Abstract
Background:
Juniperus communis Linn. is an important plant in India traditional system of medicine which is widely used by different tribes in many countries.
Objective:
In the present study, the antioxidant, cytotoxic and hepatoprotective activities of Juniperus communis leaves were investigated against various models.
Materials and Methods:
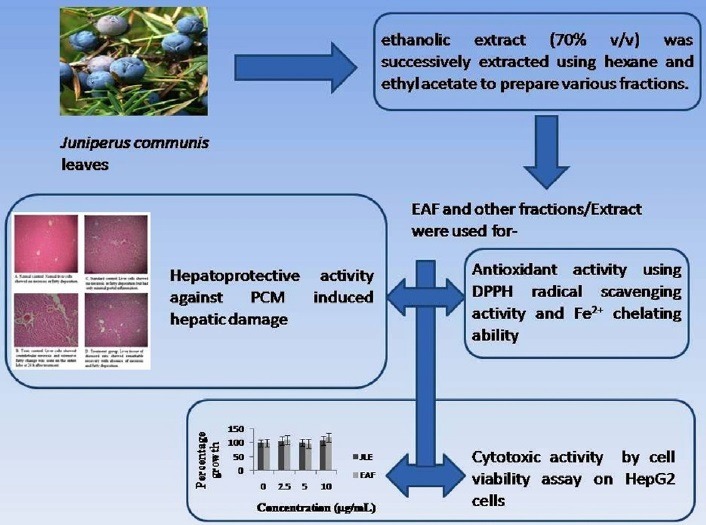
ethanolic extract (70% v/v) of J. communis leaves was successively extracted using hexane and ethyl acetate to prepare various fractions. Total phenol content was resolute by the Folin-Ciocalteau's process. The antioxidant properties of the different fractions/extract of leaves of J. communis were examined by 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity and Fe2+ chelating ability. Cytotoxic activity was examined by cell viability assay on HepG2 cells. Hepatoprotective activity of ethyl acetate fraction (EAF) evaluated against PCM-Paracetamol-induced hepatic damage in Wistar albino rats.
Results:
Total phenol content was found maximum 315.33 mg/GAE/g in EAF. Significant scavenging activity were found for EAF (IC50 = 177 μg/ml) as compared to standard BHT (IC50 = 138 μg/ml), while EAF showed good Fe2+ chelating ability having an IC50 value of 261 mg/ML compared to standard ethylenediaminetetraacetic acid (7.7 mg/mL). It was found that EAF treated group shows remarkable decrease in serum Aspartate aminotransferase, serum Alanine aminotransferase, total bilirubin, direct bilirubin, and alkaline phosphatase level in treatment group as compared to the hepatotoxic group.
Conclusion:
EAF of J. communis leaves is found to be potent antioxidant and hepatoprotective without any cytotoxicity and it can also be included in nutraceuticals with notable benefits for mankind or animal health.
SUMMARY
Phenol-rich fraction (PRF) and other fractions/extract of Juniperus communis leaves were screened for antioxidant, cytotoxic, and hepatoprotective activity.
Significant antioxidant and hepatoprotective activity without any cytotoxicity were found while treating with ethyl acetate fraction (EAF).
Abbreviations used: HepG2: Liver hepatocellular carcinoma, BHT: Butylated hydroxytoluene, PCM: Paracetamol, IC50: Half maximal inhibitory concentration, RSA: Radical Scavenging Activity, WST: Water-soluble tetrazolium.
Keywords: Juniperus communis, Acetaminophen, Antioxidant, Cytotoxic, Hepatoprotective
INTRODUCTION
Juniperus is one of the most important genus belongs to the Cuppressaceae family.[1] The Juniperus communis Linn. is an evergreen coniferous dioeciously shrub widely distributed throughout the Artic and temperate region of Northern hemisphere.[2,3,4] Throughout India, it is widely distributed across the Himalayas from Kumaun region at an altitude of 1700-4200 meters.[5] The important phytoconstituents from Juniperus communis leaves contain a pair of atropisomer, (M)- and (P)-cupressuflavone 4-O-β-d-glucoside and its oil contain monoterpene hydrocarbons such as α-pinene (51.4%), β-pinene (5.0%), sabinene (5.8%), myrcene (8.3%), limonene, imbricatolic acid, junicedral, and trans-communic acid.[6,7] It also contains diterpene compounds such as isocupressic acid and aryltetralin lignin deoxypodophyllotoxin.[8,9,10] J. communis is useful as folk medicine such as appetizer, flavoring agent, abortifacient, antiseptic, contraceptive, diuretic, as a remedy for urinary tract infections, scrofula, chest complaints, diabetes, rheumatism, backache, chest troubles, and tuberculosis.[11,12,13,14,15,16,17,18,19,20] The whole plant of J. communis is scientifically proven to have anti-inflammatory, anti-pyretic, analgesic, antidiabetic, antihyperlipidemic, antioxidant, anticataleptic, and antimicrobial activities.[21,22,23,24,25,26]
The hepatotoxicity can be produced by alcohol, chemicals, and xenobiotics. At healing doses, acetaminophen (PCM) is considered to be nontoxic for liver. Although, when given at overdose, it is the foremost cause of hepatocytes, nephrons, and other cells damage in both humans and animals.[27] It is rapidly metabolized in the liver by conjugation with glucuronic acid (40-67%) and sulfates (20-46%) and also metabolized by cytochrome P450 isoenzymes to the extremely toxic substance N-acetyl-p-benzoquinoneimine (NAPQI).[28,29,30] PCM-induced toxicity in animal is one of the most commonly experimental model to evaluate the hepatoprotective activity. However, there is no single report yet demonstrated on J. communis leaves for potent hepatoprotective potential against PCM induced liver toxicity. Moreover, J. communis was demonstrated for anti-oxidant potential against different animal models. Consequently, combining both the individual action for counteracting the liver pathogenesis would be novel challenge in liver diseases.[31] Therefore, the objective of this study was to investigate hepatoprotective activity of phenol-rich fraction (PRF) of J. communis leaves using PCM-induced liver damage in Wistar albino rats as well as assay of antioxidant activity of the different fractions/extract of J. communis leaves by two in-vitro methods such as 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical and ferrous ion-chelating tests.
MATERIALS AND METHODS
Plant material: collection and authentication
The plant material J. communis leaves were collected from Botany Department of Kumaun University, Nainital, India, and authenticated by pharmacognostic, phytochemical, and other studies, whereas voucher sample was deposited for future reference.
Extraction: preparation of PRF
The weighted quantity 100 g of powdered leaves of J. communis was soaked in 70% ethanol (1:5 w/v) at 25°C. After 24 h, the supernatants were decanted and the residues were re-soaked in respective fresh solvent. This procedure was repeated three times for absolute extraction. Supernatants were then collected separately, followed by filtration and centrifugation at 5000 rpm for 10 min at 4°C. Solution was then lyophilized and the dried extracts were preserved in hermetically sealed dark bottles at 4°C. Five grams of crude extract was dissolved in 100 mL water and successively extracted thrice using 100 mL hexane to obtain hexane fraction (HF) and then with 100 mL ethyl acetate to obtain ethyl acetate fraction (EAF).
Determination of total phenol content
In brief, 150 µL of extract/fractions of leaves of J. communis, 2400 µL of triple distilled water and 150 µL of 0.25 N Folin-Ciocalteau's reagent were combined and mixed well. The fusion was allowed to react for 3 min, and then 300 µL of 1 N Na2CO3 solution was added and mixed well. The solution was incubated at 25°C in the dark for 2 h. The absorbance was determined at 725 nm using a double beam spectrophotometer and the results were expressed in milligrams of gallic acid equivalents (GAE) per gram of extract/fraction.[32]
Antioxidant activity
Antioxidant activity by radical-formation method: DPPH free radical scavenging activity
DPPH free radical scavenging method is an antioxidant assay based on electron-transfer that produces a violet solution in ethanol.[33,34] Five different concentrations (100-500 μg/mL) of ethanolic extract (JLE), aqueous extract (WF), and EAF of J. communis leaves were used for DPPH assay. The samples were reacted with the stable DPPH radical in an ethanolic solution. The reaction mixture consisted of 0.5 mL of sample, 3 mL of absolute ethanol and 0.3 mL of DPPH radical solution (0.5 mM in ethanol). When DPPH reacts with an antioxidant compound, which can donate hydrogen, it is reduced. The changes in color (from deep violet to light yellow) were read (absorbance) at 517 nm after 100 min of reaction using a UV-VIS spectrophotometer. The mixture of ethanol (3.3 mL) and sample (0.5 mL) served as blank. The control solution was prepared by mixing ethanol (3.5 mL) and DPPH radical solution (0.3 mL). The radical scavenging activity (RSA) was calculated as a percentage of DPPH discoloration, using the following equation:
% RSA = (ADPPH − AS)/ADPPH] × 100, where AS is the absorbance of the solution when the sample extract is added at a particular level and ADPPH is the absorbance of the DPPH solution.[35] The results were obtained from the average of three independent experiments and are expressed as mean % RSA ± SD and as mean IC50 value.
Antioxidant activity by iron-related methods: ferrous ion-chelating effect
Different concentrations of each extract/fraction (100-500 μg/mL) of J. communis leaves in 1 mL solvent were mixed with 0.5 mL of distilled water and 0.05 mL of 2 mM FeCl2. The reaction was initiated by the addition of 0.1 mL of 5 mM ferrozine. Then the mixture was shaken vigorously and left standing at room temperature for 10 min. The absorbance of the solution was measured spectrophotometrically at 562 nm. The control contains FeCl2 and ferrozine, complex formation molecules. Ethylenediaminetetraacetic acid (EDTA) was used as reference standard. The results were obtained from the average of three independent experiments and are expressed as mean percentage (%) of inhibition of the ferrozine-(Fe2+) complex formation ± SD and as mean IC50.[36]
Cytotoxic activity: cell viability assay on HepG2 cells
The effect of extracts on HepG2 cell viability was evaluated using a WST-1 Cell Proliferation Kit (Roche Applied Science, Mannheim, Germany). HepG2 human hepatocellular liver carcinoma cells were routinely cultured as monolayers in RPMI-Roswell Park Memorial Institute medium supplemented with 20% fetal bovine serum. Cells were seeded in 96-well culture plates and allowed to adhere to the plate surface for 36 h before being exposed to various concentrations (0-10 μg/mL) of extract/fractions of leaves of J. communis for 24 h. The extracts/fractions were diluted in complete media to reach the final concentrations, and 0.01% of methanol (final concentration) was used as control. WST-1 (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate) reagent was then added to each well and incubated for 40 min in a humidified atmosphere (37°C, 5% CO2). Formazan dye produced by metabolically active cells was measured at 450 nm by microplate ELISA reader. The results were obtained from the average of three independent experiments and data were expressed as mean cell growth (%) ± SD. Statistical comparison of the results was carried out by using one-way analysis of variance (ANOVA) followed by Dunnett's post hoc test.
Biological activity
Animals
Healthy adult male Wistar albino rats (180-220 g) were obtained from NBRI (CSIR), Lucknow, India. Animals will be selected from an inbred colony maintained under the controlled condition of temperature (23 ± 2°C), humidity (50 ± 5%), and light (12 and 12 h of light and dark, respectively). The animals will have free access to sterile food and water. All animal experiments were carried out after the approval by the institutional ethical committee for research on animals and performed as per approved protocols (CPCSEA Reg. No. 222/2000).
Acute toxicity study
The acute toxicity of EAF of J. communis was determined using Wistar albino rats (180-220 g), maintained under standard husbandry conditions. Acute toxicity was calculated as per OECD-Organization for Economic Co-operation and Development guidelines No. 420 by fixed dose method. After administration of dose up to 2 g/kg body weight of EAF of J. communis leaves, the mortality with each dose was noted after 14 days.
Hepatoprotective activity
A total of 42 Wistar albino rats were divided into seven groups of six rats each. The first group received saline 0.5 mL/kg per oral for 1 week (Normal control). The group II received 2 g/kg body weight PCM orally for 1 week (Disease control). The groups III received 100 mg/kg body weight per oral Silymarin (Reference control) for 7 days. Group IV, V, VI, and VII received the different concentrations (50-200 mg/kg body weight orally) of EAF of leaves of J. communis. On the fifth day, after the administration of the respective treatments, all the animals of test groups were administered with PCM 2 g/kg by oral route. On the seventh day after 2 h of respective treatments, the blood samples were collected for the estimation of biochemical marker enzymes by reported methods to assess liver functions.
Experimental design
Group I Normal control: Normal saline, 0.5 mL/kg body weight per oral for 7 days.
Group II Disease control: PCM, 2 g/kg body weight per oral for 7 days.
Group III Reference control: Silymarin, 100 mg/kg body weight per oral for 7 days.
Group IV EAF, 50 mg/kg body weight per oral for 7 days.
Group V EAF, 100 mg/kg body weight per oral for 7 days.
Group VI EAF, 150 mg/kg body weight per oral for 7 days.
Group VII EAF, 200 mg/kg body weight per oral for 7 days.
Biochemical estimation
Aspartate aminotransferase (AST)
An aliquot of 1 mL of substrate (2 mM α-ketoglutarate and 0.2 M D, L-aspartate) was incubated with 0.2 mL of serum sample for 1 h at 40°C. Then the reaction was clogged by addition of 1 mL of dinitrophenyl hydrazine (1 mM). After 20 min, 10 mL of 0.4 N NaOH was added. The absorbance of the solution was measured at 505 nm after 30 min and distilled water kept as a blank.[37]
Alanine aminotransferase (ALT)
An aliquot of 1 mL of substrate and 0.2 M L-alanine (for AST) was incubated with 0.2 mL of serum sample for 1 h at 40°C. Then the reaction was stopped up by addition of 1 mL of dinitrophenyl hydrazine (1 mM). Then after 20 min, 10 mL of 0.4 N NaOH was added. The absorbance of the solution was considered at 505 nm after 30 min and distilled water kept as a blank.
Alkaline phosphatase (ALP)
A single vial Monotest Alkaline Phosphatase (AMP buffer) Kit was used to measure the total alkaline phosphatase activity in serum.
Bilirubin (total and direct)
0.4 mL of serum diluted with 3.6 mL of distilled water in two separate tubes, one treated with 0.5% HCL (blank) and other with diazo reagent (0.5% sodium nitrite and 0.1% sulfanilic acid) and incubated for 30 min at room temperature. After incubation absorbance at 540 nm was calculated against blank.[38]
Histopathological studies
The animals were sacrificed and abdomen was cut open to remove the liver. The liver was fixed in Boucin's solution (mixture of 75 mL of saturated picric acid, 25 mL of 40% formaldehyde and 5 mL of glacial acetic acid) for 12 h, and then embedded in paraffin using conventional methods and cut into 5 μm thick sections and stained using haematoxylin-eosin dye and finally mounted in di-phenyl xylene. The sections were then observed under microscope for histopathological changes in liver architecture and their photomicrographs were taken and shown in Figure 2A–D.
Figure 2.
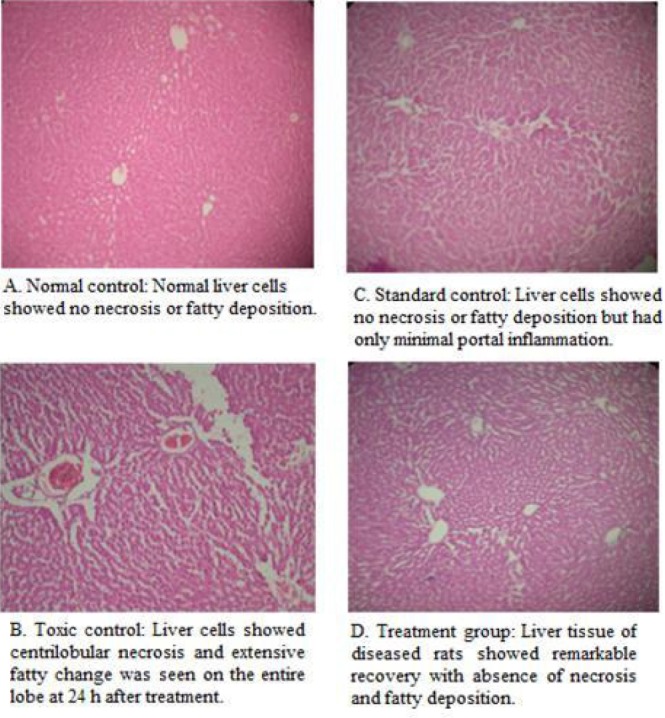
Photomicrograph of rat liver of Normal control, Toxic control, Standard control and treated with EAF, 200 mg/kg body weight.
Statistical analysis
The mean values ± SD were calculated for each parameter. For determining the significant inter-group difference, each parameter was analyzed separately, and ANOVA was carried out. Then the individual comparisons of the group mean values were done using Dunnett's test procedure. All the analysis was carried out using Graph pad Prism software.
RESULTS AND DISCUSSION
Phenol-rich fraction
Extractive value of 70% ethanolic extract of J. communis leaves was found 21.76%. From this crude extract, PRF was prepared by sequential extraction using hexane and ethyl acetate. Total phenol content of ethanol extract, hexane fraction, EAF, and aqueous fraction were found to be 238.78, 189.65, 315.33, and 205.33 mg/GAE/g extract/fraction, respectively. It was found that EAF contained the maximum amount of phenolic compounds. Therefore, EAF is considered as PRF and it was used for cytotoxic, hepatoprotective, and antioxidant studies.
Acute toxicity
Over the study duration of 14 days, no mortality was seen up to dose of 2 g/kg body weight of the EAF of leaves of J. communis orally. During the observation time animals did not produce any changes in the general appearance.
Antioxidant activity
There are numerous methods for assessment of antioxidant activity of plant extract/fraction, but every method has its individual limitations. Antioxidant activity of extract/fraction cannot be practically validated by a particular method due to the composite nature of phytochemicals and their interactions, thus it becomes essential to use multi assay system with diverse indices.[39] In view of that, DPPH radical scavenging and ferrous ion-chelating assays were performed to verify in vitro antioxidant activity of the extract/fractions of J. communis leaves.
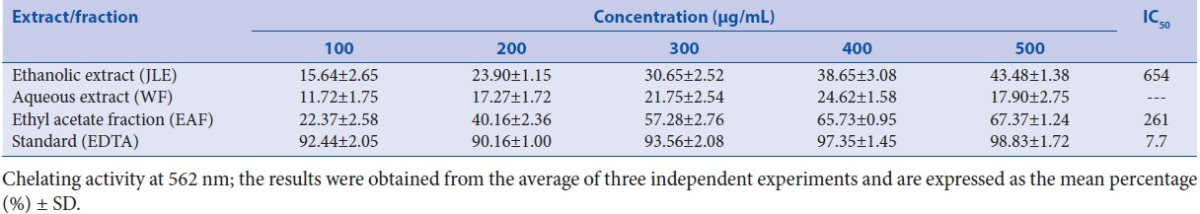
The antioxidant property of the JLE, EAF, HF, and WF of J. communis leaves were studied for DPPH RSA according to the method described and the outcomes of the test are revealed in Table 1. Among all of the fractions/extract evaluated, significant RSA was found to be for EAF (IC50 = 177 μg/mL) as compared to standard BHT (IC50 = 138 μg/mL). Elevated iron levels may perform catalytically to generate reactive oxygen species, with a harmful impact on the structure and function of the cells. Iron can excite lipid peroxidation using the Fenton reaction and hasten the peroxidation of the lipid breakdown pathway of hydroperoxides into peroxyl and alkoxyl radicals that can abstract hydrogen and propagate the chain reaction of lipid peroxidation. Thus, metal chelating activity specifies antioxidant property. The reduced absorbance in the reaction mixture point toward higher metal chelating ability.[40] Different fractions/extract such as JLE, WF, and EAF showed Fe2+ chelating ability; based on the IC50 values, the activity was higher for EAF (261 mg/mL) and JLE (654 mg/mL), although fractions/extract exerted lower chelating effects on ferrous ions than those of the standard EDTA (7.7 mg/mL) as shown in Table 2.
Table 1.
Effect of fractions/extract of J. communis leaves using DPPH scavenging assay
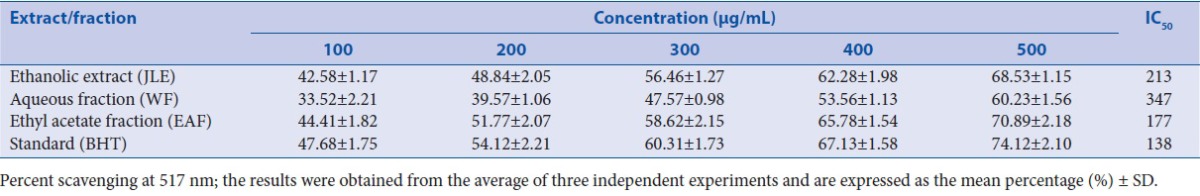
Table 2.
Effect of fractions/extract of J. communis leaves using ferrous ion-chelating assay
Cytotoxic activity: cell viability assay on HepG2 cells
A growing body of literature data based on cell models suggests that Juniperus genus could represent a source of bioactive compounds with potential anticancer activity.[41,42,43,44,45,46,47] In vitro studies of various Juniperus species have been found to exert cytotoxic effects against human cancer cell lines from different origins (liver, HepG2; breast, MCF-7/AZ; cervix, HeLa; stomach, HGC-27). However, the cytotoxicity of J. communis leaves extract against human tumor cell lines has not been investigated till date. We aimed to test the effect of JLE and EAF on cancer cell viability by using a human hepatocellular liver carcinoma (HepG2) cell line. Results obtained from the WST-1 proliferation assay clearly show that EAF did not affect HepG2 cell viability after treatment for 24 h at all concentration tested (0-10 μg/mL) as shown in Figure 1.
Figure 1.
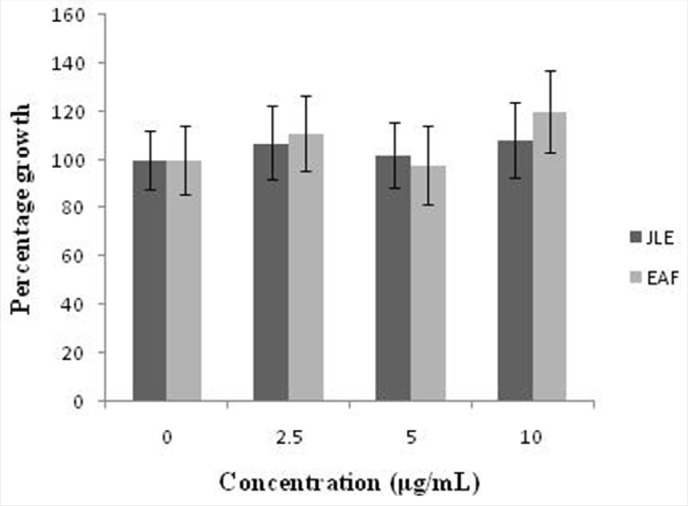
Effect of ethanol extract (JLE) and ethyl acetate fraction (EAF) on the cell proliferation of HepG2 cells, as measured by the WST-1 assay. The results were obtained from the average of three independent experiments and are expressed as the mean cell growth (%) ± SD.
Hepatoprotective activity: biochemical markers
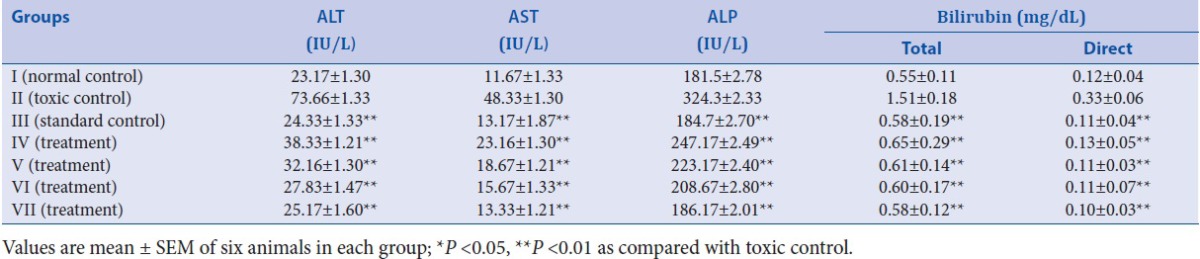
To assess liver injury, biochemical markers (ALT, AST, ALP, TB, and DB) levels are measured. In this study, the hepatotoxicity due to treatment of animals with PCM (disease control) was confirmed by increased levels of ALT (73.66 ± 1.33 IU/L), AST (48.33 ± 1.30 IU/L), and ALP (324.3 ± 2.33 IU/L), as compared to normal control group ALT (23.17 ± 1.30 IU/L), AST (11.67 ± 1.33 IU/L), and ALP (181.5 ± 2.78 IU/L). In hepatotoxicity, the transport function of liver cells is compromised, causing leakage of plasma membrane, therefore resulting in leakage of these enzymes leading to an elevation of their serum level. The levels of ALT (24.33 ± 1.33 IU/L), AST (13.17 ± 1.87 IU/L), and ALP (184.7 ± 2.70 IU/L) significantly (**P < 0.01) reduced on treatment with the standard drug Silymarin in reference control group. The EAF at the dose of 50, 100, 150, and 200 mg/kg body weight oral were significantly (**P < 0.01) reduce the increased levels of ALT, AST, and ALP in PCM-induced liver damage in rats. The EAF showed maximum hepatoprotective potential (ALT = 25.17 ± 1.60 IU/L; AST = 13.33 ± 1.21 IU/L; and ALP = 186.17 ± 2.01 IU/L) at 200 mg/kg body weight and is comparable to the standard drug Silymarin. Similarly, in normal control group of animals, levels of total bilirubin (TB) and direct bilirubin (DB) were found to be normal (0.55 ± 0.11 and 0.12 ± 0.04 mg/dL, respectively), but when treated with PCM, the level of TB and DB were increased (1.51 ± 0.18 and 0.33 ± 0.06 mg/dL, respectively) which may be due to excessive heme destruction and block of bile duct within the liver leading to mass inhibition of conjugation reaction and release of unconjugated bilirubin from damaged liver cells. After treatment with reference drug Silymarin in standard group animals, the levels of TB and DB were significantly (**P < 0.01) decreased (0.58 ± 0.19 and 0.11 ± 0.04 mg/dL, respectively). The EAF showed maximum potency in decreasing the levels of TB and DB at 200 mg/kg body weight, which was found to be 0.58 ± 0.12 and 0.10 ± 0.03 mg/dL, respectively. Hepatoprotective effect of EAF obtained from ethanol extract of leaves of J. communis at the dose of 200 mg/kg body weight was found to be comparable in effect with Silymarin for reduction in all biochemical parameters (ALT, AST, ALP, TB, and DB) as shown in Table 3.
Table 3.
Effect of EAF of J. communis leaves on levels of ALT, AST, ALP, TB, and DB in acetaminophen induced hepatotoxicity in rats
Histopathological studies
Photomicrographs of liver section of Wistar rats of normal group, disease control (PCM-induced hepatic damage), Silymarin treated (reference control) and EAF treated (200 mg/kg body weight) are shown in Figure 2A–D. Histopathology of liver sections revealed that the normal liver architecture was disturbed by PCM. The liver sections of the rat treated with the 200 mg/kg body weight of EAF showed the normal cellular architecture (arrangement of portal triads and central veins) and comparable with the standard Silymarin group, hence confirming the significant hepatoprotective effect of PRF of J. communis leaves.
CONCLUSION
The present study has demonstrated that the phenol-rich ethyl acetate fraction of the ethanolic extract of the leaves of J. communis has shown the potent hepatoprotective activity against PCM-induced hepatotoxicity in rats without any cytotoxicity. The hepatoprotective potential may be due to their anti-oxidant potential against reactive oxygen and nitrogen species, which prevent lipid peroxidation that ultimately results in prevention of necrosis or apoptosis of the liver cells. The results suggested a high potential of application for EAF of J. communis leaves as an antioxidant and hepatoprotective agent. It can also be included in nutraceuticals with notable benefits for mankind or animal health.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
None.
REFERENCES
- 1.Moein MR, Ghasemi Y, Moein S, Nejati M. Analysis of antimicrobial, antifungal and antioxidant activities of Juniperus excelsa M. B subsp. Polycarpos (K. Koch) Takhtajan essential oil. Pharmacognosy Res. 2010;2:128–31. doi: 10.4103/0974-8490.65505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen D, Hatfield G. An Ethnobotany of Britain and Ireland. Cambridge: Timber Press; 2004. Medicinal Plants in Folk Tradition. [Google Scholar]
- 3.Nakanishi T, Iida N, Inatomi Y, Murata H, Inada A, Murata J, et al. Neolignan and flavonoid glycosides in Juniperus communis var. depressa. Phytochemistry. 2004;65:207–13. doi: 10.1016/j.phytochem.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Seca ML, Silva AMS. The chemical composition of the Juniperus genus (1970-2004), recent progress in medicinal plants. Phytomedicines. 2005;16:402–522. [Google Scholar]
- 5.Khare P. Indian Medicinal Plants: An Illustrated Dictionary. Springer References. 2007:348–49. [Google Scholar]
- 6.Clifton SJ, Ward LK, Ranner DS. The status of Juniper communis L. in north east England. Biol Conserv. 1997;79:67–77. [Google Scholar]
- 7.Kailash C, Chaudhari BG, Dhar BP, Joseph GVR, Mangal AK, Dabur R, et al. Database on Medicinal Plants Used in Ayurved. Centre Council for Research in Ayurveda and Siddha. 2007:8. [Google Scholar]
- 8.Bais SN, Gill SN, Rana Shandil S. A Phytopharmacological Review on a Medicinal Plant: Juniperus communis. International Scholarly Research Notices. 2014 doi: 10.1155/2014/634723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonny M, Cavaleiro C, Salgueiro L, Casanova J. Analysis of Juniperus communis subsp. alpina needle, berry, wood and root oils by combination of GC, GC/MS and 13C-NMR. Flavour Fragrance J. 2006;21:99–106. [Google Scholar]
- 10.Innocenti M, Michelozzi M, Giaccherini C, Leri F, Vincieri FF, Mulinacci N. Flavonoids and biflavonoids in Tuscan berries of Juniperus communis L.: Detection and quantitation by HPLC-DAD-ESI-MS. J Agric Food Chem. 2007;55:6596–602. doi: 10.1021/jf070257h. [DOI] [PubMed] [Google Scholar]
- 11.Darwin T. The Scots Herbal-The Plant Lore of Scotland. Edinburgh: Mercat Press; 2000. [Google Scholar]
- 12.Foster S. Tyler's Honest Herbal: A Sensible Guide to the Use of Herbs and Related Remedy. New York: The Haworth Herbal Press; 1999. [Google Scholar]
- 13.Tilford GL. Edible and Medicinal Plants of the West. Missoula: Mountain Press Publishing Company; 1997. [Google Scholar]
- 14.Stanic G, Samarzija I, Blazevic N. Time-dependent diuretic response in rats treated with Juniper berry preparations. Phytotherapy Res. 1998;12:494–97. [Google Scholar]
- 15.Ritch KEM, Thomas S, Turner NJ, Towers GHN. Carrier herbal medicine: Traditional and contemporary plant use. J. Ethnopharmacol. 1996;52:85–94. doi: 10.1016/0378-8741(96)01392-x. [DOI] [PubMed] [Google Scholar]
- 16.Fujita E, Sezik M. Tabata Traditional medicine in Turkey Folk medicine in middle and west black sea regions. Econ Bot. 1995;7:406–22. [Google Scholar]
- 17.Barjaktarovic B, Sovilj M, Knez Z. Chemical composition of Juniperus communis L. fruits supercritical CO2 extracts dependence on pressure and extraction time. J Agric Food Chem. 2005;53:2630–36. doi: 10.1021/jf048244g. [DOI] [PubMed] [Google Scholar]
- 18.Seca AML, Silva AMS. The chemical composition of the Juniperus genus 1970-200. Recent Progress Med Plants Source. 2007;16:401–522. [Google Scholar]
- 19.Kirtikar KR, Basu BD. Indian Medicinal Plants. In: Kirtikar KR, Basu BD, editors. 2nd ed. Vol. 3. Dehra Dun, India: International book distributors; 1987. pp. 2061–2062. [Google Scholar]
- 20.Cutcheon AR, Stokes RW, Thorson LM. Antimycobacterial screening of British Columbian medicinal plants. Intl J Pharmacognosy. 1997;35:77–83. [Google Scholar]
- 21.Chatterjee TK, Ghosh C. Antibacterial efficacy of Juniperus communis Linn. leaf extract in-vitro. Indian J Microbiol. 1993;33:273–75. [Google Scholar]
- 22.Chatterjee TK, Ghosh C, Raychaudhuri P. Anti-inflammatory and anti-pyretic action of Juniperus communis Linn. leaf extract in rats. Indian Drugs. 1991;28:430. [Google Scholar]
- 23.Banerjee S, Mukherjee A, Chatterjee TK. Evaluation of Analgesic activities of methanolic extract of medicinal plant Juniperus communis Linn. Int J Pharm Pharm Sci. 2012;4:547–50. [Google Scholar]
- 24.Banerjee S, Singh H, Chaterjee TK. Evaluation of anti-diabetic and anti-hyperlipidemic potential of methanolic extracts of Juniperus communis Linn. Intl J Pharma Biosci. 2013;4:10–17. [Google Scholar]
- 25.Akdogan M, Koyu A, Ciris M, Yildiz K. Anti-hypercholesterolemic activity of J. communis Oil in rats: A biochemical and histopathological investigation. Biomed Res. 2012;23:321–28. [Google Scholar]
- 26.Nikam S, Nikam P, Ahaley SK, Sontakke AV. Oxidative stress in Parkinson's disease. Indian J Clin Biochem. 2009;24:98–101. doi: 10.1007/s12291-009-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace JL. Acetaminophen hepatotoxicity: NO to the rescue. Br J Pharmacol. 2004;143:1–2. doi: 10.1038/sj.bjp.0705781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hung OL, Nelson L. Tintinalli's Emergency Medicine: A Comprehensive Study Guide. New York: McGraw-Hill; 2004. [Google Scholar]
- 29.Jollow DJ, Thorgeirsson SS, Potter WZ. Acetaminophen- induced hepatic necrosis VI. Metabolic disposition of toxic and nontoxic doses of acetaminophen. Pharmacology. 1974;12:251–71. doi: 10.1159/000136547. [DOI] [PubMed] [Google Scholar]
- 30.Hinson JA, Reid AB, McCullough SS, James LP. Acetaminophen-induced hepatotoxicity: Role of metabolic activation, reactive oxygen/nitrogen species, and mitochondrial permeability transition. Drug Metab Rev. 2004;36:805–22. doi: 10.1081/dmr-200033494. [DOI] [PubMed] [Google Scholar]
- 31.Jayadevaiah KV, Bhat KI, Joshi AB. Hepatoprotective activity of Desmodium oojeinense (Roxb.) H. Ohashi against paracetamol induced toxicity. Asian J Pharm Health Sci. 2012;2:312–15. [Google Scholar]
- 32.Singleton VL, Rossi J. Colorimetry of total phenolics with phosphomolibdic-phosphotungtic acid reagents. Am J Enol Viticult. 1965;16:144–58. [Google Scholar]
- 33.Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assay. J Agric Food Res. 2005;53:1841–856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 34.Brand WW, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebenson Wiss Technol. 1995;28:25–30. [Google Scholar]
- 35.Oktay M, Gulcin I, Kufrevioglu OI. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extract. Lebensm Wiss Technol. 2003;36:263–71. [Google Scholar]
- 36.Miceli N, Trovato A, Marino A. Phenolic composition and biological activities of Juniperus drupacea Labillberries from Turkey. Food Chem Toxicol. 2011;49:2600–08. doi: 10.1016/j.fct.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 38.Raghuramlu N, Madhavanm NK, Kalayanasundaram S. A Manual of Laboratory Techniques. HyderabadNational Institute of Nutrition. 1983:32–33. [Google Scholar]
- 39.Perez JJ, Arranz S, Tabernero M. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res Int. 2008;41:274–85. [Google Scholar]
- 40.Gulcin Elias R, Gepdiremen A. Antioxidant activity of bisbenzylisoquinoline alkaloids from Stephania rotunda: cepharanthine and fangchinoline. J Enzyme Inhib Med Chem. 2010;25:44–53. doi: 10.3109/14756360902932792. [DOI] [PubMed] [Google Scholar]
- 41.Shokrzadeh M, Azadbakht M, Ahangar N. Comparison of the cytotoxic effects of Juniperus sabina and Zataria multiflora extracts with Taxus baccata extract and Cisplatin on normal and cancer cell lines. Pharmacogn Mag. 2010;6:102–5. doi: 10.4103/0973-1296.62894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moujir L, Seca AM, Silva AM, Barreto MC. Cytotoxic activity of diterpenes and extracts of Juniperus brevifolia. Planta Med. 2008;74:751–53. doi: 10.1055/s-2008-1074529. [DOI] [PubMed] [Google Scholar]
- 43.Muto N, Tomokuni T, Haramoto M. Isolation of apoptosis-and differentiation-inducing substances toward human promyelocytic leukemia HL-60 cells from leaves of Juniperus taxifolia. Biosci Biotechnol Biochem. 2008;72:477–84. doi: 10.1271/bbb.70570. [DOI] [PubMed] [Google Scholar]
- 44.Van SS, Daniels AL, Hooten CJ. Effect of crude aqueous medicinal plant extracts on growth and invasion of breast cancer cells. Oncol Rep. 2007;17:1487–92. [PubMed] [Google Scholar]
- 45.Bayazit V. Cytotoxic effects of some animal and vegetable extracts and some chemicals on liver and colon carcinoma and myosarcoma. Saudi Med J. 2004;25:156–63. [PubMed] [Google Scholar]
- 46.Wang WS, Li EW, Jia ZJ. Terpenes from Juniperus przewalskii and their antitumor activities. Pharmazie. 2002;57:343–45. [PubMed] [Google Scholar]
- 47.Ali AM, Mackeen MM, Intan IS. Antitumour-promoting and antitumour activities of the crude extract from the leaves of Juniperus chinensis. J Ethnopharmacol. 1996;153:165–69. doi: 10.1016/0378-8741(96)01434-1. [DOI] [PubMed] [Google Scholar]


