
- Kornberg A. DNA replication. J Biol Chem. 1988 Jan 5;263(1):1–4. [PubMed] [Google Scholar]
- Koschinsky T., Bünting C. E., Rütter R., Gries F. A. Increased growth stimulation of human vascular cells by serum from patients with primary hyper-LDL-cholesterolemia. Atherosclerosis. 1987 Jan;63(1):7–13. doi: 10.1016/0021-9150(87)90076-1. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Hino A., Yasumasu I., Kato J. Stimulation of protein synthesis in round spermatids from rat testes by lactate. J Biochem. 1981 Apr;89(4):1309–1315. [PubMed] [Google Scholar]
- Nakaya K., Kumakawa N., Iinuma H., Nakamura Y. Purification of a low molecular weight factor that induces differentiation and inhibits growth in myeloid leukemia cells. Cancer Res. 1988 Aug 1;48(15):4201–4205. [PubMed] [Google Scholar]
- Namiki H., Ogata H., Koga N., Kusunoki S. A growth-promoting factor existing on the body surface of rats. Life Sci. 1986 Jan 6;38(1):27–31. doi: 10.1016/0024-3205(86)90271-7. [DOI] [PubMed] [Google Scholar]
- Pitlick F. A. This is FASEB. American Association of Pathologists. FASEB J. 1990 Jan;4(1):121–124. doi: 10.1096/fasebj.4.1.2403950. [DOI] [PubMed] [Google Scholar]
- Rognstad R., Clark D. G. Effects of aminooxyacetate on the metabolism of isolated liver cells. Arch Biochem Biophys. 1974 Apr 2;161(2):638–646. doi: 10.1016/0003-9861(74)90348-8. [DOI] [PubMed] [Google Scholar]
- Sens D. A., Hochstadt B., Amos H. Effects of pyruvate on the growth of normal and transformed hamster embryo fibroblasts. J Cell Physiol. 1982 Mar;110(3):329–335. doi: 10.1002/jcp.1041100319. [DOI] [PubMed] [Google Scholar]
- Swendeman S., Thorley-Lawson D. A. The activation antigen BLAST-2, when shed, is an autocrine BCGF for normal and transformed B cells. EMBO J. 1987 Jun;6(6):1637–1642. doi: 10.1002/j.1460-2075.1987.tb02412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J. E., Goldman N. D., Tosato G. Biochemical and biological analysis of human interleukin 6 expressed in rodent and primate cells. Cytokine. 1990 Sep;2(5):363–374. doi: 10.1016/1043-4666(90)90067-4. [DOI] [PubMed] [Google Scholar]
- Taylor C. M., Weiss J. B., McLaughlin B., Kissun R. D., Garner A. Increased procollagenase activating angiogenic factor in the vitreous humour of oxygen treated kittens. Br J Ophthalmol. 1988 Jan;72(1):2–4. doi: 10.1136/bjo.72.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosato G., Marti G. E., Yarchoan R., Heilman C. A., Wang F., Pike S. E., Korsmeyer S. J., Siminovitch K. Epstein-Barr virus immortalization of normal cells of B cell lineage with nonproductive, rearranged immunoglobulin genes. J Immunol. 1986 Sep 15;137(6):2037–2042. [PubMed] [Google Scholar]
- Tosato G., Seamon K. B., Goldman N. D., Sehgal P. B., May L. T., Washington G. C., Jones K. D., Pike S. E. Monocyte-derived human B-cell growth factor identified as interferon-beta 2 (BSF-2, IL-6). Science. 1988 Jan 29;239(4839):502–504. doi: 10.1126/science.2829354. [DOI] [PubMed] [Google Scholar]
- Tosato G., Tanner J., Jones K. D., Revel M., Pike S. E. Identification of interleukin-6 as an autocrine growth factor for Epstein-Barr virus-immortalized B cells. J Virol. 1990 Jun;64(6):3033–3041. doi: 10.1128/jvi.64.6.3033-3041.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P. W., Frinak S., Bicher H. I. Heterogeneous oxygen partial pressure and pH distribution in C3H mouse mammary adenocarcinoma. Cancer Res. 1981 May;41(5):2008–2013. [PubMed] [Google Scholar]
- Wakasugi H., Rimsky L., Mahe Y., Kamel A. M., Fradelizi D., Tursz T., Bertoglio J. Epstein-Barr virus-containing B-cell line produces an interleukin 1 that it uses as a growth factor. Proc Natl Acad Sci U S A. 1987 Feb;84(3):804–808. doi: 10.1073/pnas.84.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz W., Mukerji S. Lactate production and release in cultured astrocytes. Neurosci Lett. 1988 Apr 12;86(3):296–300. doi: 10.1016/0304-3940(88)90499-5. [DOI] [PubMed] [Google Scholar]


