Abstract
Background:
In Korea, Aloe is routinely ingested as a traditional medicine or as a component of health beverages.
Objective:
To research the inhibition of indoleamine 2, 3-dioxygenase (IDO) activities of components from Aloe.
Materials and Methods:
the compounds were isolated by a combination of silica gel and YMC Rp-18 column chromatography, and their structures were identified by analysis of spectroscopic data (1D, 2D-NMR, and MS). All of the isolated compounds were examined for their ability to inhibit IDO, which actively suppresses immune functions by catalyzing the rate limiting reaction in the conversion of tryptophan to kynurenine.
Results:
In this phytochemical study, 18 known compounds were isolated from aqueous dissolved Aloe exudates. All of the isolated compounds were examined for their ability to inhibit IDO activities for a series of anthraquinone derivatives (1-7) isolated from the Aloe extract; the IC50 values of these compounds ranged from 39.41 to 53.93 µM. Enzyme kinetic studies of their modes of inhibition indicated that all of the compounds were uncompetitive inhibitors.
Conclusion:
The aqueous dissolved Aloe exudate can be used as a source of novel natural IDO inhibitors and merit testing as therapeutic agents in the treatments of cancer and immunopathologic diseases, such as autoimmune, inflammatory, and allergic disorders.
SUMMARY
In this study, 18 known compounds were isolated from aqueous dissolved Aloe exudates. All of the isolated compounds were examined for their ability to inhibit indoleamine 2, 3-dioxygenase (IDO) activities for a series of anthraquinone derivatives (1−7) isolated from the Aloe extract.
Abbreviation used: IDO: inhibit indoleamine 2, 3-dioxygenase, TMS: tetramethylsilane, HMQC: heteronuclear multiple quantum correlation, HMBC: heteronuclear multiple bond correlation, COSY: 1H-1H correlation spectroscopy, ESI-MS: Electrospray ionization mass spectrometry, DMSO: dimethyl sulfoxide
Keywords: Aloe, anthraquinone derivatives, Asphodelaceae, indoleamine 2, 3-dioxygenase (IDO)
INTRODUCTION
Indoleamine 2, 3-dioxygenase (IDO) is an intracellular enzyme that catalyzes the transformation of L-tryptophan to N-formylkynurenine, which is the first and rate-controlling step in the kynurenine pathway.[1] The role of IDO in immunomodulation has been reported in previous studies, including preclinical studies of allograft tolerance, inflammation, and cancer.[2] Both animal and human studies have demonstrated that IDO-expressing cells function as immunosuppressors by increasing T-lymphocyte tolerance. These observations strongly suggest that IDO plays an important role in the regulation (suppression) of the adaptive immune response and thus provides an excellent therapeutic target for anticancer immunotherapy.[3]
Aloe is a short-stemmed succulent herb what belongs to the Asphodelaceae family. The seven genera of this family include approximately 650 species.[4] Of these, the 400 species of the genus Aloe typically grow in temperate and subtropical regions of Africa.[5,6,7] Based on morphological characteristics,[8] the genus Aloe has been divided into 20 subgroups, ranging from grass to tree aloes. In addition to its use in traditional medicines for the treatment of various diseases, aloe is an ingredient in many cosmetics and health foods. A previous phytochemical study revealed that the main constituents of Aloe are phenolic compounds, including anthraquinones, chromones, and pyrones.[9,10,11] Aqueous Aloe exudates and several monomeric compounds isolated from Aloe exert anti-inflammatory, antioxidant, anticancer, and antidiabetic properties.[12,13,15] However, to our knowledge, the IDO inhibitory activity of the plant has not been reported. In the present study, 18 compounds isolated from aqueous dissolved Aloe exudates were evaluated for their IDO inhibitory activities. Our findings have medical and pharmacological applications.
MATERIALS AND METHODS
General experimental procedures
The NMR spectra were recorded using a JEOL ECA 600 spectrometer (1H, 600 MHz and 13C, 150 MHz), with tetramethylsilane as an internal standard. Heteronuclear multiple quantum correlation, heteronuclear multiple bond correlation, and 1H- 1H correlation spectroscopy spectra were recorded using a pulsed field gradient. The ESI-MS: Electrospray ionization mass spectrometry and DMSO: dimethyl sulfoxide, spectra were obtained by using an Aglient 1200 LC-MSD Trap spectrometer. Melting points were determined using an Electro thermal IA-9200 system. Column chromatography was performed using a silica gel (Kieselgel 60, 70-230, and 230-400 mesh, Merck, Darmstadt, Germany), YMC RP-18 resins, and thin layer chromatography was performed using pre-coated silica-gel 60 F254 and RP-18 F254S plates (both 0.25 mm, Merck, Darmstadt, Germany), the spots were detected under UV light and using 10% H2SO4.
Plant material
The dried exudates of Aloe were purchased from herbal company, Naemome Dah, Ulsan, Korea in April 2014, and identified by Prof. Young Ho Kim, College of Pharmacy, Chungnam National University. A voucher specimen (CNU14105) was deposited at the herbarium of the College of Pharmacy, Chungnam National University in Korea.
Extraction and isolation
The dried exudation of Aloe (200 g) were dissolved in H2O three times under ultrasonication at ambient temperature and then filtered. The filtrate was concentrated and partitioned with CHCl3, EtOAc, and n-BuOH to yield CHCl3 (2.0 g), EtOAc (24.0 g), and n-BuOH (20.0 g) fractions. The EtOAc fraction (24.0 g) was subjected to silica gel (10 × 30 cm) column chromatography with a gradient of CHCl3-MeOH-H2O (1:0:0, 30:1:0, 15:1:0, 9:1:0, 5:1:0.1, 1:1:0.1; 2.0 L for each step) to give seven fractions (Fr. E1–E7). Fraction E2 was further chromatographed on a reverse-phase (RP; 1.0 × 80 cm) column chromatography using a gradient of MeOH-H2O (1:4, 1:2; 500 mL each step) to give compounds 1 (37.5 mg), 10 (3.0 mg), and 11 (16.8 mg). The fraction E3 was chromatographed on an RP (1.5 × 80 cm) column with MeOH-H2O (1:5, 1:3.5, 1:1.5, 1:1; 800 mL each step) to obtain four subfractions (E3.1–E3.4), further purification of the subfraction E3.3 by silica gel (1.0 × 60 cm) column with CHCl3-MeOH (20:1, 15:1; 800 mL each step) led to compounds 4 (2.0 mg), 9 (3.0 mg), and 16 (15.0 mg). Subfraction E3.4 was separated using a silica gel (2.0 × 80 cm) column chromatography with a gradient of CHCl3-MeOH (15:1, 10:1; 1 L each step) to give compounds 8 (100.0 mg) and 14 (16.0 mg). Fraction E5 was column chromatographed over RP (3.0 × 80 cm), eluting with MeOH-H2O (1:4, 1:2, 1:1, 2:1; 1500 mL each step) to obtain five subfractions (E5.1–E5.5), then subfraction E5.3 was further chromatographed on a silica gel (1.0 × 60 cm) column chromatography with a gradient of CHCl3-MeOH (10:1; 800 mL) to give compound 2 (50.0 mg). Subfraction E5.5 was chromatographed on a silica gel (1.0 × 80 cm) column chromatography with CHCl3-MeOH-H2O (6.5:1:0.1; 800 mL) to yield compound 3 (100.0 mg).
The n-BuOH fraction (20.0 g) was subjected to silica gel (10.0 × 30 cm) column chromatography with a gradient of CHCl3-MeOH-H2O (15:1:0, 10:1:0, 7:1:0.1, 4:1:0.1, 2:1:0.2; 2.0 L for each step) to give six fractions (Fr. B1–B6). The fraction B3 was subjected to RP (2.5 × 60 cm) column chromatography with a gradient of acetone-MeOH-H2O (0.025:0.025:1, 0.1:0.1:1, 0.2:0.2:1. 0.3:0.3:1; 1.5 L for each step) to give five fractions (Fr. B3.1–B3.5). The fraction B3.2 was subjected to an silica gel (1.0 × 80 cm) column chromatography with a CHCl3-MeOH-H2O (7:1:0.1; 800 mL) to give compounds 13 (2.0 mg) and 15 (6.5 mg). The fraction B3.4 was separated using an RP (1.0 × 80 cm) column chromatography with an MeOH-H2O (1:2; 800 mL) elution solvent to give compound 12 (15.0 mg). The fraction B5 was subjected to RP (2.5 × 60 cm) column chromatography with a gradient of acetone-MeOH-H2O (0.025:0.025:1, 0.1:0.1:1, 0.2:0.2:1. 0.3:0.3:1, 0.4:0.4:1; 2.0 L for each step) to give six fractions (Fr. B5.1–B5.6). Compound 18 (20.0 mg) was isolated from fraction B5.3 using a silica gel (2.0 × 80 cm) column chromatography with CHCl3-MeOH-H2O (4:1:0.1; 1.0 L). Subfraction B5.5 was subjected to a silica gel (1.5 × 80 cm) column chromatography with a CHCl3-MeOH-H2O (5:1:0.1; 1.0 L for each step) elution solvent to give compound 17 (20.4 mg). The fraction B6 was subjected to RP (2.5 × 60 cm) column chromatography with a gradient of acetone-MeOH-H2O (0.025:0.025:1, 0.05:0.05:1, 0.2:0.2:1. 0.3:0.3:1, 0.4:0.4:1; 1.0 L for each step) to give seven fractions (Fr. B6.1–B6.7). Subfraction B6.5 was further purified by chromatography column over silica gel (1.0 × 80 cm) to obtain compounds 5 (15.0 mg) and 6 (3.0 mg). Compound 7 (8.0 mg) was isolated from fraction B6.7 using a silica gel (1.0 × 80 cm) column chromatography with CHCl3-MeOH-H2O (6.5:1:0.1; 800 mL).
IDO assay and determination of inhibition pattern of IDO inhibitors
IDO assays were performed mainly as described previously by Nakano et al.[16] Briefly, a compound serially diluted in DMSO was mixed with 1 μg of purified human IDO in an IDO assay buffer (50 mM potassium phosphate buffer, pH 6.5). Then L-(+)-ascorbic acid, methylene blue, and catalase and L-tryptophan were added to the enzyme-compound mixture to final concentrations of 50 mM, 20 mM, 10 mM, 100 μg/mL, and 200 μM, respectively. The enzyme reaction mixture was incubated at 37°C for 1 hr. After incubation, the reaction mixture was supplemented with 40 μL of 30% trichloroacetic acid and heated for 15 min at 65Β °C followed by centrifugation to remove the precipitate. The supernatant taken after centrifugation was mixed with an equal volume of Ehrlich's reagent (2% p-dimethylaminobenzaldehyde in acetic acid) and incubated at RT. The intensity of the color developed, which represents the concentration of L-kynurenine produced during the enzyme reaction, was measured by reading the absorbance at 480 nm wavelength.
Inhibition patterns of IDO inhibitors were determined with Lineweaver-Burk plot, for which IDO assays were run at five different L-tryptophane concentrations and four different inhibitor concentrations (0, 25, 50, 100 μL), respectively. Ki of IDO inhibitors were calculated from the y-intercept of Lineweaver-Burk plot, which is denoted by (1 + [I]/Ki)/Vmax.[17,18]
Statistical analysis
All measurements were performed independently at least triplicate. Data were expressed as the mean ± standard deviation (SD). Statistical significance is determined by one-way analysis of variance followed by Dunnett's multiple comparison test, P < 0.05.
RESULTS
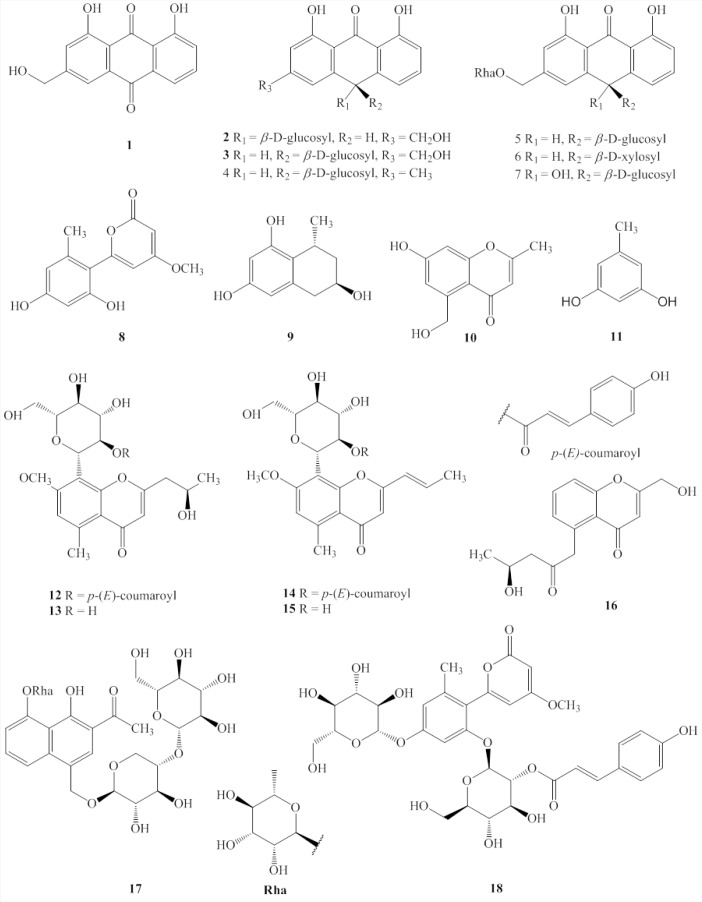
In this study, 18 compounds (1-18) were isolated from the aqueous dissolved Aloe exudate. The structures of compounds 1-18 were identified based on spectroscopic data, chemical evidence, and comparisons with previously reports [Figure 1]. Their structures were elucidated as aloe emodin (1),[19] aloin A (2),[20] aloin B (3),[20] desoxyaloin (4),[21] aloinoside B (5),[22] aloinoside C (6),[23] aloinoside D (7),[19] aloenin aglycone (8),[24] feroxidin (9),[25] 7-hydroxy-5-(hydroxymethyl)-2-methylchromone (10),[24] 5-methylresorcinol (11),[26] aloe resin D (12),[11] 7-O-methylaloesinol (13),[11] aloeresin G (14),[27] C-2’-decoumaroylaloeresin G (15),[14] 5-((S)-2’-oxo-4’-hydrosypentyl)-2-hydroxymethylchromone (16),[24] aloveroside A (17),[28] and aloenin B (18).[28]
Figure 1.
Structures of compounds 1–18 from Aloe.
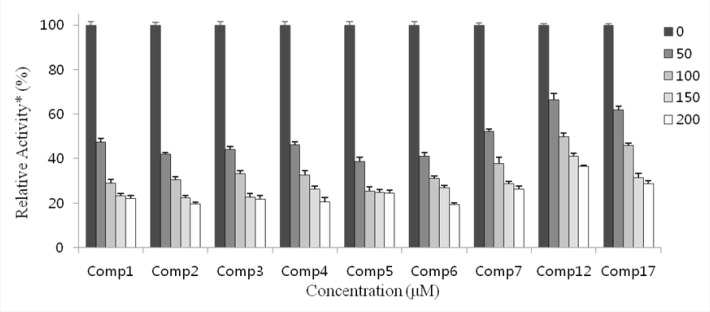
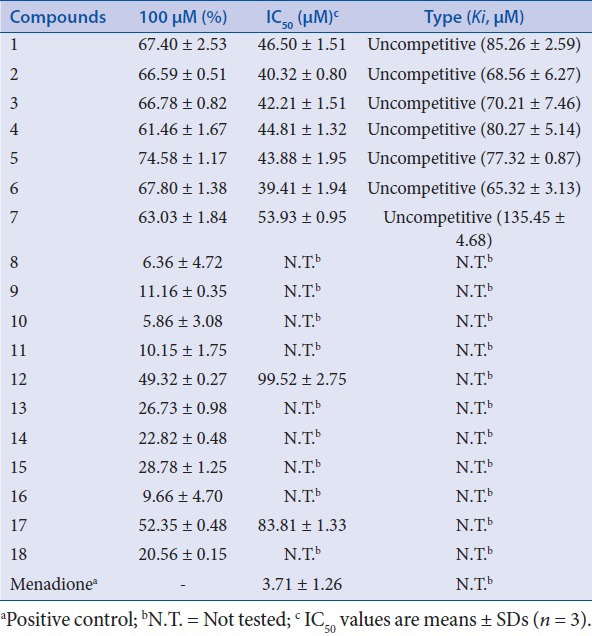
The inhibitory activities of the 18 isolated compounds against IDO were evaluated. Menadione served as the positive control (IC50: 3.71 ± 1.26 μM). The IDO inhibition of compounds 1–18 were tested at concentration of 100 μM. At this concentration, the effects of several compounds were minimal, although compounds 1–7 showed over 60% inhibition at 100 μM. The activities of these seven compounds were examined further at lower concentrations to determine their respective IC50 values. Potent inhibitory activities with IC50 values of 46.50 ± 1.51, 40.32 ± 0.80, 42.21 ± 1.51, 44.81 ± 1.32, 43.88 ± 1.95, 39.41 ± 1.94, and 53.93 ± 0.95 μM, were determined for compounds 1–7, respectively [Figure 2 and Table 1].
Figure 2.
IDO inhibitory effects of compounds 1–7, 12, and 17. * Relative to that obtained in the absence of IDO inhibitor were plotted. Concentrations (μM) of inhibitors used in the experiment are as shown.
Table 1.
The IDO inhibitory activities of compounds 1–18
An analysis of the structure-activity relationships of the isolated compounds showed that compounds 1-7 all have an anthraquinone core structure. It therefore seems likely that the anthracycline moiety is responsible at least in part for the observed inhibition of IDO.
DISCUSSION
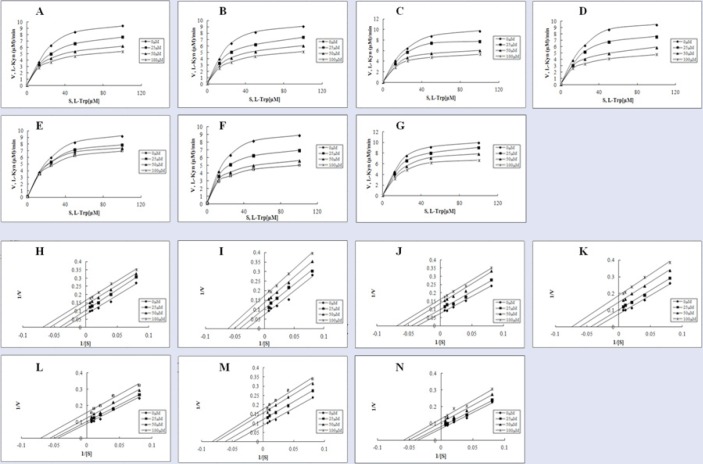
To more precisely determine the modes of inhibition of compounds 1-7, enzyme kinetics studies employing Lineweaver-Burk (double reciprocal) plots were performed. As shown in Figure 3, regardless of the compound used, Lineweaver-Burk plots generated in the absence (0 μM) or presence of three different concentrations (25, 50, and 100 μM) of the respective compounds yielded almost parallel line. These results suggest that compounds 1-7 should be uncompetitive inhibitors [Figure 3]. Based on the notion that compounds 1-7 were uncompetitive inhibitors, their inhibition constants (Ki) were also calculated using the same plots [Table 1].
Figure 3.
The inhibition pattern of compounds 1–7. Michaelis-Menten plots with data obtained in the presence (25, 50, and 100 µM, respectively) or absence of compounds 1 (A), 2 (B), 3 (C), 4 (D), 5 (E), 6 (F), and 7 (G). Lineweaver-Burk plots [1 (H), 2 (I), 3 (J), 4 (K), 5 (L), 6 (M), 7 (N)] generated after transformation of data used in (A–G).
In recent years, several studies have reported IDO inhibition by various synthetic and microbial secondary products.[29,30] However, studies on IDO inhibitors derived from plants are limited. To our knowledge, this is the first report detailing the IDO inhibitory activities of anthraquinone derivatives from Aloe. Our finding suggest that aqueous dissolved Aloe exudate can be used as a source of novel natural IDO inhibitors and merit testing as therapeutic agents in the treatments of cancer and immunopathologic diseases such as autoimmune, inflammatory, and allergic disorders. Moreover, the anthraquinone compounds identified in this study may be exploitable as templates for the synthesis of more potent and potentially clinical IDO inhibitors.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
This study was supported by the Priority Research Center Program (2009-0093815) through the National Research Foundation of Korea (NRF) and Global R and D Center program (NRF-2014K1A4A3069114) and Small Grant for Exploratory Research (NRF-2015R1D1A1A02062348) from the National Research Foundation (NRF), Republic of Korea
REFERENCES
- 1.Lob S, Konigsrainer A, Rammensee HG, Opelz G, Terness P. Inhibitors of indoleamine-2, 3-dioxygenase for cancer therapy: Can we see the wood for the trees? Nat Rev Cancer. 2009;9:445–52. doi: 10.1038/nrc2639. [DOI] [PubMed] [Google Scholar]
- 2.Mellor AL, Munn DH. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 3.Eleftheriadis T, Pissas G, Antoniadi G, Spanoulis A, Liakopoulos V, Stefanidis I. Indoleamine 2,3-dioxygenase increases p53 levels in alloreactive human T cells, and both indoleamine 2,3-dioxygenase and p53 suppress glucose uptake, glycolysis and proliferation. Int Immunol. 2014;26:673–84. doi: 10.1093/intimm/dxu077. [DOI] [PubMed] [Google Scholar]
- 4.Smith GF, Vanwyk BE, Mossmer M, Viljoen A. The taxonomy of Aloinella, Guillauminia and Lemeea (Aloaceae) Taxon. 1995;44:513–7. [Google Scholar]
- 5.Mebberley DJ. The plant book; a portable dictionary of higher plants. Cambridge, UK: Cambridge University Press; 1987. [Google Scholar]
- 6.Demissew S. The botany and chemistry of Aloes of Africa. Bull Chem Soc Ethiop. 1996;10:73–88. [Google Scholar]
- 7.Okamura N, Asai M, Hine N, Yagi A. High-performance liquid chromatographic determination of phenolic compounds in Aloe species. J Chromatogr. 1996;746:225–31. [Google Scholar]
- 8.Reynolds GW. Aloes of tropical Africa and Madagascar. Mbabane, Swaziland: Aloe Book Fund; 1996. [Google Scholar]
- 9.Conner JM, Gray AI, Waterman PG, Reynolds T. Novel anthrone anthraquinone dimers from Aloe elgonica. J Nat Prod. 1990;53:1362–4. [Google Scholar]
- 10.Okamura N, Hine N, Tateyama Y, Nakazawa M, Fujioka T, Mihashi K, et al. Five chromones from Aloe vera leaves. Phytochemistry. 1998;49:219–23. [Google Scholar]
- 11.Dur̬ L, Morelli CF, Crippa S, Speranza G. 6-Phenylpyrones and 5-methylchromones from Kenya aloe. Fitoterapia. 2004;75:520–2. doi: 10.1016/j.fitote.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Hutter JA, Salman M, Stavinoha WB, Satsangi N, Williams RF, Streeper RT, et al. Antiinflammatory C-glucosyl chromone from Aloe barbadensis. J Nat Prod. 1996;59:541–3. doi: 10.1021/np9601519. [DOI] [PubMed] [Google Scholar]
- 13.Lee KY, Weintraub ST, Yu BP. Isolation and identification of a phenolic antioxidant from Aloe barbadensis. Free Radic Biol Med. 2000;28:261–5. doi: 10.1016/s0891-5849(99)00235-x. [DOI] [PubMed] [Google Scholar]
- 14.Lv L, Yang QY, Zhao Y, Yao CS, Sun Y, Yang EJ, et al. BACE1 (beta-secretase) inhibitory chromone glycosides from Aloe vera and Aloe nobilis. Planta Med. 2008;74:540–5. doi: 10.1055/s-2008-1074496. [DOI] [PubMed] [Google Scholar]
- 15.Abdissa N, Induli M, Fitzpatrick P, Alao JP, Sunnerhagen P, Landberg G, et al. Cytotoxic quinones from the roots of Aloe dawei. Moleculers. 2014;19:3264–73. doi: 10.3390/molecules19033264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakano S, Takai K, Isaka Y, Takahashi S, Unno Y, Ogo N, et al. Identification of novel kynurenine production-inhibiting benzenesulfonamide derivatives in cancer cells. Biochem Biophys Res Commun. 2012;419:556–61. doi: 10.1016/j.bbrc.2012.02.059. [DOI] [PubMed] [Google Scholar]
- 17.Kudo Y, Boyd CA. Human placental indoleamine 2,3- dioxygenase: Cellular localization and characterization of an enzyme preventing fetal rejection. Biochim Biophys Acta. 2000;1500:119–24. doi: 10.1016/s0925-4439(99)00096-4. [DOI] [PubMed] [Google Scholar]
- 18.Dolusic E, Larrieu P, Moineaux L, Stroobant V, Pilotte L, Colau D, et al. Tryptophan 2,3-dioxygenase (TDO) inhibitors. 3-(2-(Pyridyl)ethenyl)indoles as potential anticancer immunomodulators. J Med Chem. 2011;54:5320–34. doi: 10.1021/jm2006782. [DOI] [PubMed] [Google Scholar]
- 19.Danielsen K, Aksnes DW. NMR study of some anthraquinones from Rhubarb. Magn Reson Chem. 1992;30:359–63. [Google Scholar]
- 20.Manitto P, Monti D, Speranza G. Studies on aloe-6-conformation and absolute configuration of aloin A and B related to 10-C-glucosyl-9-anthrones. J Chem Soc Perk T. 1990;1:1297–300. [Google Scholar]
- 21.Hata K, Baba K, Kozawa M. Chemical studies on the heartwood of Cassia garrettiana Craib. I. Anthraquinones including cassialoin, a new anthrone C-glycoside. Chem Pharm Bull. 1987;26:3792–7. [Google Scholar]
- 22.Gao J, Zhang GG, Dai R, Bi K. Isolation of aloinoside B and metabolism by rat intestinal bacteria. Pharm Biol. 2004;42:581–7. [Google Scholar]
- 23.Sun YN, Kim JH, Li W, Jo AR, Yan XT, Yang SY, et al. Soluble epoxide hydrolase inhibitory activity of anthraquinone components from Aloe. Bioor Med Chem. 2015;23:6659–65. doi: 10.1016/j.bmc.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Zhong JS, Huang YY, Ding WJ, Wu XF, Wan JZ, Luo HB. Chemical constituents of Aloe barbadensis Miller and their inhibitory effects on phosphodiesterase-4D. Fitoterapia. 2013;91:159–65. doi: 10.1016/j.fitote.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 25.Speranza G, Manitto P, Monti D, Lianza F. Feroxidin a novel 1-methyltetralin derivative isolated from cape aloe. Tetrahedron Lett. 1990;31:3077–80. [Google Scholar]
- 26.Lee YR, Wang X. First concise synthesis of biologically interesting nigrolineabenzopyran A, (±)-blandachromene II, and (±)-daurichromene D. B Korean Chem Soc. 2007;28:2061–4. [Google Scholar]
- 27.Xiao ZY, Chen DH, Si JY, Tu GZ, Ma LB. The chemical constituents of Aloe vera L. Acta Pharmaceutica Sinica. 2000;35:120–3. [Google Scholar]
- 28.Yang QY, Yao CS, Fang WS. A new triglucosylated naphthalene glycoside from Aloe vera L. Fitoterapia. 2010;81:59–62. doi: 10.1016/j.fitote.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Oh WK, Lee HS, Kim BY, Ahn SC, Kang DO, Kim YH, et al. CRM-51005, a new phospholipase C inhibitor produced by unidentified fungal strain MT51005. J Antibiot. 1997;50:1083–5. doi: 10.7164/antibiotics.50.1083. [DOI] [PubMed] [Google Scholar]
- 30.Jang JP, Jang JH, Oh M, Son S, Kim SM, Kim HM, et al. Inhibition of indoleamine 2,3-dioxygenase by thielavin derivatives from a soil fungus, Coniochaeta sp. 10F058. J Antibiot. 2014;67:331–3. doi: 10.1038/ja.2013.134. [DOI] [PubMed] [Google Scholar]