Abstract
Degeneration of intervertebral discs (IVDs) results in an overall alteration of the biomechanics of the spinal column and becomes a major cause of low back pain. In this study, an injectable hydrogel composite is fabricated and characterized as a potential scaffold for the treatment of degenerated IVDs. Crosslinking of type II collagen-hyaluronic acid (HA) hydrogel with 1-ethyl-3 (3-dimethyl aminopropyl) carbodiimide (EDC) increases the gel stability against collagenase digestion and reduces water uptake in comparison with non-crosslinked gel. Cell viability assay exhibits the proliferation of human nucleus pulposus (HNP) cells in hydrogels. The cells in non-crosslinked gel and the gel crosslinked with a low concentration of EDC (0.1 mM) show superior cell viability and morphology compared with cells in gels crosslinked with higher concentration of EDC. Quantitative PCR assay demonstrates the gene expression of extracellular matrix (ECM) by cells cultured in the gels. The expression of ECM genes by HNP cells in the gels demonstrated the phenotypic change of the cells. This study suggests that the type II collagen-HA hydrogel and crosslinked hydrogel (0.1 mM EDC) are permissive matrix for the growth of HNP cells and can be potentially applied in NP repair.
Keywords: Nucleus pulposus cells, hydrogel, type II collagen, hyaluronic acid
INTRODUCTION
Intervertebral discs (IVDs) are elastic structures situated between adjacent vertebrae in the spine. They are composed of fibrocartilage material that includes the external annulus fibrosus (AF) and inner nucleus pulposus (NP). The extracellular matrix of the gelatinous NP comprises type II collagen, aggrecan, and the aggregating hydrated proteoglycan.1–5 The elastic NP functions to distribute hydraulic pressure to the extracellular matrix (ECM) within each disc under compressive loads. Degeneration of the NP and breakdown of the AF result in low back pain.6–8 Intervertebral disc degeneration is characterized by increased cell death, swelling of the disc, immune privilege unbalance, and aberrant gene expression.9,10 During degeneration, the resulting dehydration leads to a decrease in the size of the NP and increased stress on the AF with a compensatory increase in functional size. The hydrated gelatinous ECM is condensed and replaced by a more fibrous structure.4,5
Advances in the research of biomaterials provide a promising approach to regenerating degenerated and damaged intervertebral discs. Biomaterial scaffolds have been investigated for in vitro cell-biomaterial interaction and in vivo transplantation into IVDs.11–16 A few types of synthetic and natural hydrogels, such as collagen, hyaluronic acid (HA), fibrin, gelatin, polyglycolic acid, alginate, and chitosan, have been investigated for their potential to replace and regenerate the NP.11–15 Hydrogels of natural biomaterials have exhibited the ability to maintain NP cell viability and provide natural cues to cells that may stimulate regenerative response. The density of NP cells considerably declines during childhood and gradually remains at a low level in adulthood. In adult human NP (HNP), the cell density is around 1–5 million cells/ml.17, 18 Though the cell number is low in the NP, these cells express high levels of aggrecan and collagen type II that are essential for the maintenance of NP tissue.19 During the process of degeneration and aging as the result of damage and stress, cells lose their ability to proliferate and be replaced by entering a state of cell senescence. NP cells are not readily capable of self-repair.20–22 Intervertebral disc degeneration is characterized by alterations at the cellular and molecular level, including increased cell death, swelling of the disc, gene polymorphism, immune privilege unbalance, and aberrant gene expression.9,10 Transplantation of rabbit NP cells into an animal IVD prevented degeneration of the disc.23 Studies have been performed to test the feasibility of HNP cell transplantation for the therapy of degenerated rabbit and human discs, and results were encouraging.24, 25 Injectable biomaterials that have the ability of self-assembly in vivo can deliver NP cells to the IVD for NP regeneration.11, 26–28 It was also reported that biomaterials such as alginate hydrogel and lyophilized chitosan-gelatin scaffolds can support HNP cell growth.29
Type II collagen and HA, being major components of IVDs, have been studied for their potential as biomaterial grafting into the NP. They can be remodeled by endogenous or transplanted cells without producing toxic byproducts. One previous study showed that type II collagen-HA hydrogel can support the growth of rat mesenchymal stem cells or bovine nucleus pulposus cells.11,12 We also showed that collagen neural conduits crosslinked with 1-ethyl-3 (3-dimethyl aminopropyl) carbodiimide (EDC) can significantly support peripheral nerve regeneration.30 In addition, we reported that collagen microspheres crosslinked with EDC can support oligodendrocyte precursor growth for myelination in vitro.31 However, whether an injectable type II collagen-HA hydrogel crosslinked with EDC can support the growth and biological function of HNP has not been reported. In this study, we fabricated an injectable type II collagen-HA hydrogel that was crosslinked with a low amount of EDC. We characterized the material properties of the hydrogel composite and investigated the long-term growth and gene expression of HNP cells in the hydrogel. The outcomes of this study suggest that type II collagen-HA hydrogel and the gel crosslinked with a low concentration of EDC are a suitable matrix for HNP cell growth and can serve as cell carriers for HNP cell transplantation into an IVD.
MATERIALS AND METHODS
Fabrication of type II collagen-HA hydrogels
Type II collagen was extracted from fetal bovine cartilage and dissolved in 0.01 M acetic acid at a final concentration of 9 mg/ml. Hyaluronic acid sodium (Sigma-Aldrich, St. Louis, MO) was dissolved in Dulbecco’s modified eagles medium (DMEM) (Lifetechnology, Grand Island, NY) complemented by 0.4 M NaCl at 1.57 mg/ml. Type II collagen (5 mg/ml) and an HA-sodium solution were mixed together following the weight ratio of 9:1. The pH of the resulting solution was adjusted to 7 by adding 1 M NaOH aqueous solution and 5× phosphate-buffered saline (PBS) solution. The hydrogel was then crosslinked with different concentrations of EDC (Sigma-Aldrich, St. Louis, MO) (0.1, 0.5, and 1 mM). Hydrogel without adding any crosslinker was used as the control.
HNP cell culture and cell growth in type II collagen-HA hydrogel
Intervertebral disc tissues from three patients with discogenic low back pain were obtained during discectomy surgery. The tissue of the central portion of NP was minced to 1 mm3 segments and digested with 0.25% trypsin for 30 minutes followed by the addition of 0.2% collagenase for 4 hours with agitation. After filtration through a 100 um nylon mesh (Fisher Scientific, Cat. no. 22363549) to eliminate tissue debris, the isolated cells were pelleted by centrifugation at 500 g for 10 minutes. The cells were grown in a DMEM/nutrient mixture F12 (Ham) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Lifetechnology, Grand Island, NY). The medium was changed every 2–3 days, and the cell culture was incubated with 5% CO2 at 37°C. The cells of passage 2, 3, and 4 were used in the studies. Independent experiments were performed on cells from each patient.
The type II collagen (0.5 ml, 9 mg/ml) and HA solution were added to the wells of a 48-well plate, and the pH of the mixture was adjusted to 7 by adding NaOH. A crosslinker (EDC) in different concentrations was added to the gel and mixed thoroughly using a pipette. The HNP cells were seeded into the crosslinked and non-crosslinked collagen-HA solution and incubated for 15 minutes at 37°C to allow for gel formation, and then the medium was added into each cell culture well.
Cell viability assay
The cell viability assay (LIVE/DEAD® Cell Vitality Assay, Lifetechnology, Grand Island, NY) was performed after 10 days or after 2 months of cell culture in the hydrogel. Reagents for the LIVE/DEAD® assay were ethidium homodimer-1 (Ethd-1), with a molecular weight of 856.77, and Calcein AM, with a molecular weight 994.87. Solutions of the assay were removed from the freezer and allowed to warm to room temperature. EthD-1 stock solution (2 μl, 2 mM) and Calcein AM stock solution (0.5 μl of 4 mM) were added to sterile PBS solution (1 ml) and vortexed. The solution (300 μl) was added directly to each cell culture well and incubated for 30 minutes at room temperature. The cells were then viewed under a fluorescent microscope. At least 3 independent experiments were performed in this study. Four images of cells within the gel were recorded in each experiment. The live and dead cells in the images were counted, and the ratios of live cells to all cells were quantified.
AlamarBlue® assay
The viability and proliferation of HNP cells in the hydrogel were studied by monitoring their metabolic activity using the alamarBlue® assay (Pierce Biotechnology, Rockford, lL). To perform this assay, HNP cells with a density of 50,000 cells were seeded in the hydrogel and cultured for 10 days or 2 months. These cells were then incubated with a cell culture medium containing 10% (v/v) alamarBlue® reagent for 4 hours. Absorbance was measured at wavelengths of 570 nm and 600 nm in a microplate reader (Synergy Mx Monochromator-Based Multi-Mode Microplate Reader, Winooski, VT).
Infrared spectroscopy
Fourier transform infrared spectroscopy (FTIR) was performed to study the chemical bond formation between the HA and the type II collagen using EDC. Spectra were obtained with a Spectrum 100 FT-IR Spectrometer Perkin Elmer (PerkinElmer, Waltham, MA). To obtain an IR spectrum, the specimen (hydrogel) was loaded onto a salt plate and placed in a mounting plate in the sample loading compartment. The parameters were adjusted. In FTIR, the infrared beam enters the compartment, passes through the sample, and finally enters the detector area, resulting in a spectrum with peaks.
Swelling test
For the swelling test, crosslinked type II collagen-HA hydrogel specimens and non-crosslinked control samples were prepared, and the weight of each specimen was measured. The hydrogels were soaked in PBS solution and incubated for 2 hours at 37°C. Then the PBS solution was removed, and the hydrogels were held over a paper filter until all PBS had dripped off. The samples were then observed and weighed again. The weights obtained for each type of crosslinked and non-crosslinked hydrogels after soaking in the PBS solution were compared.
Degradation test
Non-crosslinked hydrogels and hydrogel specimens crosslinked with 0.1, 0.5 or 1 mM EDC were freeze-dried, after which the weight of each sample was measured. Then the samples were digested with collagenase from clostridium histolyticum (Sigma-Aldrich, St. Louis, MO), which was prepared in 0.1 mM Tris (pH 7.4) containing 0.05 M calcium chloride (CaCl2). The samples were incubated in the digestion solution for 5 hours or 11 hours at 37°C. Then the samples were centrifuged, the supernatant was removed, and the pellets were lyophilized. The weight of each sample was measured again. Mass loss was calculated by analyzing the initial mass (Wi) with the final mass (Wf) according to the following equation: .
Scanning electron microscopy
Cells cultured in the collagen-HA hydrogel were fixed in a 2% glutaraldehyde-PBS solution for 30 minutes. The samples were broken into small pieces, dehydrated with graded ethanol, and dried with hexamethyldisilazane. Then they were air-dried and subsequently coated with gold. The images of the scaffolds with cells were taken by scanning electron microscopy (SEM) using a Zeiss Sigma VP (Carl Zeiss Microscopy, LLC, Thornwood, NY).
Real-time polymerase chain reaction
In order to study the quantitative reverse transcription polymerase chain reaction (qRT-PCR), 120,000 cells were seeded in each collagen-HA hydrogel. The total ribonucleic acid (RNA) of the HNP cells that were cultured for 8 days or 30 days was extracted using an RNeasy Micro Kit (Qiagen, Germantown, MD) according to the supplier’s protocol. The amount of RNA was determined using a NanoDrop 2000c spectrophotometer (Thermo Scientific, Waltham, MA). The complementary deoxyribonucleic acid (cDNA)was reverse-transcribed from the total RNA using a high-capacity cDNA Reverse Transcription Kit (Lifetechnology, Grand Island, NY) according to the manufacturer’s protocol. The qRT-PCR was performed using Power SYBR® Master Mix by the StepOnePlus™ qRT-PCR System (Lifetechnology, Grand Island, NY) at 95°C for 10 minutes, and then 40 cycles at 95°C for 15 seconds, followed by 60°C for 60 seconds. Gene transcription was normalized in relation to transcription of the housekeeping rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The 2−ΔΔCt method was used to calculate the relative gene expression for each target gene. Primers used in the qRT-PCR are provided in Table 1.
Table 1.
Primers for qRT-PCR
| Gene | Oligonucleotide (5′-3′) |
|---|---|
| Type II collagen A1 (COL2A1) | F: CCGGGCAGAGGGCAATAGCAGGTT R: CAATGATGGGGAGGCGTGAG |
| Aggrecan | F: CCAGTGCACAGAGGGGTTTG R: TCCGAGGGTGCCGTGAG |
| Sox9 | F: CATGAGCGAGGTGCACTCC R: TCGCTTCAGGTCAGCCTTG |
| Type I collagen A1 (COLIA1) | F: CGATGGCTGCACGAGTCACAC R: CAGGTTGGGATGGAGGGAGTTTAC |
| GAPDH | F: CGAGATCCCTCCAAAATCAA R: TTCACACCCATGACGAACAT |
STATISTICS
Statistical analysis was done using the two-tailed Student’s t-test, where data are expressed as the mean ± standard deviation. Statistical significance was placed at p < 0.05.
RESULTS
EDC-crosslinking reduced degradation rate and controlled swelling of type II collagen-HA hydrogel
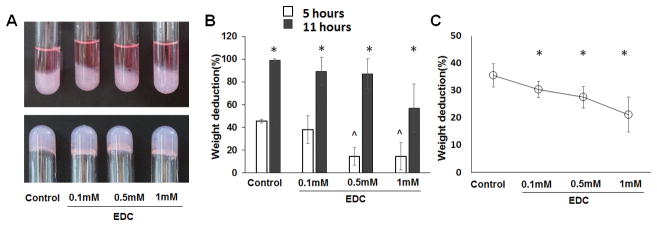
The type II collagen-HA hydrogels with and without EDC crosslinking were fabricated, and all were stable in the cell culture medium (Figure 1A). The increase of EDC concentration for crosslinking the type II collagen-HA hydrogel increased the resistance of the gels to degradation by collagenase. After incubation with collagenase for 5 hours and 11 hours, the degradation rate of the freeze-dried scaffolds decreased from 45.4 ± 1.4% and 99.2 ± 1.2% to 14.6 ± 11.9% and 56.1 ± 21.3%, respectively, when the EDC amount increased from 0 mM to 1 mM (Figure 1B). The swelling tests for the control gel and crosslinked gels were assessed by the measure of relative PBS solution uptake. The PBS solution uptake for the non-crosslinked gel was 35.5 ± 4.23 mg. The PBS solution uptakes of the hydrogels crosslinked with 0.1 mM EDC, 0.5 mM EDC and 1 mM EDC were 30.33 ± 2.86 mg, 27.5 ± 3.94 mg, and 21.16 ± 6.33 mg, respectively (Figure 1C). Results indicate that EDC crosslinking significantly reduced uptake of the PBS solution by the hydrogel.
Figure 1.
Crosslinking of type II collagen-HA hydrogel with EDC showing reduced gel degradation rate and water uptake. (A) Type II collagen-HA hydrogels formed in glass tubes with cell culture medium and remain stable at bottom of glass tubes after removal of medium; (B) Weight deduction of the type II collagen- HA hydrogel after 5 hours and 11 hours of collagenase treatment. *, p < 0.05, compared with the corresponding gels of 10 days. ^, p < 0.05, compared with the non-crosslinked gel. (C) Relative PBS solution uptake of type II collagen-HA hydrogels compared with non-crosslinked hydrogel and hydrogels crosslinked with EDC at different concentrations. *, p < 0.05, compared with the non-crosslinked gel.
FTIR was employed to determine the functional group of type II collagen and hyaluronic acid (Figure 2). Results show the spectra obtained from FTIR for the different crosslinked and non-crosslinked type II collagen-HA hydrogels. The formation of amide bonds was observed when the hydrogel was crosslinked with EDC.
Figure 2.
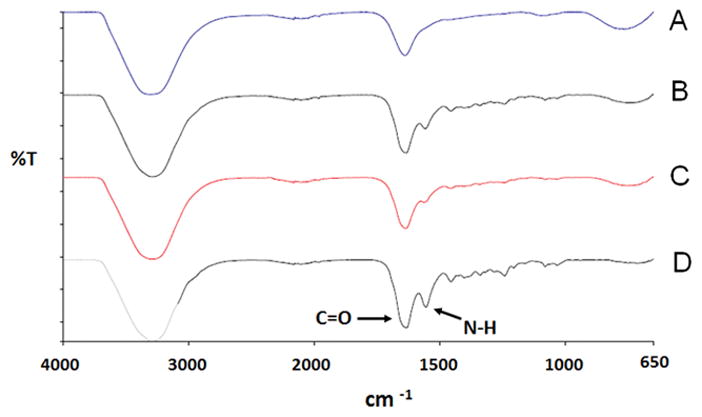
FTIR spectra of type II collagen/HA hydrogels. (A) non-crosslinked hydrogel; (B) gel crosslinked with 0.1 mM EDC; (C) gel crosslinked with 0.5 mM EDC; (D) gel crosslinked with 1mM EDC.
HNP cells grow and proliferate in type II collagen-HA hydrogel
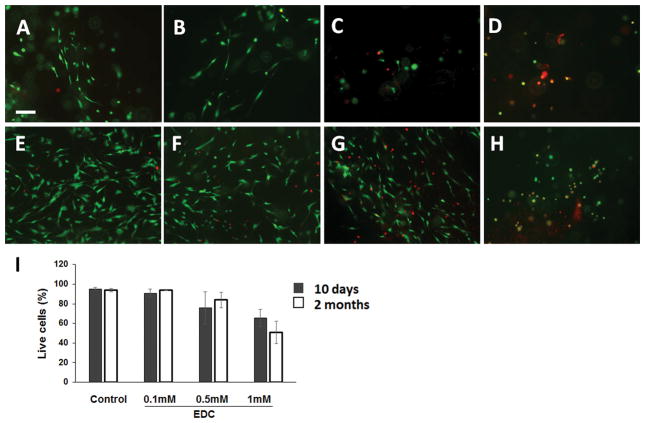
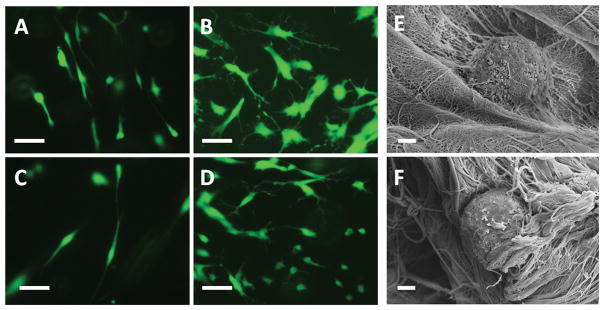
Cell viability was determined by using a LIVE/DEAD® cell vitality assay kit and viewed under a fluorescent microscope (Figure 3). For cells cultured for 10 days, most cells in the control gel (95.21 ± 1.33 %) and hydrogel crosslinked with 0.1 mM EDC (90.92 ± 3.90%) were alive, while 76.03 ± 16.34% and 65.69 ± 8.78% of the cells cultured in the hydrogel crosslinked with 0.5 mM and 1 mM EDC, respectively, were alive. For cells cultured for 2 months, the live cells in the control gel and hydrogel crosslinked with 0.1 mM EDC were 93.86 ± 1.50% and 93.72 ± 0.39%, respectively, while the live cells in the hydrogel crosslinked with 0.5 mM EDC and 1 mM EDC were 83.92 ± 8.02% and 50.90 ± 11.60%, respectively. Cells cultured in the control gel (Figure 4A) and gel crosslinked with 0.1 mM EDC (Figure 4C) after 10 days showed elongated morphology. Cells cultured in the control gel (Figure 4B) and gel crosslinked with 0.1 mM EDC (Figure 4D) after 2 months showed multiple processes. SEM images indicated the presence of multiple processes of cultured HNP cells in the gel (Figure 4E).
Figure 3.
LIVE/DEAD® cell vitality assay for HNP cells grown for 10 days in gel: (A)–(D) LIVE/DEAD® cell vitality assay for HNP cells grown in gel for 10 days. (A) non-crosslinked hydrogel, (B) hydrogel crosslinked with 0.1 mM EDC, (C) hydrogel crosslinked with 0.5 mM EDC, and (D) hydrogel crosslinked with 1 mM EDC. (E) – (H)LIVE/DEAD® cell vitality assay for HNP cells grown for 2 months in gel: (E) non-crosslinked hydrogel, (F) hydrogel crosslinked with 0.1 mM EDC, (G) hydrogel crosslinked with 0.5 mM EDC and (H) hydrogel crosslinked with 1 mM EDC. Live cells labeled with calcein AM (green). Dead cells labeled with ethidium homodimer-1 (red). (I) Quantification of live cells in the gels. Scale bar: 100 μm.
Figure 4.
Morphology of HNP cells in hydrogel: (A) HNP cells in non-crosslinked type II collagen-HA hydrogel for 10 days; (B) HNP cells in non-crosslinked type II collagen-HA hydrogel for 2 months; (C) HNP cells in crosslinked type II collagen-HA hydrogel (0.1 mM) for 10 days; (D) HNP cells in crosslinked type II collagen-HA hydrogel (0.1 mM) for 2 months. Cells labeled with calcein AM. Scale bar: 50 μm; (E)–(F) SEM images of HNP cells in crosslinked type II collagen-HA hydrogel (0.1 mM). Scale bar: 2 μm.
The alamarBlue® assay showed that HNP cells proliferated in both the control collagen-HA gel and the crosslinked collagen-HA gel. The reduction of alamarBlue®reagent for HNP cells cultured for 2 months was significantly higher than that of HNP cells cultured for 10 days in all the gels. At 10 days, the reduction of alamarBlue® reagent of HNP cells in the control gel (22.43 ± 3.11%) was significantly higher than that of HNP cells in the gel crosslinked with 0.1 mM EDC (17.5 ± 1.76%) or the gel crosslinked with 1 mM EDC (14.37 ± 0.79%). At 30 days, the reduction of alamarBlue® reagent for HNP cells cultured in the gel crosslinked with 1 mM EDC (24.483 ± 0.74%) was significantly lower than the other groups (34.28 ± 6.05%, 0.1 mM EDC; 32.31 ± 0.67%, 0.1 mM EDC; 8.44 ± 4.488%, 0.5 mM EDC).
HNP cells express ECM genes in type II collagen-HA hydrogel
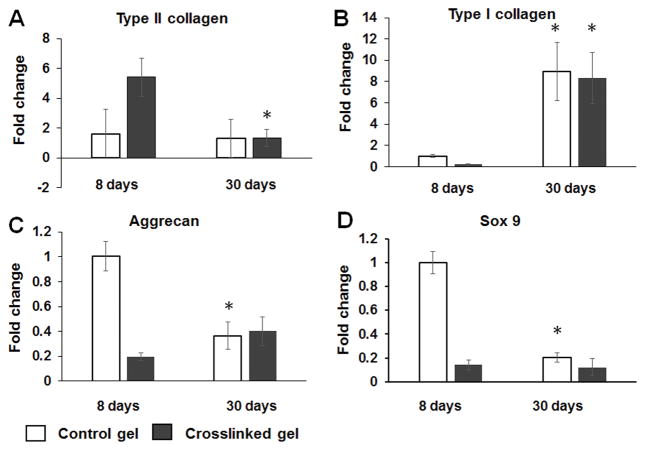
While cell viability assays were performed after the cells were cultured for a long time (2 months), the gene expression was measured after the cells were cultured for a relatively short time. In this assay, we detected the change of cell biological function after culturing for 1 month. The expression of type II collagen, type I collagen, Sox9, and aggrecan genes of HNP cells in the collagen-HA hydrogel were studied after they were cultured for 8 days or 30 days (Figure 6). The type II collagen gene expression of HNP cells cultured for 8 days in the gel crosslinked with 0.1 mM EDC (5.42 ± 1.28) was higher than that of cells cultured for 30 days (1.32 ± 0.59) (Figure 6A). The expression of type I collagen gene in HNP cells cultured for 8 days in the control gel (1.00 ± 0.11) or gel crosslinked with 0.1 mM EDC (0.22 ± 0.08) were significantly lower compared to that of HNP cells cultured for 30 days (8.95 ± 2.75, control gel; 8.32 ± 2.38, crosslinked gel) (Figure 6C). The aggrecan gene expression of HNP cells cultured for 8 days (1.00 ± 0.12) in the control gel was significantly higher than that of HNP cells cultured for 30 days (0.36 ± 0.11) (Figure 6B). The Sox9 gene expression of HNP cells cultured for 8 days (1.00 ± 0.12) in the control gel was significantly higher than that of HNP cells cultured for 30 days (0.36 ± 0.11) (Figure 6B).
Figure 6.
Results of qRT-PCR for chondrocytic cell marker genes Sox-9, type II collagen and aggrecan, and the fibroblastic marker gene type I collagen. Expressed as fold change compared with HNP cells in non-crosslinked type II collagen-HA hydrogel. Each target gene normalized to GAPDH. *, p < 0.05, compared with corresponding result at 8 days.
DISCUSSION
Injectable materials that can generate minimal injury to animals have attracted considerable attention in IVD regeneration studies. Though natural materials allow the process of natural remodeling without the production of toxic byproducts, these biomaterials are not easily manipulated. Chemical crosslinking is a common way to enhance the physical and chemical properties of natural materials. EDC is a water-soluble zero-length crosslinker and has been shown to be less toxic than these bridge-linking crosslinkers. However, the toxicity of EDC is obvious at a high level. Previous studies have shown that rat mesenchymal stem cells (MSCs) can grow and proliferate in an EDC-crosslinked type II collagen-HA hydrogel.12 The crosslinking of hydrogel with a high level of EDC (8 mM) significantly increased the stiffness of the gel. However, the hydrogel must be washed to remove EDC residues in the gel before the cells are seeded in the gel. It is difficult to evenly mix cells and the gel, and repeated washing can also remove HA in the gel. In this study, we crosslinked the type II collagen-HA hydrogel with a low amount of EDC that allowed the even mixture of the cells and the collagen-HA solution before the gel was formed. Because the toxicity of the gel is low, the mixture of the type II collagen-HA solution and cells can potentially be injected into the disc without a washing procedure. The hydrogel will form in vivo after injection and support NP cell growth.
The biological therapy approach attempts to regenerate degenerated tissue and accounts for a long-term cure by preventing or reversing degeneration. Effective regeneration would rely on the remodeling of the ECM of NP tissue by the biological activity of cells. A number of studies have been performed to investigate autologous NPC transplantation in animal models of degenerated tissue.23,28,33 This approach has also been applied in clinical trials.34 The application of biomaterial hydrogel that delivers therapeutic cells for the regeneration of NP provides the initial permissive environment for the cell to cope with the hostile environment. The laminin-111 functionalized poly(ethylene glycol) (PEG-LM111) hydrogel was developed as a biomaterial carrier for NP cell delivery to the IVD.15 Poly(ethylene glycol) (PEG) hydrogels have been widely used in tissue engineering applications because of their non-toxic and hydrophilic properties.35 The incorporation of biological signals such as a laminin peptide into the PEG significantly improved its biological function of serving as a carrier for NP cell delivery.
Collagen type II is the ligand of cell membrane collagen receptors such as integrin β1. The interaction between receptors and collagen type II mediates NP cell proliferation, migration, and adhesion. Additionally, the binding of HA with its cell membrane receptors may also mediate the cell phenotype response to the hydrogel.36 A previous study showed that type II collagen-HA hydrogel crosslinked with 4-arm polyethylene glycol succinimidyl glutarate Mw10,000 (4S-StarPEG) was not toxic to bovine NP cells that grew in the gel.11 However, the gene expression of major ECM components, such as type II collagen, type I collagen, and aggrecan, all decreased after culturing for 7 days, compared with cells at day 0. In this study, the gene expression of ECM for cells in the non-crosslinked gel and the gel crosslinked with a low concentration of EDC (0.1 mM) was studied because the cells showed better viability and morphology in these gels compared with those crosslinked with higher EDC (0.5 mM and 1 mM) in alamarBlue® assay and LIVE/DEAD® cell viability assay.
Since the ratio of type II collagen and HA is 9:1 (w/w) in native NP tissue, a hydrogel that mimics the native ECM constituents of NP tissue may produce an optimal environment for NP cell growth.32 A previous work studied the growth of porcine NP cells in the type II collagen-HA hydrogel.11 However, the behavior of HNP cells in the type II collagen hydrogel for a long-term culture has not been reported. In this study, we isolated NP cells from patients with degenerated IVDs and studied the cell viability in the type II collagen-HA hydrogel. Most HNP cells survived in the type II collagen-HA hydrogel and the hydrogel crosslinked with EDC (0.1 mM) after 2 months of cell culture. The NP cells showed an elongated cell shape at the early stage of cell culture, and the cells developed multiple processes after culturing for 2 months in the hydrogel. The development of multiple processes suggested that the cells settled down and generated more contacts with ECM after the longer-term cell culture.
In this study, we investigated the cell viability and ECM gene expression at two different time points. The study of the ability of hydrogels to support cell growth was performed on the cells that were cultured for a long time (2 months). Since this may change after culturing for a relatively short time, the ECM gene expression of NP cells was measured after the cells were cultured for 1 month using the qRT-PCR. We found that although HNP cells proliferated in the type II collagen-HA hydrogel and the hydrogels maintained cell viability after culturing for 2 months, the qPCR assay showed the phenotypic drift of NP cells after culturing for 1 month. The increased expression of the type I collagen gene was observed after cells were cultured for 30 days compared with cells cultured for 8 days. We also observed a significant change of type II collagen gene expression of NP cells in the crosslinked gel (0.1 mM EDC) and the aggrecan and Sox9 genes of NP cells in the non-crosslinked gel after a long-term cell culture. The results of gene expression studies may indicate the adaption of HNP cells to the 3D cell culture condition. The increased type I collagen expression may be due to the cells from degenerated discs, which is a limitation of the study of NP cell function in the gel. Though the type II collagen-HA hydrogel provides a permissive condition for NP cell growth, mechanical stimulation may be necessary to maintain the cell phenotype and restore healthy NP tissue in vivo.
The mechanical properties of materials such as stiffness may affect cell behavior including cell attachment and proliferation.37, 38 EDC crosslinking may change the mechanical property of the collagen-HA hydrogel and thereby affect HNP cell behavior. In this study, we found that increasing the EDC concentration can increase the toxicity to cells. Additionally, HNP cells in the hydrogel may also modify the gel composition and mechanical properties after long-term cell culturing. In the future study, we will determine the effect of material stiffness on HNP cell behavior in the gel. Further experiments are required to differentiate the effects of mechanical properties and toxicity on the cells, and the cell culture time should be shortened.
CONCLUSION
In this study, we fabricated an injectable type II collagen-HA hydrogel that was crosslinked with EDC, and then studied the growth and gene expression of HNP cells in the hydrogel. We found that the optimal conditions of the hydrogel for HNP cell growth are the non-crosslinked type II collagen-HA gel and the type II collagen-HA gel crosslinked with 0.1 mM EDC. Most cells in these hydrogels were survival cells and developed multiple processes after culturing for 2 months. A quantitative PCR study revealed the gene expression profile of HNP cells in the hydrogels after culturing for 8 days and 30 days. The differential gene expression of the cells for short- or long-term culturing showed that the cell phenotype changed in the hydrogel. The change of gene expression of the cells may be the result of their adaption to the hydrogel at different cell culture stages. Results of this study suggest that the type II collagen-HA hydrogel and the gel crosslinked with a low concentration of EDC are a suitable matrix for HNP cell growth and can serve as cell carriers for HNP cell transplantation into the IVD.
Figure 5.
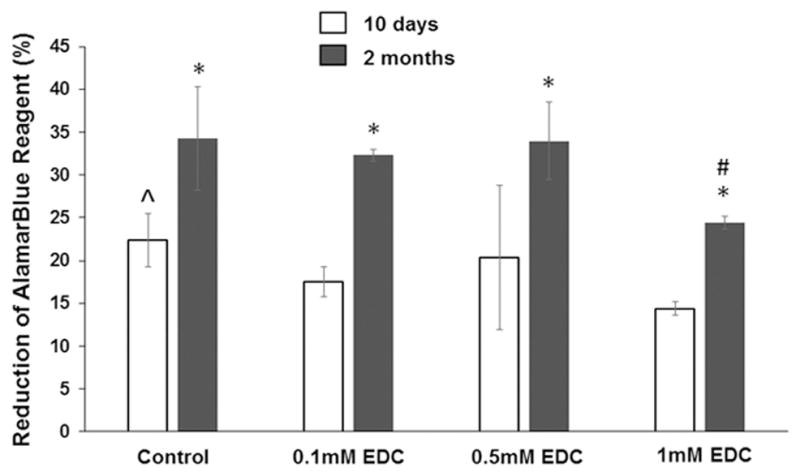
AlamarBlue® cell viability assay of HNPs growing in non-crosslinked and crosslinked collagen type II-HA hydrogel. *, p < 0.05, compared with the corresponding gels of 10 days. ^, p < 0.05, compared with the gels crosslinked with 0.1mM and 1mM EDC. #, p < 0.05, compared with the control gel and the gels crosslinked with 0.1mM and 0.5mM EDC.
Acknowledgments
We acknowledge Li Yao’s start-up funding, Wichita State University, the National Institute of General Medical Sciences (P20 GM103418) with the National Institutes of Health, and Wichita Medical Research & Education Foundation (WMREF) for support (NP cell isolation) of this work.
References
- 1.Humzah MD, Soames RW. Human intervertebral disc: Structure and function. Anat Rec. 1988;20:337–356. doi: 10.1002/ar.1092200402. [DOI] [PubMed] [Google Scholar]
- 2.Pooni JS, Hukins DW, Harris PF, Hilton RC, Davies KE. Comparison of the structure of human intervertebral discs in the cervical, thoracic and lumbar regions of the spine. Surg Radiol Anat. 1986;8:175–182. doi: 10.1007/BF02427846. [DOI] [PubMed] [Google Scholar]
- 3.Bradford DS, Oegema TR, Jr, Cooper KM, Wakano K, Chao EY. Chymopapain, chemonucleolysis, and nucleus pulposus regeneration. A biochemical and biomechanical study. Spine. 1984;9:135–147. doi: 10.1097/00007632-198403000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Lv F, Leung VY, Huang S, Huang Y, Sun Y, Cheung KM. In search of nucleus pulposus-specific molecular markers. Rheumatology (Oxford, England) 2014;53:600–610. doi: 10.1093/rheumatology/ket303. [DOI] [PubMed] [Google Scholar]
- 5.Phillips KL, Chiverton N, Michael AL, Cole AA, Breakwell LM, Haddock G, Bunning RA, Cross AK, Le Maitre CL. The cytokine and chemokine expression profile of nucleus pulposus cells: implications for degeneration and regeneration of the intervertebral disc. Arthritis Res Ther. 2013;15:R213. doi: 10.1186/ar4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker MH, Anderson DG. Molecular basis of intervertebral disc degeneration. Spine J. 2004;4:158S–166S. doi: 10.1016/j.spinee.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Goins ML, Wimberley DW, Yuan PS, Fitzhenry LN, Vaccaro AR. Nucleus pulposus replacement: an emerging technology. Spine J. 2005;5:317S–324S. doi: 10.1016/j.spinee.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 8.Masuda K, An HS. Prevention of disc degeneration with growth factors. Eur Spine J. 2006;15:S422–432. doi: 10.1007/s00586-006-0149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Z, Wan ZY, Guo YS, Wang HQ, Luo ZJ. FasL on human nucleus pulposus cells prevents angiogenesis in the disc by inducing Fas-mediated apoptosis of vascular endothelial cells. Int J Clin Expl Pathol. 2013;6:2376–2385. [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YF, Zhang YZ, Zhang WL, Luan GN, Liu ZH, Gao Y, Wan ZY, Sun Z, Zhu S, Samartzis D, Wang CM, Wang HQ, Luo ZL. Insights into the hallmarks of human nucleus pulposus cells with particular reference to cell viability, phagocytic potential and long process formation. Int J Med Sci. 2013;10:1805–1816. doi: 10.7150/ijms.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collin EC, Grad S, Zeugolis DI, Vinatier CS, Clouet JR, Guicheux JJ, Weiss P, Alini M, Pandit AS. An injectable vehicle for nucleus pulposus cell-based therapy. Biomaterials. 2011;32:2862–2870. doi: 10.1016/j.biomaterials.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Calderon L, Collin E, Velasco-Bayon D, Murphy M, O’Halloran D, Pandit A. Type II collagen-hyaluronan hydrogel—a step towards a scaffold for intervertebral disc tissue engineering. Eur Cells Mater. 2010;20:134–148. doi: 10.22203/ecm.v020a12. [DOI] [PubMed] [Google Scholar]
- 13.Park SH, Cho H, Gil ES, Mandal BB, Min BH, Kaplan DL. Silk-fibrin/hyaluronic acid composite gels for nucleus pulposus tissue regeneration. Tissue Eng Part A. 2011;17:2999–3009. doi: 10.1089/ten.tea.2010.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renani H, Ghorbani M, Beni BH, Karimi Z, Mirhosseini M, Zarkesh H, Kabiri A. Determination and comparison of specifics of nucleus pulposus cells of human intervertebral disc in alginate and chitosan-gelatin scaffolds. Adv Biomed Res. 2012;1:81. doi: 10.4103/2277-9175.102996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francisco AT, Mancino RJ, Bowles RD, Brunger JM, Tainter DM, Chen YT, Richardson WJ, Guilak F, Setton LA. Injectable laminin-functionalized hydrogel for nucleus pulposus regeneration. Biomaterials. 2013;34:7381–7388. doi: 10.1016/j.biomaterials.2013.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Endres M, Abbushi A, Thomale UW, Cabraja M, Kroppenstedt SN, Morawietz L, Casalis PA, Zenclussen ML, Lemke AJ, Horn P, Kaps C, Woiciechowsky C. Intervertebral disc regeneration after implantation of a cell-free bioresorbable implant in a rabbit disc degeneration model. Biomaterials. 2010;31:5836–5841. doi: 10.1016/j.biomaterials.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 17.Maroudas A, Stockwell RA, Nachemson A, Urban J. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120:113–130. [PMC free article] [PubMed] [Google Scholar]
- 18.Liebscher T, Haefeli M, Wuertz K, Nerlich AG, Boos N. Age-related variation in cell density of human lumbar intervertebral disc. Spine. 2011;36:153–159. doi: 10.1097/BRS.0b013e3181cd588c. [DOI] [PubMed] [Google Scholar]
- 19.Johnstone B, Bayliss MT. The large proteoglycans of the human intervertebral disc. Changes in their biosynthesis and structure with age, topography, and pathology. Spine. 1995;20:674–684. doi: 10.1097/00007632-199503150-00008. [DOI] [PubMed] [Google Scholar]
- 20.Chon BH, Lee EJ, Jing L, Setton LA, Chen J. Human umbilical cord mesenchymal stromal cells exhibit immature nucleus pulposus cell phenotype in a laminin-rich pseudo-three-dimensional culture system. Stem Cell Res Ther. 2013;4:120. doi: 10.1186/scrt331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kregar Velikonja N, Urban J, Frohlich M, Neidlinger-Wilke C, Kletsas D, Potocar U, Turner S, Roberts S. Cell sources for nucleus pulposus regeneration. Eur Spine J. 2014;23:S364–374. doi: 10.1007/s00586-013-3106-9. [DOI] [PubMed] [Google Scholar]
- 22.Mern DS, Beierfuss A, Thome C, Hegewald AA. Enhancing human nucleus pulposus cells for biological treatment approaches of degenerative intervertebral disc diseases: a systematic review. J Tissue Eng Regen Med. 2014;8:925–936. doi: 10.1002/term.1583. [DOI] [PubMed] [Google Scholar]
- 23.Feng G, Zhao X, Liu H, Zhang H, Chen X, Shi R, Liu X, Zhao X, Zhang W, Wang B. Transplantation of mesenchymal stem cells and nucleus pulposus cells in a degenerative disc model in rabbits: a comparison of 2 cell types as potential candidates for disc regeneration. J Neurosurg Spine. 2011;14:322–329. doi: 10.3171/2010.11.SPINE10285. [DOI] [PubMed] [Google Scholar]
- 24.Iwashina T, Mochida J, Sakai D, Yamamoto Y, Miyazaki T, Ando K, Hotta T. Feasibility of using a human nucleus pulposus cell line as a cell source in cell transplantation therapy for intervertebral disc degeneration. Spine. 2006;31:1177–86. doi: 10.1097/01.brs.0000217687.36874.c4. [DOI] [PubMed] [Google Scholar]
- 25.Mochida J, Sakai D, Nakamura Y, Watanabe T, Yamamoto Y, Kato S. Intervertebral disc repair with activated nucleus pulposus cell transplantation: a three-year, prospective clinical study of its safety. Eur Cell Mater. 2015;29:202–12. doi: 10.22203/ecm.v029a15. [DOI] [PubMed] [Google Scholar]
- 26.Wang B, Wu Y, Shao Z, Yang S, Che B, Sun C, Ma Z, Zhang Y. Functionalized self-assembling peptide nanofiber hydrogel as a scaffold for rabbit nucleus pulposus cells. J Biomedical Mater Res Part A. 2012;100:646–653. doi: 10.1002/jbm.a.33300. [DOI] [PubMed] [Google Scholar]
- 27.Richardson SM, Hughes N, Hunt JA, Freemont AJ, Hoyland JA. Human mesenchymal stem cell differentiation to NP-like cells in chitosan-glycerophosphate hydrogels. Biomaterials. 2008;29:85–93. doi: 10.1016/j.biomaterials.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Zhou Y, Huang B, Liu LT, Liu MH, Wang J, Li CQ, Zhang ZF, Chu TW, Xiong CJ. Utilization of stem cells in alginate for nucleus pulposus tissue engineering. Tissue Eng Part A. 2014;20:908–920. doi: 10.1089/ten.TEA.2012.0703. [DOI] [PubMed] [Google Scholar]
- 29.Renani HB, Ghorbani M, Beni BH, Karimi Z, Mirhosseini M, Zarkesh H, et al. Determination and comparison of specifics of nucleus pulposus cells of human intervertebral disc in alginate and chitosan-gelatin scaffolds. Adv Biomed Res. 2012;1:81. doi: 10.4103/2277-9175.102996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao L, de Ruiter GC, Wang H, Knight AM, Spinner RJ, Yaszemski MJ, Windebank AJ, Pandit A. Controlling dispersion of axonal regeneration using a multichannel collagen nerve conduit. Biomaterials. 2010;31:5789–5797. doi: 10.1016/j.biomaterials.2010.03.081. [DOI] [PubMed] [Google Scholar]
- 31.Yao L, Phan F, Li Y. Collagen microsphere serving as a cell carrier supports oligodendrocyte progenitor cell growth and differentiation for neurite myelination in vitro. Stem Cell Res Ther. 2013;4:109. doi: 10.1186/scrt320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halloran DO, Grad S, Stoddart M, Dockery P, Alini M, Pandit AS. An injectable cross-linked scaffold for nucleus pulposus regeneration. Biomaterials. 2008;29:438–447. doi: 10.1016/j.biomaterials.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Ruan DK, Xin H, Zhang C, Wang C, Xu C, Li C, He Q. Experimental intervertebral disc regeneration with tissue-engineered composite in a canine model. Tissue Eng Part A. 2010;16:2381–2389. doi: 10.1089/ten.TEA.2009.0770. [DOI] [PubMed] [Google Scholar]
- 34.Ganey TM, Meisel HJ. A potential role for cell-based therapeutics in the treatment of intervertebral disc herniation. Eur Spine J. 2002;11:S206–214. doi: 10.1007/s00586-002-0494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 36.Chopra A, Murray ME, Byfield FJ, Mendez MG, Halleluyan R, Restle DJ, Raz-Ben Aroush D, Galie PA, Pogoda K, Bucki R, Marcinkiewicz C, Prestwich GD, Zarembinski TI, Chen CS, Pure E, Kresh JY, Janmey PA. Augmentation of integrin-mediated mechanotransduction by hyaluronic acid. Biomaterials. 2014;35:71–82. doi: 10.1016/j.biomaterials.2013.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai L, Lu J, Sheen V, Wang S. Optimal poly(L-lysine) grafting density in hydrogels for promoting neural progenitor cell functions. Biomacromolecules. 2012;13:1663–1674. doi: 10.1021/bm300381d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai L, Lu J, Sheen V, Wang S. Promoting nerve cell functions on hydrogels grafted with poly(L-lysine) Biomacromolecules. 2012;13:342–349. doi: 10.1021/bm201763n. [DOI] [PMC free article] [PubMed] [Google Scholar]