Abstract
Context:
Sea buckthorn (Hippophae rhamnoides L.) as a traditional Chinese medicinal plant has various uses in Xinjiang.
Objective:
A reversed-phase rapid-resolution liquid-chromatography method with diode array detector was developed for simultaneous determination of protocatechuic acid, rutin, quercetin, kaempferol, and isorhamnetin in the pulp and seed of sea buckthorn, a widely used traditional Chinese medicine for promoting metabolism and treating scurvy and other diseases.
Settings and design:
Compounds were separated on an Agilent ZORBAX SB-C18 column (4.6 mm × 250 mm, 5 μm; USA) with gradient elution using methanol and 0.4% phosphoric acid (v/v) at 1.0 mL/min. Detection wavelength was set at 280 nm.
Materials and Methods:
The fruits of wild sea buckthorn were collected from Wushi County in Aksu, Xinjiang Province.
Statistical performances:
The RSD of precision test of the five compounds were in the range of 0.60-2.22%, and the average recoveries ranged from 97.36% to 101.19%. Good linearity between specific chromatographic peak and component qualities were observed in the investigated ranges for all the analytes (R2 > 0.9997).
Results:
The proposed method was successfully applied to determine the levels of five active components in sea buckthorn samples from Aksu in Xinjiang.
Conclusions:
The proposed method is simple, fast, sensitive, accurate, and suitable for quantitative assessment of the pulp and seed of sea buckthorn.
SUMMARY
Quantitative analysis method of protocatechuic acid, rutin, quercetin, kaempferol, and isorhamnetin in the extract of sea buckthorn pulp and seed is developed by high-performance liquid chromatography (HPLC) diode array detection.
This method is simple and accurate; has strong specificity, good precision, and high recovery rate; and provides a reliable basis for further development of the substances in the pulp and seed of sea buckthorn.
The method is widely used for content determination of active ingredients or physiologically active components in traditional Chinese medicine and its preparation
Abbreviation used: PR: protocatechuic acid, RU: rutin, QU: quercetin, KA: kaempferol, IS: isorhamnetin, HPLC: high-performance liquid chromatography, HPLC-DAD: high performance liquid chromatographydiode array detector, LOD: linearity and limit of detection, LOQ: limit of quantitation, RSD: relative standard deviation
Keywords: Content determination, HPLC-DAD, pulp, seed, sea buckthorn
INTRODUCTION
Sea buckthorn (Hippophae rhamnoides L.) belongs to the genus Hippophae of the family Elaeagnaceae and also known as hippophae fruit, acid thorn, black thorn, and Ji han (Uygur name).[1] Sea buckthorn has a warm, sour taste.[2] In ancient times, doctors commonly use sea buckthorn as traditional Chinese medicine to treat diseases. Sea buckthorn contains abundant nutrients and biologically active ingredients.[3] Sea buckthorn plays an important role in promoting metabolism and has antifatigue, antiaging, antiatherosclerosis, antiradiation, and antiscurvy properties; hence, this plant has high medicinal value.[4] At present, China is the world's largest distributor of sea buckthorn plants and has the most abundant resource of sea buckthorn, accounting for the world's total area of more than 95%. Therefore, China is well known as the “sea of sea buckthorn.[5]” The northeast, north, northwest, and southwest regions of China are the main distribution areas of sea buckthorn. The genus Hippophae possesses a strong ecological adaptability and drought resistance, and demonstrates sand fixing capabilities.[6] This genus is also nontoxic and pollution free. Sea buckthorn has become one of the main afforestation tree species in soil erosion that improves soil fertility and ecological environment, thereby gaining increasing attention.[7] Sea buckthorn has wide distribution and variety. It has become an important raw material for domestic and foreign drugs, health food, and cosmetics.[8] Sea buckthorn is an edible plant resource that has long been the source of income of the People's Republic of China Pharmacopoeia.[9]
The chemical compositions of sea buckthorn have attracted considerable attention worldwide.[10] Medicinal herbs may contain hundreds of complex active components, and identifying all these substances by quantitative analysis is often impractical.[11] In this study, we divided sea buckthorn fruit into two parts: pulp and seed. The chemical compositions in the sea buckthorn pulp and seed are bioactive constituents, which are good for our health and have antioxidants, hepatoprotective, and immunomodulatory properties.[12] Protocatechuic acid (PR), rutin (RU), quercetin (QU), kaempferol (KA), and isorhamnetin (IS) are five compounds in the extract of sea buckthorn pulp and seed that have similar molecular structures as shown in Fig. 1. Several chromatographic methods have been documented for the determination of chemical compositions present in sea buckthorn.[13] Among all the chromatographic methods, the rapid-resolution liquid-chromatography method is widely applied.[14] Therefore, this method is important for simultaneous determination of the five aforementioned compounds in sea buckthorn pulp and seed extracts. No report on simultaneous determination of these five compounds in sea buckthorn pulp and seed by high-performance liquid chromatography (HPLC) method has been presented.
Figure 1.
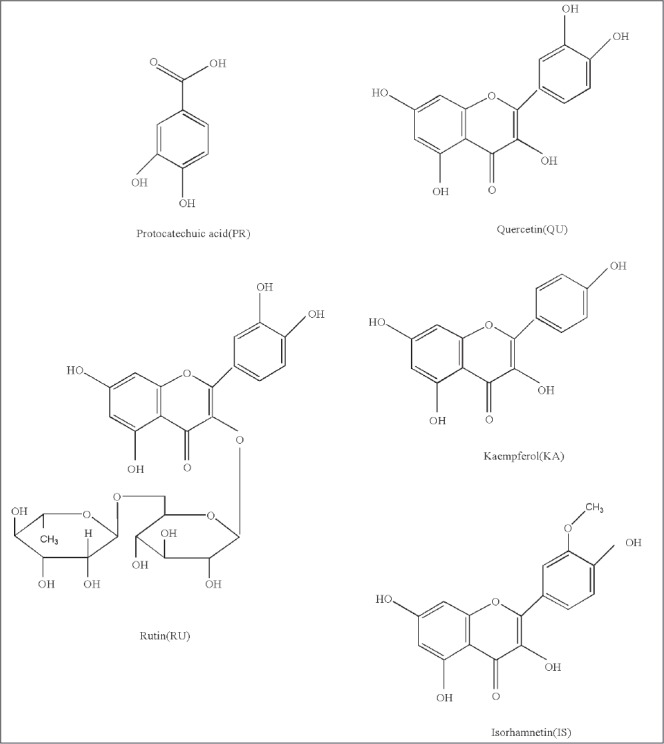
Chemical structures of the five bioactive components in sea buckthorn.
In this study, we developed an HPLC with diode array detect (DAD) method that allows for simultaneous determination of at least five of the putative bioactive ingredients: PR, RU, QU, KA, and IS. This method may form the basis for a more efficient analytical procedure to assess the medicinal quality of H. rhamnoides L. samples and serve as guide for future studies on therapeutic mechanisms.
MATERIALS AND METHODS
Materials and equipment
Materials
The fruit of wild sea buckthorn were collected from Wushi county in Aksu, Xinjiang Province in September 2014. We divided the fruit of wild sea buckthorn into two parts: pulp and seed. We smashed the pulp and seed into powder, through 40 mesh sieve, respectively.
Equipment
Agilent 1220 liquid chromatograph, Agilent 1220 diode array detector, Agilent ZORBAX SB-C18 column (4.6 mm × 250 mm, 5 μm, USA).
Reagents
Methanol was of HPLC grade was supplied by Fisher Scientific (USA) and ultrapure water was obtained from a Millipore Q3 ultrapure water system (USA). Phosphoric acid was of analytical grade (Chengdu Kelong Chemical Reagents Company, China). PR, QU standards were purchased from Shanghai Yuanye Biological Technology Company; RU standard was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China); KA, IS standards were purchased from Chengdu Must Bio-technology Company.
Preparation of standard solutions
Standard stock solutions of five compounds were prepared in methanol at concentrations of 0.51 mg/mL for PR, 0.69 mg/mL for RU, 0.50 mg/mL for QU, 0.42 mg/mL for KA, and 1.09 mg/mL for IS. All standard solutions were filtered through a 0.45 μm membrane filter and then injected directly.
Preparation of sample solutions
The pulp and seed powder were dried at 60°C. Extraction was carried out using 1 g of powdered pulp and seed with 30 mL of 70% ethanol at 80°C in a reflux device for 1 h. The samples were then filtered at a high temperature and evaporated to dryness on a water bath. The residue was dissolved with 25 mL of water and 3.5 mL of hydrochloric acid, heated and hydrolyzed in the water bath for 30 min, and cooled immediately. Afterward, the samples were shaken twice with 20 mL of ethyl acetate, combined with ethyl acetate, and washed thrice with 10 mL of water. The ethyl acetate extracts were dried with 2 g of anhydrous sodium carbonate. Finally, the extracts were evaporated to dryness, and the residue was dissolved with 2 mL of methanol. Thus, we can obtain the pulp and seed solutions. All sample solutions were filtered through a 0.45 μm membrane filter; the extract was injected directly.
Chromatographic conditions
Chromatographic analyses were carried out using an Agilent ZORBAX SB-C18 column (4.6 mm × 250 mm, 5 μm) on an Agilent 1200 system with DAD. The detection wavelength was set at 280 nm. Flow rate and injection volume were 1.0 mL/min and 10 μL, respectively. All chromatographic operations were conducted at ambient temperature. The mobile phase was methanol (B)-water containing 0.4% phosphoric acid (A), using gradient elution: 0-10 min, 80-70% A; 10-20 min, 70-60% A; 20-30 min, 60-50% A; 30-40 min, 50-40% A; 40-45 min, 40-30% A. The mobile phase was filtered through a 0.45 μm membrane filter and then denaturized ultrasonically prior to use.
RESULTS AND DISCUSSION
Optimization of sample preparation
Reflux and ultrasonication (all with 70% methanol as extractants, pulp, and seed powder dried under 60°C, 40 mesh sifter) were respectively used to extract five compounds of the pulp and seed in the sea buckthorn. Results showed that the extraction yield by ultrasonication was evidently low, and the five compounds cannot be separated effectively, whereas the extraction yield by reflux was higher and the five compounds can be separated effectively in the chromatogram. Therefore, in this work, reflux extraction was considered a simpler and more effective method for extraction of five compounds of the pulp and seed of sea buckthorn, and consequentially used in the following tests.
Optimization of chromatographic conditions
Chromatographic conditions were optimized, including the mobile phase, flow rate, injection volume, column temperature, and detection wavelength, to ensure a chromatogram with well-separated peaks and minimal analysis time per run. The appropriate mobile phase, which can adjust the retention time, can also improve the chromatographic peak profile[15] According to several references,[16,17] various mixing ratios of water (with 0.4% phosphoric acid) to methanol as the mobile phase were used; however, the five compounds cannot be separated effectively using isocratic elution. Optimization of the separation conditions of the gradient elution is important in chromatography. Hence, gradient elution was used throughout this study.
The choice of appropriate wavelength is crucial to ensure that all the compounds are detected.[18] The five standard solutions were injected into the Infinity LC system, and the UV spectra were measured over the range of 200-400 nm by DAD. The DAD detector can provide an optimized condition of wavelength in this study. Synthesis of the spectra of each of the five compounds showed the same absorption maximum at 280 nm. Other chromatographic variables were also optimized. Finally, optimal separation was achieved at a flow rate of 1.0 mL/min, injection volume of 10 μL, and ambient column temperature.
A typical Infinity LC chromatogram is shown in Fig. 2. The retention times for the five compounds were 7.61 min (PR), 27.73 min (RU), 37.20 min (QU), 42.71 min (KA), and 43.84 min (IS)
Figure 2.
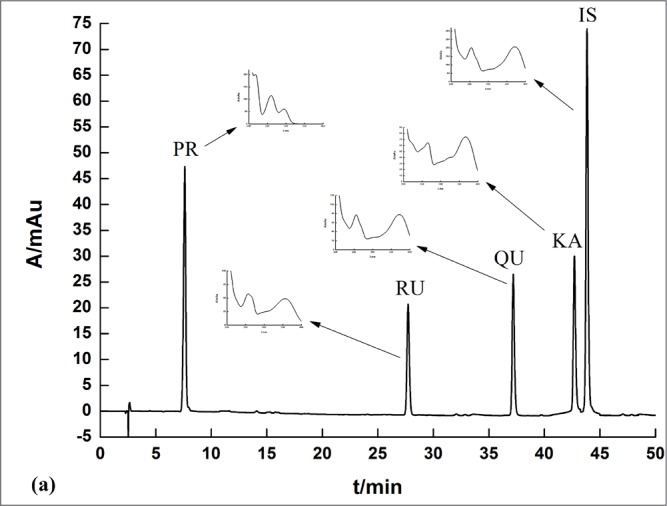
A typical rapid-resolution liquid-chromatography chromatogram and UV spectra of standard solutions.
System suitability
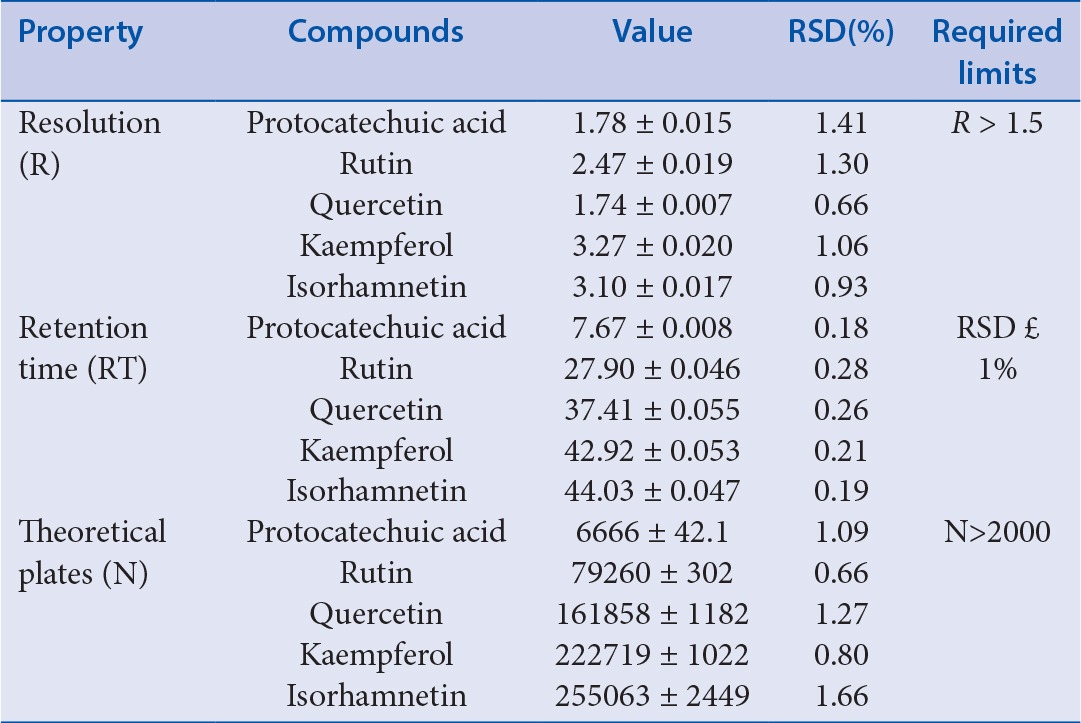
System suitability tests are an integral part of liquid chromatographic methods and have been proven to be adequate for analysis for the chromatographic system.[19] Retention time, resolution, and number of theoretical plates were evaluated in five replicate injections of the standard solutions. As shown in Table 1, all parameters were within acceptable limits. Therefore, under the selected conditions, several conditions, such as column length, carrier performance, and column filling, are all in line with the requirements, and the effect of determination is good.
Table 1.
System-suitability data
Method validation tests
Linearity and limit of detection
Regression analyses were performed using a data-processing software. Correlation coefficient r2 and linear regression equations were also computed by a data-processing software. The linear standard curves were constructed from five different concentrations of five standard solutions; assays were performed three times. The peak area of the five standard solutions included Y as the longitudinal coordinate and X (g) as the cross coordinate for the quality of the corresponding reference; the linear regression equation was Y=aX + b. The results of the regression analyses and the calculated correlation coefficients (r2) are listed in Table 2. Good linearity between peak areas (Y) and quality (X) was observed in the investigated ranges for all the analytes.
Table 2.
Statistical performances of linear regression-equation analysis in the determination of the five investigated compounds
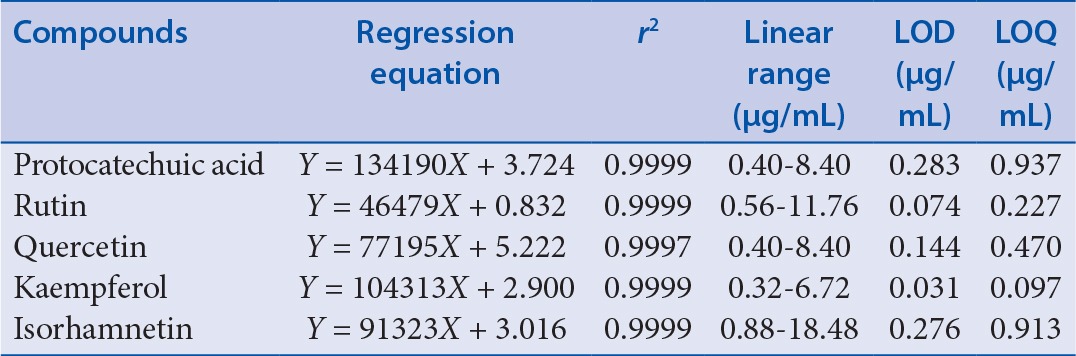
LOD was considered to be the minimum detectable concentration, referring to the signal-to-noise ratio (S/N) of 3. Limit of quantitation (LOQ) was considered to be the lowest concentration, referring to signal-to-noise ratio (S/N) of 10.[20,21] Detection limits and quantitative limits are expressed as the concentration of the analytes, with the unit of µg/mL. The LOQ and LOD values for the three chemical components are also listed in Table 2.
Precision and sample analyses
The interday precision of the method was determined by analyzing the mixture of five standard solutions continuously within 1 day. The relative standard deviation (RSD) values of the peak area for six times were in the range of 0.60–2.22%, indicating good precision of the instruments.
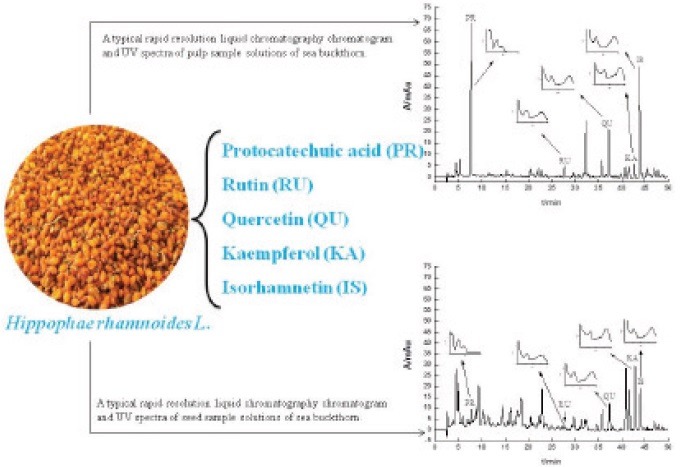
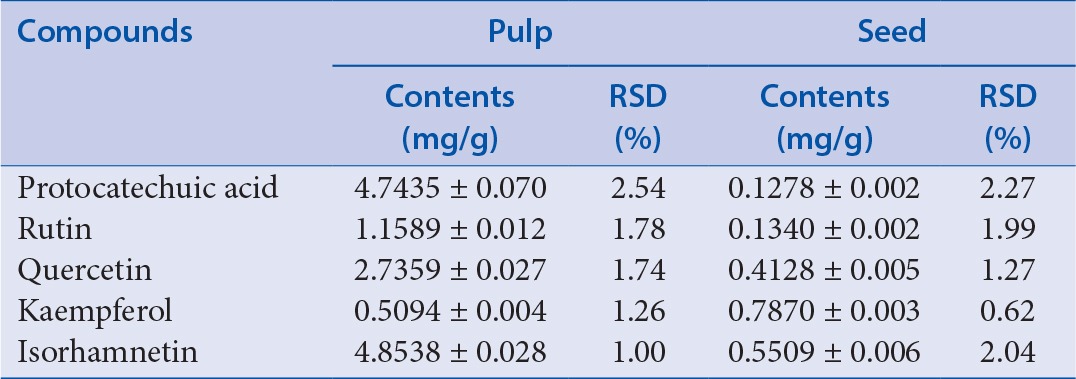
Methanol solutions in the extract of the sea buckthorn pulp and seed were injected directly and separated completely under the optimum condition mentioned earlier. The typical chromatograms in the sea buckthorn pulp and seed extracts are shown in Figures 3 and 4. The calculated contents of the five compounds are given in Table 3.
Figure 3.
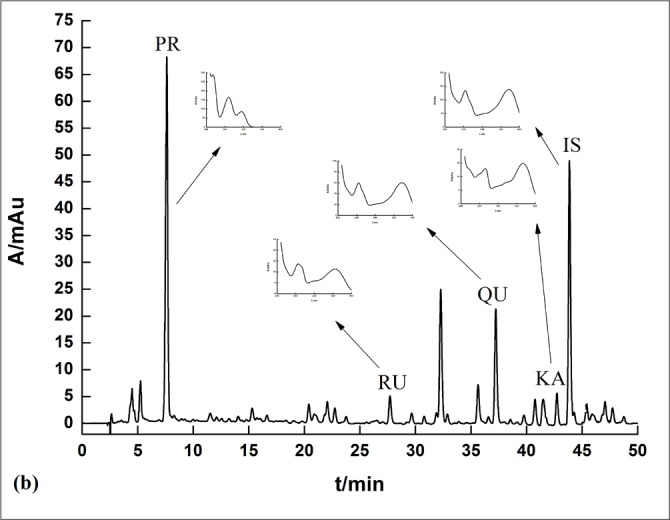
A typical rapid-resolution liquid-chromatography chromatogram and UV spectra of pulp sample solutions of sea buckthorn.
Figure 4.

A typical rapid-resolution liquid-chromatography chromatogram and UV spectra of seed sample solutions of sea buckthorn.
Table 3.
Contents of the five compounds in pulp and seed samples of sea-buckthorn (n = 3)
Reproducibility and stability
The stability test of the method was assessed by analyzing the same sample solution (Sample: pulp and seed solution) at 0, 2, 4, 6, 8, 10, and 12 h. The RSD values of the peak areas of the pulp and seed of sea buckthorn were in the ranges of 0.74-2.38% and 0.44-2.52%, respectively. The result indicated that the two sample solutions were stable within 12 h.
Repeatability tests of the six independently prepared solutions of the sample solution (Sample: pulp and seed solution) were conducted and analyzed. The RSD values of the peak area of the pulp and seed of sea buckthorn were in the ranges of 0.74-2.38% and 0.44-2.52%, respectively. The results showed good reproducibility of the method.
Recovery
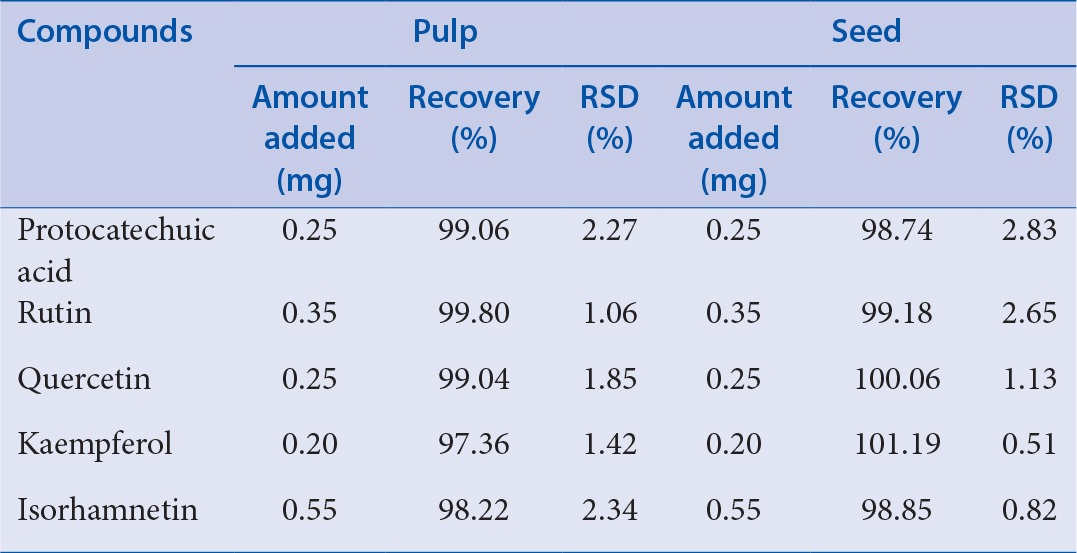
The recovery test of the method was determined by standard addition method. The experiments of the five compounds were performed by adding PR, RU, QU, KA, and IS standards to the sea buckthorn pulp and seed extracts, which were treated according to the procedure described in Section “Preparation of sample solutions” for six times. The recovery test results are summarized in Table 4. The average recovery rates of the five compounds of the pulp and seed of sea buckthorn were in the ranges of 97.36-99.80% and 98.74-101.19%, respectively; their RSD values were in the ranges of 1.06-2.34% and 0.51-2.83%, respectively. Hence, the obtained results indicated that the HPLC method was reproducible with high accuracy and sensitive enough for simultaneous quantitative evaluation of the five compounds of sea buckthorn. Satisfactory quantitative analysis was achieved.
Table 4.
Recoveries of the five compounds in the extract of pulp and seed in sea-buckthorn (n = 5)
CONCLUSION
HPLC is widely used for content determination of active ingredients or physiologically active components in traditional Chinese medicine and its preparation. In this study, HPLC was used to determine the five compounds from pulp and seed of sea buckthorn, with 280 nm as detection wavelength and methanol-0.4% phosphoric acid solution (gradient elution) as mobile phase under chromatographic conditions; the five compounds were well separated.
This study established a method for determination of the five compounds in the pulp and seed of sea buckthorn. The method is in line with the quality standard requirements of traditional Chinese medicine as indicated in the validation tests. The data obtained indicate that the PR, RU, QU, KA, and IS contents of the pulp were more than those of the seed of sea buckthorn. The IS content in the pulp was higher than the other compounds; however, the KA content in the seed was the highest among the other compounds. The five compounds, whether in pulp or in seed, had very high medicinal value because of their synergistic effect.
This method is simple and accurate; has strong specificity, good precision, and high recovery rate; and provides a reliable basis for further development of the substances in the pulp and seed of sea buckthorn. Furthermore, this method can be used as quality control for the five compounds in sea buckthorn and will serve as guide for the determination of the five compounds in other medicinal plants or pharmaceutical preparations.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest
Acknowledgement
This work was financially supported by the China Russian France International Cooperation Research Project (Grant no:87E2A0313397).
REFERENCES
- 1.Khan BA, Akhtar N, Mahmood TA. Comprehensive review of a magic plant, Hippophae rhamnoides. Pharmacogn J. 2010;2:65–8. [Google Scholar]
- 2.Song LR, Hu L, Hong X. Chinese material medica. Uygur Medicine Volume. 2005; Shanghai Scientific and Technical Publishers. 2005:183–5. [Google Scholar]
- 3.Buyukokuroglu ME, Gulcin I. In vitro antioxidant and antiradical properties of Hippophae rhamnoides L. Pharmacogn Mag. 2009;5:189. [Google Scholar]
- 4.Arimboor R, Kumar KS, Arumughan C. Simultaneous estimation of phenolic acids in sea buckthorn (Hippophae rhamnoides) using RP-HPLC with DAD. J Pharm Biomed Anal. 2008;47:31–8. doi: 10.1016/j.jpba.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Xu XM, Chen Y, Yu MY, Wen FY, Zhang H. Identification, quantification and antioxidant activity of acylated flavonol glycosides from sea buckthorn (Hippophae rhamnoides ssp. sinensis) Food Chem. 2013;141:1573–9. doi: 10.1016/j.foodchem.2013.03.092. [DOI] [PubMed] [Google Scholar]
- 6.Suryakumar G, Gupta A. Medicinal and therapeutic potential of sea buckthorn (Hippophae rhamnoides L.) J Ethnopharmacol. 2011;138:268–78. doi: 10.1016/j.jep.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Zu Y, Li C, Fu Y, Zhao C. Simultaneous determination of catechin, rutin, quercetin kaempferol and isorhamnetin in the extract of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD. J Pharm Biomed Anal. 2006;41:714–9. doi: 10.1016/j.jpba.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 8.Bal LM, Meda V, Naik SN, Satya S. Sea buckthorn berries: A potential source of valuable nutrients for nutraceuticals and cosmoceuticals. Food Res Int. 2011;44:1718–27. [Google Scholar]
- 9.Xing J, Yang B, Dong Y, Wang B, Wang J, Kallio HP. Effects of sea buckthorn (Hippophae rhamnoides L.) seed and pulp oils on experimental models of gastric ulcer in rats. Fitoterapia. 2002;73:644–50. doi: 10.1016/s0367-326x(02)00221-6. [DOI] [PubMed] [Google Scholar]
- 10.Tian S, Yu Q, Xin L, Zhou ZS, Upur H. Chemical fingerprinting by RP-RRLC-DAD and principal component analysis of Ziziphora clinopodioides from different locations. Nat Prod Commun. 2012;7:1181–4. [PubMed] [Google Scholar]
- 11.Weon JB, Yang HJ, Ma JY, Ma CJ. A HPLC-DAD method for the simultaneous determination of five marker components in the traditional herbal medicine Bangpungtongsung-san. Pharmacogn Mag. 2011;7:60. doi: 10.4103/0973-1296.75903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo XF, Yue YD, Tang F, Wang J, Yao X, Sun J. Simultaneous determination of seven flavonoids in Dan Bamboo Phyllostachys glauca McClure leaf extract and in commercial products by HPLC-DAD. J Food Biochem. 2013;37:748–57. [Google Scholar]
- 13.Chen C, Zhang H, Xiao W, Yong ZP, Bai N. High-performance liquid chromatographic fingerprint analysis for different origins of sea buckthorn berries. J Chromatogr A. 2007;1154:250–9. doi: 10.1016/j.chroma.2007.03.097. [DOI] [PubMed] [Google Scholar]
- 14.Yun BR, Weon JB, Lee J, Eom MR, Ma CJ. Simultaneous determination of 11 bioactive compounds in Jaeumganghwa-tang by high performance liquid chromatography-diode array detection. Pharmacogn Mag. 2014;10:S256. doi: 10.4103/0973-1296.133267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brito SM, Coutinho HD, Talvani A, Coronel C, Barbosa AG. Analysis of bioactivities and chemical composition of Ziziphus joazeiro Mart. using HPLC-DAD. Food Chem. 2015;186:185–91. doi: 10.1016/j.foodchem.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 16.Summa S, Magro SL, Armentano A, Muscarella M. Development and validation of an HPLC/DAD method for the determination of 13 sulphonamides in eggs. Food Chem. 2015;187:477–84. doi: 10.1016/j.foodchem.2015.04.070. [DOI] [PubMed] [Google Scholar]
- 17.Weon JB, Ma JY, Yang HJ, Lee B, Yun BR, Ma CJ. Qualitative and quantitative analysis of nine major compounds in the Bozhougyiqi-Tang using a high-performance liquid chromatography coupled with a diode array detector and electrospray ionization mass spectrometer. Pharmacogn Mag. 2013;9:271. doi: 10.4103/0973-1296.113291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Remane D, Grunwald S, Hoeke H, Mueller A, Roeder S, von Bergen M, et al. Validation of a multi-analyte HPLC-DAD method for determination of uric acid, creatinine, homovanillic acid, niacinamide, hippuric acid, indole-3-acetic acid and 2-methylhippuric acid in human urine. J of Chromatogr B. 2015;998:40–44. doi: 10.1016/j.jchromb.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Tian S, Yu Q, Wang D, Upur H. Development of a rapid resolution liquid chromatography-diode array detector method for the determination of three compounds in Ziziphora clinopodioides Lam from different origins of Xinjiang. Pharmacogn Mag. 2012;8:280. doi: 10.4103/0973-1296.103653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu LL, Hou S, Zheng TT, Zhang XL, Wei JH. Simultaneous determination of five hydrophilic and lipophilic components from roots of Salvia miltiorrhiza by HPLC. Chin Herbal Med. 2015;1:013. [Google Scholar]
- 21.Lee B, Weon JB, Yun BR, Lee J, Eom MR, Ma CJ. Simultaneous determination of five major compounds in the traditional medicine Pyeongwee-San by high performance liquid chromatography-diode array detection and liquid chromatography-mass spectrometry/mass spectrometry. Pharmacogn Mag. 2014:10–S22. doi: 10.4103/0973-1296.127335. [DOI] [PMC free article] [PubMed] [Google Scholar]


