Abstract
Context:
Dendrobium candidum (D. candimum) widely is a functional drug. The curative effect of D. candidum on lupus nephritis has been studied in vivo.
Materials and Method:
The DBA/2 and B6D2F1 mice were used for this in vivo experiment. The 50% effective dose (ED50) was used to check the effective concentration for this study. Then the SCr, BUN, TC, TG, IL-6, IL-12, TNF-α, and IFN-γ levels were determined by kits. The output of urine protein was determined by means of Coomassie Brilliant Blue, and the auto-antibody dsDNA was determined with titer plate technology and indirect immunofluorescence. The NF-κB, IκB-α, TGF ‘β1, Fas, and FasL expressions were measured by RT-PCR and western blot assay. The component analysis of D. candidum was determined by nuclear magnetic resonance.
Results:
Based on the ED50 result at 329 mg/kg, 200 and 400 mg/kg doses were chosen for this study. SCr, BUN, TC and TG levels of 400 mg/kg D. candidum mice were lower than control mice, TP and ALB levels were higher than control mice. The control and 400 mg/kg treated mice tested positive for dsDNA at the end of sixth and tenth week after the experiment began. The glomerular number of 400 mg/kg treated mice was more than control group. Treatment with 400 mg/kg D. candidum reduced IL-6, IL-12, TNF-α and IFN-γcytokine levels as compared to control mice. D. candidum decreased NF-κb, TGF ‘β1, Fas, FasL and increased IκB-α expressions in kidney tissue. There were 11 compounds in dry D. candidum, these compounds might make the curative effects of lupus nephritis.
Conclusion:
D. candidum showed a potential curative effect on lupus nephritis. It could be used as a health medicine on lupus nephritis.
SUMMARY
D. candidum reduced the SCr, BUN, TC, TG serum levels and raised the TP, ALB levels compared to control group.
The glomerular number of D. candidum treated mice was more than control group.
D. candidum treated mice showed lower IL-6, IL-12, TNF-α and IFN-γ cytokine levels than control mice.
D. candidum decreased NF-κb, TGF-β1, Fas, FasL and increased IκB-α expressions in kidney tissue.
Abbreviations used: LN: Lupus nephritis, SLE: systemic lupus erythematosus, D. candidum: Dendrobium candidum; IL-6: interleukin-6, IL-12: interleukin-12, TNF-α: tumor necrosis factor alpha, IFN-γ: Interferon-gamma, SCr: serum creatinine, BUN: blood urea nitrogen, TC: total cholesterol, TG: triglyceride, TP: total protein, ALB: albumin.
Keywords: Cytokine, dendrobium candidum, inflammation, lupus nephritis, RT-PCR
INTRODUCTION
Lupus nephritis (LN) is systemic lupus erythematosus (SLE). It is a kind of immune complex nephritis caused by exhausting the kidney. Its clinical features include all features of glomerulonephritis.[1] The murine lupus nephritis model is an internationally recognized and widely applied animal model and the experimental conditions are easy to control. The renal pathological lesions appear after the induction of mice cells solutions. The lesion is similar to SLE occurring in human beings. The main features include lymphonodular hyperplasia, the formation of autoantibodies, and infection with glomerulonephritis.[2] Renal morphologic changes include the segmental or pervasive proliferation of glomerular, mesangial, membranous nephritis, and glomerular sclerosis, which is likely to happen in patients with more serious cases.[3]
Dendrobium is a plant of Orchidaeae genu (Dendrobium family). This category plant contains about 1,200 species, including Dendrobium candidum Wall. ex Lindl. [4] It is a traditional Chinese health drug herb, that is used raw or processed for health care products in China.[5] D. candidum contains water-soluble polysaccharides, phenanthrenes, and various amino acids.[6] These acids could be used to treat nephrotic patients as medicine supplements.[7]
B-cell and T-cell excessive activation could cause the SLE. This state showed the similar clinical manifestations like most SLE do. The GVHD (graft-versus-host disease) LN mice model is an international recognized experiment model for lupus nephritis examination. The kidneys of mice were injured and the mice would show the symptoms of SLE patients. The male DBA/2 mice and female B6D2F1 could successfully construct the lupus nephritis and used the model for the curative effects of drugs checking.[8] In the present study, the preventative effect on lupus nephritis of D. candidum was also determined by this model. The serum levels and inflammation-related cytokines levels were used to determine the curative effect of D. candidum on lupus nephritis mice. The glomerular situation tissues were checked by histology, and the mRNA and protein gene expressions in tissues were also determined for explaining the curative effect. At last, the chemical analysis (NMR assay) of D. candidum was used for the chemical composition identifying.
MATERIALS AND METHODS
Preparations of D. candidum
D. candidum was purchased at Shanghai Pharmacy Co., Ltd. (Shanghai, China). This sample was verified by Prof. Xingjia Ming (Chongqing Academy of Chinese MateriaMedica, Chongqing City, China) in June, 2013. The 500 g D. candidum was stored at -80°C and freeze-dried to produce a powder. A 20-fold volume of boiling water was added to the powdered sample and extracted twice by stirring overnight. The aqueous extract was evaporated and concentrated using a rotary evaporator (Eywla, N-1100, Tokyo, Japan).
Lupus nephritis experiment
Eight-week-old male DBA/2 mice (n=20) and female B6D2F1 (C57BL/6 J × DBA/2) hybrid mice(n=40) were purchased from the Experimental Animal Center of Third Military Medical University (Chongqing, China). They were maintained in a temperature-controlled facility (temperature 23 ± 1°C, relative humidity 50 ± 5%) with a 12h light/dark cycle. The mice had unlimited access to a standard mouse chow diet and water. The spleen, thymus, and lymphonodus of DBA/2 mice were isolated in a germ-free state, then were grinded and cut into pieces in normal saline. Cells were isolated using lymphocyte isolation medium. A single-cell suspension was made with D-Hanks solution. The ratio of spleen, thymus, and lymphonodus was 3:2:1. The cell suspension was blended with 2.5 × 108 cells/mL of living cells on the 0 day, the 3rd day, the 7th day, the 10th day, respectively to B6D2F1 mice in the control group and the D. candidum treated groups. Cell suspension (0.2 mL) was injected into the body of B6D2F1 (C57BL/6J × DBA/2) hybrid mice by means of tail vein injection, and then D-Hanks solution was injected into mice in the normal group. The control and normal groups B6D2F1 mice received no D. candidum treatment. After the single-cell suspension was injected on the first day, the D. candidum groups B6D2F1 mice received oral administration of 50, 100, 200 or 400 mg/kg D. candidum everyday for 2 weeks, and the urinary protein excretions were checked for ED50 and text concentration choosing. Then, the treatment group mice were treated with 200 and 400 mg/kg D. candidum for 12 weeks.[9] These experiments followed a protocol approved by the Animal Ethics Committee of Chongqing Medical University (SCXK (Yu) 2012-0001, Chongqing, China).
Urinary protein excretion test
Since the first injection of lymphocytes, mice of different groups were fed in metabolism cages every 2 weeks before the experiment. They drank and ate freely in cages. Their urine was collected between 8:00 am and 8:00 am the next. The output of urine protein during 24 h was determined by means of Coomassie Brilliant Blue.
Analysis of inflammation-related cytokines in serum by enzyme-linked immunosorbent assay (ELISA)
For the serum cytokine assay, blood from the inferior vena cava was collected in a tube and centrifuged at 3000 rpm, 4°C for 10 min. The serum was aspirated and assayed as described below. Concentrations of inflammatory-related cytokines IL-6, IL-12, TNF-α, and IFN-γ in serum were measured by ELISA according to the manufacturer's instructions (Biolegend, San Diego, CA, USA). Briefly, biotinylated antibody reagent was added to 96-well plates, then supernatants of homogenized serum were added and the plates were incubated at 37°C in CO2 for 2 h. After washing with PBS, streptavidin-horseradish peroxidase (HRP) solution was added and the plate was incubated for 30 min at room temperature. The absorbance was measured at 450 nm using a microplate reader (iMark; Bio-Rad, Hercules, CA, USA).[10]
Serum levels of SCr (serum creatinine), BUN (blood urea nitrogen), TC (total cholesterol), TG (triglyceride), TP (total protein) and ALB (albumin) determination
The blood was drawn from the eye orbit with a glass capillary syringe to prepare blood serum every 2 weeks since the injection of lymphocyte for the first time. The mouse blood was collected in a tube and centrifuged at 730 x g, 4°C for 10 min. Then the SCr, BUN, TC (total cholesterol), TG, TP and ALB levels of the serum were determined using commercially available kits (Unison Biotech Inc, Hsinchu, Taiwan).
Auto-antibody dsDNA assay
Blood was drawn from the eye orbit with a glass capillary syringe to prepare blood serum every two weeks since the first injection of lymphocytes. The auto-antibody dsDNA was determined with titer plate technology and indirect immunofluorescence, and was observed with a fluorescence microscope (BX50, Olympus, Tokyo, Japan).
Histology assay
The renal tissue specimens were fixed with 10% neutral formalin. Following the paraffin embedding, 3 μm sections were then stained with HE. The total number of cell nuclei in the cross section of glomerulus was counted. The hardening exponent of the glomerulus was determined by a semi-quantitative scoring method. Glomeruli 25 of each animal were counted and were averaged.
Reverse transcription-polymerase chain reaction (RT-PCR) of inflammation-related gene expression in the kidney tissue
The total RNA from kidney tissue was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's recommendations. The RNA was digested with RNase-free DNase (Roche, Basel, Switzerland) for 15 ± 1 min at 37 ± 1°C and purified using a RNeasy kit (Qiagen, Hilden, Germany), according to the manufacturer's protocol. cDNA was synthesized from 2 μg of total RNA by incubation at 37 ± 1°C for l h with avian myeloblastosis virus reverse transcriptase (GE Healthcare, Little Chalfont, United Kingdom) with random hexanucleotides, according to the manufacturer's instruction. The primers used to specifically amplify the genes of interest were: NF-κB forward: 5′ -CAC TTA TGG ACA ACT ATG AGG TCT CTG G-3′ and reverse: 5′ -CTG TCT TGT GGA CAA CGC AGT GGA ATT TTA GG -3′ IκB-α forward: 5′ -GCT GAA GAA GGA GCG GCT ACT -3′ and reverse: 5′ -TCG TAC TCC TCG TCT TTC ATG GA -3′ TGF -β1 forward: 5′ -CTT CAG CTC CAC AGA GAA GAA CGT C -3′ and reverse: 5′ -CAC GAT CAT GTT GGA CAA CTG CTC -3′ Fas forward: 5′ -GAA ATG AAATCC AAA GCT -3′ and reverse: 5′ -TAATTT AGA GGC AAA GTG GC -3′ FasL forward: 5′ -GGATTG GGC CTG GGG ATG TTT CA -3′ and reverse: 5′ -TTG TGG CTC AGG GGC AGG TTG TTG -3′. The internal control gene of GAPDH was amplified using the primers: forward: 5′ -CGG AGT CAA CGG ATT TGG TC -3′ and reverse: 5′ -AGC CTT CTC CAT GGT CGT GA -3′. Amplification was performed in a thermal cycler (Eppendorf, Hamburg, Germany). The polymerase chain reaction (PCR) products were separated in 1.0% agarose gels and visualized with an ethidium bromide staining.[11]
Protein extraction and western blot analysis in the kidney tissue
The total kidney tissue protein was obtained with RIPA buffer as previously described.[11] Protein concentrations were determined with a Bio-Rad protein assay kit (Hercules, CA, USA). For the western blot analysis, aliquots of the lysate containing 30-50 μg protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then electrotransferred onto a nitrocellulose membrane (Schleicher and Schuell, Keene, NH, USA). The membranes were subjected to immunoblot analysis and the proteins were visualized by an enhanced chemiluminescence (ECL) method (GE Healthcare). The cell lysates were separated by 12% SDS-PAGE, transferred onto a polyvinylidene fluoride membrane (GE Healthcare), blocked with 5% skimmed milk and hybridized with primary antibodies (diluted 1:1,000). The antibodies against NF-κB, IκB-α, TGF-β1, Fas, and FasL were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). The blots were then incubated with the horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology Inc.) for 1 h at room temperature. The blots were washed three times with PBS-T and then developed by enhanced chemiluminescence (Amersham Life Science, Arlington Heights, IL, USA).
Component analysis by nuclear magnetic resonance
Dried D. candidum was refluxed and extracted 3 times with 10 times amount of ethyl acetate. The ethyl acetate extract was collected after 1 h for every reflux extraction and decompress concentrating extraction. The total ethyl acetate extract was extracted by anhydrous ethanol 3 times. The ethanol extract was suspended in water, and extracted by petroleum ether, chloroform, butanol extraction, respectively. The ethyl acetate extract was treated by gradient elution in silica gel column with petroleum ether-ethyl acetate system. Then, the chloroform extract was treated by gradient elution in silica gel column with petroleum chloroform-methanol system. Butanol extract was dissoluted by sonication in water, and the extracting solution was obtained after filtering. The extract was then eluted by HP2MGL macroporous resin column with water, 10% ethanol, 30% ethanol, and 60% ethanol, respectively. After elution, the different solvents contained different compounds, and their composition could then be identified by nuclear magnetic resonance (Varian INOVO 400, Varian Inc., Palo Alto, CA, USA). The nuclear magnetic resonance was set at a 1H frequency of 300 MHz, a temperature of 25°C, pulse length in 8 μs, spin speed in 20 Hz, and scan for 64 times. The 1H NMR spectra were recorded using a standard high-resolution magic-angle spinning probe with magic-angle gradient.
Statistical analysis
The data are presented as mean ± standard deviation (SD). Differences between the mean values for individual groups were assessed with one-way analysis of variance (ANOVA) with Duncan's multiple range test. p<0.05 was considered to indicate a statistically significant difference. SAS version 9.1 (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis.
RESULTS
Urinary protein excretion of mice
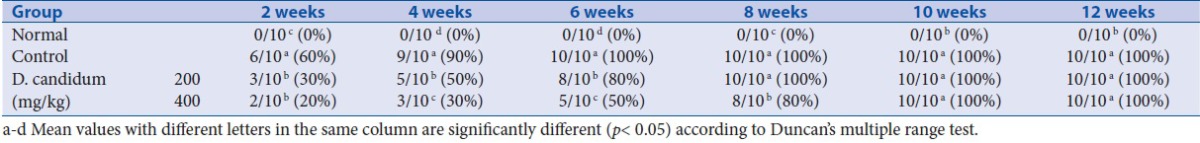
The output of urine protein in normal mice showed no significant change during the experiment, but mice from the other groups displayed increased urine protein as time prolonged. After 2 weeks, at the concentrations of 50, 100, 200 and 400 mg/kg of D. candidum, there were 9, 8, 7 and 4 mice who showed protein urine, while all control group mice showed protein urine. The ED50 of D. candidum was 329 mg/kg D. candidum dose (y = -0.0145x + 9.775; R² = 0.9917), 200 and 400 mg/kg doses were chose to other test. After 12 weeks, the urine protein contents in lupus nephritis mice were higher than those in D. candidum treated mice and normal mice [Table 1]. The output of urine protein in mice treated with a high concentration of 400 mg/kg dose D. candidum was closest to the normal mice.
Table 1.
Output of urine protein of D. candidum treated lupus nephritis mice (n =10, mg/24 h)
Serum SCr, BUN, TC, TG, TP and ALB levels
The SCr, BUN, TC and TG serum levels in control mice were higher than in the other groups, and these levels in normal mice were significantly decreased [p<0.05, Table 2]. The mice treated with D. candidum also had decreased levels of SCr, BUN, TC and TG levels compared to the control mice, but higher than in normal mice. D. candidum at 400 mg/kg could bring levels of SCr, BUN, TC, and TG close to normal levels. TP and ALB serum levels in this experiment showed the reverse trends, the levels in each group from high to low were the normal group, 400 mg/kg D. candidum treated group, 200 mg/kg treated D. candidum group and control group.
Table 2.
Serum SCr, BUN, TC, TG, TP and ALB levels of lupus nephritis mice treated with D. candidum
Glomerular number and glomerular sclerosis index by histological analysis
At the end of this experiment, through the H&E histological analysis, there were 33 ± 4 glomeruli in one kidney section for each normal mouse, compared with a much lower glomerular number of 11 ± 2 in control mice. D. candidum could help to reduce the glomerular change, and the 400 mg/kg D. candidum treatment showed a stronger effect [number of glomerular = 28 ± 5; glomerular sclerosis index = 1.26 ± 0.41, Table 3]. Glomerular sclerosis index of control mice was highest, and the sclerosis indexes of D. candidum groups mice were reduced corresponding to the concentrations.
Table 3.
Glomerular number and glomerular sclerosis index of D. candidum treated lupus nephritis mice (n=10, mg/24 h)
dsDNA positive rate during the experiment
The autoantibody dsDNA was measured by indirect immunofluorescence at the end of 2nd, 4th, 6th, 8th, 10th and 12th week following treatment. The dsDNA antibody titer of serum was observed with a fluorescence microscope. With the threshold titer being 1:20, the result shows that all mice of the control group tested positive since the end of 6th week, and the LN induction to of them, is successful. Mice from the low-concentration D. candidum group tested positive at the end of 8th week and mice from the high-concentration D. candidum group tested positive at the end of 10th week, which means that the D. candidum slowed down the speed than the mice who were were infected with LN [Table 4].
Table 4.
ds-DNA positive rate of D. candidum treated lupus nephritis mice (n=10, titer=1:20)
Cytokine IL-6, IL-12, TNF-α and IFN-γ levels
The normal mice showed the lowest IL-6, IL-12, TNF-α, and IFN-γ levels; however, these levels of control mice were more than three times of normal mice [p<0.05, Table 5]. The levels of IL-6, IL-12, TNF-α, and IFN-γ in 200 or 400 mg/kg D. candidum treated mice were lower than control mice. And the 400 mg/kg D. candidum treated mice had the lowest levels of IL-6, IL-12, TNF-α and IFN-γ.
Table 5.
Serum IL-6, IL-12, TNF-α and IFN-γ levels of lupus nephritis mice treated with D. candidum

Gene expression of NF-κB, IκB-α, TGF-β1, Fas and FasL
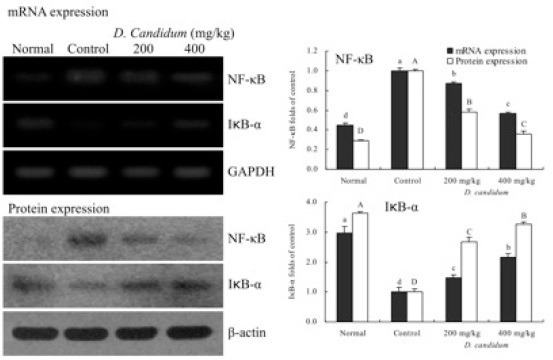
RT-PCR and western blots were used to determine whether the inflammatory actions of D. candidum were associated with inhibition of NF-κB, IκB-α, TGF-β1, Fas and/or FasL gene expression. As shown in [Figure 1], mRNA and protein expression of NF-κB and IκB-α were changed in mice treated with D. candidum. mRNA and protein expression of NF-κB were significantly decreased, while IκB-α mRNA and protein levels were increased (p<0.05). D. candidum significantly decreased mRNA and protein expression of TGF-β1, and the TGF-β1 level mice treated with the high concentration of 400 mg/kg D. candidum were lower than those treated with the 200 mg/kg D. candidum [Fig. 2]. With the D. candidum treatment, mRNA and protein expressions of Fas and FasL were gradually elevated [Fig. 3] NF-κB, TGF-β1, Fas and FasL gene expression of normal mice showed the lowest levels, control mice showed the highest levels, and the IκB-α of normal and control mice showed the opposite tendency. Overall, the results of this experiment showed that D. candidum had a potent anti-inflammatory effect on lupus nephritis.
Figure 1.
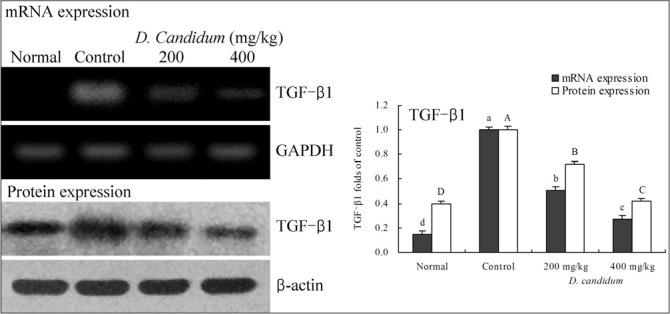
Effects of D. candidum on the mRNA and protein expression of NF-κB and IκB-α in lupus nephritis mice. Fold-ratio: gene expression / GAPDH (β-actin) × control numerical value (control fold ratio: 1).a-d, A-D Mean values with different letters over the bars are significantly different (p< 0.05) according to Duncan's multiple range test.
Figure 2.
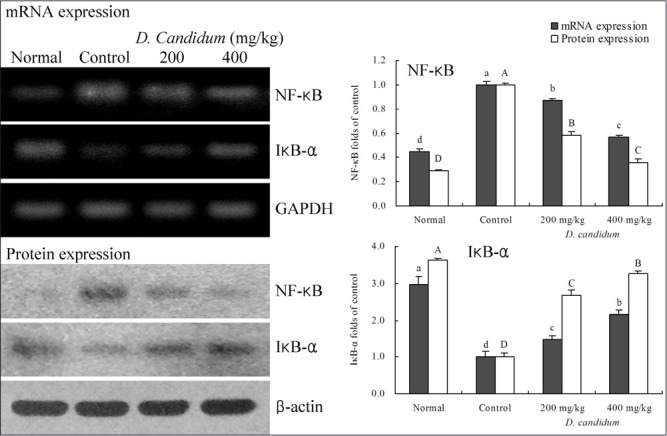
Effects of D. candidum on the mRNA and protein expression of TGF-β1 in lupus nephritis mice. Fold-ratio: gene expression / GAPDH (β-actin) × control numerical value (control fold ratio: 1).a-d, A-D Mean values with different letters over the bars are significantly different (p< 0.05) according to Duncan's multiple range test.
Figure 3.
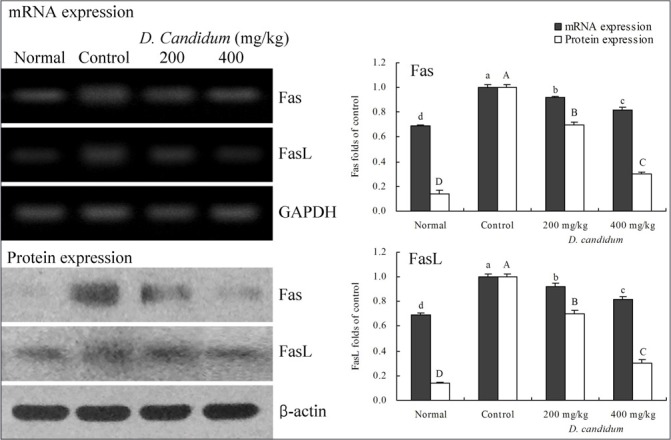
Effects of D. candidum on the mRNA and protein expression of Fas and FasL in lupus nephritis mice. Fold-ratio: gene expression / GAPDH (β-actin) × control numerical value (control fold ratio: 1).a-d, A-D Mean values with different letters over the bars are significantly different (p< 0.05) according to Duncan's multiple range test.
Content of the D. candidum leaf
After the compound assay, 11 compounds were isolated and identified in D. candidum leaf. Compound 1 was obtained as a clear crystal, the 1H-NMR spectrum of this compound exhibited at δ 6.92 (2H, d), 6.62 (2H, d), 6.06 (2H, s), 6.03 (1H, s), 2.65 (4H, m), it can be ensured that material 1 is dihydrogen resveratrol. Compound 2 was obtained as a white powder, the 1H-NMR spectrum of this compound exhibited at δ 6.98 (2H, d), 6.74 (2H, d), 6.62 (1H, s), 6.47 (1H, d), 4.83 (1H,d), 4.63 (1H, d), 3.1-3.8 (12H), 3.73 (3H, s), 3.69 (3H, s), 2.74 (4H, m), it can be ensured that material 2 is dendromoniliside E. Compound 3 was obtained as a black red needle, the 1H-NMR spectrum of this compound exhibited at δ 11.00 (1H, s), 8.15 (1H, d), 6.06 (2H, s), 8.07 (1H, d), 6.95 (1H, s), 6.83 (1H, s), 6.15 (1H, s), 3.96 (3H, s), 3.93 (3H, s), it can be ensured that material 3 is denbinobin. Compound 4 was obtained as a colorless needle, the 1H-NMR spectrum of this compound exhibited at δ 4.72 (2H, m), 3.85 (1H, d), 6.06 (2H, s), 2.53 (1H, d), 2.49 (1H, t), 2.39 (1H, dd), 2.21 (1H, dd), 1.64 (1H, m), 1.35 (3H, s), 1.03 (3H, d), 0.95 (3H, d), it can be ensured that this material is aduncin. Compound 5 was obtained as a white needle, the 1H-NMR spectrum of this compound exhibited at δ 8.25 (1H, s), 8.10 (1H, s), 5.90 (1H, d), 4.66 (1H, dd), 3.5 - 4.2 (4H, m), it can be ensured that material 5 is adenosine. Compound 6 was obtained as a white powder, the 1H-NMR spectrum of this compound exhibited at δ 7.95 (1H, d), 5.85 (1H, d), 5.66 (1H, d), 3.2-4.3 (5H, m), it can be ensured that the material 6 is uridine. Compound 7 was obtained as a clear crystal, the 1H-NMR spectrum of this compound exhibited at δ 10.60 (1H, s), 7.92 (1H, s), 6.45 (2H, s), 5.66 (1H, d), 3.4-4.4 (5H, m), it can be ensured that the material 7 is guanosine. Compound 8 was obtained as a white powder, the 1H-NMR spectrum of this compound exhibited at δ 7.65 (1H, d), 7.41 (2H, d), 6.85 (2H, d), 6.33 (1H, d), 4.17 (2H, t), 1.69 (2H, m), 1.25 (54H, m), 0.85 (3H, t), it can be ensured that material 8 is defuscin. Compound 9 was obtained as a white powder, the 1H-NMR spectrum of this compound exhibited at δ 7.45 (2H, d), 6.82 (2H, d), 6.81 (1H, d), 5.83 (1H, d), 4.16 (2H, t), 1.67 (2H,m), 1.23 (54H, m), 0.88 (3H, t), it can be ensured that material 9 is n-triacontylcis-p-coumarate. Compound 10 was obtained as a white powder, the 1H-NMR spectrum of this compound exhibited at δ 2.35 (2H, t), 1.62 (2H, m), 1.25 (24H, m), 0.88 (3H, t), it can be ensured that the material 10 is hexadecanoic acid. Compound 11 was obtained as a white powder, the 1H-NMR spectrum of this compound exhibited at δ 3.85 (2H, t), 1.75 (2H, m), 1.45 (2H, m), 1.22 (54H, m), 0.85 (3H, t), it can be ensured that the material 11 is hentriacontane.
DISCUSSION
Although D. candidum has been used as a traditional Chinese medicine, there were not many scientific studies regarding how it functions or what active ingredients it contains. D. candidum has been recently reported to have some functional effects on numerous pathologic conditions such as inflammation, immunity, hyperglycemia, and cancer.[12] The lupus nephritis could be replicated in mice, the lupus nephritis animal model could be used for the scientific research. In this study, the curative effect of D. candidum was first determined by the lupus nephritis animal model.
Serum creatinine and urea nitrogen are nitrogenous organic compounds and the end products of protein metabolism. When renal function was normal, these small molecules are filtered from the glomerulus. When the kidney suffers from lesions, the filtering capability of the glomerulus decreases, and the content of serum creatinine and urea nitrogen increases. This increase in serum levels could be used as an index for the clinical diagnosis of renal damage.[13] Too much cholesterol and triglycerides results in hyperlipemia. When nephropathy is deteriorated to a certain degree, the features of hyperkalemia would co-exist. Therefore, cholesterol and triglyceride could also be considered as an index to renal hypofunction and renal lesion.[14]
The protein in urine was lost for a long time in nephrotic syndrome. The total protein in serum was drastically reduced.[15] Albumin is the most prevalent protein in serum. It serves to cure serious diseases including edema caused by clinically curative nephropathy. It was thus evident that keeping the amount of total protein and albumin in serum is an important way to maintain normal renal function. The decrease of total protein and albumin are the manifestations of poor renal function.[14]
The final stage of lupus nephritis is represented by Global sclerosis involving most of the glomeruli, and represents healing prior to inflammatory injury. The sclerosis aggravated glomeruli parenchymal cells reduction.[15]
A high-concentration of antibody to dsDNA seemed to be seen only in SLE. Therefore, this antibody to dsDNA was specific to SLE, and is closely related to the disease activity index, especially to LN. It could also be used as an index to diagnose systematic lupus erythematosus.[16,17]
IL-12 plays an important role in LN autoimmune response, and the level of IL-12 in the active stage of LN rises. One of the features of LN was the emergence of large amounts of autoantibody. It was proven, that IL-12 can promote cells to produce this autoantibody directly. The increase in the content of IL-12 led to the large amount of production of this autoantibody.[18] As an inflammatory mediator, IFN-γ takes part in the whole immune inflammatory process of nephritis. The level IFN-γ in glomerulonephritis is substantially increased.[19] After the occurrence of nephritis, cytokines related to inflammation changed. The content of cytokines related to inflammation, like IL-6, IL-12, TNF-α and IFN-γ, in blood would also obviously increase.[4]
NF-κB is an important transcription factor serving to convey signals in the cell. It's found to be decisive in the gene transcription regulation of numerous factors in the aforementioned immune inflammation. The nephridial tissue of normal mice appeared with a few mesangial cell and NF-κB, which indicates that moderate NF-κB activity might be necessary to adjust physiological function of normal mixed renal cells.[20] Guijarro et al.[21] found that activation of NF-κB in mesangial cell nurtured in vitro can enhance the reproduction of mesangial cell and serves to fulfill centering control function to multiple chemokines secreted by mesangial cells. According to scientific research,[22] when interstitial nephritis was accompanied by large amount of albuminuria, NF-κB in the renal cortex was activated and expression of TNF-α, IL-6, TGF-β increased. After treatment, the activation of NF-κB and damage of renal interstitium was decreased, and IL-6 and TGF-β expression also reduced. By the expression quantity of TGF-β mRNA in kidneys of the BXSB mice assay, TGF-β played an important role in the pathological accumulation of extracellular matrix in lupus nephritis and the formation process of glomerular sclerosis.[23] The experiment results showed that TNF-α and IL-6 levels in serum of the D. candidum treated groups decrease greatly compared with the LN control group, which came to the same conclusion with that of Rangan et al.[22] D. candidum serves to reduce the content of these cytokines in blood. Thereby, D. candidum has an obvious curative effect to LN. In this research, the expression of NF-κB and TGF-β in mice in the LN control group is higher than that in mice of the normal group and the D. candidum treated groups. The better the curative effect of D. candidum, the weaker the expression of NF-κB and TGF-β. Meanwhile, IκB expression showed the opposite tendency. Fas and FasL are the molecules that cause the death of cells. Fas is a member of the ErbB of TNF nerve growth factors and exists in the surface of active T/B leukomonocytes and hematopoietic cells, as well as other tissues.[20] Fas induced the death of cells through oxidative stress after interacting with its natural ligand.[24] It was proven that the apoptosis rate of monocytes of LN patients is faster than that of normal people. FasL is a member of TNF family. In clinical checks, it was found that no FasL is obviously detected in the tissues of normal people while there is palpable FasL in LN patients. The increase of FasL level is likely to be caused by the change of LN.[21]
Li et al.[12] found that there are 20 compounds in D. candidum, and 11 compounds were also found in this study. The contents of plants from different areas lead to variation, as seen between this study and the previous report of Li et al.[12] These compounds had many functional effects. Resveratrol is recommended for inflammation prevention, as it is known to act as an antioxidant and to fight colonic inflammation,[22] and those anti-inflammatory effects might help to prevent lupus nephritis. Aduncin is a special component only found in Dendrobium, although it may have anti-inflammatory effect, its functional effects need to be researched, such as preventive effect of lupus nephritis. Adenosine has both pro- and anti-inflammatory effects and acts on inflammatory and resident immune cells and antioxidant enzymes.[23] Uridine appeared to affect the tumour necrosis factor (TNF) levels in lung inflammation SD rats, and it had anti-inflammatory effects in vivo.[25] Defuscin, n-triacontylcis-p-coumarate, hexadecanoic acid, and hentriacontane also showed many functional activities in human health.[19] These compounds showed anti-inflammatory activities in the studies, and it may be the reason why D. candidum had the strong curative effects on lupus nephritis in vivo. From The 11 compounds reported in this study had anti-inflammatory effects, but these effects were not very strong. The anti-inflammatory effect of D. candidum was found to be strong in lupus nephritis treatment, but the 11 compounds of D. candidum might have composite effects. These effects might promote the effect of certain compound by other compounds.
In summary, the lupus nephritis curative effect of D. candidum was evaluated by various in vivo experimental methods, including serum cytokine assays of IL-6, IL-12, TNF-α and IFN-γ; an assay for serum levels of SCr, BUN, TC, TG, TP, and ALB; an auto-antibody dsDNA assay, analysis of urinary protein excretion, a histology assay, tissue RT-PCR, and western blot assays for checking the inflammatory related genes of NF-κB, IκB-α, TGF-β1, Fas, and FasL. The 11 functional compounds of D. candidum might cause the medical effects of lupus nephritis in mice. An analysis of the various mice treatment groups revealed that D. candidum had a curative effect on lupus nephritis, indicating that D. candidum represents a potentially useful agent for the treatment or prevention of lupus nephritis in mice.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest
Acknowledgement
Qiang Wang and Peng Sun contributed equally to this work. This study was supported by Natural Science Foundations of China (No. 31401559), Program for Innovative Research Team in Chongqing University of Education (No. KYC-cxtd03-20141002), Construction Program of Chongqing Engineering Research Center (cstc2015yfpt_gcjsyjzx0027) and Program for Innovation Team Building at Institutions of Higher Education in Chongqing (CXTDX201601040), People's Republic of China.
REFERENCES
- 1.Zhang JJ. Progress in diagnosis and treatment of lupus nephritis. J Kunming Med Univ. 2008;S1:200–4. [Google Scholar]
- 2.Ma HL, Zhang XG, Zhang XZ, Xu Y. Effect of Esculentoside A on therapeutic and cytokines secretion of lupus nephritis in BXSB mice. Chongqing Med J. 2012;41:2954–55. [Google Scholar]
- 3.Bruijn JA, van Elven EH, Hogendoorn PC, Corver WE, Hoedemaeker PJ, Fleuren GJ. Murine chronic graft-versus-host disease as a model for lupus nephritis. Am J Pathol. 1988;130:639–41. [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Q, Sun P, Li GJ, Zhu K, Wang C, Xin Zhao. Inhibitory effects of Dendrobium candidum Wall ex Lindl. onazoxymethane- and dextran sulfate sodium-induced colon carcinogenesis in C57BL/6 mice. Oncol Lett. 2014;7:493–98. doi: 10.3892/ol.2013.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao F, Zhang JZ. First report of Fusarium oxysporum causing wilt of Dendrobium candidum in Zhejiang province, China. Plant Dis. 2012;96:1377. doi: 10.1094/PDIS-03-12-0304-PDN. [DOI] [PubMed] [Google Scholar]
- 6.Shao H, Zhang LQ, Li JM, Wei RC. Advances in research of Dendrobium of ficinal. Chinese Tradit Herbal Drug. 2004;35:109–111. [Google Scholar]
- 7.Walser M, Hill S, Tomalis EA. Treatment of nephrotic adults with a supplemented, very low-protein diet. Am J Kidney Dis. 1996;28:354–64. doi: 10.1016/s0272-6386(96)90492-8. [DOI] [PubMed] [Google Scholar]
- 8.Hou CM, Zhang JL, Li Y, Shen BF, Feng JN, Xiao H. Establishment of a murine lupus nephritis model. Bull Acad Mil Med Sci. 2009;33:151–52. [Google Scholar]
- 9.Bo YH, Jiang F, Su W, Ling YL, Bi ZQ. The protective effect of idiotypic peptide on kidney in the GVHD lupus nephritis mice. J Cap Med Univ. 2007;28:770–73. [Google Scholar]
- 10.Zhao X, Kim SY, Park KY. Bamboo salt has in vitro anticancer activity in HCT-116 cells and exerts anti-metastatic effects in vivo. J Med Food. 2013;16:9–19. doi: 10.1089/jmf.2012.2316. [DOI] [PubMed] [Google Scholar]
- 11.Chen LH, Song JL, Qian Y, Zhao X. Increased preventive effect on colon carcinogenesis by use of resistant starch (RS3) as the carrier for polysaccharide of Larimichthyscrocea swimming bladder. Int J Mol Sci. 2014;15:817–29. doi: 10.3390/ijms15010817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Wang CL, Wang FF, Dong HL, Guo XX, Yang JS. Chemical constituents of Dendrobium candidum. China J Chin Mater Med. 2010;35:1715–19. doi: 10.4268/cjcmm20101314. [DOI] [PubMed] [Google Scholar]
- 13.Li XY, Kong FY, Zhang HQ, Tang RX, Zheng KY. The duplication and 445 identification of anti-glomerular basement membrane (GBM) nephritis model in mice. Acta Acad Med Xuzhou. 2011;31:527–30. [Google Scholar]
- 14.Chou A, Zhou JY, Zhou Y, Hua J, Wu JB. Therapeutic effect of zhenwu decoction on chronic glomerulonephritis rat model induced by cationization bovine serum albumin osmotic pump. Tradit. Chinese Drug Res Chin Pharm. 2012;23:626–30. [Google Scholar]
- 15.Chen W. Changes of urine protein in patients with chronic glomerulonephritis after different doses of irbesartan treatment. Chinese J Arterioscl. 2010;18:909–10. [Google Scholar]
- 16.Du J, Wang QS, Jia RH. Glomerular cell proliferation and apoptosis experimental glomerulosclerosis. J Prac Med. 2005;21:1623–25. [Google Scholar]
- 17.Wang MF. Significance of anti-double stranded DNA in system lupus erythematosus. Chinese J Health Lab Technol. 2010;20:606–7. [Google Scholar]
- 18.Uhm WS, Na K, Song GW, Jung SS, Lee T, Park MH, et al. Cytokine balance in kidney tissue from lupus nephritis patients. Rheumatology (Oxford) 2003;42:935–38. doi: 10.1093/rheumatology/keg255. [DOI] [PubMed] [Google Scholar]
- 19.Xiang L, Gao XX, Pan JR. Serum levels of interferon-gamma and interleukin-10 in patients with chronic glomerulonephritis and their clinical significance. Clin Med J China. 2006;13:269–71. [Google Scholar]
- 20.Philip S, Bulbule A, Kundu GC. Matrix metalloproteinase-2: mechanism and regulation of NF-κB-mediated activation and its role in cell motility and ECM-invasion. Glycocorj J. 2004;21:429–41. doi: 10.1007/s10719-004-5533-7. [DOI] [PubMed] [Google Scholar]
- 21.Guijarro C, Kim Y, Kasiske BL. Central role of the transcription factor NF-κB in mesangial cell production of chemokines. Contrib Nephrol. 1997;120:210–18. doi: 10.1159/000059839. [DOI] [PubMed] [Google Scholar]
- 22.Rangan GK, Wang Y, Tay YC, Harris DC. Cytokine gene expression in Adriamycin nephropathy: effects of antioxidant nuclear factor kappaB inhibitors in established disease. Nephron. 2000;86:482–90. doi: 10.1159/000045838. [DOI] [PubMed] [Google Scholar]
- 23.Zhou P, Chen XR, Li SM, Tu SQ. Expression of insulin-like growth gactor-I, interleukin-1 and tumor necrosis factor-α genes in kidneys of BXSB lupus mice. Chin J Derm Venereol. 1999;13:77–78. [Google Scholar]
- 24.Yao CW, Tang DS, Liang D. Expression and significance of NF-κB in mouse renal tissue with lupus nephritis-prone BXSB. J Guangdong Med Coll. 2005;23:493–95. [Google Scholar]
- 25.Li ZJ, Li YJ, Yang QQ, Yang X, Xu YW, Yu XQ. Significance of levels IL-12 and IgG in patients with lupus nephritis. J New Med. 2002;33:19–20. [Google Scholar]


