Abstract
Background:
The Bile-processed Rhizoma coptidis (BRC), which has a colder drug property than Rhizoma coptidis (RC), is widely used for the treatment of heat syndrome. We compared the pharmacokinetics of the protoberberine-type alkaloids in BRC and RC in rats with heat syndrome to elucidate the bile-processing mechanism.
Material and Methods:
We established a rapid and sensitive method for simultaneously determining three alkaloids: berberine, palmatine, and jatrorrhizine, in rat plasma based on ultra-performance liquid chromatography/tandem mass spectrometry. The separation was carried out on a Waters ACQUITY BEA C18 column. The mobile phase consisted of acetonitrile (containing 0.1% formic acid) and water (containing 0.1% formic acid and 10 mmol/L ammonium acetate) and carbamazepine was used as an internal standard. The detection was carried out in a multiple reaction monitoring mode (MRM) using electrospray ionization in the positive ion mode.
Results:
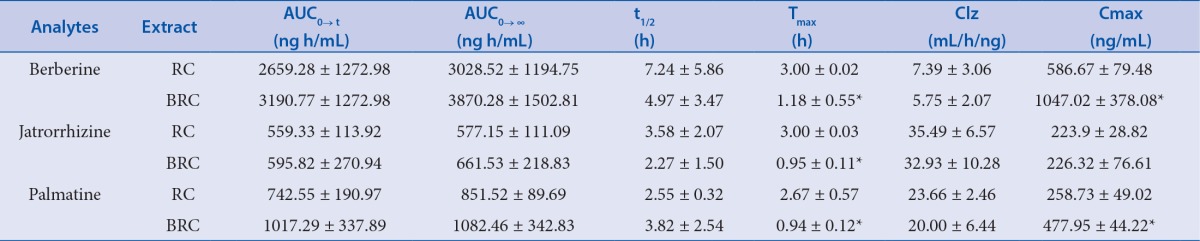
Pharmacokinetic profiles indicated that the Cmax of berberine and palmatine increased two times and the Tmax of the three alkaloids decreased three times after bile processing. AUC0→∞ and AUC0→t of the alkaloids were similar between RC and BRC.
Conclusion:
The results suggest that bile processing could increase the absorption rate of alkaloids. This study broadens our understanding of Chinese herbal medicine processing.
SUMMARY
Contents of berberine, palmatine and jatrorrhizine, in heat syndrome rats’ plasma between the raw and bile-processed Rhizoma coptidis (RC) were determined by UPLC-MS/MS.
The whole pharmacokinetic profiles of three alkaloids in the bile-processed Rhizoma coptidis (BRC) were similar to those of RC.
The shorter Tmax and increased 2-fold Cmax were obtained after RC bile-processing.
Bile-processing could promote the absorption rate of alkaloids in a certain degree.
Abbreviation Used: RC: Rhizoma coptidis, BRC: Bile-processed Rhizoma coptidis, HPLC: high-performance liquid chromatography, UPLC-MS/MS: ultra-performance liquid chromatography-mass spectrometry/ mass spectrometry, LC-MS: liquid chromatography-mass spectrometry, MRM: multiple reaction monitoring mode, QC: quality control, RE: relative error, RSD: relative standard deviation, Cmax: maxium of drug concentration, Tmax: time for maxium of drug concentration, AUC: area under concentration-time curve, LLOQ: Linearity and lower limits of quantification, t1/2: half-life, Clz: body clearance
Keywords: Bile-processed, heat syndrome, pharmacokinetic profiles, protoberberine-type alkaloids, Rhizoma coptidis
INTRODUCTION
Rhizoma coptidis (RC), known as Huang Lian, has been broadly applied in traditional Chinese medicine to treat intestinal infection,[1] inflammation,[2] and fever.[3] According to the classical theory of traditional Chinese medicine, RC has a special property of cold. It has long been used to dispel heat and purge fire. The evidence has shown that bile-processed RC (BRC) dispels heat more effectively than RC because of its colder property. In Yilin Zhuanyao and Lianzi Huang lian Wan, BRC has been typically used instead of RC for the treatment of infantile heat syndrome with fever. Recently, a great deal of research and trials have focused on the pharmacological effect, chemical constituents, and processing technology of BRC.[4,5] Our previous research has shown that BRC has an increased antipyretic effect and total alkaloid content compared with RC;[6,7] however, the effect of bile-processing technology on the pharmacokinetic behavior of BRC has not been elucidated.
The different processing technologies, originating from traditional Chinese medicine, can be associated with variations in drug properties and pharmacokinetic behaviors of active constituents compared to the crude herb. The previous research has compared the pharmacokinetic parameters of icariin derived from crude and mutton-fat processed Epimedii Folium. The Cmax and AUC0→t of icariin in the mutton-fat processed group was significantly higher than that in the crude Epimedii Folium group, possibly because the mutton-fat processing accelerated the absorption of icariin from Epimedii Folium to rat plasma.[8] When ten alkaloids were measured by UPLC-MS/MS in the plasma of rats treated with crude RC and wine-processed RC aqueous extract, it was found that wine processing increased the bioavailability of certain alkaloids.[9] These results show that it is necessary to study the differences in pharmacokinetic behavior between crude and processed herbs.
The modern chemical and pharmaceutical research showed that protoberberine-type alkaloids are the main active compounds in RC and BRC, including berberine, palmatine, epiberberine, and jatrorrhizine. These alkaloids are associated with a variety of biological activities including anti-inflammatory,[10] antipyretic,[11] and immuno suppressive[12] properties. Novel, simple high-performance liquid chromatography (HPLC)-UV, and liquid chromatography-mass spectrometry (LC-MS) methods have been developed to determine the concentrations of protoberberine alkaloids in biological samples.
In this work, we describe a rapid and accurate ultra-performance liquid chromatography tandem mass-spectrometry (UPLC-MS/MS) method to simultaneously measure the alkaloids berberine, jatrorrhizine, and palmatine in the blood plasma of rats with heat syndrome treated with RC and BRC. By comparing the pharmacokinetic parameters of RC and BRC, we explored whether pigs’ bile could affect the pharmacokinetic behavior of the alkaloids. The results of this study will help elucidate the mechanism of bile processing of RC and its effect on drug properties.
MATERIALS AND METHODS
Chemicals, solvents, and herbal materials
HPLC-grade acetonitrile and methanol were purchased from Merck Inc. (Darmstadt, Germany). HPLC-grade formic acid and ammonium acetate (purity 98%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All the other chemicals were of analytical grade and purchased from Lvye Biotechnology (Jiangshu, China). Dry yeast was purchased from Anqi Yeast Co. LTD (Hubei, China). Ultra pure water was produced using a Milli-Q system (Millipore, Bedford, MA, USA). The reference standards of berberine, jatrorrhizine, palmatine, and carbamazepine (internal standard, IS) were purchased from the National Institute for Control of Pharmaceutical and Biological Products (Beijing, China).
Rhizome coptidis (RC, Batch No.: 130612), Cinnamomum cassia Presl (Batch No.: 140426), dried ginger (Batch No.: 140120), and Radix Aconiti Lateralis Preparata (Batch No.: 140305) were all from Dalian Quanjian Traditional Chinese Medicine Co. LTD (Dalian, China). The fresh pig's bile was purchased from Dalian Changqing supermarket. The bile-processed Rhizome coptidis (BRC) was prepared according to the Chinese Pharmacopoeia (Chinese Pharmacopoeia Commission, 2010). Briefly, 100 g of raw RC was soaked in 40 mL pig's bile solution (3 g bile for every 20 mL water) for 1h, and then fried at 105°C for about 20 min until completely dry.
The decoction of herbs for heat syndrome modeling was prepared as follows: Cinnamomum cassia Presl, dried ginger, and Radix Aconiti Lateralis Preparata (100 g, 1:1:1, w/w) were refluxed with water (1:10, w/v) twice for 1h each. The extraction solution was combined for filtration with silk cloth, and then the solution was evaporated at 40°C in a negative pressure system. The residue was dissolved in water to 100 mL.
Preparation of RC extract and BRC extract
Rhizome coptidis (100g) was extracted twice by refluxing with water (1:10,w/v) for 1h each. The extraction solutions were combined for filtration, and then evaporated at 40°C in a negative pressure system. The residue was dissolved in the water to 500 mL. The same procedure was used for BRC (100g). Both extracts were stored at 4°C before use.
Detection of three protoberberine-type alkaloids in RC extract and BRC extract
To calculate the administered dose, the contents of berberine, jatrorrhizine, and palmatine in the extracts were quantitatively measured. The aqueous extracts of RC and BRC were diluted 5 times with methanol, and the diluted solution was centrifuged for 10 min at 5,000 rpm. The supernatant was filtered through a 0.45-μm Millipore filter before HPLC analysis. A 10 μL sample was injected into the HPLC system with a Diamonsil C18 column (4.6 mm × 250 mm, 5μm). The mobile phase consisted of acetonitrile-0.025 mol/L KH2PO4 containing SDS 1.7 g/L (40:60) with the flow rate of 1.0 mL/min at 30°C. The detection wavelength was at 345 nm. The concentrations of berberine, jatrorrhizine, and palmatine in RC extract were 31.56, 11.23, and 8.60 mg/g respectively, and 36.50, 12.24, and 10.73 mg/g, respectively, in the BRC extract.
Animals
The animal experiments were conducted according to the guidelines for the Care National Institutes of Health. The female Wistar rats (weighing 200 ± 20 g, aged 10 weeks) were obtained from the Liaoning Changsheng Biological Technology Co., LTD (certificate no. SCXK(LN)2010-0001). The rats were kept in a room at 22 ± 1°C with a light/dark cycle of 12 h for three days (Laboratory Animal Center of Liaoning University of Traditional Chinese Medicine). The rats’ rectal temperatures were measured twice daily with a digital thermometer during this period. The temperature difference of all the rats selected for the study was less than 0.5°C.
Animal modeling
The aqueous extract of Cinnamomum cassia Presl, dried ginger, and Radix Aconiti Lateralis Preparata was administered to 16 rats (1 mL extract /100 g rat's weight) for 15 days. The rats began to embody typical behavior of heat syndrome with red and purple tongue, longer and thicker arteries and veins under the tongue, red and purple claw color, and red ear flap edge, although the body temperature of these rats had no significant elevation during this period. The yeast injection could induce a fever symptom that was an alogous to heat syndrome. Therefore, on the 16th day, heat syndrome was induced in these rats by an intraperitoneal injection of 20% dried yeast suspension (10 mL/kg).
Preparation of stocks, calibration samples, and quality control samples
The stock solutions (I) of berberine, jatrorrhizine, and palmatine at concentration of 0.2 mg/mL for each were separately prepared by dissolving the accurately weighed standard reference compounds in methanol. The stock solutions (II) were obtained by mixing all three stock solutions above, to a final concentration of 20 μg/mL for berberine and 10 μg/mL for jatrorrhizine and palmatine. A 1 μg/mL solution of carbamazepine (IS) was also prepared in methanol. All the solutions were stored at−20 °C.
The analytical standard and quality control (QC) samples were prepared as follows:
In the analytical standard sample, the blank plasma was spiked with appropriate amounts of working solutions (5% of the total plasma sample volume) and then vortexed for 30s. The final calibration samples were prepared at concentrations of 0.4, 0.6, 1.92, 4.8, 12, 38.4, 96, 192, 240, 480, 2400 ng/mL for berberine, and 0.4, 0.8, 2, 5, 16, 40, 80, 100, 200, 500, 1000 ng/mL for jatrorrhizine and palmatine. The QC samples were prepared at concentrations of 0.6, 12, 480 ng/mL for berberine, 0.8, 16, 500 ng/mL for jatrorrhizine and palmatine.
Sample preparation
Ten microliters of IS solution (1 μg/mLcarbamazepine in methanol) was added to 30 μL of the plasma sample. The mixture was vortexed for 30s and 50 μL of acetonitrile was added. The mixture was vortexed again for 3 min and then centrifuged at 13,000 rpm for 10 min at 4°C. Ten microliters of the supernatant was injected into the UPLC-MS/MS system for analysis.
Analytical conditions of liquid chromatography and mass spectrometry
The UPLC-MS/MS analysis was carried out with an Agilent 1290 liquid chromatograph and a 6460 triple quadrupole mass spectrometer with anelectrospray ionization (ESI) source. Chromatographic separation was performed on a Waters ACQUITY BEA C18 (1.7 μm, 2.1 mm×50 mm) column at 35°C. The mobile phase A was water containing 10 mmol/L ammonium acetate and 0.1% formic acid, and B was acetonitrile with 0.1% formic acid. The elution was programmed as follows: 5%-50% B for 0-3 min, 50%-95% B for 3-4 min, kept at 95% for 3 min, 95%-5% B for 7-7.1 min, and kept 5% for 2.9min. The flow rate was 0.3 mL/min.
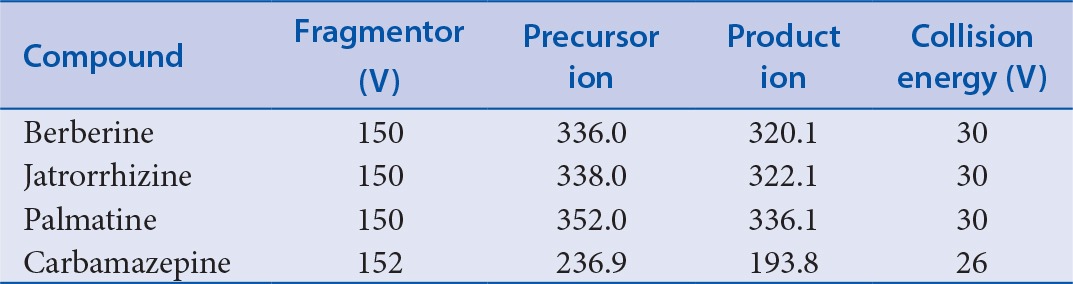
The mass spectrometer was operated in positive ion mode. The data acquisition was performed using multiple reaction monitoring (MRM) to detect the mass transitions for each compound. Compound-dependent parameters are listed in Table 1. Other ESI parameters were set as follows: positive ion mode, sheath gas flow rate 11 L/min, sheath gas temperature 250°C, nebulizer pressure 45 psi, auxiliary sweep gas flow rate 5 L/min, source spray voltage 5 kV, capillary temperature 300°C, and capillary voltage 3500 V.
Table 1.
The MS/MS transitions and parameters for the detection of the analytes and internal standards
Validation of the analytical method
Selectivity
The selectivity of the method was investigated by analyzing the chromatograms of blank plasma samples from healthy rats, spiked plasma samples at the concentrations of the lower limit of quantification (LLOQ), and plasma samples after administration of BRC extract.
Linearity and lower limits of quantification (LLOQ)
The calibration curve consisted of eleven concentration levels. The linearity of each calibration curve was determined by plotting the peak area ratio (y) of analyte to IS versus the nominal concentration (x) of analyte with weighted (1/X2) least square linear regression. The LLOQ was defined as the lowest concentration of the calibration curve (signal/noise =10) at which the accuracy was within ± 20% relative error (RE) and the precision was below 20% relative standard deviation (RSD), evaluated by analyzing six replicate samples.
Precision and accuracy
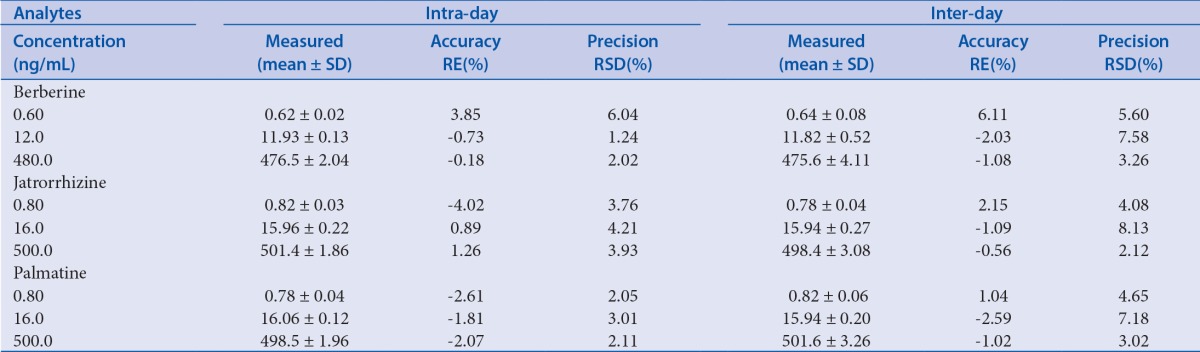
The precision and accuracy of the method were assessed by analyzing three validation batches of the QC samples (low, medium and high concentration levels) on three consecutive days. The intra-and inter-day precision were expressed as the RSD, and the accuracy was expressed as RE.
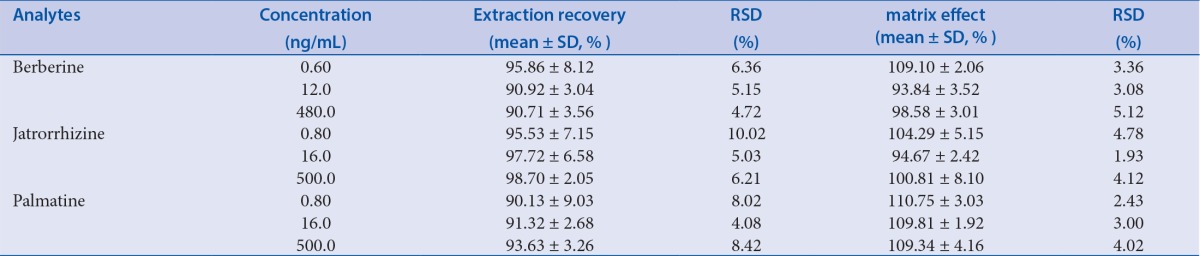
Extraction recovery and matrix effect
The extraction recovery of the analytes at three QC levels was determined by comparing the areas of the QC samples with those of the spike-post-extracted samples. The matrix effect was determined by comparing the peak areas of the blank sample where the extracted matrix was spiked with standard solutions with those of the sample in the mobile phase.
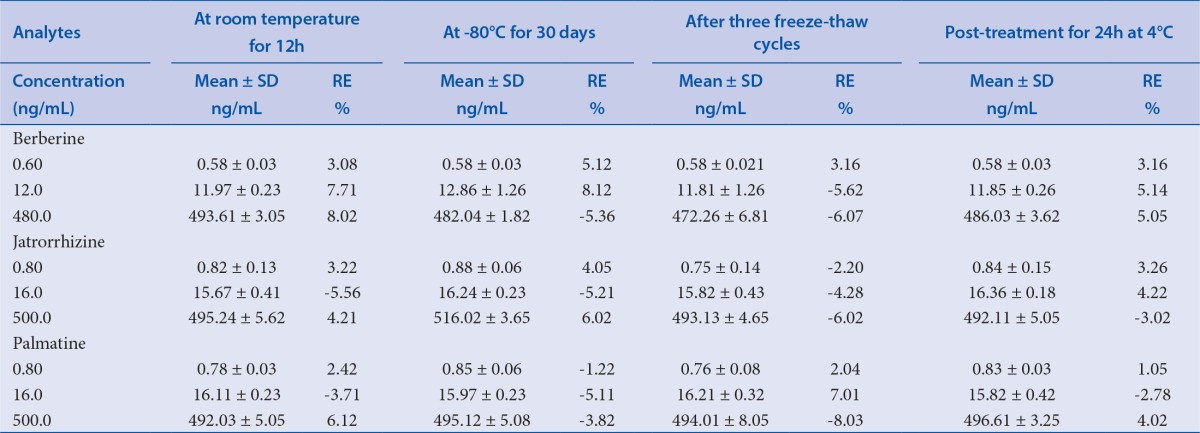
Stability
The stability of the analytes in rat plasma was evaluated by analyzing the stability of the QC samples under different conditions. Pre-treatment stability was determined by placing QC samples at room temperature for 12h. Post-treatment stability was determined by placing the extracted samples in the auto sampler at 4°C for 24h. The freeze-thaw cycle stability was evaluated after three freeze-thaw cycles (from - 80°C to 25°C) on three consecutive days. The long-term stability was determined by placing QC samples at - 80°C for 30 days.
Pharmacokinetic study
The heat syndrome model rats were randomly divided into two groups: the RC treated group (n=8) and the BRC treated group (n=8), and then they were immediately administered RC or BRC extract at a dose of 2 g crude herb/kg, respectively. Blood samples (0.1 mL) were collected from the eyes under ether anesthesia at 0.25, 0.5, 0.75, 1, 2, 3, 4, 6, 8, 12, and 24h after oral administration of RC or BRC. The samples were then placed into 1.5-mL heparinized centrifuge tubes and centrifuged for 5 min at 5000 rpm. The separated plasma samples were stored at-80°C until analysis.
Data analysis
The pharmacokinetic parameters were performed using the DAS2.1.1 software (Mathematical Pharmacology Professional Committee of China). The statistics of the results were calculated by the SPSS 11.5 software.
RESULTS AND DISCUSSIONS
Validation of the analytical method
Selectivity
Figure 1 shows the MRM chromatograms for berberine, jatrorrhizine, palmatine, and IS of the plasma sample, the spiked plasma sample, and the plasma sample from a rat 1h after the administration of BRC extract. No endogenous peaks at the retention time positions of the analytes were observed.
Figure 1.
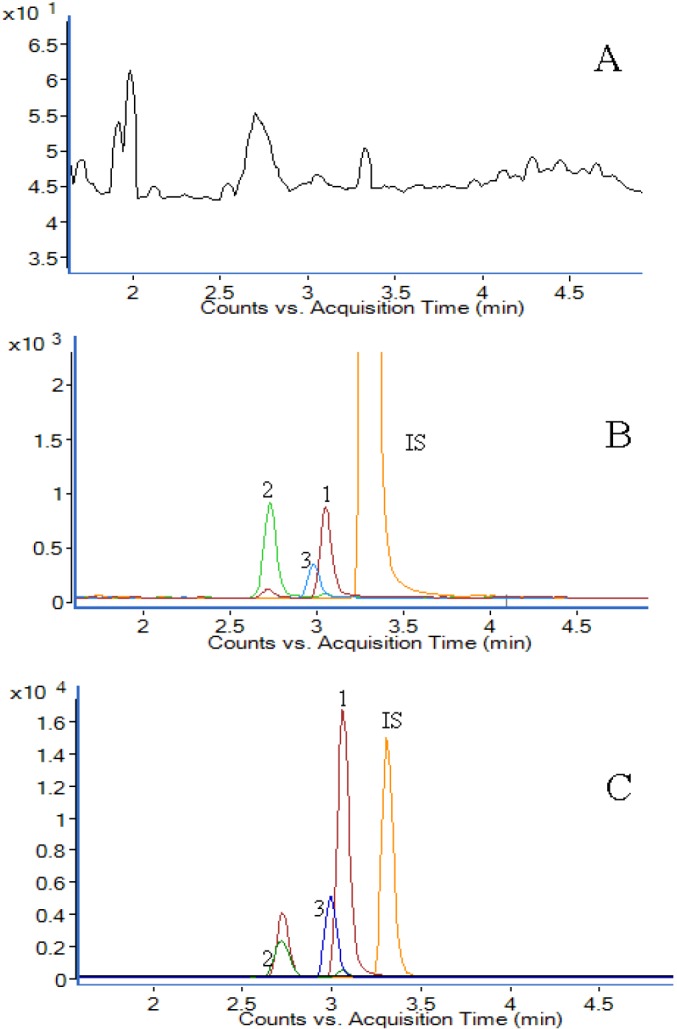
UPLC-MS/MS chromatograms of (A) blank plasma, (B) blank plasma spiked with berberine (0.4 ng/mL), jatrorrhizine (0.4 ng/mL), palmatine (0.4 ng/mL) at LLOQ and carbamazepine (IS, 1μg/mL), (C) plasma sample obtained 1h after a single oral administration of BRC extract. (1) berberine, (2) jatrorrhizine, (3) palmatine, (IS) carbamazepine. The retention time of berberine, jatrorrhizine, palmatine and IS was 3.03 min, 2.69 min, 2.96 min and 3.27 min, respectively.
Linearity and LLOQ
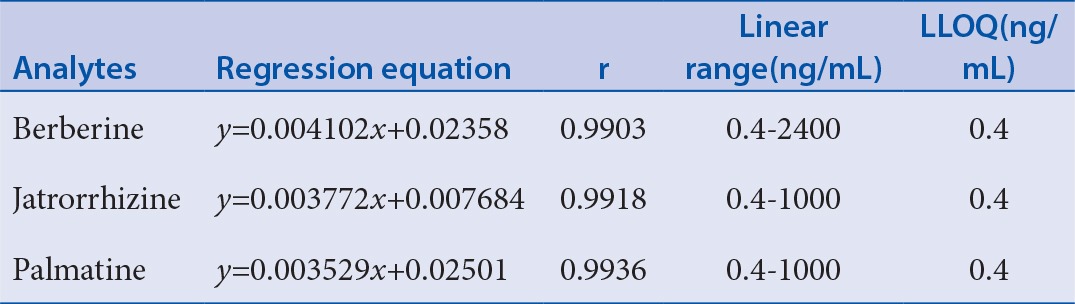
Table 2 shows the regression equations of the calibration curve. The correlation coefficients for the standard curves ranged from 0.990 to 0.994. The validated concentration range was from 0.4 to 2400 ng/mL for berberine and 0.4 to 1000 ng/mL for jatrorrhizine and palmatine. The LLOQs of berberine, jatrorrhizine, and palmatine were all 0.4 ng/mL.
Table 2.
The regression equations and lower limit of quantification of the three analytes
Precision and accuracy
The intra-and inter-day precisions and accuracies of berberine, jatrorrhizine, and palmatineare are summarized in Table 3. The intra- and inter-day precisions for each QC sample were less than 15%, and the accuracy was within ± 10%. These results indicate that the precision and accuracy of the method were acceptable for the quantitative analysis of the blood plasma samples.
Table 3.
Accuracy and precision of the analytes in rat plasma at low, medium and high concentration levels (n = 3 days, 6 replicates per day).
Extraction recovery and matrix effect
The extraction recoveries for three concentration levels of the analytes ranged from 90.13% to 98.70%, as shown in Table 4. These results indicate that the extraction efficiency of the method was within the acceptance criteria. The matrix effect values were between 93.84% and 110.75% for the analytes at three QC concentration levels, indicating no significant matrix effect.
Table 4.
The extraction recovery and matrix effect of the analytes in rat plasma at low, medium, high concentration levels (n = 6)
Stability
The stability results are summarized in Table 5. Relative error was between-8.03% and 8.12% for the analytes under the four conditions. The results demonstrated that the three analytes were stable under these storage conditions; no significant degradation occurred.
Table 5.
The stability of the analytes in rat plasma (n = 6)
Application to pharmacokinetic study
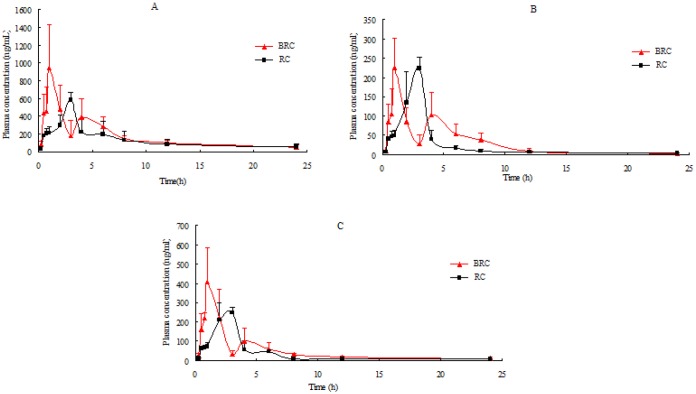
We successfully applied a UPLC-MS/MS method to the pharmacokinetic study of berberine, jatrorrhizine, and palmatinein rat plasma after oral administration of RC and BRC extracts. The mean plasma concentration-time profiles of berberine, jatrorrhizine, and palmatine are shown in Figure 2. We calculated the pharmacokinetic parameters using non-compartmental model analysis [Table 6].
Figure 2.
The mean (± SD, n=8) plasma concentration-time profiles of the three analytes in heat syndrome rats after the oral administration of RC and BRC extract. (A) Berberine; (B) Jatrorrhizine; and (C) Palmatine.
Table 6.
The pharmacokinetic parameters of the three analytes in rats after the oral administration of Rhizoma Coptidis and pigs’ bile processed Rhizoma Coptidis extract (n = 8)
The overall pharmacokinetic profiles of the three alkaloids in rats treated with BRC were similar to those of rats treated with RC [Figure 2]. In both groups, bimodal phenomena appeared owing to re-absorption and enterohepatic circulation of RC.[13] As shown in Table 6, there were no significant differences between RC and BRC in parameters AUC0→∞, AUC0→t, t1/2, and Clz, indicating that bile processing did not affect the bioavailability of the three alkaloids. However, Tmax of the alkaloids in rat plasma after oral administration of BRC extract was one-half that of RC. The observed shorter Tmax shows that the alkaloids were absorbed by rats more rapidly after the bile processing treatment. Compared with those in the RC treated group, Cmax of berberine and palmatine in the BRC group increased about two-fold, indicating that the absorption of the alkaloids was accelerated after bile processing treatment, which was associated with increases in the contents of three alkaloids after it was processed. The contents of berberine, jatrorrhizine, and palmatine were 31.56, 11.23, and 8.60 mg/g in RC and 36.50, 12.24, and 10.73 mg/g in BRC, respectively. After RC was processed with pig's bile, the contents of the three alkaloids in the extract increased significantly. Our previous research has indicated that transfer rates of total alkaloids from water decoctions of BRC were higher than those of RC were.[7] These results show that the pig's bile could affect the contents of the alkaloids in RC prepared aqueous extract, as well as the absorption of the alkaloids after the extracts were orally administration to rats.
The alkaloids in RC have therapeutic actions even at low concentrations.[14] The processing adjuvant of pig's bile mainly consists of a number of bile acids, including hyodeoxycholic acid, chenodeoxycholic acid, and lithocholic acid. The protoberberine-type alkaloids, which have alkaline and hydrophobic characteristics, formed a salt with bile acids and then easily dissolved in water. Kirana et al.[15] tested the suitability of pig's bile–derived micelles and proved that pig's bile was a convenient source of micelles for cholesterol micelle solubility and cellular uptake assay systems. The effect of surfactant from bile acids could accelerate the solubility of protoberberine-type alkaloids in RC, thus improving the absorption of alkaloids from RC in the intestinal tract, and promoting the absorption of alkaloids by rats.
The rapid absorption rate of alkaloids observed with oral administration of BRC could alter the herb's therapeutic effects. In a previous study, we observed an antipyretic effect of BRC, and the antipyretic effect at 3h after the oral administration of BRC was significantly superior to that of RC, but there was no significant difference between BRC and RC groups at the 6h and 9h time points after the herb therapy.[6] The antipyretic effect of BRC was better than that of RC in the early period of treatment, which was related with the shorter Tmax and higher Cmax of BRC.
The comparative results of pharmacokinetic behaviors of alkaloids between RC and BRC indicated that bile processing could promote the absorption rate of alkaloids. We hope to further explore how the processing technology of traditional Chinese medicine affects the pharmacokinetic behaviors of herbs in future research.
CONCLUSIONS
In this study, we established a method to measure berberine, jatrorrhizine, and palmatine in rat plasma by using UPLC-MS/MS. Using this method, we found differences in the pharmacokinetic behavior of Rhizoma coptidis and pig's bile processed Rhizoma coptidis. We found that rats treated with an aqueous extract of bile-processed Rhizoma coptidis had a two-fold increase in Cmax and a decreased Tmax for berberine, jatrorrhizine, and palmatine, compared to the control group that received an aqueous extract of the crude herb. There was no significant difference between the groups in AUC0→∞, AUC0→t, t1/2, and Clz. The bile processing appears to increase the absorption rate of alkaloids in Rhizoma coptidis. This study contributes to our understanding of the relationship between the pharmacokinetic behavior of herbs and herb processing technology.
Financial support and sponsorship
This work was financially supported by the National Natural Science Foundation of China. (No. 81303225, No. 81303205)
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Li HM, Wang YY, Wang HD, WJ Cao, Yu XH, Lu DX, et al. Berberine protects against lipopolysaccharide-induced intestinal injury in mice via alpha 2 adrenoceptor-independent mechanisms. Acta Pharmacologica Sinca. 2011;32:1364–72. doi: 10.1038/aps.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu J, Wang JS, Kong LY. Anti-inflammatory effects of Huang-Lian-Jie-Du decoction, its two fractions and four typical compounds. Journal of Ethnopharmacology. 2011;134:911–8. doi: 10.1016/j.jep.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 3.Wan HY, Kong XY, Li XM, Zhu HW, Su XH, Lin N, et al. Effect of traditional Chinese medicines with different properties on thermoregulation and temperature-sensitive transient receptor potentialion channel protein of rats with yeast-induced fever. China Journal of Chinese Materia Medica. 2014;39:3813–8. [PubMed] [Google Scholar]
- 4.Shen XQ, Zhang F, Jia TZ. Studied on antipyretic effect before and after Rhizoma coptidis processed with pig's bile. Chin Med Pharmaco Clinic. 2013;29:118–22. [Google Scholar]
- 5.Liu F, Zhang ZQ, Lai JY, Hu B. Determination of four kinds of alkaloids from Rhizoma Coptis and processed Rhizoma Coptis by HPLC. Chin Tradit Pat Med. 2010;32:1925–8. [Google Scholar]
- 6.Wang J, Chen Y, Yuan ZM. Influence on serum thyroid hormone and body temperature before and after Coptidis Rhizoma processed with the pig's bile on the febrile rats. Chin J Mod Appl Pharm. 2015;12:1417–9. [Google Scholar]
- 7.Wang J, Lv J, Yuan ZM, Chen Y. Effect of being processed on dissolution of alkaloids in CoptidisRhizoma with pig's bile. Chin J Exp Tradit Med. 2016;22:5–9. [Google Scholar]
- 8.Zhou YF, Hu CJ, Zhang XJ, Yu LY, Chen L, Gao Y, et al. Pharmacokinetic of Epimedii Folium with and without processing in rats. Chin Tradit Pat Med. 2013;35:2717–20. [Google Scholar]
- 9.Qian XC, Zhang L, Tao Y, Huang P, Lia JS, Chai C, et al. Simultaneous determination of ten alkaloids of crude and wine-processed Rhizoma Coptidis aqueous extracts in rat plasma by UHPLC-ESI-MS/MS and its application to a comparative pharmacokinetic study. Journal of Pharmaceutical and Biomedical Analysis. 2015;105:64–73. doi: 10.1016/j.jpba.2014.11.049. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto K, Takase H, Abe K, Saito Y, Suzuki A. Pharmacological studies on antidiarrheal effects of a preparation containing berberine and geraniiherba, Nihon yakurigakuzasshi. Folia Pharmacologica Japonica. 1993;101:169–75. doi: 10.1254/fpj.101.3_169. [DOI] [PubMed] [Google Scholar]
- 11.Küpeli E, Kosar M, Yesilada E, Baser KHC, Baser C. A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species. Life Sciences. 2002;72:645–57. doi: 10.1016/s0024-3205(02)02200-2. [DOI] [PubMed] [Google Scholar]
- 12.Mirska I, Kedzia H, Kowalewski Z, Kedzia W. The effect of berberine sulfate on healthy mice infected with Candida albicans. Archivum Immunologiae et The rapiae Experimentalis. 1972;20:921–9. [PubMed] [Google Scholar]
- 13.Deng Y, Liao Q, Li S, Bi K, Pan B, Xie Z. Simultaneous determination of berberine, palmatine and jatrorrhizine by liquid chromatography-tandem mass spectrometry in rat plasma and its application in a pharmacokinetic study after oral administration of coptis-evodia herb couple. Journal of Chromatography. B, Analytical technoligies in the biomedical and life sciences. 2008;863:195–05. doi: 10.1016/j.jchromb.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 14.Ma BL, Yao MK, Zhong J, Ma YM, Gao CL, Wu JS, et al. Increased systemic exposure to Rhizoma Coptidis alkaloids in lipopolysaccharide-pretreated rats attributable to enhanced intestinal absorption. Drug Metabolism and Disposition. 2012;40:381–8. doi: 10.1124/dmd.111.041152. [DOI] [PubMed] [Google Scholar]
- 15.Kirana C, Rogers PF, Bennett LE, Abeywardena MY, Patten GS. Naturally derived micelles for rapid in vitro screening of potential cholesterol-lowering bioactives. Journal of Agriculture and Food Chemistry. 2005;53:4623–7. doi: 10.1021/jf050447x. [DOI] [PubMed] [Google Scholar]