Abstract
Aim:
The present study evaluated the effect of ethanolic extract of Nardostachys jatamansi roots (NJet) on MYCN mediated regulation of expression of MDM2 and p53 proteins in neuroblastoma cell lines, IMR-32 and SK-N-MC.
Materials and Methods:
The effect of NJet on cell viability was determined by MTT; and on growth kinetics was evaluated by trypan blue dye exclusion method and soft agar assay. The expression of p53, MDM2 and MYCN proteins in response to NJet treatment was evaluated by immunoblotting.
Results:
NJet decreased the viability of neuroblastoma cells without affecting the viability of non-cancerous, HEK-293 cells. It altered the growth kinetics of the cancer cells in a dose-dependent manner. NJet down regulated the expression of MYCN and MDM2 proteins with a simultaneous increase in the expression of tumor suppressor protein p53.
Conclusions:
The present data demonstrated that NJet regulated the growth of IMR-32 and SK-N-MC through reduction in MYCN expression that lead to down regulation of MDM2 protein and increase in p53 expression. These preliminary results warrant further in depth studies to explore the therapeutic potential of Nardostachys jatamansi in the management of neuroblastoma.
SUMMARY
NJet reduced the viability of human neuroblastoma cell lines without affecting the viability of non-cancerous, HEK-293 cells.
NJet regulated the growth kinetics of the cancer cells.
NJet decreased the expression of MYCN and MDM2 proteins and simultaneously increased the expression of tumor suppressor protein p53.
Abbreviation used: NJet: Ethanolic extract of Nardostachys jatamansi MTT: 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide HPTLC: High performance thin layer chromatography
Keywords: Neuroblastoma, Nardostachys jatamansi, MYCN, p53, MDM2
INTRODUCTION
Nardostachys jatamansi (Valerianaceae), commonly known as muskroot, is indigenous to the Himalayan regions of India.[1] In Ayurveda, N. jatamansi has been used traditionally since centuries for treating various neurological disorders. It has been used as a sedative, an antispasmodic, for mind rejuvenation, promotion of sleep, alleviating mental diseases and as a brain tonic.[2] It has also been used for treating headache, insomnia, epilepsy; disorders of cardiovascular system; flatulence and intestinal colic as well as menopausal symptoms.[2] N. jatamansi has been shown to possess anti-inflammatory,[3] anti-oxidant,[4] anti-bacterial,[5] anticonvulsant[6] and hepatoprotective activities.[7] It has been shown to exhibit anti-tumour activity in lung, liver, ovary, breast and prostate cancers.[4,6] Recently, the higher doses of alcoholic and n-hexane extract of N. jatamansi were shown to significantly inhibit the growth of neurobalstoma cell lines, IMR 32 and SK-N-SH.[4]
In the present study, we have shown that ethanolic extract of N. jatamansi roots (NJet) significantly altered the growth kinetics of IMR-32 and SK-N-MC neuroblastoma cells at lower doses through down regulation of MYCN and MDM2 proteins and upregulation of p53.
MATERIALS AND METHODS
Drugs and chemicals
Tissue culture plasticware was purchased from BD Biosciences (CA, USA). DMEM powder, penicillin and streptomycin were obtained from Invitrogen (Grand Island, NY, USA). Fetal bovine serum (FBS), 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenylthiazolium bromide (MTT), Valerinic acid and primary antibody against MYCN were purchased from Sigma-Aldrich (St. Louis, MO). Primary antibody against p53 (DO-1), MDM2, tubulin (B-7) and donkey anti-mouse IgG antibody were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Plant material, extract preparation and preliminary phytochemical analysis
N. jatamansi roots were purchased from Green Pharmacy (Pune, Maharashtra, India). The sample was authenticated and validated macroscopically and microscopically at the Department of Botany, Agharkar Research Institute (ARI), Pune (Maharashtra, India) (ref no.1045). Voucher specimen sample (Number R-154) has been deposited at the Department.
N. jatamansi roots were weighed, powdered and extracted in ethanol using soxhlet apparatus. The resulting extract was centrifuged at 13000 rpm for 15 min to remove the particulate matter. The supernatant was filter-sterilized using swiney filter (pore size, 0.45 µm) and the resultant filtrate was stored in aliquots at -80°C until use.
Cell culture
The cell lines (IMR-32, SK-N-MC and HEK-293) used in the present study were obtained from National Centre for Cell Science (NCCS), Pune, India. The cells were grown in DMEM containing 2 mM L-glutamine supplemented with 10% fetal bovine serum and 20 U/ml of penicillin-streptomycin and incubated in a humidified 5% CO2 incubator at 37°C.
Cell viability
IMR-32, SK-N-MC and HEK-293 were seeded at a density of 1 × 105 cells/ ml in a 96-well plate. The cells were treated with different concentrations of NJet (0-160 µg/ml) in triplicates for 24 h. Cell viability was determined by MTT assay as described previously.[8,9]
Cell growth analysis
IMR-32 and SK-N-MC were seeded at a density of 5 × 104 cells/well in 24-well plate. The cells were treated with different concentrations of NJet (0-20 µg/ml) in triplicates for 24, 48 and 72 h. The effect of NJet on cell growth was determined by trypan blue dye exclusion method as described previously.[8,9]
Soft agar assay
IMR-32 and SK-N-MC cells (5 × 103 cells/ml) treated with different concentrations of NJet (0-20 μg/ ml) were mixed with culture medium containing 0.35% agarose and plated over a previously gelled layer of 0.5% agarose as described previously.[8,9] The colonies were manually counted and photographed directly using an Axiovert 200 M microscope (Carl Zeiss, Germany).
Western blotting
IMR-32 and SK-N-MC cells were plated at a seeding density of 5 × 105 cells/well in a 6-well plate and incubated for 24 h at 37°C in CO2 incubator. Next day, the cells were treated with different concentrations of NJet (0-20 μg/ml) for 24 h. The cells were lysed as previously described.[8,9] The protein was estimated using Bradford reagent (Biorad Laboratories Inc, CA, USA). Equal amount of protein was loaded on a 10% SDS-polyacrylamide gel and transferred electrophoretically to Amersham Hybond-P PVDF membrane (GE Healthcare, UK) in sodium phosphate buffer (pH 6.8). The membrane was blocked in 5% BSA in TBST and incubated at room temperature for 3 h with mouse monoclonal primary antibodies against p53, MDM2 and tubulin (Santacruz, CA, USA) at 1:2000 and MYCN (Sigma-Aldrich, St. Louis, MO) at 1:500 dilutions respectively. The blots were washed in TBST and incubated with donkey anti mouse IgG HRP conjugate at 1:4000 (for p53, MDM2, tubulin) and 1:2000 (for MYCN) dilutions. Proteins were visualized using a chemiluminescence kit (Amersham ECL Advance western blotting detection kit, GE Healthcare, UK) and densitometric analysis of X-ray films was performed using the Image J gel analysis tool.
Measurement of apoptosis
The cells were plated at a seeding density of 5 × 105 cells/well in a 6-well plate and treated with different concentrations of NJet (0-20 μg/ml). After 24 h of treatment, the cells were harvested and washed with PBS twice. Cells were stained with Annexin V-FITC following the manufacturer's instructions (Annexin V-FITC apoptosis kit #3, Invitrogen) and analyzed for apoptosis by FACS using Cell Quest Software.[8]
Statistical analysis
All the results were obtained from three independent experiments, each performed in triplicates and the values have been presented as mean ± SD. Differences among means were tested for statistical significance using one-way analysis of variance (ANOVA). The analyses were carried out using Graph-pad prism 5 software (San Diego, CA, USA). *p < 0.05; **p < 0.01; ***p < 0.001 were considered to be statistically significant.
RESULTS
Chromatographic analysis of NJet
HPTLC chromatographic examination of NJet was carried out to authenticate N. jatamansi root extract based on its marker compound, valerinic acid [Figure 1]. The finger print profile was developed in toluene: ethylacetate: formic acid (8:2:0.5) solvent system and the peaks corresponding to valerinic acid were recorded at Rf 0.75. Similar peaks were found in chromatographic profile of NJet at the same Rf value of 0.75. The calibration curves were linear in the range of 200-1200 ng of valerinic acid. The amount of valerinic acid in NJet was found to be 41.58 mg/g.
Figure 1.
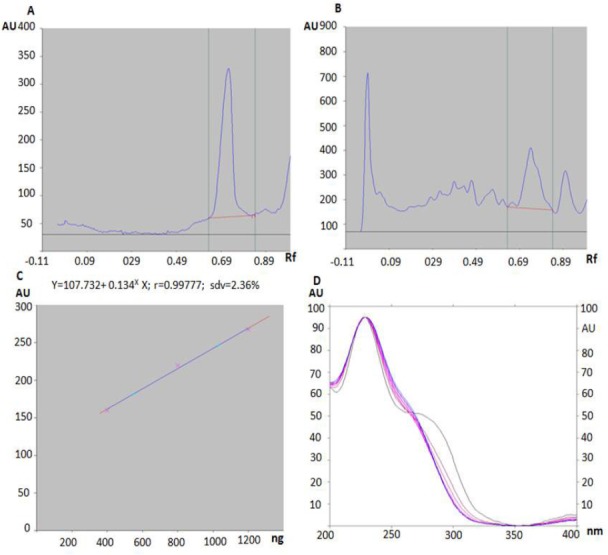
HPTLC images and standardization of NJet. HPTLC densitogram of (A) marker compound valerinic acid and (B) NJet. (C) Calibration curves in linear range of 200, 400, 800, 1000, 1200 ng of standard valerinic acid. (D) UV spectra of standard valerinic acid and samples of NJet obtained by HPTLC spot scanning from 200 to 400 nm.
NJet altered growth kinetics of neuroblastoma cell lines
In IMR-32 and SKNMC, NJet altered growth kinetics of neuroblastoma cell lines exhibited IC50 values of 61.43 and 63.42 µg/ml, respectively [Figure 2]. Contrarily, in non-cancerous HEK-293 cells, IC50 was not obtained upto 160 µg/ml concentration of NJet [Figure 2]. Thus, NJet significantly reduced the viability of neuroblastoma cells at lower doses without affecting the viability of the non-cancerous cells. Further studies were carried out at non-cytotoxic doses (0-20 µg/ml) of NJet to analyze its effect at cellular and molecular level.
Figure 2.
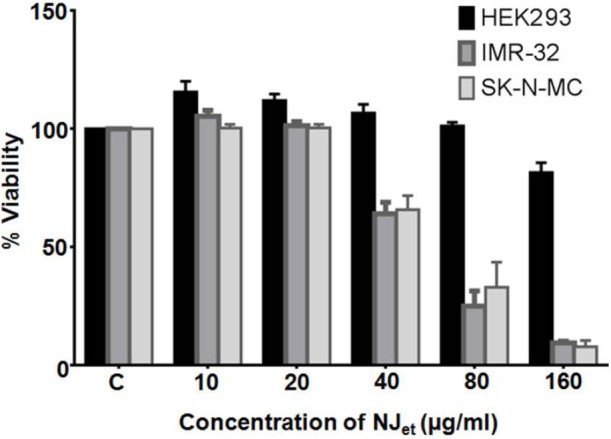
Effect of NJet on cell viability. IMR-32, SK-NMC and HEK-293 cells were treated with different concentrations (0-160 μg/ml) of N. jatamansi root extract. The cell viability was determined by MTT dye uptake method and the data represents mean ± SD of three independent experiments, each performed in triplicates.
In IMR-32, NJet reduced the cell proliferation at 20 µg/ml dose by ~2-fold at 24 h and by ~1.5-folds at 48 and 72 h (p = < 0.001) [Figure 3A]. On the other hand, in SK-N-MC, the proliferation was reduced by~2- (p = <0.001), 3-(p = < 0.001) and 2- folds (p = < 0.001), respectively at 24, 48 and 72 h compared to the untreated control cells [Figure 3B]. The effect of NJet on the cell growth was further evaluated by soft agar assay that showed a dose dependent decrease in the number of soft agar colonies. At 20µg/ml, there was ~2.5- (p ≤ 0.001) and 3.5-folds (p ≤ 0.001) reduction in the number of colonies in IMR-32 and SK-N-MC, respectively, compared to the untreated control cells [Figure 3C]. These data demonstrated that NJet significantly regulated the proliferation and growth kinetics of IMR-32 and SK-N-MC cells.
Figure 3.
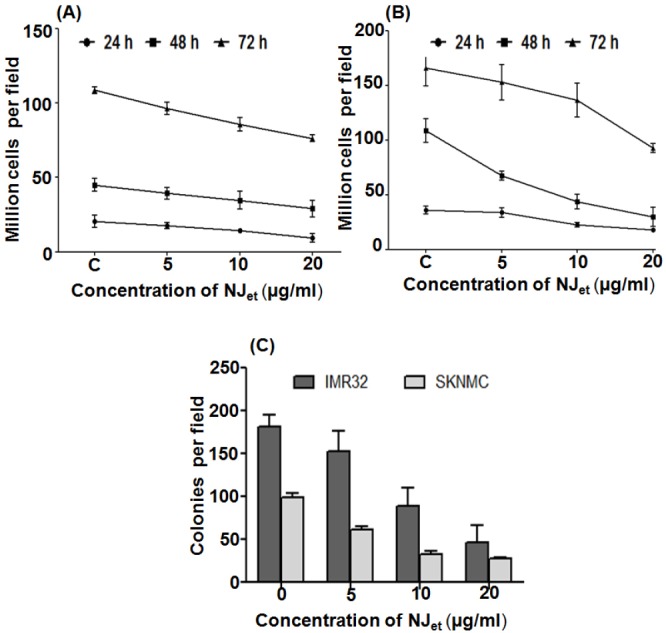
Effect of NJet on growth kinetics of neuroblastoma cells. (A) IMR-32 and (B) SK-N-MC cell lines were treated with NJet (0-20 μg/ml) for 24, 48 and 72 h and the number of viable cells were counted by trypan blue dye exclusion method. Data represent mean± SD of three independent experiments (C) IMR-32 and SK-N-MC cell lines were treated with NJet (0-20 μg/ml) and grown in soft agar for two weeks. Colonies were counted from at least 10 different areas and the average of each has been plotted. The data represents mean ± SD of five independent experiments.
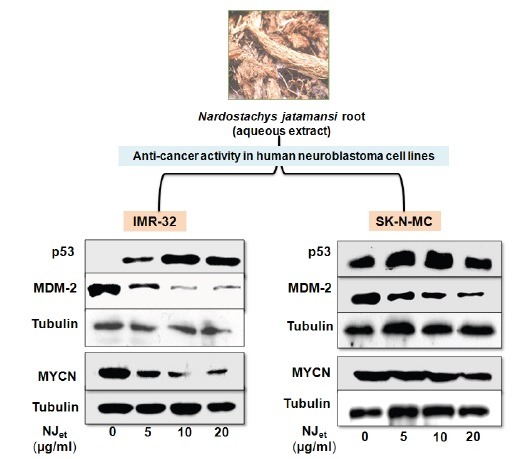
NJet increased the expression of p53 through down regulation of MYCN and MDM2
It was found that NJet up regulated p53 expression in a dose-dependent manner in IMR-32 and SK-N-MC [Figure 4A and B, respectively] cells. At 20µg/ml, there was~1.5-(p < 0.05) and 18.36-folds (p < 0.05) increase in p53 expression in IMR-32 and SK-N-MC cells, respectively, compared to the untreated control cells [Figure 4C]. Contrarily, a dose dependant decrease in the expression of MDM2 and MYCN proteins was observed in IMR-32 and SK-N-MC cells compared to the untreated control cells [Figure 4A and B, respectively]. In IMR-32 and SK-N-MC cells, at 20 µg/ml dose of NJet, there was ~4- and 9-folds (p < 0.05) decrease in the expression of MDM2 (p < 0.05) [Figure 4D], respectively, as well as ~1.7- and 4.4-folds decrease in MYCN expression, respectively, compared to the untreated control cells [Figure 4E].
Figure 4.
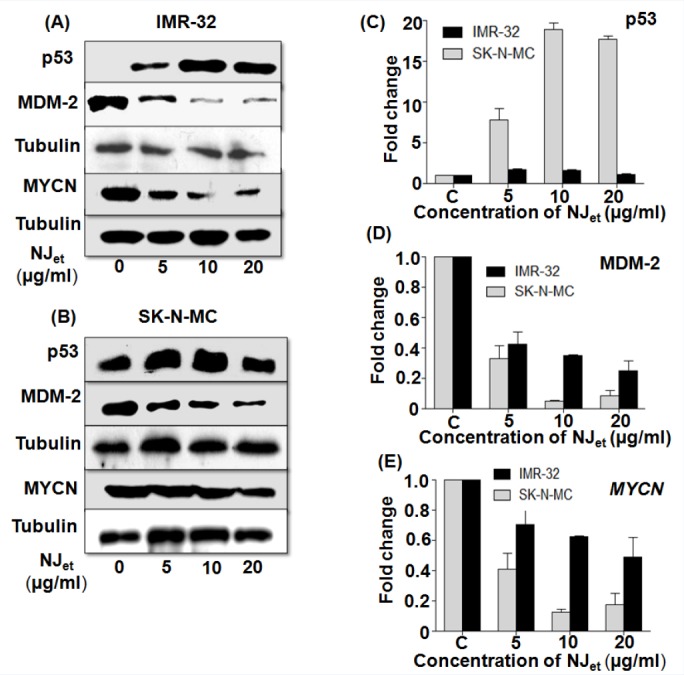
Effect of NJet on the expression of tumor regulatory markers. (A-E) IMR-32 and SK-N-MC cells were analyzed for the expression of p53, MDM2 and MYCN proteins by western blotting. Tubulin was used as a loading control.
DISCUSSION
The present study reported the mechanism of anti-cancer potential of N. jatamansi root extract (NJet) in the human neuroblastoma cell lines, IMR-32 and SK-N-MC. NJet reduced the viability of neuroblastoma cell lines at lower doses (40 μg/ml onwards) compared to the non-cancerous cells. At 80 µg/ml dose, NJet reduced the cell viability of IMR-32 and SK-N-MC by 70 and 62%, respectively, with IC50 values of 61.43 and 63.42 µg/ml, respectively. It negatively regulated the growth kinetics of neuroblastoma cells in a significant manner and effectively reduced their proliferation. Previously, Monga and Kumar in 2013 had reported the anti- proliferative potential of alcoholic extract and n-hexane fraction of roots of N. jatamansi against neuroblastoma cell lines, IMR-32 and SK-N-SH.[2] They had shown that at 100 μg/ml dose, the alcoholic extract reduced the proliferation of IMR-32 and SK-N-SH by 71 and 85%, respectively, whereas at the same dose hexane fraction reduced the proliferation by 91 and 82% respectively. Interestingly, in our study NJet reduced the viability at much lower doses compared to that reported by Monga and Kumar, which is excellent for drug development point of view. The difference in these results could be due to time of collection of the roots, method of preparation of the extract and the method of cell proliferation assay. Various reports have shown anticancer activity of herbal formulations against neuroblastoma cell lines. PienTze Huang, a popular Chinese medicine for liver diseases, has been reported to decrease survival of SH-SY5Y neuroblastoma cell line.[10] The leaf extracts of Holarrhena antidysenterica have been reported to be cytotoxic to SK-N-MC neuroblastoma cells.[11] Calotropisprocera, Ocimum sanctum and Cannabis sativa have been shown to be cytotoxic to IMR-32.[12] However, neither of the studies has delineated the molecular mechanisms underlying the anticancer activity of these medicinal plants.
In the present work, we have shown the preliminary mechanism of action of NJet in neuroblastoma cell lines. We found that NJet significantly down regulated the expression of MYCN, the major negative prognostic marker in neuroblastoma with significant role in the pathogenesis and clinical onset of this aggressive malignancy.[13] MYCN has been reported to activate proliferative cellular pathways and inhibition of p53-mediated apoptosis has been considered as the prerequisite for MYC-driven tumorigenesis.[14] Interestingly, NJet not only decreased the expression of MYCN but simultaneously upregulated the expression of tumor suppressor protein p53. Moreover, NJet significantly reduced the expression of MDM2, the negative regulator of p53. MDM2 is amplified in a variety of human cancers, including neuroblastoma.[15] It has been reported to be a predictor for poor treatment outcome in neuroblastoma patients.[16] Thus, NJet regulated the growth of the neuroblastoma cells through disruption of MYCN and MDM2 expression and stabilization of p53. These results lead us to study the mechanism of cell death induced by NJet, which was found to induce apoptosis in IMR-32 and G2/M arrest in SK-N-MC cells (data not shown).
All these data suggested the potential of ethanolic extract of N. jatamansi (NJet) in regulating the growth of neuroblastoma cells.
CONCLUSIONS
The present study has evaluated the mechanism of anticancer activity of NJet in IMR-32 and SK-N-MC neuroblastoma cell lines. However, further in-depth studies are warranted against other neurablastoma cell lines as well as in vivo studies need to be done to establish the chemopreventive potential of N. jatamansi.
Financial support and sponsorship
This work was supported by funding from the Interactive Research School for Health Affairs (IRSHA), Bharati Vidyapeeth University.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors thank Director of IRSHA for his generous support and encouragement. The authors would like to acknowledge Dr. Anand Zanwar, Department of Food and Nutrition Lab, for helping in HPTLC profiling of the extract.
REFERENCES
- 1.Raina AP, Negi KS. Essential oil composition of Valerianajatamansi jones from himalayan regions of India. Indian J Pharm Sci. 2015;77:218–22. doi: 10.4103/0250-474x.156614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monga AK, Kumar S. A phytopharmacological review on Jatamansi. Pharm Res. 2013;9:21–32. [Google Scholar]
- 3.Singh RK, Vaishali Panda SK, Murthy PN, Panigrahi G, Sharma PK, Gupta RK. Evaluation of anti-inflammatory potential of Nardostachys jatamansi rhizome in experimental rodents. Jcoastlife med. 2014;2:38–43. [Google Scholar]
- 4.Chaudhary S, Chandrashekar KS, Pai KS, Setty MM, Devkar RA, Reddy ND, et al. Evaluation of antioxidant and anticancer activity of extract and fractions of Nardostachys jatamansi DC in breast carcinoma. BMC Complement Altern Med. 2015;15:50. doi: 10.1186/s12906-015-0563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bengal Das MP, Banerjee A Anu. Comparative study on In vitro antibacterial and antifungal properties of five medicinal plants of west. Asian J Plant Sci. 2013;3:107–11. [Google Scholar]
- 6.Bhagat M, Pandita RM, Saxena AK. In vitro and In vivo Biological Activities of Nardostachys jatamansi roots. MAP. 2013;2:6. [Google Scholar]
- 7.Ali S, Ansari KA, Jafry MA, Kabeer H, Diwakar G. Nardostachys jatamansi protects against liver damage induced by thioacetamide in rats. J Ethnopharmacol. 2000;71:359–63. doi: 10.1016/s0378-8741(99)00153-1. [DOI] [PubMed] [Google Scholar]
- 8.Choudhari AS, Suryavanshi SA, Kaul-Ghanekar R. The aqueous extract of Ficusreligiosa induces cell cycle arrest in human cervical cancer cell lines SiHa (HPV-16 Positive) and apoptosis in HeLa (HPV-18 positive) PLoS One. 2013;8:e70127. doi: 10.1371/journal.pone.0070127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koppikar SJ, Choudhari AS, Suryavanshi SA, Kumari S, Chattopadhyay S, Kaul-Ghanekar R. Aqueous cinnamon extract (ACE-c) from the bark of Cinnamomum cassia causes apoptosis in human cervical cancer cell line (SiHa) through loss of mitochondrial membrane potential. BMC Cancer. 2010;10:210. doi: 10.1186/1471-2407-10-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lü L, Wai MS, Yew DT, Mak YT. PienTze Huang, a composite Chinese traditional herbal extract, affects survival of neuroblastoma cells. Int. J Neurosci. 2009;119:255–62. doi: 10.1080/00207450802324770. [DOI] [PubMed] [Google Scholar]
- 11.Sharma V, Hussain S, Bakshi M, Bhat N, Saxena AK. In vitro cytotoxic activity of leaves extracts of Holarrhena antidysenterica against some human cancer cell lines. Indian J Biochem Biophys. 2015;1:46–51. [PubMed] [Google Scholar]
- 12.Sundaram S, Verma SK, Dwivedi PR. In vitro cytotoxic activity of Indian medicinal plants used traditionally to treat cancer. Asian J Pharm Clin Res. 2011;4:27–9. [Google Scholar]
- 13.Slack A, Chen Z, Tonelli R, Pule M, Hunt L, Pession A, et al. The p53 regulatory gene MDM2 is a direct transcriptional target of MYCN in neuroblastoma. Proc Natl Acad Sci. 2005;102:731–6. doi: 10.1073/pnas.0405495102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z, Lin Y, Barbieri E, Burlingame S, Hicks J, Ludwig A, et al. Mdm2 deficiency suppresses MYCN-driven neuroblastoma tumorigenesis in vivo. Neoplasia. 2009;11:753–62. doi: 10.1593/neo.09466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He J1, Gu L, Zhang H, Zhou M. Crosstalk between MYCN and MDM2-p53 signal pathways regulates tumor cell growth and apoptosis in neuroblastoma. Cell Cycle. 2011;10:2994–3002. doi: 10.4161/cc.10.17.17118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carol H, Reynolds CP, Kang MH, Keir ST, Maris JM, Gorlick R, et al. Initial testing of the MDM2 inhibitor RG7112 by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2013;60:633–41. doi: 10.1002/pbc.24235. [DOI] [PMC free article] [PubMed] [Google Scholar]


