Abstract
Background:
Gentiana crassicaulis ( ) is an important traditional Chinese herb. Like other herbs, its chemical compounds vary greatly by the environmental and genetic factors, as a result, the quality is always different even from the same region, and therefore, the quality evaluation is necessary for its safety and effective use. In this study, a comprehensive method including HPLC quantitative analysis and fingerprints was developed to evaluate the quality of Cujingqinjiao and to classify the samples collected from Lijiang City of Yunnan province. A total of 30 common peaks including four identified peaks, were found, and were involved for further characterization and quality control of Cujingqinjiao. Twenty-one batches of samples from Lijiang City of Yunnan Province were evaluated by similarity analysis (SA), hierarchical cluster analysis (HCA), principal component analysis (PCA) and factor analysis (FA) according to the characteristic of common peaks.
) is an important traditional Chinese herb. Like other herbs, its chemical compounds vary greatly by the environmental and genetic factors, as a result, the quality is always different even from the same region, and therefore, the quality evaluation is necessary for its safety and effective use. In this study, a comprehensive method including HPLC quantitative analysis and fingerprints was developed to evaluate the quality of Cujingqinjiao and to classify the samples collected from Lijiang City of Yunnan province. A total of 30 common peaks including four identified peaks, were found, and were involved for further characterization and quality control of Cujingqinjiao. Twenty-one batches of samples from Lijiang City of Yunnan Province were evaluated by similarity analysis (SA), hierarchical cluster analysis (HCA), principal component analysis (PCA) and factor analysis (FA) according to the characteristic of common peaks.
Results:
The obtained data showed good stability and repeatability of the chromatographic fingerprint, similarity values were all more than 0.90. This study demonstrated that a combination of the chromatographic quantitative analysis and fingerprint offered an efficient way to quality consistency evaluation of Cujingqinjiao. Consistent results were obtained to show that samples from a same origin could be successfully classified into two groups.
Conclusion:
This study revealed that the combinative method was reliable, simple and sensitive for fingerprint analysis, moreover, for quality control and pattern recognition of Cujingqinjiao.
SUMMARY
HPLC quantitative analysis and fingerprints was developed to evaluate the quality of Gentiana crassicaulis
Similarity analysis, hierarchical cluster analysis, principal component analysis and factor analysis were employed to analysis the chromatographic dataset.
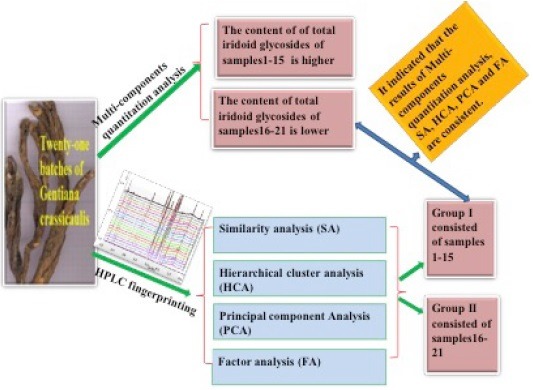
The results of multi-components quantitation analysis, similarity analysis, hierarchical cluster analysis, principal component analysis and factor analysis were consistent.
All samples could be classified into two groups, which could to some extent reflect the quality differences of theses samples.
Abbreviations used: SA: Similarity analysis, HCA: Hierarchical cluster analysis, PCA :Principal component Analysis, FA :Factor analysis
Keywords: Gentiana crassicaulis, HPLC fingerprint, quality evaluation, multi-components quantitation
INTRODUCTION
Gentianae macrophyllae Radix, also known as Qinjiao in Chinese, has long been used as an important herb in traditional Chinese medicine. According to Chinese Pharmacopeia (2015, 1st volume), it consists of the dried root of Gentiana macrophylla Pall, Gentiana straminea Maxim, Gentiana crassicaulis Duthie ex Burk(Cujingqinjiao) and Gentiana dahurica Fisch.[1] Among these species, Cujingqinjiao was known for best quality and was officially listed in the Chinese Pharmacopoeia under the name Radix Gentianae Macrophyllae and have been frequently used to dispel rheumatism and ease pain.[2,3] Cujingqinjiao was widely distributed in the cold region of southeastern Tibet, northwest Yunnan, northwest Sichuan and Guizhou, southeastern Qinghai and southern Gansu.[4] Especially, the products from Yunnan, were regarded as higher quality than others, and was widely cultivated in Lijiang City of Yunnan Province as raw material of Radix Gentianae Macrophyllae due to the high demand of this herbal medicine.[5,6,7] The cultivated environment could seriously influence the content of the active components and yield of Chinese herbs,[8] However, for a long time, there has been no comprehensive method for the quality control of Cujingqinjiao from the genuine production area of Lijiang, which has seriously affected the development and exchange of Cujingqinjiao.
Cujingqinjiao contained a variety of chemical components, which mainly included iridoid glycoside, triterpene, sterol, flavonoids, and phenolic acids. Iridoid glycosides acknowledged to be the principal bioactive components, which include gentiopicroside (GT), loganic acid (LA), swertiamarin (ST) and sweroside (SS).[9,10,11] Comparison of the active components among different Cujingqinjiao represented a good method to achieve standardisation and quality control of them. However, it was insufficient to determine merely one or two markers for completely evaluating the inner quality of Cujingqinjiao. Therefore, a comprehensive and systematic standard for the quality assessment of Cujingqinjiao is imperative. Chromatographic fingerprinting analysis was one of the effective methods, which had been used for identification and assessment of the stability of Radix Gentianae Macrophyllae from G. macrophylla, G. straminea and Cujingqinjiao.[12,13,14,15,16] Moreover, it had been used to comparative analysis of Radix Gentianae Macrophyllae and their related substitutes.[17] But, a comparison of fingerprints for the further assessment of Cujingqinjiao from the same origin had not been reported under the same chromatographic conditions.
In order to develop a direct, rapid and reliable method for quantitative analysis and evaluation the quality of Cujingqinjiao comprehensively, in this study, we developed an optimized HPLC chromatographic fingerprint method to make a holistic and detailed comparison of the Cujingqinjiao samples; meanwhile, the contents of the major constituents (total iridoid glycosides including loganic acid, swertiamarin, gentiopicroside and sweroside) in all of the samples were simultaneously determined by HPLC quantitative analysis. According to the characteristic of common peaks in fingerprint chromatograms the SA, HCA, PCA and FA were performed to classify the samples.
MATERIALS AND METHODS
Materials and Reagents
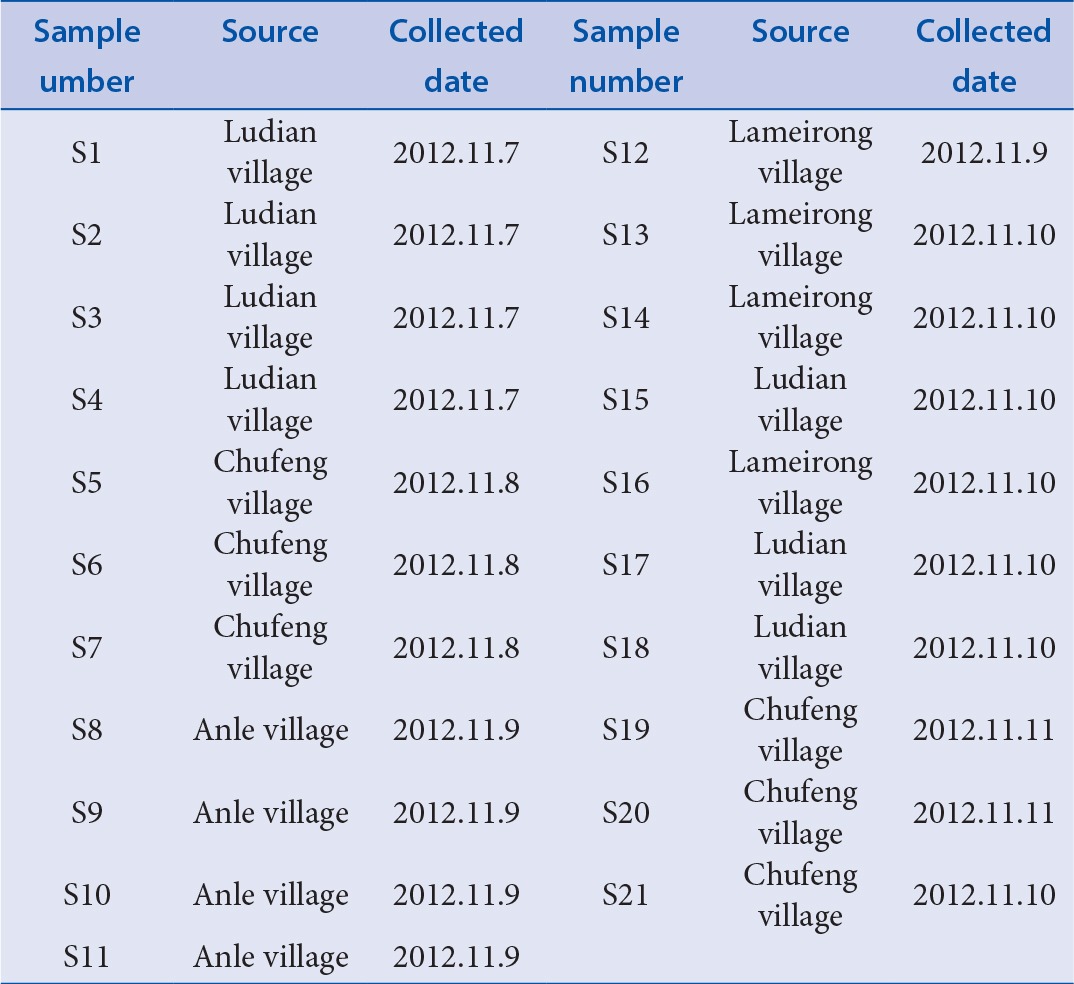
Twenty-one batches of samples of roots of Cujingqinjiao [Table 1] were collected from Lijiang City, Yunnan Province of China. The roots were washed with distilled water, dried at 45°C and powdered to homogeneous size. The samples were identified by Professor Xing-fu Chen, College of Agronomy, Sichuan Agriculture University.
Table 1.
Sources of Cujingqinjiao samples
Standard substances of swertiamarin and gentiopicroside were purchased from the Must Bio-Technology Co.Ltd (Chengdu, China), loganic acid was obtained from the Food and Drug Verification Research Institute of China (Beijing, China), sweroside was obtained from the China Pharmaceutical Biological Products Analysis Institute (Beijing, China), HPLC grade methanol and phosphoric acid were purchased from Kelong Chemical Factory (Chengdu, China), water was generated from a Millipore ultrapure water system.
Methods
Preparation of solutions
Each powdered sample (0.500g) was accurately weighted in a stopped conical flask. 10 mL ethanol–water (1:3) was added, each sample was repeated three times. Then the mixture was supersonic extracted for 30 min, and then adjusted to the initial weight by adding ethanol–water (1:3) as per the requirement. The extracts were filtered through a syringe filter (0.45 μm) prior to HPLC analysis.
Stock solutions of the reference standards (loganic acid, swertiamarin, gentiopicroside, and sweroside) were prepared by accurately weighting and dissolving them in ethanol, and then a mixed stock solution was prepared. The mixed working standard solution was then diluted to appropriate concentration ranges for the establishment of calibration curves. These solutions were all stored at 4°C and brought to room temperature before use.
Apparatus and chromatographic conditions
A Shimadzu Corporation (Japan) LC-2010A HPLC system with an UV detector was used to obtain HPLC fingerprints and quantitative chromatograms.
The chromatographic separation was carried out on a Sepax Gp-C18 column (150 mm × 4.6 mm, 5 μm; Promptar Co. Ltd, China) maintained at 25°C. The mobile phase was methanol (A) and 0.1% phosphoric acid aqueous solution (B) with a gradient program as follows: 0–35 min, linear gradient 0–15% A; 35–55 min, 15% A; 55–65 min, linear gradient 15–40% A and 65–90min, linear gradient 40–85% A. The flow rate was 1.0 mL.min-1. The UV absorbance was monitored at 254 nm. All injection volume of sample and standard solutions were 10 μL.
Data analysis
Similarity tests were performed by the software named Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine (version 2004A), The HCA was conducted by SPSS19.0 software, PCA was performed on the common chromatographic peaks in the HPLC fingerprints using software of SIMCA-P11, and factor analysis was performed by SPSS19.0 software.
RESULTS AND DISCUSSION
Optimization of extraction conditions
Extraction solvents (e.g., water, methanol and ethanol), solvent volumes (e.g., 10, 15 and 20 mL), extraction time (e.g., 20, 30, 40, and 50 min), were investigated to obtain the optimized extraction efficiency. The results showed that ethanol–water (6:4) was the most effective solvent for extracting chemical markers. 10 mL solvent volume, extraction for 30 min was the appropriate condition.
Optimization of HPLC conditions
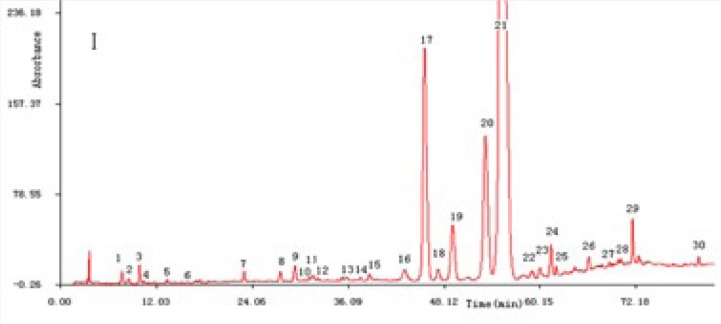
Based on the previously known procedure,[18,19] the combination of different mobile phase solvents (acetonitrile-water and methanol-water with different modifiers, including phosphoric acid and acetic acid) were used. Column temperatures (20, 25 and 5°C) and wavelength (230, 254 and 270 nm) were examined and compared. The results showed that most components had adequate absorption and no interference at wavelength 254 nm; when water contained 0.1% phosphoric acid and methanol was selected as a mobile phase with a step-linear gradient, good resolution and symmetric peak shape were obtained. Moreover, column temperature maintained at 25 °C, flow rate set at 1 mL.min-1, injection volume of 10 μL could provide good resolution and acceptable peaks parameters. Representative HPLC profiles for the active components were well separated and shown in Figure 1, the peaks 17, 19, 21 and 24 were loganic acid, swertiamarin, gentiopicroside, and sweroside, respectively.
Figure 1.
Sample of Cujingqinjiao, peaks 17, 19, 21 and 24 were loganic acid, swertiamarin, gentiopicroside and sweroside.
Method validation
Specificity
The specificity was evaluated by comparing the consistency of the retention time of each analyte between a sample and the corresponding reference standard. The integration peaks in the chromatogram of the sample solution were corresponding to the peaks in the chromatogram of the standard solution [Figure 1].
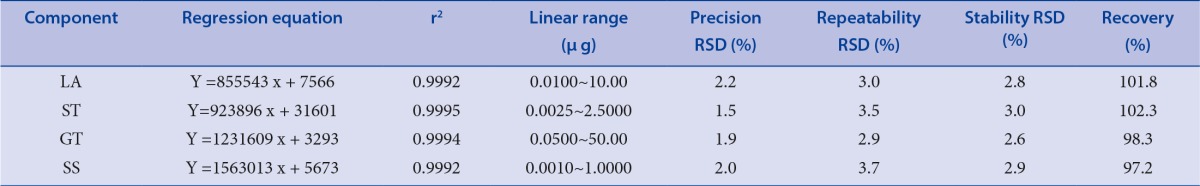
Linearity, repeatability, precision, accuracy, and stability
The calibration curves results were calculated and shown in Table 2. The four analytes had good linearity (r2 > 0.999) in a wide range of concentrations. Precision, repeatability, and stability results were listed in Table 2. The RSD values of the four compounds were all less than 3.7%, which indicated that the system was excellent. The recovery of the method was in the range of 97.2–102.3%, with RSD less than 3.9% shown in Table 2. Seven injections of the same sample solution stored for 0, 2, 4, 6, 8, 10 and 12 h were processed for the evaluation of sample stability. RSD of peaks area were less than 3.0%, indicating that the sample solution was stable within the tested time period. Seven different solutions prepared from the same sample were analyzed to confirm the repeatability.
Table 2.
Calibration curves, precision, repeatability, and recoveries of four bioactive compounds in samples (n = 7)
Contents of the four marker compounds in samples
Iridoid glycosides exist widely in species of Gentianaceae and show a wide variety of biological activities.[20] The developed analytical method was successfully applied to the simultaneous determination of the total iridoid glycosides (loganic acid, swertiamarin, gentiopicroside and sweroside) in all samples. Peaks in the chromatograms were identified by comparing the retention times with the standards.
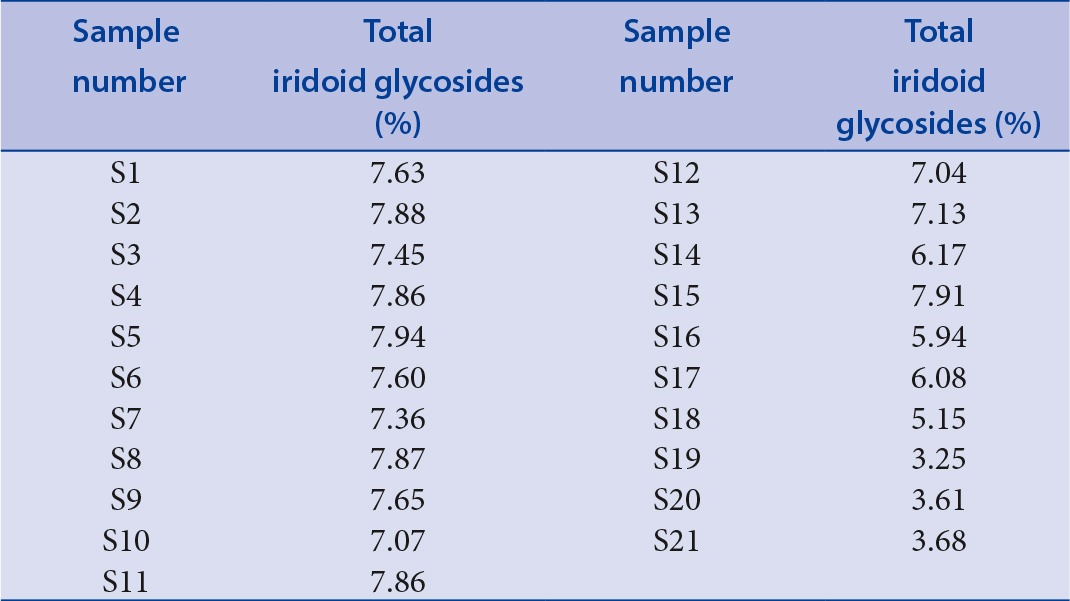
Table 3 shows the contents of the total iridoid glycosides in all samples, and the contents varied greatly among the different samples. Iridoid glycosides is the well-known major active constituents in Gentian root, and the pharmacological properties of G. macrophylla have been mainly attributed to these bioactive components.[21] The content of total iridoid glycosides ranged from 3.25% to 7.91% with an average of 6.67%, sample 15 possessed the highest content of iridoid glycosides among all the samples. All the samples were qualified according to Chinese Pharmacopoeia, as their gentiopicroside content were above 2%. However, the content of total iridoid glycosides of samples1–15 (6.17–7.91%) was higher than samples16–21 (3.25–6.08%), revealing that the quality of samples 1–15 were better than that of samples 16–21.
Table 3.
The content of total iridoid glycosides in samples
Establishment of chromatographic fingerprint of Cujingqinjiao and similarity analysis
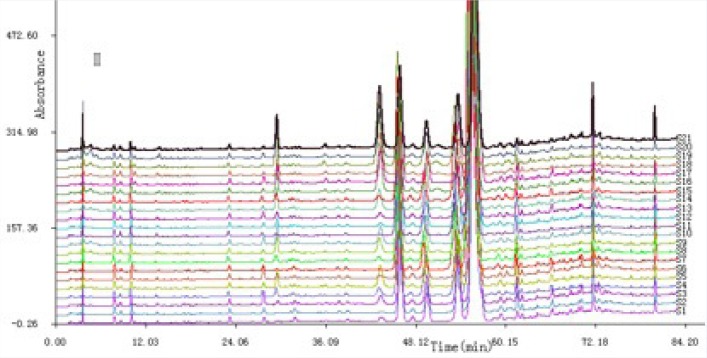
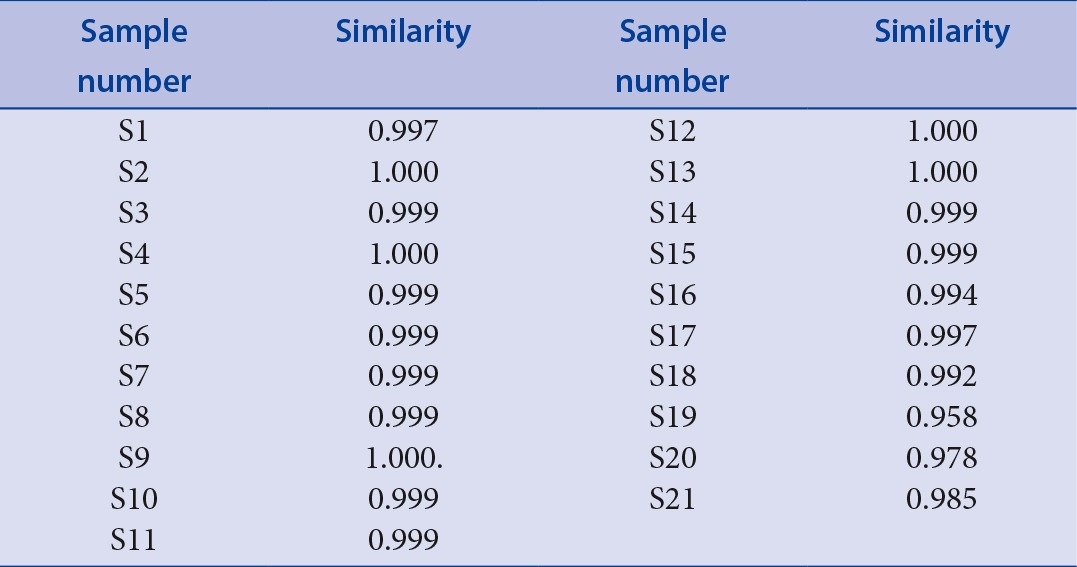
The HPLC fingerprints of the twenty-one batches of Cujingqinjiao samples were also detected at the UV absorption of 254 nm, and grouped as in Figure 2. Peaks existing in all chromatograms were assigned as “common peaks”, 30 common characteristic peaks in the 21 chromatograms were selected. It was found that these samples had similar HPLC profiles. Similarity analysis was conducted based on the standard fingerprints, and the results were shown in Table 4. Four peaks were identified, cornpared with standard compounds, including loganic acid,[17] swertiamarin,[19] gentiopicroside,[21] and sweroside.[24] If the similarity value over a certain value were regarded as the threshold for qualification, it was easy to identify the qualified samples according to the chromatographic fingerprint. The similarity values of all Cujingqinjiao samples were more than 0.959, while some small differences existed. Similarity values of samples 1–15 (0.997–1.000) were higher than that of samples 16–21 (0.959–0.997). Moreover, the contents of total iridoid glycosides of samples 1–15 were higher than that of samples 16–21 [Table 3]. The results indicated that the samples had higher similarity values, showing the internal quality of them were better.
Figure 2.
Chromatographic fingerprints of all samples
Table 4.
The similarities of chromatograms of samples
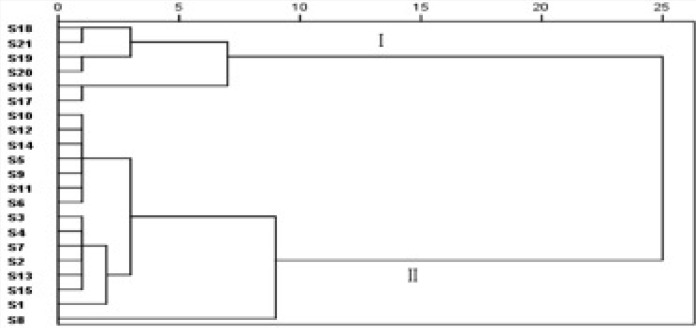
HCA of the Cujingqinjiao samples
Hierarchical cluster analysis (HCA) is a multivariate analysis technique that is used to sort samples into groups.[22] Different samples of Cujingqinjiao were analyzed by using SPSS 19.0. HCA was carried out following the basis of thirty common peaks values of the HPLC fingerprints. A dendrogram was generated [Figure 3], which revealed the relationship among the samples. The result showed clearly that these samples were classified into two quality clusters [Figure 3]. Cluster I was formed by the samples 16–21, cluster II consisted of the samples 1–15. Samples 16–21 were classified into the same group, which might be mostly due to their similarity values were relatively lower than that of samples 1–15, and their content of total iridoid glycosides were also relatively lower than that of samples 1–15. According to Figure 3, Table 3 and Table 4, it could be revealed that samples with closer similarities had a shorter Squared Euclidean distance, as well, their contents of effective components were similar. It indicated that the results of HCA were consistent with those of quantitative analysis and SA.
Figure 3.
Dendrograms of cluster analysis for the samples
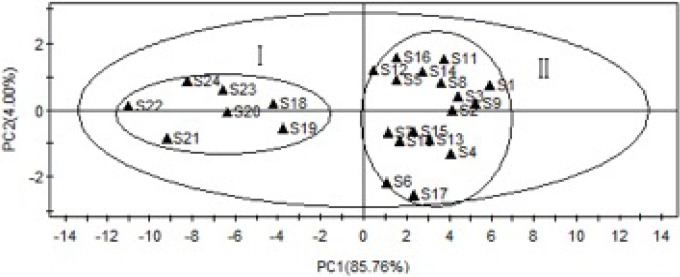
Principal component analysis of the Cujingqinjiao samples
Principal component analysis (PCA) was useful to study the relationships among the independent variables, which was used to separate inter-relationships into statistically independent,[23] here PAC was performed by using SIMCA-P11.0 software. The full 21 × 30 auto scaled data matrix was subjected to PCA analysis. The first principal component and the second principal component described 85.76 % and 4.00 % of the variability in the original observations, respectively. The first two principal components explained more than 89.00 % of the total variance [Figure 4]. Therefore, the first two principal components concentrated the multidimensional information to classify the samples. According to the difference of the PC1 values, all the samples can be classified into two groups by the scatter plots of PCA, namely group I and group II, and the result was similar to that of HCA. Moreover, the contents of total iridoid glycosides from group II were higher than group I, indicating that the same group had similar internal quality. The result was corresponding with the SA and same to HCA. These results of PCA and HCA could be validated each other and provided more references for the quality evaluation of Cujingqinjiao.
Figure 4.
PCA scatter plot scores for the samples.
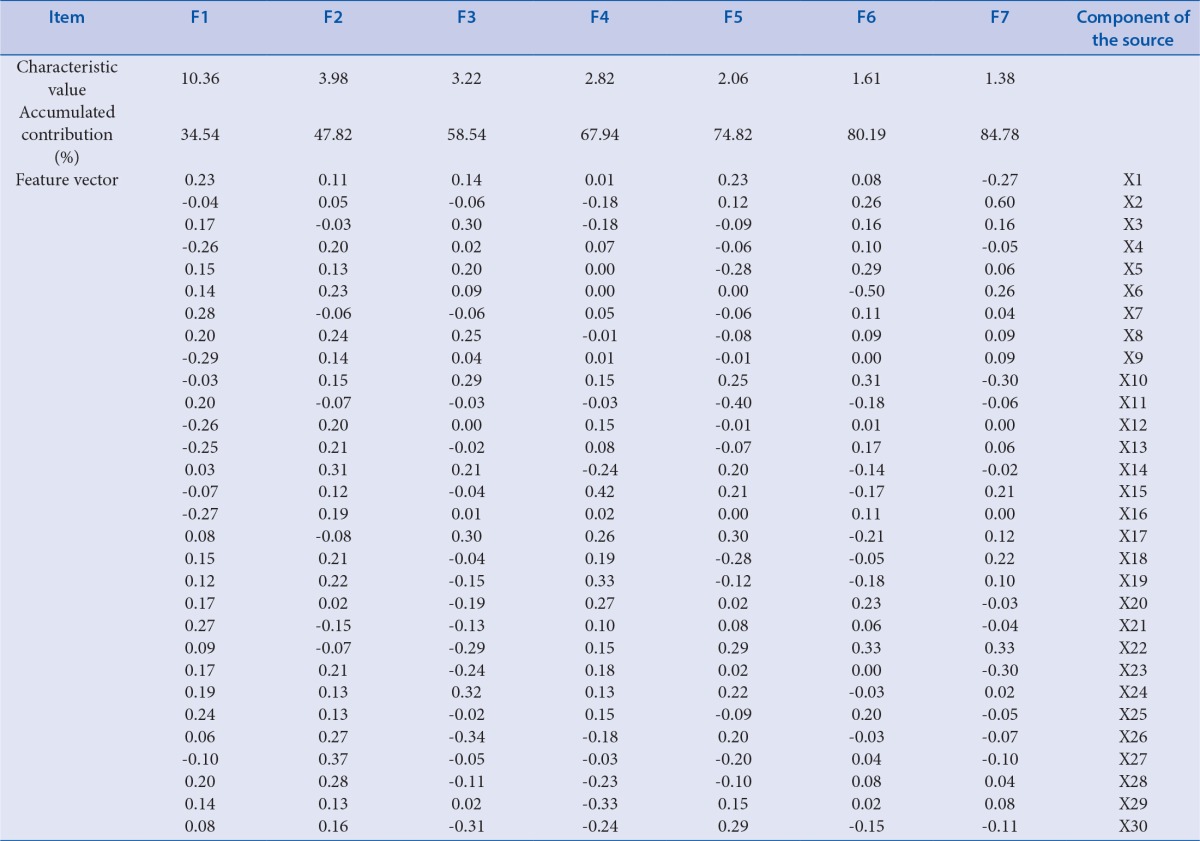
Further factor analysis was performed by SPSS19.0 for the thirty common peaks area of all samples. Factoring types include canonical factor analysis (CFA), principal component analysis (PCA) and others. Here, PCA was employed as the method of FA. The factor analysis displayed the first seven principal components (F1, F2, F3, F4, F5, F6, and F7), were chosen as the representative, and they can explained 84.78 % information of 30 active constituents, the results are shown in Table 5 (X represented the chromatographic peaks). Therefore, the seven variables could instead of the original thirty, and could be used for further quality assessment of all samples. According to the feature vector, the principal components could be severally expressed as follow:
Table 5.
Characteristic value, accumulated contribution and feature vector
F1=0.23ZX1 - 0.04ZX2 - … + 0.08ZX30
F2=0.11ZX1 + 0.05ZX2 + … + 0.16 ZXX30
F3=0.14ZX1 - 0.60ZX2 + … - 0.31ZX30
(ZXi was the standardized value of the primary variables Xi)
According to the variance contribution rate of each principal component to get the comprehensive evaluation function of principal values (F):
F=0.3454F1 + 0.1328F2 + 0.1072F3 + 0.0941F4 + 0.0688F5 + 0.0537F6 + 0.0459F7
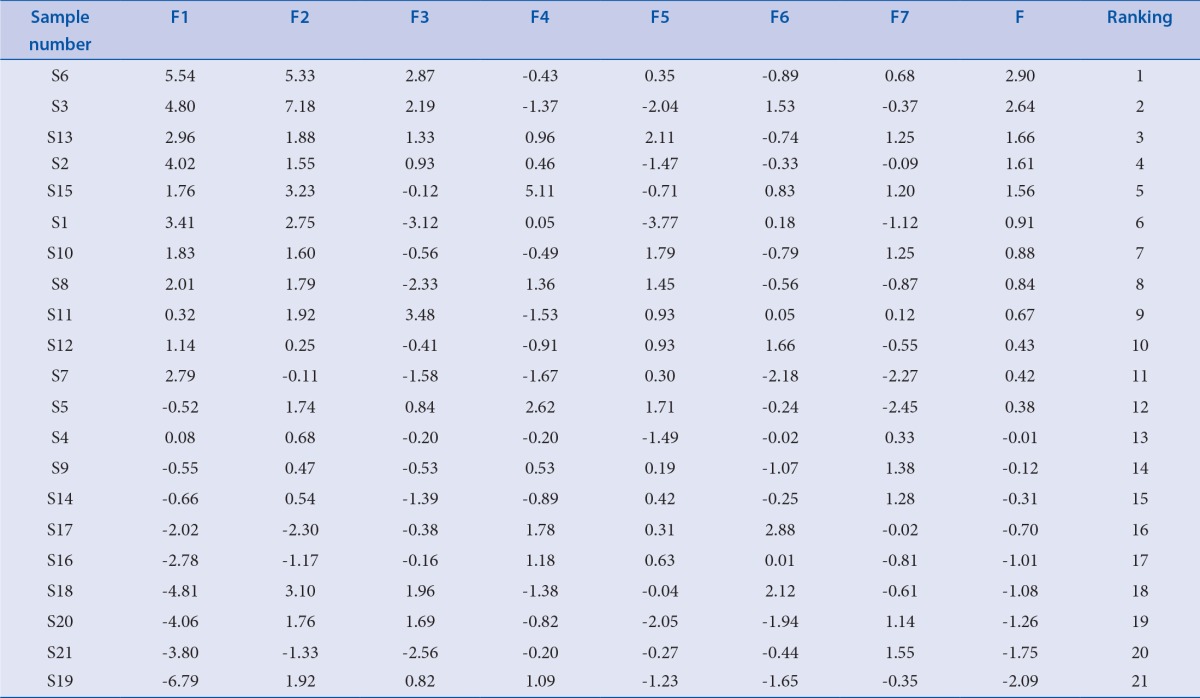
According to the above function the comprehensive principal component value (F) of each sample were calculated and sequenced, results showed in Table 6. The result indicated that samples 1–15 were ranking well and samples 16–21 were poorly, it was consistent with that of the SA, HCA and PCA.
Table 6.
Comprehensive principal value and rank of samples
CONCLUSION
In this study, an HPLC method was developed for simultaneously quantifying total iridoid glycosides and investigating the variance of chemical components among different samples of Cujingqinjiao from Lijiang City of Yunnan province. The HPLC data showed that, twenty-one batches of samples could be classified into two groups. One consisted of samples 1–15, the other consisted of samples 16–21, which could reflect the quality differences of these samples to some extent. For total iridoid glycosides, samples1–15 were better than the samples 16–21. Meanwhile, similarity values of samples 1–15 (0.997–1.000) were higher than that of samples 16–21 (0.959–0.997), the comprehensive principal component values (F) of samples 1–15 (0.31–2.90) were higher than that of samples 16–21 (-2.09–0.70). It indicated that the results of multi-components quantitation analysis, SA, HCA and PCA were consistent. Thus, it is easy to evaluate and quality control of Cujingqinjiao and its related products based on the chromatographic fingerprint.
The results revealed that quantitation of pharmacologically active components combined with chromatographic fingerprints analysis using SA, HCA, PCA and FA offers an efficient method for monitoring consistency in the quality of Cujingqinjiao, and it had been produced desirable results with high consistency. A lot of references through fingerprint combined with clustering analysis and principal component analysis to identify species or origins of Chinese medicines were reported.[24,25,26] However, it was difficult to classify the Cujingqinjiao samples that from same sources. This study provided an example for identification and quality evaluation by using a combination of HPLC fingerprint and quantitative analyses. The presented method suggested that the quality of different samples for sale was not consistent, and it might be helpful for discriminating Cujingqinjiao. Cujingqinjiao from Lijiang City of Yunnan province occupied an important place in Gentiana resource. Thus, it would be significant to classify and evaluate them.
Financial support and sponsorship
This research was supported by the Scientific Reseach Fund of Leshan Normal University (grant No. Z1516), the Scientific Reseach Fund of Leshan Normal University (grant No. Z1305), Education Department of Sichuan Province (grant No. 15ZA0283) and Education Department of Sichuan Province (grant No.14CZ0024)
Conflicts of interest
The authors have no conflict of interests.
Acknowledgement
Our special thanks are due to Ms. Qiong Tang for proofreading on the manuscript.
REFERENCES
- 1.Chinese Pharmacopoeia Commission. Pharmacopoeia of People's Republic of China. Beijing: Chemical Industry Press; 2015. pp. 253–54. [Google Scholar]
- 2.Tan RX Wolfender, JX Ma, WG Fuzzati, Marston N, A Hostettmann K. Acyl secoiridoids and antifungal constituents from Gentiana macrophylla. Phytochemistry. 1996;42:1305–13. doi: 10.1016/0031-9422(96)00149-5. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Liu JC, Zhang XN, Guo YY. Downregulation of NR2B receptors partially contributes to analgesie effects of gentiopicroside in persistent inflammatory pain. Neuropharmacology. 2008;54:1175–81. doi: 10.1016/j.neuropharm.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Li HW, S W, Annie Bligh, Leon Christine J. Chemotaxonomically significant roburic acid from section cruciata of Gentiana. Biochem Syst Ecol. 2012;43:152–55. [Google Scholar]
- 5.Wu JR, Zhao ZL, Wang ZT. Determination of organic matter and inorganic elements in radix Gentianae crassicaulis and the growing soil. Chin J Inf Tradit Chin Med. 2010;17:39–40. [Google Scholar]
- 6.Chen LY, Chen QL, Xu D, Hao JG, Michael S, Xu ZQ. Changes of gentiopicroside synthesis during somatic embryogenesis in Gentiana maceophylla. Planta Med. 2009;75:1618–24. doi: 10.1055/s-0029-1185808. [DOI] [PubMed] [Google Scholar]
- 7.Zheng P, Zhang KJ, Wang ZZ. Genetic diversity and gentiopicroside content of four Gentian a species in China revealed by ISSR and HPLC methods. Biochem Syst Ecol. 2011;39:704–10. [Google Scholar]
- 8.Yu CL, Luo SG, Peng XL, Liu YY. Effects of boron, zinc, and iron on the gentiopicroside content and yield of Gentian. Pedosphere. 2006;16:210–14. [Google Scholar]
- 9.Wu WS, Ye HY, Tang MH. Using high-performance counter-current chromatography combined withpreparative high performance liquid chromatography for the separation of bioactive compounds from the water extract of Gentiana macrophylla Pall. Sep Sci Technol. 2012;47:762–68. [Google Scholar]
- 10.Wei SH, Chen G, He WF, Chi HD, Abe HK, Yamashita Koichi, et al. Inhibitory effect of secoiridoids from the roots of Gentian a straminea on stimulus-induced superoxide generation, phosphorylation and translocation of cytosolic compounds to plasma membrane in human neutrophils. Phytother Res. 2012;26:168–73. doi: 10.1002/ptr.3496. [DOI] [PubMed] [Google Scholar]
- 11.Fan H, Zang Y, Zhang Y, Zhang HF, Zhao Z, Hu JF. Triterpenoids and iridoid glycosides from Gentiana dahurica. Helvetica Chimica ACTA. 2010;93:2439–3447. [Google Scholar]
- 12.Cao XY, Li YH, Wang ZZ. Study on HPLC fingerprint of Gentiana macrophylla. J Chin Med Mater. 2008;31:498–1. [PubMed] [Google Scholar]
- 13.Chen QL, Shi ZY, Sun WJ. A study on HPLC fingerprint of Gentiana macrophylla from Shanxi. J Northwest Univ (Natural Science Edition) 2007;27:253–56. [Google Scholar]
- 14.Wu QX, An Y, Zhang MJ, Lu YC, Bao JY, Hu SQ, et al. HPLC fingerprint of Gentiana straminea, a traditional Chinese medicine from Qinghai province. Acta Bot Boreal-Occident Sin. 2006;26:174–78. [Google Scholar]
- 15.Li XM, He XR. HPLC fingerprint of Gentiana straminea Maxim. Chin Tradit Patent Med. 2004;26:4–7. [Google Scholar]
- 16.Wang YY, Zhao ZL, Wu JR, Wang ZT. HPLC fingerprint of Radix Gentianae Crassicaulis. Chin Tradit Herb Drugs. 2009;40:121–23. [Google Scholar]
- 17.Cao XY, Wang ZZ. Simultaneous determination of four iridoid and secoiridoid glycosides and comparative analysis of Radix Gentianae Macrophyllae and their related substitutes by HPLC. Phytochem Anal. 2010;21:348–54. doi: 10.1002/pca.1206. [DOI] [PubMed] [Google Scholar]
- 18.Hao BH, Sun WJ, Zhi ZH, Wang YY. Supersonic extraction of gentiopicrin in Gentiana Macrophylla pall and determination of its content by HPLC. J Northwest Univ (Natural Science Edition) 2004;34:81–84. [Google Scholar]
- 19.Song JH, Yang WY, Meng J, Li Y, Z Y, Cheng T, et al. HPLC with switching wavelength simultaneous determination of six constituents in Gentiana crassicaulis. Chem Res Appl. 2014;26:1136–40. [Google Scholar]
- 20.Kumarasamy Y, Nahar L, Cox PJ, Jaspars M, Sarker SD. Bioactivity of secoiridoid glycosides from Centaurium erythraea. Phytomedicine. 2003;10:344–47. doi: 10.1078/094471103322004857. [DOI] [PubMed] [Google Scholar]
- 21.Wang CH, Cheng XM, He YQ. Pharmacokinetic behavior of gentiopicroside from decoction of Radix Gentianae, Gentiana macrophylla after oral administration in rats: a pharmacokinetic comaprison with gentiopicroside after oral and intravenous administration alone. Arch Pharm Res. 2007;30:1149–54. doi: 10.1007/BF02980251. [DOI] [PubMed] [Google Scholar]
- 22.Kannel PR, Lee S, Kanel SR, Khan SP. Chemometric application in classification and assessment of monitoring locations of an urban river system. Anal Chim Acta. 2007;582:390–99. doi: 10.1016/j.aca.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Wang YX, Li Q, Wang Q, Li YJ, Ling JH, Liu LL, et al. Simultaneous determination of seven bioactive components in Oolong tea camellia sinensis: quality control by chemical composition and HPLC fingerprints. Agric Food Chem. 2012;60:256–60. doi: 10.1021/jf204312w. [DOI] [PubMed] [Google Scholar]
- 24.Liu XJ, Hu J, Li ZY, Qin XM, Zhang LZ, Guo XQ. Species classification and quality assessment of chaihu (RadixBupleuri) based on high-performance liquid chromatographic fingerprint and combined chemometrics methods. Arch Pharm Res. 2011;34:961–69. doi: 10.1007/s12272-011-0613-2. [DOI] [PubMed] [Google Scholar]
- 25.Chen CY, Chen JX, Li W, Li H, Yang B. Comparative chemical and statistical analysis of cultivated and wild Radix Scutellariae. AM J Chinese Med. 2011;39:1029–41. doi: 10.1142/S0192415X1100938X. [DOI] [PubMed] [Google Scholar]
- 26.Yang L, Su ZJ, Zeng X, Li X, Wu ZF. Quality assessment of Fructus xanthii based on fingerprinting using high-performance liquid chromatography. J AOAC INT. 2012;95:1053–58. doi: 10.5740/jaoacint.11-032. [DOI] [PubMed] [Google Scholar]