Abstract
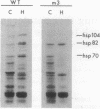
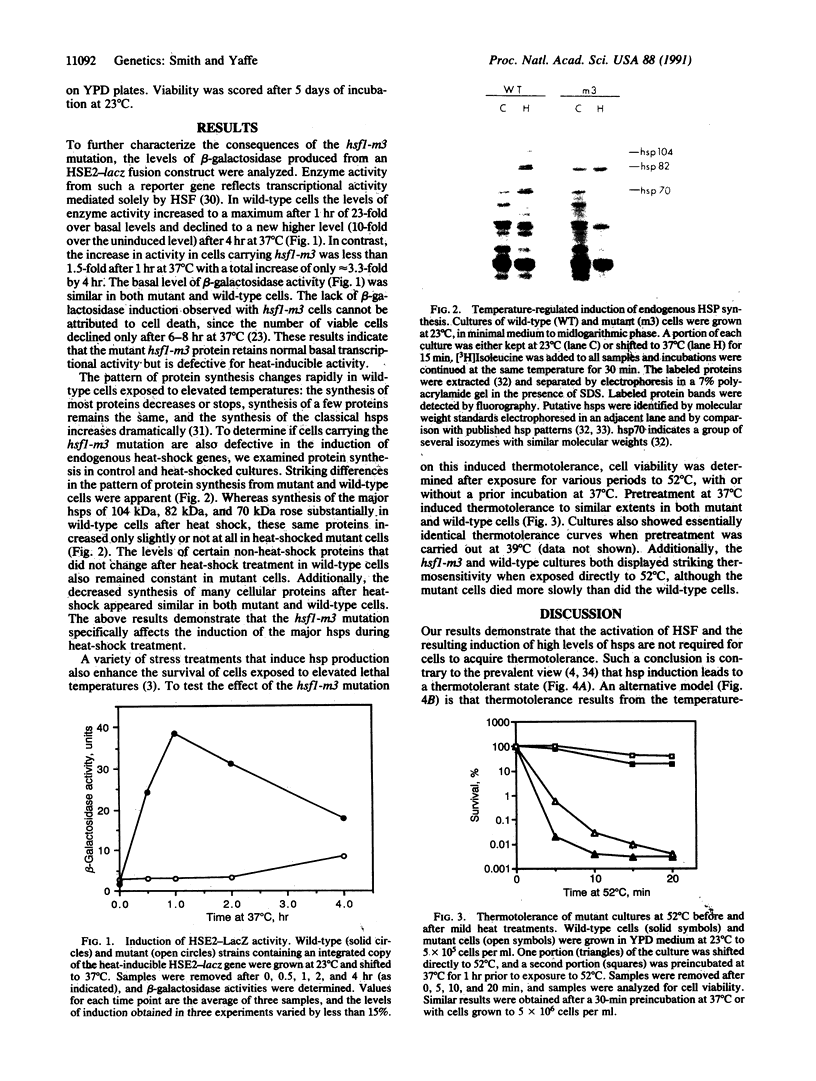
Exposure of cells to elevated temperatures causes a rapid increase in the synthesis of heat shock proteins (hsps) and induces thermotolerance, the increased ability of cells to survive exposure to lethal temperatures; however, the connection between hsp induction and the acquisition of thermotolerance is unclear. hsp induction in the yeast Saccharomyces cerevisiae is mediated by the activation of heat-shock transcription factor, and recently we have described a mutation, hsf1-m3, in heat-shock transcription factor that prevents the factor's activation. We now demonstrate that this mutation results in a general block in heat-shock induction but does not affect the acquisition of thermotolerance. Our results indicate that high-level induction of the major hsps is not required for cells to acquire thermotolerance.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman E., Kumamoto C. A., Emr S. D. Heat-shock proteins can substitute for SecB function during protein export in Escherichia coli. EMBO J. 1991 Feb;10(2):239–245. doi: 10.1002/j.1460-2075.1991.tb07943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes C. A., Johnston G. C., Singer R. A. Thermotolerance is independent of induction of the full spectrum of heat shock proteins and of cell cycle blockage in the yeast Saccharomyces cerevisiae. J Bacteriol. 1990 Aug;172(8):4352–4358. doi: 10.1128/jb.172.8.4352-4358.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Cavicchioli R., Watson K. Loss of heat-shock acquisition of thermotolerance in yeast is not correlated with loss of heat-shock proteins. FEBS Lett. 1986 Oct 20;207(1):149–152. doi: 10.1016/0014-5793(86)80030-8. [DOI] [PubMed] [Google Scholar]
- Cheng M. Y., Hartl F. U., Martin J., Pollock R. A., Kalousek F., Neupert W., Hallberg E. M., Hallberg R. L., Horwich A. L. Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature. 1989 Feb 16;337(6208):620–625. doi: 10.1038/337620a0. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Schekman R. The role of stress proteins in membrane biogenesis. Trends Biochem Sci. 1988 Oct;13(10):384–388. doi: 10.1016/0968-0004(88)90180-6. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Guarente L., Ptashne M. Fusion of Escherichia coli lacZ to the cytochrome c gene of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2199–2203. doi: 10.1073/pnas.78.4.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G. Yeast thermotolerance does not require protein synthesis. J Bacteriol. 1983 Dec;156(3):1363–1365. doi: 10.1128/jb.156.3.1363-1365.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Slater M. R., Craig E. A. Saccharomyces cerevisiae contains a complex multigene family related to the major heat shock-inducible gene of Drosophila. Mol Cell Biol. 1982 Nov;2(11):1388–1398. doi: 10.1128/mcb.2.11.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Wheeler S. A. Chromatin-associated heat shock proteins of Dictyostelium. Dev Biol. 1982 Apr;90(2):412–418. doi: 10.1016/0012-1606(82)90390-6. [DOI] [PubMed] [Google Scholar]
- McAlister L., Finkelstein D. B. Heat shock proteins and thermal resistance in yeast. Biochem Biophys Res Commun. 1980 Apr 14;93(3):819–824. doi: 10.1016/0006-291x(80)91150-x. [DOI] [PubMed] [Google Scholar]
- Meluh P. B., Rose M. D. KAR3, a kinesin-related gene required for yeast nuclear fusion. Cell. 1990 Mar 23;60(6):1029–1041. doi: 10.1016/0092-8674(90)90351-e. [DOI] [PubMed] [Google Scholar]
- Murakami H., Pain D., Blobel G. 70-kD heat shock-related protein is one of at least two distinct cytosolic factors stimulating protein import into mitochondria. J Cell Biol. 1988 Dec;107(6 Pt 1):2051–2057. doi: 10.1083/jcb.107.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolet C. M., Craig E. A. Inducing and assaying heat-shock response in Saccharomyces cerevisiae. Methods Enzymol. 1991;194:710–717. doi: 10.1016/0076-6879(91)94052-e. [DOI] [PubMed] [Google Scholar]
- Nieto-Sotelo J., Wiederrecht G., Okuda A., Parker C. S. The yeast heat shock transcription factor contains a transcriptional activation domain whose activity is repressed under nonshock conditions. Cell. 1990 Aug 24;62(4):807–817. doi: 10.1016/0092-8674(90)90124-w. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Bienz M. A synthetic heat-shock promoter element confers heat-inducibility on the herpes simplex virus thymidine kinase gene. EMBO J. 1982;1(11):1473–1477. doi: 10.1002/j.1460-2075.1982.tb01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesofsky-Vig N., Brambl R. Heat shock response of Neurospora crassa: protein synthesis and induced thermotolerance. J Bacteriol. 1985 Jun;162(3):1083–1091. doi: 10.1128/jb.162.3.1083-1091.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler I., Chiang A., Kurihara T., Rothblatt J., Way J., Silver P. A yeast gene important for protein assembly into the endoplasmic reticulum and the nucleus has homology to DnaJ, an Escherichia coli heat shock protein. J Cell Biol. 1989 Dec;109(6 Pt 1):2665–2675. doi: 10.1083/jcb.109.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Y., Lindquist S. L. HSP104 required for induced thermotolerance. Science. 1990 Jun 1;248(4959):1112–1115. doi: 10.1126/science.2188365. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J. Heat shock proteins. J Biol Chem. 1990 Jul 25;265(21):12111–12114. [PubMed] [Google Scholar]
- Smith B. J., Yaffe M. P. A mutation in the yeast heat-shock factor gene causes temperature-sensitive defects in both mitochondrial protein import and the cell cycle. Mol Cell Biol. 1991 May;11(5):2647–2655. doi: 10.1128/mcb.11.5.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P. K., Pelham H. R. Purification and characterization of a heat-shock element binding protein from yeast. EMBO J. 1987 Oct;6(10):3035–3041. doi: 10.1002/j.1460-2075.1987.tb02609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P. K., Pelham H. R. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988 Sep 9;54(6):855–864. doi: 10.1016/s0092-8674(88)91219-6. [DOI] [PubMed] [Google Scholar]
- Sorger P. K. Yeast heat shock factor contains separable transient and sustained response transcriptional activators. Cell. 1990 Aug 24;62(4):793–805. doi: 10.1016/0092-8674(90)90123-v. [DOI] [PubMed] [Google Scholar]
- Vogel J. P., Misra L. M., Rose M. D. Loss of BiP/GRP78 function blocks translocation of secretory proteins in yeast. J Cell Biol. 1990 Jun;110(6):1885–1895. doi: 10.1083/jcb.110.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson K., Dunlop G., Cavicchioli R. Mitochondrial and cytoplasmic protein syntheses are not required for heat shock acquisition of ethanol and thermotolerance in yeast. FEBS Lett. 1984 Jul 9;172(2):299–302. doi: 10.1016/0014-5793(84)81145-x. [DOI] [PubMed] [Google Scholar]
- Widelitz R. B., Magun B. E., Gerner E. W. Effects of cycloheximide on thermotolerance expression, heat shock protein synthesis, and heat shock protein mRNA accumulation in rat fibroblasts. Mol Cell Biol. 1986 Apr;6(4):1088–1094. doi: 10.1128/mcb.6.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederrecht G., Seto D., Parker C. S. Isolation of the gene encoding the S. cerevisiae heat shock transcription factor. Cell. 1988 Sep 9;54(6):841–853. doi: 10.1016/s0092-8674(88)91197-x. [DOI] [PubMed] [Google Scholar]
- Wu C., Wilson S., Walker B., Dawid I., Paisley T., Zimarino V., Ueda H. Purification and properties of Drosophila heat shock activator protein. Science. 1987 Nov 27;238(4831):1247–1253. doi: 10.1126/science.3685975. [DOI] [PubMed] [Google Scholar]
- Yaffe M. P. Analysis of mitochondrial function and assembly. Methods Enzymol. 1991;194:627–643. doi: 10.1016/0076-6879(91)94046-f. [DOI] [PubMed] [Google Scholar]