Abstract
Background:
Wheatgrass is known to have antioxidant, antiaging, and anti-inflammatory effect. However, its protective effect against hypoxia is not yet evaluated.
Objective:
In this study, we evaluated the protective and anti-inflammatory effect of wheatgrass against the hypoxia in airway epithelial cells.
Materials and Methods:
A549 human lung adenocarcinoma cells were incubated in a hypoxic condition (CO2 5%/O2 1%) for 24 hr in the presence of different concentration of wheatgrass 50, 75, 100, and 150 μg/mL, and the magnitude of each immunologic response produced by the A549 cells was compared. The mRNA expression level of mucin gene (MUC), 5A, 5B, 8, GM-CSF, TNF-α, and VEGF were evaluated by using real-time polymerase chain reaction. The MUC proteins level before and after knocking out the hypoxia-inducible factor (hif)-1α via short interfering (si) RNA transfection were assessed by immunoblot analysis. Accordingly, the involved cell signaling pathway was evaluated by immunoblot analysis.
Results:
The inflammatory cytokines (GM-CSF, TNF- α) and the expressions of MUC 5A, 5B, and 8 were augmented by hypoxia. The augmented MUC expression was decreased by the wheatgrass extract administration. Hif-1α gene expression after hypoxia exposure was decreased by wheatgrass. Knockdown of hif-1α by siRNA reduced the mucin gene expression and which was more enhanced by wheatgrass extract.
Conclusion:
Theses results suggest that wheatgrass may be useful in the treatment of sinonasal disease by inhibiting mucus hypersecretion in airway epithelium.
SUMMARY
Wheatgrass extract decreases the hypoxia-induced MUC 5A, 5B and 8 expression.
Hif-1α gene expression after hypoxia exposure was decreased by wheatgrass.
Wheatgrass inhibits p44/42 phosphorylation in hypoxia-exposed airway epithelial cells.
Abbreviations used: A549: human lung adenocarcinoma cells, GM-CSF: granulocyte-macrophage colony stimulating factor, HIF: hypoxia inducible factor, IL: interleukin, MUC: mucin, MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, TNF: tumor necrosis factors, VEGF: vascular endothelial growth factor, si RNA: short interfering RNA
Keywords: Airway epithelium, hypoxia, mucin, wheatgrass, VEGF
INTRODUCTION
The airway epithelium is lined by mucus, which is a mixture of water, glycoprotein, proteins, and lipids. Mucin is one of the components of mucus and has been implicated in several airway diseases. In the sinus, mucin gene (MUC) 5AC and MUC 5B are known to be predominant and their expression is controlled by various mediators such as interleukin (IL)-1β, IL-6, IL-13, IL-17 or tumor necrosis factor-α.[1,2,3] Recently, one study demonstrated anoxia upregulates MUC5AC by the hypoxia-inducible factor-1α (hif-1α) signaling pathway in human nasal epithelium.[4]
The roles of hypoxia in sinus, in terms of cause of inflammation, are supposed to be exist generally as at least two different ways. One way is to induce an epithelial–mesenchymal transition, which is basic feature of nasal polyps,[5] and the other is to overexpress the mucin gene. Thus, hif-1α can be a promising target for the prevention and treatment sinus inflammatory disease.
Wheatgrass refers to the young grass of the common monocot wheat plant “Triticumae stivum”. Its consumption in the Western world began in the 1930s. Today, wheatgrass is quickly becoming one of the most widely used supplemental health foods and is available in many health food stores as fresh produce, tablets, frozen juice, and powder. Wheatgrass contains vitamins, minerals, enzymes, amino acids, polysaccharides, and large amounts (70%) of chlorophyll. Several papers have indicated that wheatgrass has antitumor activities,[6] antioxidant properties,[7] and a therapeutic effect on distal ulcerative colitis.[8] In addition, wheatgrass may help prevent some disorders, including diabetes and heart disease.[9]
Considering that hypoxia has been getting an attention as one of the pathogenic mechanism of sinusitis, mucus hypersecretion induced by hypoxia could be a new therapeutic target of sinonasal disease. In this study, we have aimed to evaluate the protective and anti-inflammatory effect of wheatgrass against the hypoxia in airway epithelial cells. We found that wheatgrass extract inhibits hif-1α expression and downregulates hif-1α induced mucin overexpression in airway epithelial cells. This suggests that wheatgrass may have potential therapeutic relevance.
MATERIALS AND METHODS
SP600125 (JNK inhibitor), PD980599 (ERK inhibitor), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma–Aldrich (Munich, Germany). The other chemicals used were of the purest grade available from Sigma (St Louis, MO).
Preparation of wheat sprout sample
The wheatgrass sprout was harvested from a commercial planting located Gwang-ju, Korea in 2015.
Extracts preparation
After grinding dried wheat sprouts in a mixer, they were extracted by 80% ethanol for three days in shaking incubators. Extracting conditions were at 25Â °C, 200 rpm. They were concentrated by the rotary evaporator. We first extracted wheat sprout with 80% ethanol, and the ethanol extracts were fractionated with ethyl acetate or water. Ethyl acetate layer was further separated with n-butanol and water. Then, the butanol fraction was separated with hexane and water.
Cell cultures
A549 human lung adenocarcinoma cells were maintained in RPMI 1640 (Gibco BRL, Gnd Island, NY) media supplemented with 1% penicillin/streptomycin (gibco/Invitrogen, Carlsbad, CA) and 10% fetal calf serum (Capricorn) (w/v). The cells were grown to 60% confluence in 100 mm culture plates and kept at 37°C in a carbon-dioxide-enriched (95% air, 5% CO2) humidified atmosphere.
Cells were pre-treated with different concentration of wheatgrass extract (50–150 μg/mL) for 30 min and then incubated in modular incubator chamber (hypoxic, 5% CO2/1% O2) (Billups-Rotheberg, Del Mar, CA) or in normoxic condition (5% CO2/20% O2) for additional 24 hr.
Cell cytotoxicity assays
Cell proliferation was measured by a colorimetric assay using MTT. In brief, a549 cells cultured overnight on 96-well plates were administered media containing different concentrations of wheatgrass (50–-150 μg/mL) and incubated for 24 hr under hypoxic condition. 10 μL of MTT stock solution (5 mg/mL) were added to each well, followed by incubation for an additional 4 hr. Blue formazans were eluted from cells by the addition of 100 μL of DMSO with gentle shaking for 10 min at room temperature. Absorbances were measured at 570 nm using an enzyme-linked immunosorbent assay reader (Spectra MAX; Molecular Devices, Sunnyvale, CA).
Real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from the cells using TRIzol reagent (Invitrogen) according to manufacturer's instructions, and reverse transcribed into cDNA with the Quantitect Reverse Transcription kit (Qiagen, Venlo, NLD). qRT-PCR analyses were performed using a 7500 FAST qRT-PCR System (Applied Biosystems, Foster City, CA). Each reaction mixture contained 10 μL of SYBR® Green PCR Master Mix (Applied Biosystems), 4 pmol of forward and reverse primers each, and 1 μL of cDNA in a final volume of 20 μL. Reaction mixtures were incubated at 95 °C for 5 min to activate FastStartTaq DNA Polymerase, followed by amplification for 40 cycles. Data were analyzed using Sequence Detection Software version 1.9.1 (Applied Biosystems). Target mRNA expression was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression, and calculated using the comparative Ct method. Primers constructed were shown in Table 1.
Table 1.
Primers for real-time polymerase chain reaction

si RNA transfection
Cells were transfected with short interfering (si) RNA corresponding to human hif-1α or control siRNA using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's protocol. The si-RNA was #1068432V for hif-1α (Bioneer, Daejeon, Korea). After incubation for 4 h, media were changed with complete medium containing 10% serum and antibiotics.
Nuclear protein extract
The nuclear protein was extracted by using the NE-PER® Nuclear and Cytoplasmic Extraction Reagents (Pierce, Rockford, IL) according to the manufacture's instruction.
Immunoblot analysis
After incubation in hypoxic condition, media was removed and cells were washed with phosphate-buffered saline (10 mM, pH 7.4). Then, cells were lysed with lysis buffer (50 mM Tris pH 7.7, 150 mM NaCl, 1% NP-40, 5 mM EGTA, 50 mM glycerophosphate, 20 mM NaF, 1 mM Na3VO4, 2 mM phenylmethyl sulfonyl fluoride, 10 mg/mL leupeptin, and 10 mg/mL aprotinin) and incubated for 20 min at 4°C. After sonication briefly, the cells were centrifuged at 13,000 g for 10 min at 4°C. The supernatant that contained the total cell lysate was collected. Protein concentration of the lysates was measured by Bio-Rad Protein Assay (Bio-Rad, Hercules, CA). Equal amounts of protein were mixed with sample buffer, boiled for 5 min, and separated by electrophoresis on 10%-12% Tris-HCl gels. The protein content of the gels was transferred to a PVDF membrane (Amersham, Buckinghamshire, UK), and the membranes were blocked with TBS-T (20 mM Tris, 500 nM NaCl, with 0.1% Tween-20) containing 5% (w/v) skim milk for 1 hr at room temperature. The membrane was incubated overnight at 4°C with a specific primary antibody of MUC 8 (Sigma-Aldrich, St. Louis, MO), Hif-1a (Novus, Littleton, Co) p38, phospho-p38, p42/44, phospho-p42/44 (Cell Signaling Technology, Danvers, MA), and GAPDH (Santa Cruz Biotechnology, Dallas, TX) followed by peroxidase-conjugated antimouse IgG or antirabbit IgG (Jackson Immuno Research, West Grove, PA). The membranes were developed using the enhanced chemiluminescent analysis system (SuperSignal® West Pico Chemiluminescent Substrate, Pierce, Waltham, MA) and the signal was captured on an image reader (LAS4000; Fuji Photo Film, Tokyo, Japan). Results were obtained from three independent experiments.
Statistical analysis
All data are expressed as mean standard deviation (SD). Statistical analysis was performed using student's t-test of variance. p < 0.05 for the null hypothesis was accepted as indicating a statistically significant difference. Statistical analyses were performed using SPSS for Windows (Ver. 12.0, SPSS Inc., Chicago, IL).
RESULTS
Non-cytotoxic effects of wheatgrass extract on airway epithelial cells
To examine the effect of wheatgrass, we used MTT assays to measure the viabilities of cells that had been exposed to different concentration of wheatgrass for 24 hr under hypoxic condition. Wheatgrass per se did not show toxicity of cells at various concentration of 50–150 μg/mL [Figure 1].
Figure 1.
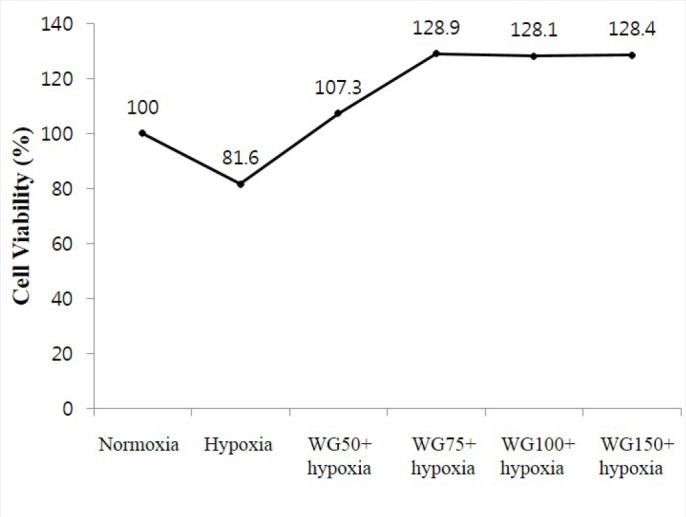
MTT assay to determine cytotoxic effects of hypoxia and wheatgrass. WG, wheatgrass. Wheatgrass per se did not have cytotoxic effect.
Wheatgrass extract decreases mucin gene expression induced by hypoxia exposure
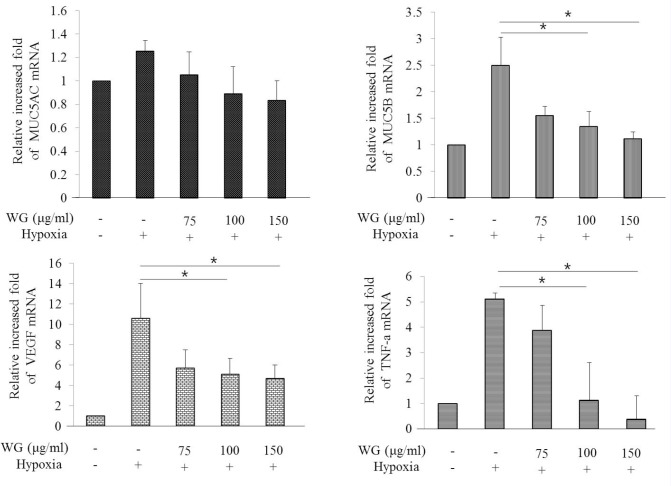
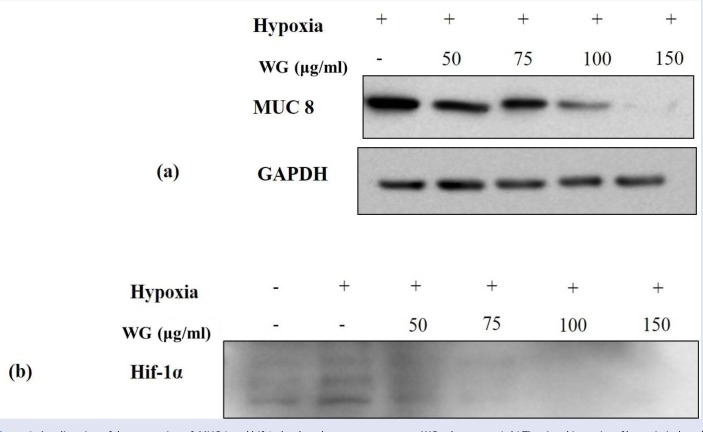
We investigate the effects of wheatgrass extract on MUC gene production in hypoxia-exposed airway epithelial cells. The mRNA expression of MUC 5AC and MUC8 was increased when cells were exposed to hypoxia for 24 hr [Figure 2]. To elucidate the effect of wheatgrass, we treated wheatgrass extract at various concentration of 50, 75, 100, and 150 μg/mL to cells 30 min before hypoxia exposure. There after, cells were incubated in a hypoxic condition for 24 hr. MUC 5AC and MUC 8 mRNA expression were increased after hypoxia exposure, and decreased after wheatgrass administration. It was confirmed at protein level for MUC 8 gene. Hypoxia-induced MUC production was inhibited by wheatgrass in a dose-dependent manner which was confirmed by western blotting Figure 3a. Control GAPDH was constitutively expressed and was not affected by wheatgrass treatment.
Figure 2.
Hypoxia induces the expression of MUC mRNA in A549 cells. WG, wheatgrass; VEGF, vascular endothelial growth factor hypoxia-induced MUC mRNA or VEGF, TNF-α was decreased by wheatgrass administration in a dose-dependent pattern.
Figure 3.
Amelioration of the expression of MUC 8 and hif-1α by the wheatgrass treatment. WG, wheatgrass. (a,b) The signal intensity of hypoxia-induced MUC 8 and hif-1α was diminished as the concentration of wheatgrass rose.
Inhibition of hif-1α activation by wheatgrass extract in hypoxia-exposed airway epithelial cells
Hypoxia is known to induce MUC5AC production via hif-1α signaling pathway.[4] The expression of hif-1α was increased when cells were exposed to hypoxia for 24 hr. The increased hif-1α after hypoxia exposure was decreased dose-dependently by the wheatgrass administration [Figure 3b].These results showed that wheatgrass extract decreased hif-1α gene expression, which was known to be involved in MUC gene expression.
Knockdown of hif-1α reduced the production of mucin gene expression
Next, to further evaluate the effect of wheatgrass extract on hif-1α expression, we transiently knock downed hif-1α gene by using si RNA-hif-1α. After si RNA-hif-1α transfection, cells were incubated in a hypoxic (5% CO2/1% O2) or normoxic condition for 24 hr. The transfection efficiency was confirmed at the protein level. As shown in [Figure 4], signal intensity of MUC 8 protein in cells treated with wheatgrass extract (150 μg/mL) for 30 min and then exposed to hypoxia for 24 hr was similar with that in cells transiently hif-1α gene silenced and then exposed to hypoxia for 24 hr. Moreover, its signal was as low as the normoxic control when cells were transiently transfected and then treated with wheatgrass extract (150 μg/mL). These results suggest that hypoxia-induced increase in mucin gene expression in airway epithelial cells is dependent of hif-1α transcription factor and wheatgrass acts in a way decreasing hif-1α transcriptional factor, thus reduces the mucin gene expression.
Figure 4.
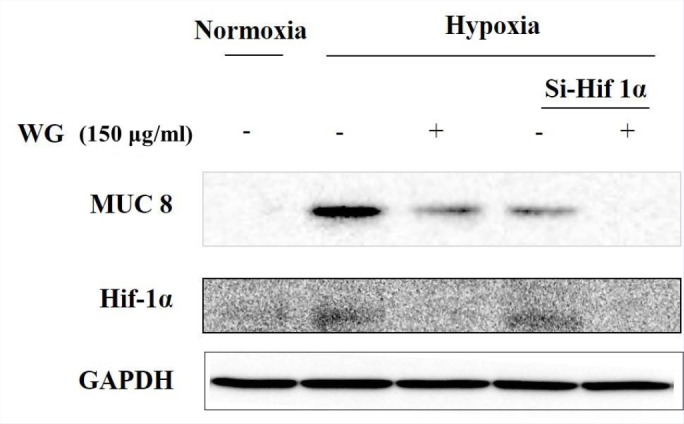
Hif-1α is involved in the regulation of MUC 8 expression by the wheatgrass treatment. WG, wheatgrass. Wheatgrass acts in a way decreasing hif-1α transcriptional factor expression and reducing the mucin gene expression like small interfering hif-1a RNA.
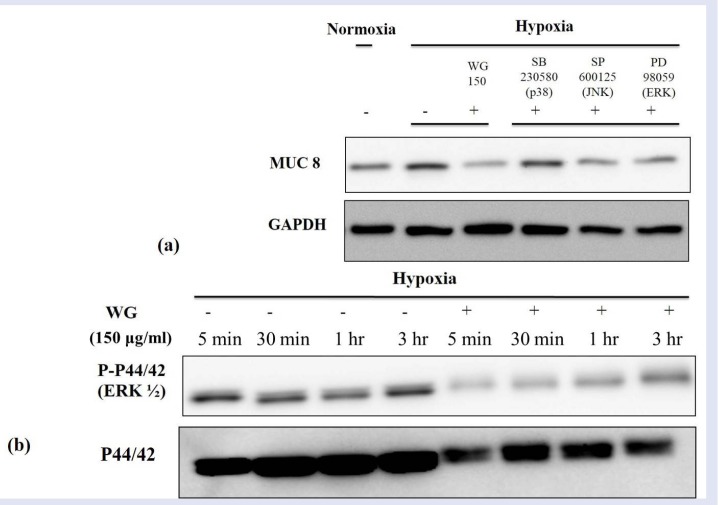
Inhibition of p44/42 (ERK1/2) phosphorylation by wheatgrass in hypoxia-exposed airway epithelial cells
To evaluate the down stream signal pathway for hif-1α transcriptional factor, we used the SB230580 (a p38 inhibitor), SP600125 (a JNK inhibitor), and PD98059. PD98059 is a specific inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase 1 (MEK-1), which is responsible for ERK1/2 (p44/42) activation. Cells treated with wheatgrass extract demonstrated a similar signal density of MUC 8 protein with cells treated the SP600125 or PD98059, whereas SB203580 did not show a definite inhibitory effect [Figure 5a]. These observations indicate a possible involvement of JNK or ERK in the wheatgrass-induced anti-hypoxic effect. Since p44/42 kinase is important for hif-1α downstream pathway in airway epithelial cells and a possible target of wheatgrass extract, we further determined the role p44/42 in hypoxia-induced MUC gene production. So, cells were treated with wheatgrass extract and exposed hypoxia for indicated time [Figure 5b]. A kinetic study showed that hypoxia-induced phosphorylation of p44/42 peaked at 5 min, was maintained until 3 hr. When cells were treated with wheatgrass extract and exposed hypoxia for the same indicated time, the signal intensity of phosphorylation of p44/42 was decreased as shown in Figure 5b. These results suggest that the p44/42 kinase pathway plays an important role in the regulation of MUC gene production of wheatgrass extract in relation with hypoxia in airway epithelial cells.
Figure 5.
JNK/ERK MAPK signal pathway is involved in the regulation of MUC 8 expression by the wheatgrass treatment. WG, wheatgrass. (a) The expression of MUC 8 was decrease by the treatment of SP600125 or PD98059. (b) The signal intensity of phosphorylation of p44/42 was decreased by the wheatgrass administration.
DISCUSSION
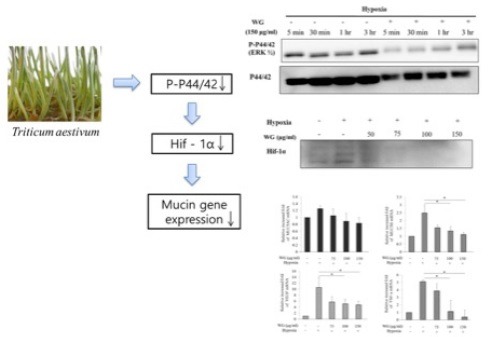
Since hypoxia has been demonstrated to be involved in chronic rhinosinusitis and nasal polyp,[5] targeting hif-1α represents an important therapeutic strategy for sinonasal disease. We sought to identify a natural compound capable of inhibiting hypoxia-mediated inflammatory responses. Wheatgrass is known to have antioxidant, antiaging, and anti-inflammatory effect.[7,8] However, its immune-modulatory effect is not yet evaluated. In this study, we evaluated the protective effect of wheatgrass against hypoxia in airway epithelial cells. We demonstrated that wheatgrass extract inhibits the p44/42 (ERK1/2) pathway in hypoxia-exposed airway epithelial cells and that decreases MUC gene expression in response to hypoxia.
Hif-1α is important for maintaining oxygen homeostasis by transcriptional activation of erythropoietin, vascular endothelial growth factor, and transferrin.[4] Several lines of evidence demonstrated that hif-1α plays crucial roles not only in cancers but also in benign lesions.[10,11] Hif-1α is induced in normal organ under systemic hypoxia.[12,13] Under hypoxia conditions, stable hif-1α dimerizes with hif-1β and binds to the hypoxia-response element for transcriptional activation.[4] The expression of hif-1α in nasal polyp was reported previously and it was also noticed that hif-1α is expressed in nasal epithelium.[14,15] Moreover, ERK activity is required for hif-1α transcriptional factor activation. ERK has been reported to be involved in hif-1α-mediated transcription,[16] indicating that ERK could regulates mucin gene expression by increasing the transactivation capacity of hif-1α.
Wheatgrass extract contains chlorophyll, phenolic compounds, and flavonols. Since it was known to have anti-oxidative effect, it has been mainly studied in cancer-relative topics.[17,18] In this study, we found that wheatgrass extract acts similarly with PD98059 which selectively inhibit the ERK 1/2 pathway, and that decreases MUC gene expression in response to hypoxia.
In summary, these experiments demonstrate that wheatgrass inhibits hypoxia-induced MUC gene expression in airway epithelial cells. Based on our findings, the most likely mechanism that can account for this biological effect involves inhibition of the ERK 1/2 kinase pathway and hif-1α activity. Further research such as in vitro study using primary human nasal epithelial cells or other airway epithelial cell lines, or in vivo study using animal model would be necessary for clinical implication of wheatgrass in sinonasal disease.
CONCLUSION
Considering the importance of hif-1α in sinonasal disease, wheatgrass may be useful in the treatment of sinonasal disease by inhibiting mucus hypersecretion in airway epithelium.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflict of interest.
Acknowledgement
This work was supported by a grant from the Clinical Medicine Research Institute of the Chosun University Hospital (2014).
REFERENCES
- 1.Koo JS, Kim YD, Jetten AM, Belloni P, Nettesheim P. Overexpression of mucin genes induced by interleukin-1 beta, tumor necrosis factor-alpha, lipopolysaccharide, and neutrophil elastase is inhibited by a retinoic acid receptor alpha antagonist. Exp Lung Res. 2002;28:315–32. doi: 10.1080/01902140252964393. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem. 2003;278:17036–43. doi: 10.1074/jbc.M210429200. [DOI] [PubMed] [Google Scholar]
- 3.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, et al. Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol. 2004;31:382–94. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim YJ, Cho HJ, Shin WC, Song HA, Yoon JH, Kim CH. Hypoxia-mediated mechanism of MUC5AC production in human nasal epithelia and its implication in rhinosinusitis. PLoS One. 2014;9:e98136. doi: 10.1371/journal.pone.0098136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin HW, Cho K, Kim DW, Han DH, Khalmuratova R, Kim SW, et al. Hypoxia-inducible factor 1 mediates nasal polypogenesis by inducing epithelial-to-mesenchymal transition. Am J Respir Crit Care Med. 2012;185:944–54. doi: 10.1164/rccm.201109-1706OC. [DOI] [PubMed] [Google Scholar]
- 6.Alitheen NB, Oon CL, Keong YS, Chuan TK, Li HK, Yong HW. Cytotoxic effects of commercial wheatgrass and fiber towards human acute promyelocytic leukemia cells (HL60) Pak J Pharm Sci. 2011;24:243–50. [PubMed] [Google Scholar]
- 7.Das A, Raychaudhuri U, Chakraborty R. Effect of freeze drying and oven drying on antioxidant properties of fresh wheatgrass. Int J Food Sci Nutr. 2012;63:718–21. doi: 10.3109/09637486.2011.644769. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Arye E, Goldin E, Wengrower D, Stamper A, Kohn R, Berry E. Wheat grass juice in the treatment of active distal ulcerative colitis: A randomized double-blind placebo-controlled trial. Scand J Gastroenterol. 2002;37:444–9. doi: 10.1080/003655202317316088. [DOI] [PubMed] [Google Scholar]
- 9.Shermer M. Wheatgrass juice and folk medicine. Sci Am. 2008;299:42. doi: 10.1038/scientificamerican0808-42. [DOI] [PubMed] [Google Scholar]
- 10.Rankin EB, Rha J, Unger TL, Wu CH, Shutt HP, Johnson RS, et al. Hypoxia-inducible factor-2 regulates vascular tumorigenesis in mice. Oncogene. 2008;27:5354–8. doi: 10.1038/onc.2008.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adunka O, Gstoettner W, Knecht R, Kierner AC. Expression of hypoxia inducible factor 1 alpha and Von Hippel Lindau protein in human middle ear cholesteatoma. Laryngoscope. 2003;113:1210–5. doi: 10.1097/00005537-200307000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Stroka DM, Burkhardt T, Desbaillets I, Wenger RH, Neil DA, Bauer C, et al. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. 2001;15:2445–53. doi: 10.1096/fj.01-0125com. [DOI] [PubMed] [Google Scholar]
- 13.Wiesener MS, Jurgensen JS, Rosenberger C, Scholze CK, Horstrup JH, Warnecke C, et al. Widespread hypoxia-inducible expression of hif-2alpha in distinct cell populations of different organs. FASEB J. 2003;17:271–3. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- 14.Chien CY, Tai CF, Ho KY, Kuo WR, Chai CY, Hsu YC, et al. Expression of hypoxia-inducible factor 1alpha in the nasal polyps by real-time RT-PCR and immunohistochemistry. Otolaryngol Head Neck Surg. 2008;139:206–10. doi: 10.1016/j.otohns.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Hsu YC, Kuo WR, Chen YY, Tai CF, Tsai CJ, Wang LF. Increased expression of hypoxia-inducible factor 1alpha in the nasal polyps. Am J Otolaryngol. 2007;28:379–83. doi: 10.1016/j.amjoto.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Movafagh S, Crook S, Vo K. Regulation of hypoxia-inducible factor-1a by reactive oxygen species: new developments in an old debate. J Cell Biochem. 2015;116:696–703. doi: 10.1002/jcb.25074. [DOI] [PubMed] [Google Scholar]
- 17.Shakya G, Balasubramanian S, Rajagopalan R. Methanol extract of wheatgrass induces G1 cell cycle arrest in a p53-dependent manner and down regulates the expression of cyclin D1 in human laryngeal cancer cells-an in vitro and in silico approach. Pharmacogn Mag. 2015;11:S139–47. doi: 10.4103/0973-1296.157715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu LC, kong CK, Ooi VE. The chlorophyllin induced cell cycle arrest and apoptosis in human breast cancer MCF 7 cells is associated with ERK deactivation and Cyclin D1 depletion. Int J Mol Med. 2005;16:735–40. [PubMed] [Google Scholar]