Abstract
Background:
Proliferation and migration of keratinocytes are essential for the repair of cutaneous wounds. Hibiscus syriacus L. has been used in Asian medicine; however, research on keratinocytes is inadequate.
Objective:
To establish the dermatological properties of absolute from Hibiscus syriacus L. flower (HSF) and to provide fundamental research for alternative medicine.
Materials and Methods:
We identified the composition of HSF absolute using gas chromatography-mass spectrometry analysis. We also examined the effect of HSF absolute in HaCaT cells using the XTT assay, Boyden chamber assay, sprout-out growth assay, and western blotting. We conducted an in-vivo wound healing assay in rat tail-skin.
Results:
Ten major active compounds were identified from HSF absolute. As determined by the XTT assay, Boyden chamber assay, and sprout-out growth assay results, HSF absolute exhibited similar effects as that of epidermal growth factor on the proliferation and migration patterns of keratinocytes (HaCaT cells), which were significantly increased after HSF absolute treatment. The expression levels of the phosphorylated signaling proteins relevant to proliferation, including extracellular signal-regulated kinase 1/2 (Erk 1/2) and Akt, were also determined by western blot analysis.
Conclusion:
These results of our in-vitro and ex-vivo studies indicate that HSF absolute induced cell growth and migration of HaCaT cells by phosphorylating both Erk 1/2 and Akt. Moreover, we confirmed the wound-healing effect of HSF on injury of the rat tail-skin. Therefore, our results suggest that HSF absolute is promising for use in cosmetics and alternative medicine.
SUMMARY
Hisbiscus syriacus L. flower absolute increases HaCaT cell migration and proliferation.
Hisbiscus syriacus L. flower absolute regulates phosphorylation of ERK 1/2 and Akt in HaCaT cell.
Treatment with Hisbiscus syriacus L. flower induced sprout outgrowth.
The wound in the tail-skin of rat was reduced by Hisbiscus syriacus L. flower absolute
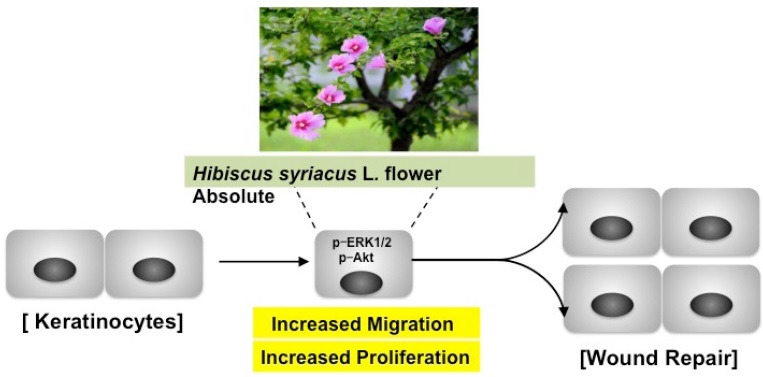
Abbreviations used: HSF: Hibiscus syriacus L. flower, Erk 1/2: extracellular signal-regulated kinase 1/2, EGF: epidermal growth factor, GC/MS: gas chromatography-mass spectrometry, DMEM: dulbecco's modified eagle medium, FBS: fetal bovine serum, BSA: bovine serum albumin, p-Akt: phosphorylation of Akt, p-Erk 1/2: phosphorylation of Erk 1/2.
Keywords: absolute, hibiscus syriacus L., HaCaT cell, skin regeneration, wound healing
INTRODUCTION
The outermost layer of the human integument system is covered with skin, which is one of the multiple layers of ectodermal tissue such as the epidermis.[1] The various risk factors for skin damage include pathogenic microorganisms, physical injury, and metabolic disease.[2,3,4] Chronic wounds are considered to be a serious medical problem because wounds in type 2 diabetes can lead to the development of chronic leg and foot ulcers.[5] Wound healing is the reconstruction and regeneration of damaged tissue, which is associated with complex biochemical processes, as well as a variety of cell activities such as collagen deposition, reepithelialization, and angiogenesis.[6,7,8] As a predominant cell type in the epidermis, keratinocytes contribute to the repair of cutaneous wounds by inducing the proliferation and migration of keratinocytes into the wound area via growth factors.[9]
Epidermal growth factor (EGF)-signal pathways play an important role in the wound healing process in cell growth, proliferation, and differentiation.[10] The downstream of the EGF activation signal regulates extracellular signal-regulated kinase 1/2 (Erk 1/2). In addition, hyperactivation of the Akt signal in the migration and proliferation of keratinocytes during wound healing is associated with the EGF pathway.[11]
Hibiscus syriacus L. found in eastern and southern Asia, is an ornamental shrub. It has been widely cultivated as a decorative shrub because its flower (HSF) is the national flower of Korea. The extracts of several parts from Hibiscus syriacus L. have been used as a prescription and effective alternative medicine in Asia.[12] Recent studies have evaluated natural substances and natural products and their dermatological properties for improving wound healing. Herbal plants or alternative medicine may exhibit lower toxicity with fewer side effects.[13] Therefore, scientific validation of the medicinal effect of plants used in traditional medicine is important.
In this study, we evaluated the dermatological properties of HSF absolute to provide fundamental research for alternative medicine. To determine the biological activity of HSF absolute in wound healing, its chemical compounds were analyzed by gas chromatography–mass spectrometry (GC/MS) and its effect on proliferation and migration in keratinocytes was tested.
MATERIALS AND METHODS
Chemicals
Recombinant human EGF was purchased from R&D Systems (Minneapolis, Minnesota, USA) and Dulbecco's modified eagle medium (DMEM), fetal bovine serum (FBS), and penicillin/streptomycin were purchased from either Hyclone (Logan, Utah, USA) or Invitrogen (Carlsbad, California, USA). Bovine serum albumin (BSA) from Sigma (St. Louis, Missouri, USA) and the EZ-CyTox kit from DAEIL LAB Service (Seoul, South Korea) were used. The antibodies used include Akt, phosphorylation of Akt (p-Akt), Erk 1/2, phosphorylation of Erk 1/2 (p-Erk 1/2) (Cell-Signaling Technology, Beverly, Massachusetts, USA), and β-actin from Sigma.
Extract and analysis of Hibiscus syriacus L. flower absolute
HSF was obtained from Hoseo University Asan city Chungnam, South Korea. HSF was identified by Jong-Cheol Yang, Division of Forest Biodiversity and Herbarium, Korea National Arboretum, Korea. A voucher specimen (No. HS-001) has been deposited at the Herbarium of the College of Life and Health Science, Hoseo University. The absolute was extracted from HSF by solvent extraction. Fresh flowers (2 kg) were kept in contact with n-hexane by complete immersion for 1 h at room temperature. The combined extracts upon solvent removal in a rotary evaporator in vacuo at 25°C produced a deep yellow waxy residue (concrete). Concrete was treated by mixing with ethanol and incubated at -20°C overnight. Filtration through a sintered funnel and evaporation of ethanol at 35°C led to the formation of a light yellow dewaxed absolute. Finally, 1.5 mL of oil (v/w, 0.075%) was obtained from HSF. The composition of the HSF absolute was identified by GC/MS.
Analysis and identification of compounds from absolute
Components of HSF were analyzed by the Korean Basic Science Institute (Seoul, Korea) and identified by GC/MS. GC/MS analysis was performed using an Agilent 6890N GC/5975i MS instrument (Palo Alto, California, USA) and DB5-MS capillary column (30 m × 250 μm, 0.25 μm film thickness). The carrier gas used was helium at a flow rate of 1 mL/min. The injector port and interface temperatures were 280 and 300°C, respectively. The gas chromatography oven was kept at 40°C for 2 min and increased to 230°C at a rate of 5°C/min, and then kept constant at 300°C for 5 min. The split ratio was 1 : 10. The mass ranges were from m/z 40–800. The retention indices for all compounds were determined according to the Kovats method using standard C7-C40n-alkanes. The compounds were identified by comparing their retention indices with those of Kovats indices[14] and by matching their fragmentation patterns in the mass spectra with those of the Wiley7NIST0.5L Mass Spectral library and catalogs for mass spectra. GC/MS data were reanalyzed according to Adams.[15]
Cell culture and proliferation assay
HaCaT cells were obtained from Daegu Gyeongbuk Institute for Oriental Medicine Industry (Daegu, South Korea) and maintained in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. The cells were incubated in a humidified 95% air 5% CO2 atmosphere at 37°C. The cells were grown to 70–80% confluence for use in each experiment. The proliferation of HaCaT cells was measured using the EZ-CyTox kit. HaCaT cells (density of 2 × 103 cells) were seeded into 96-well microtiter plates and treated with various concentrations of HSF (dissolved and diluted in DMEM containing 0.5% DMSO) for 48 h. The cells were incubated with EZ-CyTox reagent (10 μL) for 30 min at 37°C. Cell proliferation levels were measured using an ELISA reader (Synergy 2, Bio-Tek Instruments, Winooski, Vermont, USA) at 450 nm.
Migration assay
Migration was measured using a 48-well Boyden microchemotaxis chamber (Neuro Probe Inc., Gaithersburg, Maryland, USA). The lower chamber wells were loaded with various concentrations of HSF (dissolved and diluted in DMEM containing 0.5% DMSO) in medium containing 0.1% BSA. The membrane was laid over the medium of the lower chamber wells. HaCaT cells (5 × 104 cells/50 μL) were loaded into the chamber wells with medium containing 0.1% BSA. The chambers were then incubated at 37°C for 3 h. The membrane was fixed and stained using Diff-Quick (Baxter Healthcare, Miami, Florida, USA). Cells migrating through the membrane were counted using a microscope (× 200).
Western blot analysis
Total proteins (150 μg/lane) were separated using SDS-PAGE on 10% acrylamide gels. The proteins were then transferred onto a polyvinylidene difluoride membrane at 4°C, which was then blocked for 2 h in 3% nonfat dry milk at the room temperature. Subsequently, the membrane was washed using phosphate-buffered saline containing 0.05% Tween 20, followed by incubation with Akt, p-Akt, Erk 1/2, p-Erk 1/2, and β-actin antibodies (1: 1000–10,000 dilutions). The immunoreactive bands were visualized using a chemiluminescent substrate and exposed to photographic film.
Ex-vivo sprouting assay
The ex-vivo migration and proliferation of HaCaT cells were measured by a collagen sprout assay. HaCaT cells (7.5 × 104 cells/mL) were mixed with type I collagen, 10 × DMEM, and 1N NaOH (pH 7.2), and a dot in a plate was made. After drying, the spots were treated with or without HSF absolute and incubated at 37°C. After 72 h, the spots were fixed and stained using Diff-Quik. Images of the spot were obtained and photographed using microscopy (× 100) and the lengths of the sprouts were analyzed using Scion Image software (Frederick, Maryland, USA).
Wound healing assay
The animal assay was approved by the Hoseo University review board and animals were treated according to NIH guidelines (NIH publication No. 85-23). A full-thickness wound in the tail skin of male Sprague-Dawley (7-week-old, 200–220 g) rats was made by excisional surgery at, approximately, 10 mm2. The wound was evaluated at the end of the observation period (7, 14 and 21 days). The evaluated data recorded using a CCD camera when the wound areas were recalculated and analyzed using image J software.
Statistical analysis
Data were expressed as the mean ± standard error of the mean of the indicated number of experiments. Statistical analysis of the data was performed using Student's t tests for comparisons between pairs of groups and by ANOVA for multiple comparisons. P values less than 0.05 were considered to indicate a significant difference.
RESULTS
Chemical composition of Hibiscus syriacus L. flower absolute
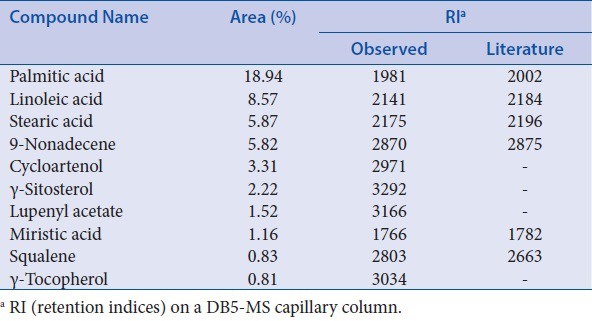
To analyze the major compounds of HSF absolute, GC/MS was used to identify the constituents. A total of 10 constituents of HSF were detected and these major compounds are listed in Table 1, which included palmitic acid (18.94%), linoleic acid (8.57%), stearic acid (5.87%), 9-nonadecene (5.82%), cycloartenol (3.31%), γ-sitosterol (2.22%), lupenyl acetate (1.52%), miristic acid (1.16%), squalene (0.83%), and γ-tocopherol (0.81%).
Table 1.
Composition of absolute from Hibiscus syriacus L. flower
Hibiscus syriacus L. flower absolute increases proliferation of HaCaT cells
The proliferation of keratinocytes plays an important role in wound healing;[1] therefore, the proliferation of HaCaT cells was evaluated using the XTT assay. Significant proliferation of HaCaT cells was induced by 166.25 ± 3.03% at 50 ng/mL of EGF. HSF absolute increased HaCaT cell proliferation in a dose-dependent manner (0.001–0.1 mg/mL). In particular, similar levels of cell proliferation were observed for both 50 ng/mL of EGF and 0.1 mg/mL (154.27 ± 2.92% of control) of HSF absolute [Figure 1].
Figure 1.
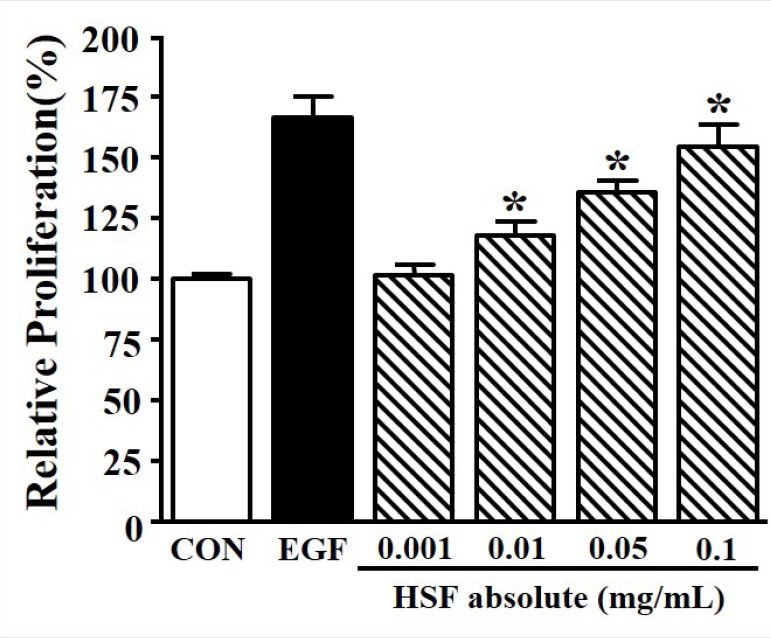
Effects of Hibiscus syriacus L. flower (HSF) absolute on proliferation in HaCaT cells. HaCaT cells were seeded (2 × 103 cells) and then incubated with recombinant human epidermal growth factor (EGF; 50 ng/mL) or HSF absolute (0.001–0.1 mg/mL) for 48 h. Cell proliferation was tested using the XTT assay. The quiescent state of the cells was expressed as 100% (n = 4). EGF was used as a positive control. Data are expressed as the mean ± SE. *P value less than 0.05 versus quiescent state.
Hibiscus syriacus L. flower absolute induced migration of HaCaT cells
The migration of HaCaT cells is also an important process in the wound healing of skin;[5] therefore, the migration of HSF absolute-induced HaCaT cells was measured using the Boyden chamber assay. Significant migration of HaCaT cells was induced by 218.79 ± 5.40% at 5 ng/mL of EGF. HaCaT cells were treated with HSF absolute (0.001–0.1 mg/mL) for 3 h. Cell migration was significantly increased by HSF absolute in a dose-dependent manner, and reached a maximal response at a concentration of 0.1 mg/mL (200.64 ± 5.17% of control) [Figure 2].
Figure 2.
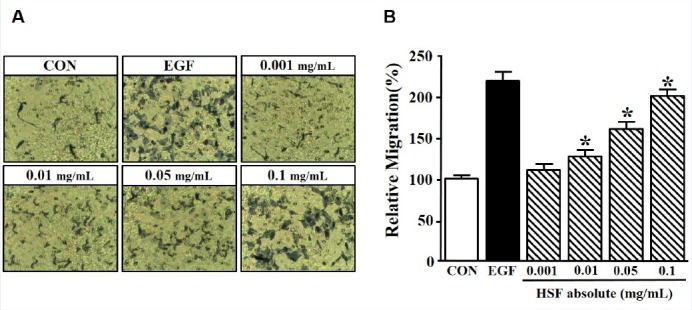
Effects of Hibiscus syriacus L. flower (HSF) absolute on migration in HaCaT cells. The cells were stimulated with recombinant human epidermal growth factor (EGF; 5 ng/mL) or HSF absolute (0.001–0.1 mg/mL) for 3 h. Migration was examined using a Boyden chamber assay. (a) The membrane was stained using Diff-Quick solution and then images were obtained using microscopy (× 200). (b) Migration in the quiescent state is expressed as 100% (n = 4). EGF was used as a positive control. Data are expressed as the mean ± SE. *P value less than 0.05 versus nonstimulated group.
Hibiscus syriacus L. flower absolute increases the phosphorylation of extracellular signal-regulated kinase 1/2 and Akt in HaCaT cells
HaCaT cell migration and proliferation are related to Erk 1/2 and Akt.[16] Therefore, the effect of HSF absolute on HaCaT cells was analyzed by western blotting. HaCaT cells were treated with HSF absolute (0.001–0.1 mg/mL) for 10 min. As shown in Figure 3 HSF absolute induced p-Erk 1/2 and p-Akt in HaCaT cells in a dose-dependent manner. p-Erk 1/2 and p-Akt were increased to 334.12 ± 2.72% and 403.16 ± 3.66% by 0.1 mg/mL of HSF absolute, respectively.
Figure 3.
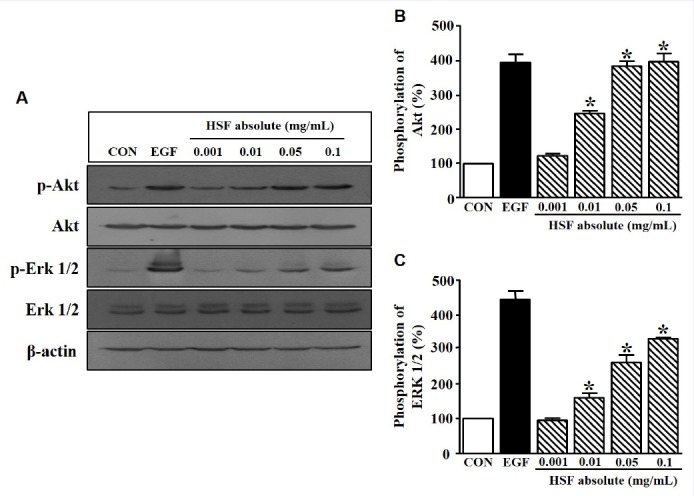
Change in protein kinase level in Hibiscus syriacus L. flower (HSF) absolute-treated HaCaT cells. (a) HaCaT cells were seeded and incubated in serum-free medium for 24 h. The cells were then treated with recombinant human epidermal growth factor (EGF; 5 ng/mL) or HSF absolute (0.001–0.1 mg/mL) for 10 min. The cell lysates were immunoblotted with each kinase antibody (p-Akt, Akt, p-Erk 1/2, and Erk 1/2). (b and c), Statistical graphs were obtained from panel A. The basal level of p-Akt and p-Erk 1/2 in HaCaT cells in the quiescent state is expressed as 100% (n = 4). EGF was used as a positive control. Data are expressed as the mean ± SE. *P value less than 0.05 versus the nonstimulated group.
Hibiscus syriacus L. flower absolute stimulates sprout outgrowth
The proliferation and migration of HSF absolute-stimulated HaCaT cells was confirmed using the ex-vivo sprouting assay. Sprout outgrowth was increased following treatment with 0.1 mg/mL HSF absolute for 72 h, and the maximum outgrowth was reached at a concentration of 0.1 mg/mL of HSF absolute. In addition, a similar values for the sprout outgrowth of keratinocytes were observed for both 50 ng/mL of EGF (506.79 ± 6.71% of control) and 0.1 mg/mL of HSF absolute (458.0 ± 6.38% of control) [Figure 4].
Figure 4.
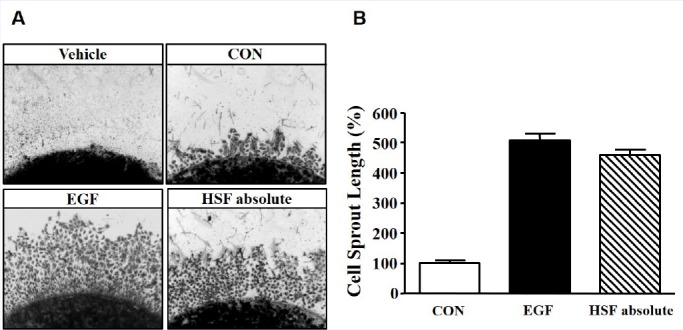
Effects of Hibiscus syriacus L. flower (HSF) absolute on sprout formation on HaCaT cells. (a) Cells were mixed with collagen and then placed into 24-well plates. Spots were treated with recombinant human epidermal growth factor (EGF; 50 ng/mL) or HSF absolute 0.1 mg/mL for 72 h. Spots and sprout outgrowth cells were stained with Diff-Quick solution and then images were obtained using microscopy (× 100). EGF was used as a positive control. (b) The statistical results were obtained from panel A. The level in the quiescent state is expressed as 100% (n = 4). Data are expressed as the mean ± SE.
Hibiscus syriacus L. flower absolute diminishes wound area
We next explored whether the effect of HSF is involved in skin reproduction in vivo using the wound-healing assay at surgical excision sites in the rat-tail skin. The injury area was treated with HSF (0.05 and 0.1 mg/mL) for 7, 14, and 21 days. As shown in Figure 5, HSF significantly reduced the surgical-induced wound area in the tail skin of rats. The best effect was observed at 0.1 mg/mL of HSF.
Figure 5.
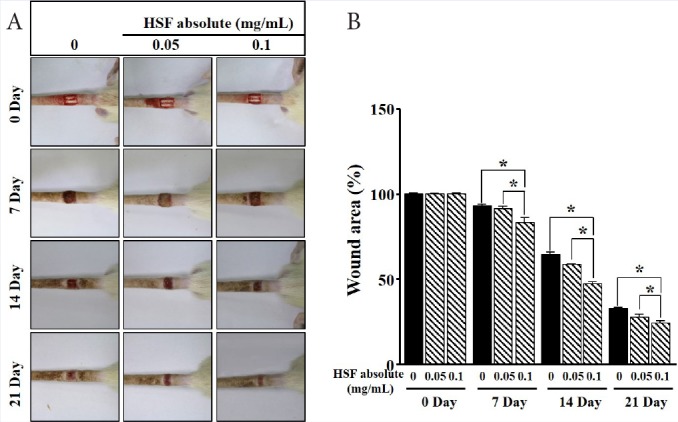
Effects of Hibiscus syriacus L. flower (HSF) absolute on wound in vivo. (a) The photographs show that HSF (0.05 and 0.1 mg/mL) was treated for the indicated number of days (7, 14, and 21) on the wound in the tail-skin of rat. (b) The statistical results were obtained from panel A. The level in the quiescent state as well as untreated group at 0 day is expressed as 100% (n = 4). Data are expressed as the mean ± SE. *P value less than 0.05.
DISCUSSION
According to a recent estimate, more than 2.5 million American patients suffer from chronic wounds. Thus, finding a new agent for wound healing from natural products such as those used in alternative medicine is very important. The wound healing process requires the rapid migration and proliferation of keratinocytes.[10] In the current study, we characterized the effect of HSF absolute on the migration and proliferation of HaCaT cells. Therefore, we performed wound healing assays both in vitro and in vivo and determined the expression of signaling molecules. Our results indicate that HSF absolute induced significant proliferation and migration of HaCaT cells through p-Erk 1/2 and p-Akt.
In this study, we identified 10 active compounds involved in the migration and proliferation of keratinocytes in HSF absolute. As reported in previous studies, palmitic acid, linoleic acid, and stearic acid can induce responses by Erk 1/2 and Akt.[17,18,19,20] Therefore, these molecules may be involved in the activation of p-Erk 1/2 and p-Akt, which may modulate basic biological processes such as the proliferation and migration of keratinocytes. In addition, the excessive migration and proliferation of keratinocytes induced oxidative stress through excessive ROS generation.[21] We also identified antioxidants present in HSF absolute using GC/MS; gamma-tocopherol, sitosterol, and squalene, which are strong antioxidants, were found.[22,23,24] Therefore, we suggest HSF absolute may also prevent excessive production of ROS in keratinocytes.
Various growth factors, cytokines, and G protein-coupled receptors can activate the Erk 1/2 pathway, a multifunctional protein kinase intracellular signaling molecule involved in the proliferative effect of diverse cells.[25,26,27] EGF also induced cellular signal transduction such as the proliferation, migration, and differentiation of diverse cells[9,28]. Simultaneously, EGF receptor is involved in EGF-bind downstream cellular signal transduction involving mitogen-activated protein kinase. Our results showed that EGF induced significant cell proliferation and migration of HaCaT cells. Similarly, the proliferative and motility effects showed equal values for both EGF and HSF absolute. Moreover, p-Erk 1/2 was expressed at similar levels in response to EGF and HSF absolute. Furthermore, the proliferative and motility effect of EGF was associated with p-Akt, an EGF downstream signal translocated from the cell membrane to its target genes, supporting the complex regulatory network.[29] As described in our results, HSF absolute may activate Akt. Therefore, HSF absolute may contribute to wound healing signaling through Erk 1/2 kinase and Akt kinase responses.
Woodely et al.[30] reported that type I collagen strongly induced the migration of keratinocytes. The activation of matrix metalloproteinase is a key function in cell migration.[31] Our data also demonstrated the ex-vivo proliferation and migration of keratinocytes by type I collagen. In detail, the sprout-out growth of HaCaT cells from the mixed type I collagen was increased by EGF and HSF absolute. Notably, we confirmed the detailed properties of HSF absolute, as well as the wound-healing effect on surgically excised rat-tail skin. Thus, the biological activity of HSF absolute is involved in the dermatological properties of wound-healing.
CONCLUSION
We identified the properties of HSF absolute for the recovery of cutaneous wounds. Therefore, HSF absolute may be a promising material for wound healing cosmetics, although further studies are needed to verify the efficacies associated with wound healing and skin regeneration.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest
Acknowledgememt
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare (Grant No.: A103017, HN12C0054) and Korea Institute for Advancement of Technology (Grant No.: N0001657), Republic of Korea.
REFERENCES
- 1.Takazawa Y, Ogawa E, Saito R, Uchiyama R, Ikawa S, Uhara H, et al. Notch down-regulation in regenerated epidermis contributes to enhanced expression of interleukin-36α and suppression of keratinocyte differentiation during wound healing. J Dermatol Sci. 2015;79:10–19. doi: 10.1016/j.jdermsci.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Braff MH, Zaiou M, Fierer J, Nizet V, Gallo RL. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect Immun. 2005;73:6771–81. doi: 10.1128/IAI.73.10.6771-6781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan YA, Sanes JR. Non-invasive visualization of epidermal responses to injury using a fluorescent transgenic reporter. J invest dermatol. 2004;123:888–91. doi: 10.1111/j.0022-202X.2004.23486.x. [DOI] [PubMed] [Google Scholar]
- 4.Taylor KR, Costanzo AE, Jameson JM. Dysfunctional γδ T cells contribute to impaired keratinocyte homeostasis in mouse models of obesity. J invest dermatol. 2011;131:2409–18. doi: 10.1038/jid.2011.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Rregen. 2009;17:763–71. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SW, Kim SH, Kim JY, Lee Y. The effect of growth hormone on fibroblast proliferation and keratinocyte migration. J plast Recons Aes. 2010;63:364–69. doi: 10.1016/j.bjps.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 7.Lönnqvist S, Rakar J, Briheim K, Kratz G. Biodegradable gelatin microcarriers facilitate reepithelialization of human cutaneous wounds - an in vitro study in human skin. Plos One. 2015:1–10. doi: 10.1371/journal.pone.0128093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brönneke S, Brückner B, Söhle J, Siegner R, Smuda C, Stäb F, et al. Genome-wide expression analysis of wounded skin reveals novel genes involved in angiogenesis. Angiogenesis. 2015;18:361–71. doi: 10.1007/s10456-015-9472-7. [DOI] [PubMed] [Google Scholar]
- 9.Andl CD, Mizushima T, Nakagawa H, Oyama K, Harada H, Chruma K, et al. Epidermal growth factor receptor mediates increased cell proliferation, migration, and aggregation in esophageal keratinocytes in vitro and in vivo. J Biol Chem. 2003;278:1824–30. doi: 10.1074/jbc.M209148200. [DOI] [PubMed] [Google Scholar]
- 10.Seeger MA, Paller AS. The Roles of Growth Factors in Keratinocyte Migration. Adv Wound care. 2015;4:213–24. doi: 10.1089/wound.2014.0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu KP, Yin J, Yu FS, et al. Lysophosphatidic acid promoting corneal epithelial wound healing by transactivation of epidermal growth factor receptor. Invest Ophth Vis. 2007;48:636–43. doi: 10.1167/iovs.06-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu RJ, Hsu YC, Chen SP, Fu CL, Yu JC, Chang FW, et al. The triterpenoids of Hibiscus syriacus induce apoptosis and inhibit cell migration in breast cancer cells. BMC Complem Altern M. 2015;15:65–74. doi: 10.1186/s12906-015-0592-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohnishi S, Takeda H. Herbal medicines for the treatment of cancer chemotherapy-induced side effects. Front Pharmacol. 2015;6:1–5. doi: 10.3389/fphar.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovats E. Gas chromatographic characterization of organic substances in the retention index system. Adv Chromatogr. 1965;1:229–47. [Google Scholar]
- 15.Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. Allured Publishing Corporation Carol Stream, Illinois. 2007:804. [Google Scholar]
- 16.Kang J, Chen W, Xia J, Li Y, Yang B, Chen B, et al. Extracellular matrix secreted by senescent fibroblasts induced by UVB promotes cell proliferation in HaCaT cells through PI3K/AKT and ERK signaling pathways. Int J Mol Med. 2008;21:777–84. [PubMed] [Google Scholar]
- 17.Wang X, Liu JZ, Hu JX, Wu H, Li YL, Chen HL, et al. ROS-activated p38 MAPK/ERK-Akt cascade plays a central role in palmitic acid-stimulated hepatocyte proliferation. Free Radical bio med. 2011;51:539–51. doi: 10.1016/j.freeradbiomed.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Stachowska E, Kijowski J, Dziedziejko V, Siennicka A, Chlubek D. Conjugated linoleic acid regulates phosphorylation of PPARγ by modulation of ERK 1/2 and p38 signaling in human macrophages/fatty acid-laden macrophages. J Agr Food Chem. 2011;59:11846–52. doi: 10.1021/jf2014233. [DOI] [PubMed] [Google Scholar]
- 19.Bi L, Chiang JY, Ding WX, Dunn W, Roberts B, Li T, et al. Saturated fatty acids activate ERK signaling to downregulate hepatic sortilin 1 in obese and diabeticmice. J Lipid Res. 2013;54:2754–62. doi: 10.1194/jlr.M039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsuta Y, Iida T, Inomata S, Denda M. Unsaturated fatty acids induce calcium influx into keratinocytes and cause abnormal differentiation of epidermis. J Invest Dermatol. 2005;154:1008–13. doi: 10.1111/j.0022-202X.2005.23682.x. [DOI] [PubMed] [Google Scholar]
- 21.Ross C, Alston M, Bickenbach JR, Aykin-Burns N. Oxygen tension changes the rate of migration of human skin keratinocytes in an age-related manner. Exp Dermatol. 2011;20:58–63. doi: 10.1111/j.1600-0625.2010.01190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engin KN. Alpha-tocopherol: looking beyond an antioxidant. Mol Vis. 2009;15:855–60. [PMC free article] [PubMed] [Google Scholar]
- 23.Huang ZR, Lin YK, Fang JY. Biological and pharmacological activities of squalene and related compounds: potential uses in cosmetic dermatology. Molecules. 2009;14:540–54. doi: 10.3390/molecules14010540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta R, Sharma AK, Dobhal MP, Sharma MC, Gupta RS. Antidiabetic and antioxidant potential of β-sitosterol in streptozotocin-induced experimental hyperglycemia. J Diabetes 2011. 2011;3:29–39. doi: 10.1111/j.1753-0407.2010.00107.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee KP, Lee K, Park WH, Kim H, Hong H. Piperine inhibits platelet-derived growth factor-BB-induced proliferation and migration in vascular smooth muscle cells. J Med Food. 2015;18:208–15. doi: 10.1089/jmf.2014.3229. [DOI] [PubMed] [Google Scholar]
- 26.Donovan S, See W, Bonifas J, Stokoe D, Shannon KM. Hyperactivation of protein kinase B and ERK have discrete effects on survival, proliferation, and cytokine expression in Nf1-deficient myeloid cells. Cancer Cell. 2002;2:507–14. doi: 10.1016/s1535-6108(02)00214-3. [DOI] [PubMed] [Google Scholar]
- 27.Gudermann T. Multiple pathways of ERK activation by G protein-coupled receptors. Novartis Found Symp. 2001;239:68–79. doi: 10.1002/0470846674.ch7. [DOI] [PubMed] [Google Scholar]
- 28.Garcez RC, Teixeira BL, Schmitt Sdos S, Alvarez-Silva M, Trentin AG. Epidermal growth factor (EGF) promotes the in vitro differentiation of neural crest cells to neurons and melanocytes. Cell Mol Neurobiol. 2009;29:1087–91. doi: 10.1007/s10571-009-9406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakai K, Yoneda K, Moriue T, Igarashi J, Kosaka H, Kubota Y, et al. HB-EGF-induced VEGF production and eNOS activation depend on both PI3 kinase and MAP kinase in HaCaT cells. J Dermatol Sci. 2009;55:170–78. doi: 10.1016/j.jdermsci.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Woodley DT, Bachmann PM, O’Keefe EJ. Laminin inhibits human keratinocyte migration. J cell physiol. 1988;136:140–46. doi: 10.1002/jcp.1041360118. [DOI] [PubMed] [Google Scholar]
- 31.Joo CK, Seomun Y. Matrix metalloproteinase (MMP) and TGF beta 1-stimulated cell migration in skin and cornea wound healing. Cell Adhes Mignr. 2008;2:252–253. doi: 10.4161/cam.2.4.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]


