Abstract
Background and Purpose
To evaluate the frequency and outcome of haemorrhagic transformation (HT) after ischaemic stroke in patients treated with non-vitamin K antagonist oral anticoagulants (NOACs).
Methods
Patients with stroke on treatment with a NOAC were prospectively enrolled in this multicentre observational study between February 2012 and 2015. Brain imaging at admission and follow-up imaging until day 7 were reviewed for HT. Functional outcome was assessed by the modified Rankin scale (mRS) before the index event, at discharge, and at 3-months.
Results
231 patients without recanalisation therapy (no-RT), and 32 patients with RT were eligible for analysis. Any HT was present at admission in 9/231 no-RT patients (3.9%, 95% CI 2.0 to 7.3) and in none of the patients with RT. In patients with follow-up imaging (no-RT, n=129, and RT, n=32), HT was present in 14.0% (no-RT; 95% CI, 8.9 to 21.1), and 40.6% (RT, 95% CI, 25.5 to 57.8), respectively. After adjustment for stroke severity, this difference between the no-RT and RT groups became non-significant. Symptomatic ICH was observed in 1 patient per group. HT was not associated with unfavourable outcome (mRS 3-6) at 3-months in multivariable analysis. Resumption of OAC after stroke was delayed in patients with HT compared to those without (15 d [IQR, 5–26] vs. 1 d [0–4], P<0.001).
Conclusions
The frequency and severity of HT after stroke on NOAC appears similar to previous reports for vitamin K antagonists and no anticoagulation. Whether asymptomatic HT should delay resumption of preventive anticoagulation requires further investigation.
Keywords: Stroke, Anticoagulation, Haemorrhagic Transformation
Introduction
Any haemorrhagic transformation (HT) is found in approximately 8.5% (95% CI 7-10%), of non-thrombolysed acute ischaemic stroke (AIS) patients [1]. Symptomatic HT associated with clinical worsening or death occurs in 1.5% (95% CI 0.8-2.2%) [1,2]. In the randomised European Cooperative Acute Stroke Study III, the rate of any HT was notably higher in both the IV thrombolysis and placebo-arm (27.0% vs. 17.6%) [3]. However, differences in assessment criteria and HT definitions hamper direct comparisons [4]. While oral anticoagulation (OAC) with vitamin K antagonists (VKA) does not appear to affect the occurrence of HT [2,5], data regarding the frequency and severity of HT in patients treated with non-Vitamin K antagonist oral anticoagulants (NOACS) is not available. The potential effects of HT on neurological outcome and on the interval until re-initiation of anticoagulation are unknown.
We evaluated the frequency and severity of HT at admission and during the first 7 days in acute stroke patients taking NOACs, and examined effects on the management and functional outcome of AIS under NOAC treatment using data from the multicentre Registry of Acute Stroke Under New Oral Anticoagulants (RASUNOA-pilot).
Methods
Study design, setting and variables
Patients presenting between February 2012 and February 2015 with an acute ischaemic stroke and taking NOACs at the time of the event were prospectively enrolled into the RASUNOA-pilot registry, an investigator-initiated, multicentre, observational cohort study (ClinicalTrials.gov, NCT01850797). Overall, 38 neurology departments with certified stroke units across Germany participated in the registry. Inclusion criteria were age ≥18 years, acute ischaemic stroke, and current therapy with a NOAC (i.e., Apixaban, Dabigatran or Rivaroxaban) at the time of stroke onset. For the present analysis, we excluded (i) patients without any available brain imaging, and (ii) patients with transient ischaemic attack (as defined by presentation without neurological deficit according to the National Institute of Health Stroke Scale (NIHSS) at the time of admission and absence of an acute ischaemic lesion on brain imaging [6]). Due to the observational character of the registry, all diagnostic and treatment decisions were left to the discretion of the attending physicians. Patient characteristics including demographic information, clinical data and laboratory parameters were prospectively collected using a standardized case report file. The modified Rankin scale score (mRS) was used for functional assessment prior to the stroke (pmRS), at admission, and at hospital discharge. Moreover, 3-month outcome was assessed by a structured telephone follow-up. The individual stroke risk in patients with atrial fibrillation was calculated using the CHA2DS2VASc-score (excluding the index event), and the individual bleeding risk using the HAS BLED-score, respectively, with the item “labile INR” set to zero [7,8].
The analysis was done stratified for patients not undergoing thrombolysis and/or thrombectomy (no-RT) and patients who received recanalisation therapy (RT), respectively (a focused analysis of the RT group without IV-only treated patients has been previously published [9].
Neuroimaging analysis
Cranial CT and MRI examinations were assessed for signs of ischaemic infarction, as well as intracranial haemorrhage by an experienced reader (JP). Assessment was performed on scans obtained at admission, and on follow-up scans carried out until day 7 if available. Regarding diagnosis and classification of haemorrhage, findings were reviewed by a board-certified neuroradiologist (MW). Disagreement between the two readers was resolved by consensus. Readers were blinded for patient details. HT was defined as any level of hyperdensity within the area of low attenuation (CT), or hypointensities on T2* or susceptibility weighted (SWI) sequences [10] in areas corresponding to DWI/FLAIR hyperintense regions on MRI. If rapid attenuation of initial hyperdensities on CT scans was observed, this was categorized as contrast staining rather than hemorrhage [9].
Haemorrhagic transformation was classified in accordance with the ECASS-I-criteria as either haemorrhagic infarction (HI1 and 2), or parenchymal haemorrhage (PH1 and 2) [11]. Accordingly, HI1 was defined as small petechiae along the margin of the infarct, HI2 as more confluent petechiae within the infarct but without space-occupying effect, PH1 as a blood clot not exceeding 30% of the infarct area with some mild space-occupying effect, and PH2 as blood clots exceeding 30% of the infarct area with significant space-occupying effect (Figure 1B) [11]. Moreover, we recorded intracerebral haemorrhage remote from the infarct, which was defined as small or medium-sized blood clots (PHr1) or large confluent dense blood clots (PHr2). Evidence of intraventricular or subarachnoidal haemorrhage was also captured. Symptomatic intracranial haemorrhage (sICH) was defined following the ECASS-II definition [12]. The extent of infarction was quantified using the Alberta Stroke Program Early Computed Tomography Score (ASPECTS; range 0 to 10 with 1 point subtracted for any evidence of ischaemic change in each defined region on axial cuts). In case of posterior circulation stroke, the pc-ASPECTS score was calculated [13,14]. For quantification of the site and size of ischaemic infarction the classification by Paciaroni and colleagues was used [2]. Accordingly, infarct size was classified as (1) small (lesion in the anterior or posterior circulation <1.5 cm), (2) medium (lesion in a cortical superficial branch of middle cerebral artery [MCA], or lesion involving the MCA deep branch, or lesion in internal borderzone territories, or lesion in a cortical superficial branch of posterior cerebral artery [PCA], or lesion involving the PCA branch or lesion in a cortical superficial branch of anterior cerebral artery [ACA]), (3) large anterior (lesion involving complete territory of MCA, PCA or ACA or lesion involving 2 cortical superficial branches of MCA or lesion involving a cortical superficial branch of MCA associated to the MCA deep branch, or lesion involving more than 1 artery territory [e.g., MCA associated to ACA territories]) or (4) large posterior (lesion involving brain stem or cerebellum >1.5 cm) [2]. Cases without definitive evidence of acute cerebral infarction on available scans were added to the Paciaroni category 1.
Figure 1.
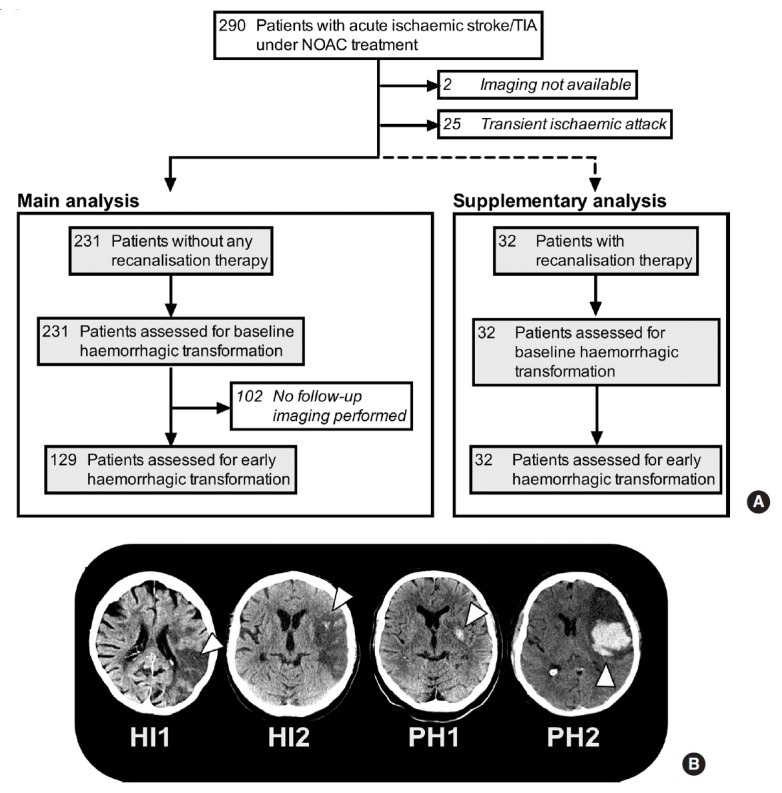
Selection of study population and classification of haemorrhagic transformation. (A) Study flow chart. (B) Representative computed tomography scans showing the four types of haemorrhagic transformation. HI, haemorrhagic infarction; PH, parenchymal haematoma.
Statistical analysis
All analyses were descriptive and exploratory. Continuous variables were described by mean and standard deviation (SD) or median and interquartile-range (IQR) for categorical variables, absolute and relative frequencies were reported. The Shapiro-Wilk test was used to ascertain distribution of data. Post-hoc, groups of patients with haemorrhagic transformation (HT) and without (non-HT) were defined. Fishers-exact test was used to compare proportions in baseline and radiological characteristics, dosing categories (standard [apixaban 5 mg BID, dabigatran 150 mg BID, rivaroxaban 20 mg QD] vs. low drug dose[apixaban 2.5 mg BID, dabigatran 110 mg BID, rivaroxaban 15 mg QD], and considering the actual dose, current renal function and age, adequate or overdosing) and outcomes, as appropriate. To compare continuous variables, t-test or non-parametric Mann-Whitney U test was used according to the skewness of the data. To calculate 95% confidence intervals (CI) of proportions, the adjusted Wald method was used [15]. Logistic regression analysis were performed to estimate odds ratio (OR) and 95% CI for potential risk factors of HT occurrence or unfavourable 3-months-outcome (mRS 3-6). In multivariable analysis the independent effect of HT on unfavourable 3-months-outcome adjusted for a subset of risk factors was assessed. Demographic variables and variables significantly related to outcome in the univariate analyses were included in the model under avoidance of possible collinearity. For variable selection the stepwise backward method with likelihood ratio test was used. Main characteristics of patients from the five best recruiting centres were compared to the standard recruiting ones in a sensitivity analysis. All statistical tests were two-sided, and P values of <0.05 were considered statistically significant. Analyses were conducted using IBM SPSS Statistics, version 23 (IBM Corp., Armonk, NY, USA). This study was performed consistent with the STROBE guidelines for observational studies.
Standard protocol approvals, registrations, and patient consents
The study was approved by the ethics committee of the Medical Faculty Heidelberg, Germany, and the ethics committees of each participating centre. Written informed consent by either the patient or a legal representative was mandatory for participation. The institutional review board at the primary study site in Heidelberg approved a waiver of consent for patients who died during initial hospitalization and for whom a legal representative could not be located.
Results
Study population and baseline characteristics
Of the 290 patients with acute ischaemic stroke/TIA under NOAC treatment enrolled in RASUNOA-pilot, 231 patients were included in the haemorrhagic transformation main analysis group (patient flow in Figure 1A) and 32 patients who were treated with recanalisation therapies (RT; n=23 endovascular therapy alone, n=5 IV thrombolysis plus thrombectomy, n=4 IV thrombolysis alone) were analysed separately (Figure 1A).
Baseline characteristics of the no-RT group are presented in Table 1, and of the RT group in the online Supplementary Table 1. In the no-RT group, the mean age of included patients was 77 (SD 8.4) years, and 46.8% were female. Median onset to first brain imaging was 3.4 hours (IQR, 2.1–9.9; exact onset of symptoms was available in n=156). Most patients were affected by minor or moderate stroke (median NIHSS 3 [IQR 2–6]). Nineteen-percent suffered from large anterior or posterior strokes (Table 2).
Table 1.
Baseline characteristics and functional outcome variables of patients with and without haemorrhagic transformation (no-RT group)
| All Patients | Admission imaging cohort |
Follow-up imaging cohort |
|||||
|---|---|---|---|---|---|---|---|
| Without early-HT | Early-HT | P value | Without early-HT | Early-HT | P value | ||
| N | 231 | 222 | 9 | 111 | 18 | ||
| Age (year) | 77.4 (± 8.4) | 77.5 (± 8.4) | 75.4 (± 8.3) | 0.45 | 77.2 (± 8.2) | 76.1 (± 5.6) | 0.47 |
| Women | 108 (46.8) | 106 (47.7) | 2 (22.2) | 0.18 | 57 (51.4) | 7 (38.9) | 0.45 |
| NOAC | 0.86 | 0.29 | |||||
| Apixaban | 36 (15.6) | 35 (15.8) | 1 (11.1) | 19 (17.1) | 1 (5.6) | ||
| Dabigatran | 62 (26.8) | 59 (26.6) | 3 (33.3) | 32 (28.8) | 4 (22.2) | ||
| Rivaroxaban | 133 (57.6) | 128 (57.7) | 5 (55.6) | 60 (54.1) | 13 (72.2) | ||
| Time since last intake NOAC (hour) | 8.7 (4.3–15.2) | 8.8 (4.3–15.4) | 5.9 (4.4–18.2) | 0.87 | 9.0 (4.2–18.1) | 12.8 (4.5–20.8) | 0.48 |
| Time until resumption of OAC (day) | 1 (0-3) | 1 (0-3) | 2 (1-15) | 0.017 | 1 (0-3) | 15 (5-26) | < .001 |
| Concomitant platelet inhibition | 24 (10.4) | 21 (9.5) | 3 (33.3) | 0.06 | 10 (9.0) | 3 (16.7) | 0.39 |
| CHA2DS2VASc score* | 5 (3–6) | 5 (3–6) | 4 (3–8) | 0.63 | 5 (4–6) | 4 (3–6) | 0.24 |
| HAS-BLED† | 4 (3–4) | 4 (3–4) | 3 (3–4) | 0.52 | 4 (3–4) | 3 (3–4) | 0.09 |
| Medical history‡ | |||||||
| Ischaemic stroke/TIA | 107 (46.3) | 102 (45.9) | 5 (55.6) | 0.74 | 53 (47.7) | 7 (38.9) | 0.61 |
| Intracranial Haemorrhage | 5 (2.2) | 5 (2.3) | 0 (0) | > 0.99 | 3 (2.7) | 0 (0) | > 0.99 |
| Atrial fibrillation | 209 (90.5) | 200 (90.1) | 9 (100) | > 0.99 | 101 (91.0) | 18 (100) | 0.36 |
| Hypertension | 193 (83.5) | 186 (83.8) | 7 (77.8) | 0.65 | 93 (83.8) | 13 (72.2) | 0.32 |
| Hyperlipidemia | 81 (35.1) | 80 (36.0) | 1 (11.1) | 0.17 | 42 (37.8) | 5 (27.8) | 0.60 |
| Diabetes mellitus | 69 (29.9) | 63 (28.4) | 6 (66.7) | 0.02 | 38 (34.2) | 6 (33.3) | > 0.99 |
| Heart failure | 53 (22.9) | 51 (23.0) | 2 (22.2) | > 0.99 | 28 (25.2) | 6 (33.3) | 0.57 |
| Peripheral vascular disease | 15 (6.5) | 14 (6.3) | 1 (11.1) | 0.46 | 8 (7.2) | 1 (5.6) | > 0.99 |
| Renal function | |||||||
| GFR < 60 mL/min | 85/ 206 (41.3) | 82/197 (36.9) | 3 (33.3) | 0.74 | 45/99 (45.5) | 8/18 (44.4) | > 0.99 |
| Creatinin level (mg/dL) | 1.0 (0.9–1.3) | 1.0 (0.9–1.3) | 1.0 (0.7–1.2) | 0.37 | 1.0 (0.8–1.3) | 1.0 (0.9–1.2) | 0.95 |
| NIHSS at admission | 3 (2–6) | 3 (2–6) | 4 (1–7) | 0.87 | 3 (2–6) | 7 (2–19) | 0.03 |
| Modified Rankin scale score | |||||||
| Pre stroke | 1 (0–2) | 1 (0–2) | 2 (1–3) | 0.14 | 1 (0–2) | 1 (1–2) | 0.13 |
| At admission | 3 (1–4) | 3 (1–4) | 4 (1–5) | 0.21 | 2 (1–4) | 5 (3–5) | 0.001 |
| At discharge | 2 (1–3) | 2 (1–3) | 3 (2–5) | 0.15 | 2 (1–3) | 4 (2–5) | 0.001 |
| At 3-months§ | 2 (1–4) | 2 (1–4) | 2 (1–6) | 0.78 | 1 (1–4) | 4 (1–6) | 0.001 |
| mRS 0-2 at 3-months | 110 (53.1) | 105 (47.3) | 5 (55.6) | > 0.99 | 60 (60.6) | 6 (33.3) | 0.04 |
| In-hospital mortality | 6/229 (2.6) | 6/220 (2.7) | 0 (0) | > 0.99 | 2 (1.8) | 2 (11.1) | 0.09 |
Data are mean (±SD), median (IQR) or n (%). Missing data for creatinin level (n=5), GFR (n=25), mRS at discharge (n=2) and at 3-months (n=24), time until resumption of anticoagulation, (n=29).
NOAC, non-vitamin K antagonist oral anticoagulant; TIA, transient ischaemic attack; GFR, glomerular filtration rate (electronic GFR as reported by the centres); NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin score [from 0 (no symptoms) to 6 (death)]; no-RT, no recanalisation therapy group.
CHA2DS2VASc score, range 0-9, from low to high risk of ischemic stroke in atrial fibrillation;
HAS-BLED score, range 0-9, from low to high risk of haemorrhage under oral anticoagulation;
Medical history, excluding the index-event;
3-months modified Rankin data available for 207/231 patients (89.6%).
Table 2.
Radiological characteristics and outcomes (no-RT group)
| Admission imaging cohort |
Follow-up imaging cohort |
|||||
|---|---|---|---|---|---|---|
| Without initial-HT | Initial-HT | P value | Without early-HT | Early-HT | P value | |
| N | 222 | 9 | – | 111 | 18 | – |
| MRI available | 118 (53.2) | 3 (33.3) | 0.32 | 76 (68.5) | 9 (50.0) | 0.18 |
| Proximal vessel occlusion* | 21/121 (17.4) | 0/2 (0) | > 0.99 | 11/69 (15.9) | 4/9 (44.4) | 0.06 |
| Infarct size | 0.001 | < 0.001 | ||||
| Small | 148 (66.7) | 0 (0) | – | 76 (68.5) | 2 (11.1) | – |
| Medium | 36 (16.2) | 4 (44.4) | – | 15 (13.5) | 6 (33.3) | – |
| Large anterior | 29 (13.1) | 4 (44.4) | – | 16 (14.4) | 8 (44.4) | – |
| Large posterior | 9 (4.1) | 1 (11.1) | – | 4 (3.6) | 2 (11.1) | – |
| Admission imaging | ||||||
| ASPECTS | 10 (9–10) | 9 (8–9) | 0.003 | 10 (9–10) | 9 (5–9) | < 0.001 |
| HI1 | 0 (0) | 6 (66.7) | – | 0 (0) | 2 (11.1) | – |
| HI2 | 0 (0) | 3 (33.3) | – | 0 (0) | 3 (16.7) | – |
| PH1 | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – |
| PH2 | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – |
| PHr1/PHr2 | 0 (0)/0 (0) | 0 (0)/0 (0) | – | 0 (0)/0 (0) | 0 (0)/0 (0) | – |
| SAH | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – |
| IVH | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – |
| sICH | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – |
| Follow-up imaging until day 7 | ||||||
| ASPECTS | – | – | – | 9 (8–10) | 5 (4–9) | 0.001 |
| HI1 | – | – | – | 0 (0)/0 (0) | 10 (55.6) | – |
| HI 2 | – | – | – | 0 (0) | 2 (11.1) | – |
| PH1 | – | – | – | 0 (0) | 0 (0) | – |
| PH2 | – | – | – | 0 (0) | 1 (5.6) | – |
| PHr1/PHr2 | – | – | – | 0 (0) | 0 (0)/0 (0) | – |
| SAH | – | – | – | 0 (0) | 0 (0) | – |
| IVH | – | – | – | 0 (0) | 0 (0) | – |
| sICH | – | – | – | 0 (0) | 1 (5.6) | – |
Data are median (IQR) or n (%). Note: In the follow-up imaging group, a total of 5 haemorrhages were present at admission, and 13 additional haemorrhages became present during follow-up imaging, resulting in a total of 18 hemorrhagic transformations in this group.
MRI, magnetic resonance imaging; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; HI1/2, haemorrhagic infarction type 1/2; PH1/2, parenchymal haemorrhage type 1/2; SAH, subarachnoid haemorrhage; IVH, intraventricular haemorrhage; sICH, symptomatic intracerebral haemorrhage (according to the ECASS-II criteria); no-RT, no recanalisation therapy group.
CT, MR or digital subtraction angiography available in 123/231 patients (53.2%).
Any follow-up imaging until day 7 was performed in 55.8% (129/231) of the no-RT group. The median time between scans was 49.6 hours (IQR, 22.4–86.0). Baseline characteristics of patients with follow-up imaging did not differ from those without (Supplementary Table 2).
Compared to patients with follow-up imaging and no recanalisation therapy (no-RT), RT-patients were more severely affected (NIHSS at admission 19 [IQR 14–22] vs. 4 [IQR 2–7], P<0.01) and suffered from larger baseline ischaemic lesions (ASPECTS 8 [IQR 7–10] vs. 10 (9–10, P<0.01; Supplementary Table 1).
Haemorrhagic transformation – No-RT group
At admission, HT was present in 9/231 patients of the no-RT group (initial-HT, 3.9%, 95% CI, 2.0 to 7.3; Table 2), and in none of the RT group. A strong trend towards a higher rate of concomitant antiplatelet therapy was observed in the initial-HT patients (33% vs. 9.5%; P=0.06; Table 1). In patients with follow-up imaging (n=129), 13 additional HTs were observed until day 7 (Table 2). In total, 18 cases of HT were registered among patients with available initial and follow-up imaging (early-HT, 18/129, 14.0%, 95% CI, 8.9 to 21.1).
There was no association between standard vs. low drug dose, or adequate or overdosing (considering actual dose and current renal function and age) and occurrence of initial-HT or early-HT in patients with AF. Univariate logistic regression revealed that infarct size had the strongest impact on the occurrence of early-HT (size small vs. medium OR 15.2, 95% CI 2.8 to 82.7; small vs. large OR 19.0, 95% CI 3.9 to 93.7, Table 3).
Table 3.
Factors associated with early haemorrhagic transformation (univariate analysis) (follow-up imaging cohort)
| OR (95 % CI) | P value | |
|---|---|---|
| Age (year) | 0.98 (0.92-1.05) | 0.57 |
| Female sex | 0.60 (0.22-1.67) | 0.33 |
| Concomitant platelet inhibition | 2.02 (0.50-8.19) | 0.33 |
| Medical history | ||
| Ischaemic stroke/TIA | 0.70 (0.25-1.93) | 0.49 |
| Hypertension | 0.50 (0.16-1.59) | 0.24 |
| Hyperlipidemia | 0.63 (0.21-1.90) | 0.41 |
| Diabetes mellitus | 0.96 (0.33-2.76) | 0.94 |
| Heart failure | 1.48 (0.51-4.32) | 0.47 |
| Peripheral vascular disease | 0.76 (0.09-6.45) | 0.80 |
| Renal function: GFR < 60 mL/min | 0.96 (0.35-2.64) | 0.94 |
| CHA2DS2VASc score* | 0.89 (0.68-1.17) | 0.41 |
| HAS-BLED score† | 0.68 (0.39-1.19) | 0.17 |
| Modified Rankin scale score‡ | ||
| Pre stroke | 1.25 (0.84-1,86) | 0.27 |
| At admission | 1.95 (1.29-2.94) | 0.002 |
| NIHSS at admission§ | 1.09 (1.03-1.16) | 0.006 |
| ASPECTS at admission | 0.61 (0.46-0.80) | < 0.001 |
| Infarct size | 0.001 | |
| Small | Ref. | |
| Medium | 15.20 (2.80-82.66) | |
| Large anterior or posterior | 19.00 (3.85-93.74) | |
| Time since last intake NOAC | 1.01 (0.95-1.08) | 0.68 |
| Time to first scan since onsetII | 1.02 (0.99-1.05) | 0.24 |
TIA, transient ischaemic attack; GFR, glomerular filtration rate (electronic GFR as reported by the centres); NIHSS, National Institutes of Health Stroke Scale; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; NOAC, non-vitamin K antagonist oral anticoagulant.
CHA2DS2VASc score, range 0-9, from low to high risk of ischaemic stroke in atrial fibrillation;
HAS-BLED score, range 0-9, from low to high risk of Haemorrhage under oral anticoagulation;
Modified Rankin scale score, from 0 (no symptoms) to 6 (death);
NIHSS, stroke related neurological deficits, from 0 (no symptoms) to 42;
Excluding cases with unknown onset.
Symptomatic HT occurred in one patient in the follow-up-imaging study population (1/129, 0.8%, 95% CI, <0.1 to 4.7%; Table 2). This patient suffered from a large MCA-territory infarction but did not show signs of HT on the initial CT-scan. The NOAC was continued after the stroke and a PH2 bleeding developed on day 2. All other observed HT remained asymptomatic.
Haemorrhagic transformation – RT group
In an unadjusted analysis, HT was more frequent in patients treated with thrombolysis and/or thrombectomy compared to those without (n=13/32, 40.6% [95% CI, 25.5 to 57.8) vs. 18/129, 14.0% [95% CI, 8.9 to 21.1], P=0.001). No HT was observed in the patients treated with IV thrombolysis only (n=4). However, differences in HT-rates are likely due to higher stroke severity (Supplementary Table 1) in patients treated with recanalisation therapy (after adjustment for stroke severity according to the admission NIHSS, logistic regression for the occurrence on HT became non-significant).
Association with functional outcome – No-RT group
Patients with early-HT were more severely affected at admission (median NIHSS 7 [IQR, 2–19] vs. 3 [IQR, 2–6], P=0.03; Table 1), and suffered more frequently from larger anterior and posterior infarctions than patients without early-HT (P<0.001; Table 2). Patients with early-HT had a significantly worse outcome compared to those without (median mRS 4 [IQR, 1–6] vs. mRS 2 [IQR 1–4], P=0.001; Table 1). However, in multivariate analysis, HT was not independently associated with unfavourable outcome (mRS 3-6) at 3-months (Table 4; for univariate analysis, see Supplementary Table 3). In-hospital mortality was not significantly increased among patients with HT.
Table 4.
Factors associated with unfavourable outcome (mRS 3-6) at 3-months follow up (multivariate analysis) (follow-up imaging cohort)
| OR (95% CI) | P value | |
|---|---|---|
| Age (year) | 1.07 (0.97-1.13) | 0.20 |
| Gender: female | 1.46 (0.52-4.14) | 0.47 |
| Medical history: Hypertension | 9.81 (1.92-50.04) | 0.006 |
| NIHSS admission (0-3 vs. ≥ 4)* | 3.33 (1.18-9.39) | 0.023 |
| Modified Rankin scale score pre stroke† | 1.97 (1.23-3.17) | 0.005 |
| Infarct size | 0.007 | |
| Small | Ref. | |
| Medium | 2.40 (0.61-9.35) | |
| Large anterior or posterior | 6.46 (2.02-20.73) | |
| Haemorrhagic transformation | 3.26 (0.66-16.04) | 0.14 |
NIHSS grouped into (0-3 vs. ≥4) based on Baird et al., 2001. [39];
Modified Rankin scale score, pre stroke from 0 (no symptoms) to 5 (severe disability).
Resumption of OAC – No-RT group
In stroke survivors, OAC was resumed during the acute inpatient stay in the majority of patients (n=184/230, 80%; thereof 97% NOAC, 3% phenprocoumon). In patients with early-HT, OAC was resumed after occurrence of HT in all but one case. Median time to recommended resumption was significantly longer in patients with early-HT (15 d [IQR, 5–26]) compared to patients without (1 d [0–4]; P<0.001). Notably, the delay was at least partly mediated through larger infarct-sizes in the early-HT group (see above), as a generally longer time until resumption of OAC was observed in patients without HT but large infarct compared to patients without HT and a small infarct (10 d [IQR, 0–15] and 1 d [IQR, 0–2]; P=0.034; data not shown).
Discussion
The new findings of our study are that (1) spontaneous HT occurs at a similar rate in acute stroke patients treated with NOAC as previously reported for non-anticoagulated and VKA-anticoagulated stroke patients [1,2,5], (2) recanalisation therapy did not increase the proportion of patients with HT after adjustment for stroke severity, (3) presence of HT on imaging appeared to delay the resumption of OAC, and (4) the presence of HT in NOAC treated patients was not independently associated to an unfavourable outcome at 3 months.
Ischaemic stroke occurred under NOAC therapy in 1 to 2% of NOAC treated patients with AF per year in large randomised controlled trials [16-19]. The incidence of strokes in patients taking NOACs is expected to increase in the future due to an increase of the prevalence of atrial fibrillation [20], higher utilisation of NOAC in the population [21], and a potential extension of indications [22]. A major advantage of NOAC compared to VKA treatment is a significant reduction of intracranial haemorrhage (ICH) [23]. Nevertheless, NOAC-related ICH has a similar poor prognosis as VKA-related ICH [24]. Three preclinical studies in mice undergoing transient middle cerebral occlusion that were anticoagulated with either warfarin or dabigatran supported the assumption that NOAC are also associated with a lower risk of HT in ischaemic stroke compared to VKA [25-27]. Moreover, Bohmann and colleagues suggested that early continuation of dabigatran might be safe in experimental ischaemic stroke [26]. In contrast to these experimental data, current expert guidance regarding the resumption of anticoagulation recommends starting oral anticoagulation in TIA and stroke patients with short or longer delay depending on the severity of the stroke [28].
According to the present analysis, 14.0% (95% CI, 8.9 to 21.1) of the NOAC anticoagulated patients with initial- and follow-up imaging developed HT, which is slightly higher than reported for VKA treated patients (10% in patients with INR <2, but no data on patients with INR >2 available) [5], and for patients without antithrombotic or thrombolysis treatment (8.5%, 95% CI 7% to 10%) [1]. In contrast, substantially higher rates of HT have been reported in the placebo arm of intravenous thrombolysis trials (21.6 to 29.6%) [29].
Although there is an ongoing debate about the relevance of both initial clinically symptomatic and asymptomatic forms of HT in terms of functional outcome [30-33], the present study highlights a more direct clinical consequence: Delayed resumption of OAC after occurrence of HT. Primary or secondary prevention of ischaemic stroke in (non-valvular) atrial fibrillation was the indication of oral anticoagulation in the majority of our patients. These patients are at a high risk of recurrent stroke, indicated by a median CHA2DS2VASc score of 5 at admission (excluding the index event). Once a patient has suffered from HT, resumption of OAC is usually postponed until HT is resolved or at least considered radiologically stable. In our study, OAC was indeed resumed significantly later in patients in whom early-HT occurred. However, larger infarct-sizes in the HT group might have contributed to this finding.
Interestingly, the median admission NIHSS of 4 in our population was relatively low compared to cardioembolic stroke [34]. A potential explanation might be that orally anticoagulated patients have less severe stroke compared to non-anticoagulated patients with AF in observational studies [35,36]. Alternatively, stroke may have been caused by another etiology of cardioembolism.
Larger scale prospective studies are needed to determine whether early restart or continued anticoagulation in non-disabling small ischaemic stroke with less severe blood-brain barrier damage is safe and beneficial. The proportion of 9% of patients who developed HT despite small-size infarction in our cohort however still warrants caution. A recent single-centre open-label non-randomised study found no increased risk of HT after initiation of dabigatran therapy within 24 hours of TIA or minor stroke (NIHSS ≤3) [37]. Another single-centre open-label study reports a progression of asymptomatic HT after administration of rivaroxaban on a median of 3 days after TIA and mild-to-moderate stroke (NIHSS ≤9) comparing baseline to follow-up MRI at day 7 [38].
Our study has some limitations. Recruitment was imbalanced among centres with six centres recruiting 57% of patients. However, a sensitivity analysis revealed no difference in terms of age, sex, and stroke severity in patients from top-recruiting versus other centres (Supplementary Table 4). As the RASUNOA pilot study did not have a simultaneously recruited control group, only indirect comparisons with other anticoagulation or no anticoagulation are possible. We do not have data regarding intercurrent antiplatelet therapy, which might have influenced the risk of HT.
Although the present study comprises the largest prospectively collected observational cohort of IS patients under NOAC treatment to our knowledge, its sample size is still limited and larger scale prospective studies are needed in order to confirm the results. Nevertheless, due to its observational character with only few exclusion criteria, we expect generalisability of the findings. Finally, neither the study design nor the sample-size allowed detection of potential differences in rate and severity of HT related to specific NOAC agents or specific doses.
Conclusions
In conclusion, we found a similar risk of HT after ischemic stroke in patients on NOAC as previously reported for VKA-anticoagulated, and non-anticoagulated patients. However, replication of the results in future prospective studies using matched control groups is therefore necessary, and current indirect comparisons should be interpreted with caution. While clinicians should carefully avoid continuation of NOAC therapy in patients with large infarcts, more evidence is needed to avoid potentially unnecessary delays of restarting anticoagulation in patients with HT of small or moderate ischaemic strokes, who are at risk of recurrent thromboembolism.
Acknowledgments
We thank all principal investigators of the RASUNOA study and participating hospitals who enrolled at least one ischaemic stroke patient (A-Z). A Binder (Kiel), M Dichgans (München), R Dziewas (Münster), K Gröschel (Mainz), M Eicke (Idar-Oberstein), M Ertl (Regensburg), MG Hennerici (Mannheim), C Hobohm (Leipzig), T Höhle (Herne), S Jander (Düsseldorf), E Jüttler (Ulm), A Khaw (Greifswald), C Kleinschnitz (Würzburg), A Kraft (Halle), M Köhrmann (Erlangen), F Meisel (Karlsruhe), T Neumann-Haefelin (Fulda), C Opherk (Heilbronn), F Palm (Ludwigshafen), S Poli (Tübingen), J Röther (Hamburg), E Schmid (Stuttgart), G Seidel (Hamburg), H Sodan (Bad Neustadt), C Tanislav (Gießen), G Thomalla (Hamburg), R Veltkamp (Heidelberg), K Wartenberg (Halle-Wittenberg), C Weimar (Essen).
JCP received travel and congress participation support from Pfizer, and personal fees from Boehringer Ingelheim, outside the submitted work. TR received consulting honoraria, speakers’ honoraria and travel support from BMS Pfizer, Boehringer Ingelheim, Bayer HealthCare and Daichii Sankyo, outside the submitted work. PK received consulting honoraria, speakers’ honoraria, travel support or research support from Boehringer Ingelheim, Bayer HealthCare, BMS Pfizer and Daiichi Sankyo. SP received honoraria from C.R. Bard, Bayer Healthcare, BeneChill, BMS-Pfizer, Boehringer-Ingelheim, Daiichi Sankyo, EMCOOLS, and ZOLL; institutional research support from BeneChill, BMS-Pfizer, Covidien, EMCOOLS, Helena Laboratories, HVM Medical, Raumedic, and ZOLL; congress/meeting traveling and accommodation costs: C.R. Bard, Bayer Healthcare, BeneChill, Boehringer-Ingelheim, EMCOOLS, and ZOLL. RD has received speaker fees and consultation honoraria from Bayer, Boehringer Ingelheim, BMS, Pfizer and Daiichi Sankyo. FP received consulting honoraria, speakers’ honoraria or travel support from Boehringer Ingelheim, BMS, Pfizer and Daiichy-Sankyo. SJ received honoraria for speaking and consultancy from Boehringer Ingelheim, Bayer Healthcare, BMS, Pfizer, and Daiichi Sankyo.PUH reports grants from BMBF, EU, Charité, Berlin Chamber of Physicians, German Parkinson Society, University Hospital Würzburg, Robert-Koch-Institute, Charité–Universitätsmedizin Berlin (within MonDAFIS; MonDAFIS is supported by an unrestricted research grant to the Charité from Bayer), University Göttingen (within FIND-AFrandomised, supported by an unrestricted research grant to the University Göttingen from Boehringer-Ingelheim), and University Hospital Heidelberg (within RASUNOA-prime; supported by an unrestricted research grant to the University Hospital Heidelberg from Bayer, BMS, Boehringer-Ingelheim, Daiichi Sankyo), outside the submitted work. RV has received speaker fees, consulting honoraria and research support from Bayer, Boehringer Ingelheim, BMS, Pfizer, Daiichi Sankyo, CSL Behring. The other authors report no competing interests.
Footnotes
The authors have no financial conflicts of interest.
Supplementary Material
Continued from the previous page
Baseline clinical and radiological characteristics of patients with and without follow up imaging
Factors associated with unfavourable outcome (mRS 3-6) at 3-months follow up (univariate analysis; follow-up imaging cohort)
Characteristics of Patients from top-recruiting centres versus standard recruiting centres
References
- 1.Lindley RI, Wardlaw JM, Sandercock PA, Rimdusid P, Lewis SC, Signorini DF, et al. Frequency and risk factors for spontaneous hemorrhagic transformation of cerebral infarction. J Stroke Cerebrovasc Dis. 2004;13:235–246. doi: 10.1016/j.jstrokecerebrovasdis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Paciaroni M, Agnelli G, Corea F, Ageno W, Alberti A, Lanari A, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke. 2008;39:2249–2256. doi: 10.1161/STROKEAHA.107.510321. [DOI] [PubMed] [Google Scholar]
- 3.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Sabin J, Maisterra O, Santamarina E, Kase CS. Factors influencing haemorrhagic transformation in ischaemic stroke. Lancet Neurol. 2013;12:689–705. doi: 10.1016/S1474-4422(13)70055-3. [DOI] [PubMed] [Google Scholar]
- 5.O’Donnell M, Oczkowski W, Fang J, Kearon C, Silva J, Bradley C, et al. Preadmission antithrombotic treatment and stroke severity in patients with atrial fibrillation and acute ischaemic stroke: an observational study. Lancet Neurol. 2006;5:749–754. doi: 10.1016/S1474-4422(06)70536-1. [DOI] [PubMed] [Google Scholar]
- 6.Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack. Stroke. 2009;40:2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 7.Lip GY, Lane DA, Buller H, Apostolakis S. Development of a novel composite stroke and bleeding risk score in patients with atrial fibrillation: the AMADEUS Study. Chest. 2013;144:1839–1847. doi: 10.1378/chest.13-1635. [DOI] [PubMed] [Google Scholar]
- 8.Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57:173–180. doi: 10.1016/j.jacc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 9.Purrucker JC, Wolf M, Haas K, Rizos T, Khan S, Dziewas R, et al. Safety of Endovascular Thrombectomy in Patients Receiving Non-Vitamin K Antagonist Oral Anticoagulants. Stroke. 2016;47:1127–1130. doi: 10.1161/STROKEAHA.116.012684. [DOI] [PubMed] [Google Scholar]
- 10.Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YC. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. Am J Neuroradiol. 2009;30:19–30. doi: 10.3174/ajnr.A1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274:1017–1025. [PubMed] [Google Scholar]
- 12.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 13.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 14.Puetz V, Sylaja PN, Coutts SB, Hill MD, Dzialowski I, Mueller P, et al. Extent of hypoattenuation on CT angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke. 2008;39:2485–2490. doi: 10.1161/STROKEAHA.107.511162. [DOI] [PubMed] [Google Scholar]
- 15.Agresti A, Coull BA. Approximate is better than “exact” for interval estimation of binomial proportions. The American Statistician. 1998;52:119–126. [Google Scholar]
- 16.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 17.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 18.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 19.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 20.Kirchhof P, Breithardt G, Bax J, Benninger G, Blomstrom-Lundqvist C, Boriani G, et al. A roadmap to improve the quality of atrial fibrillation management: proceedings from the fifth Atrial Fibrillation Network/European Heart Rhythm Association consensus conference. Europace. 2016;18:37–50. doi: 10.1093/europace/euv304. [DOI] [PubMed] [Google Scholar]
- 21.Huisman MV, Rothman KJ, Paquette M, Teutsch C, Diener HC, Dubner SJ, et al. Antithrombotic Treatment Patterns in Patients with Newly Diagnosed Nonvalvular Atrial Fibrillation: The GLORIA-AF Registry, Phase II. Am J Med. 2015;128:1306–1313. doi: 10.1016/j.amjmed.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O’Donnell MJ, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429–438. doi: 10.1016/S1474-4422(13)70310-7. [DOI] [PubMed] [Google Scholar]
- 23.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 24.Purrucker JC, Haas K, Rizos T, Khan S, Wolf M, Hennerici MG, et al. Early Clinical and Radiological Course, Management, and Outcome of Intracerebral Hemorrhage Related to New Oral Anticoagulants. JAMA Neurol. 2016;73:169–177. doi: 10.1001/jamaneurol.2015.3682. [DOI] [PubMed] [Google Scholar]
- 25.Pfeilschifter W, Spitzer D, Czech-Zechmeister B, Steinmetz H, Foerch C. Increased risk of hemorrhagic transformation in ischemic stroke occurring during warfarin anticoagulation: an experimental study in mice. Stroke. 2011;42:1116–1121. doi: 10.1161/STROKEAHA.110.604652. [DOI] [PubMed] [Google Scholar]
- 26.Bohmann F, Mirceska A, Pfeilschifter J, Lindhoff-Last E, Steinmetz H, Foerch C, et al. No influence of dabigatran anticoagulation on hemorrhagic transformation in an experimental model of ischemic stroke. PLoS ONE. 2012;7:e40804. doi: 10.1371/journal.pone.0040804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gliem M, Hermsen D, van Rooijen N, Hartung HP, Jander S. Secondary intracerebral hemorrhage due to early initiation of oral anticoagulation after ischemic stroke: an experimental study in mice. Stroke. 2012;43:3352–3357. doi: 10.1161/STROKEAHA.112.666818. [DOI] [PubMed] [Google Scholar]
- 28.Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J, et al. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2013;15:625–651. doi: 10.1093/europace/eut083. [DOI] [PubMed] [Google Scholar]
- 29.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 30.Park JH, Ko Y, Kim WJ, Jang MS, Yang MH, Han MK, et al. Is asymptomatic hemorrhagic transformation really innocuous? Neurology. 2012;78:421–426. doi: 10.1212/WNL.0b013e318245d22c. [DOI] [PubMed] [Google Scholar]
- 31.Lei C, Wu B, Liu M, Chen Y. Asymptomatic hemorrhagic transformation after acute ischemic stroke: is it clinically innocuous? J Stroke Cerebrovasc Dis. 2014;23:2767–2772. doi: 10.1016/j.jstrokecerebrovasdis.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 32.Dzialowski I, Pexman JH, Barber PA, Demchuk AM, Buchan AM, Hill MD, et al. Asymptomatic hemorrhage after thrombolysis may not be benign: prognosis by hemorrhage type in the Canadian alteplase for stroke effectiveness study registry. Stroke. 2007;38:75–79. doi: 10.1161/01.STR.0000251644.76546.62. [DOI] [PubMed] [Google Scholar]
- 33.Jickling GC, Liu D, Stamova B, Ander BP, Zhan X, Lu A, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab. 2014;34:185–199. doi: 10.1038/jcbfm.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimura K, Minematsu K, Yamaguchi T, Japan Multicenter Stroke Investigators C Atrial fibrillation as a predictive factor for severe stroke and early death in 15,831 patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2005;76:679–683. doi: 10.1136/jnnp.2004.048827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hylek EM, Go AS, Chang Y, Jensvold NG, Henault LE, Selby JV, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349:1019–1026. doi: 10.1056/NEJMoa022913. [DOI] [PubMed] [Google Scholar]
- 36.Audebert HJ, Schenk B, Schenkel J, Heuschmann PU. Impact of prestroke oral anticoagulation on severity and outcome of ischemic and hemorrhagic stroke in patients with atrial fibrillation. Cerebrovasc Dis. 2010;29:476–483. doi: 10.1159/000297963. [DOI] [PubMed] [Google Scholar]
- 37.Kate M, Gioia L, Buck B, Sivakumar L, Jeerakathil T, Shuaib A, et al. Dabigatran Therapy in Acute Ischemic Stroke Patients Without Atrial Fibrillation. Stroke. 2015;46:2685–2687. doi: 10.1161/STROKEAHA.115.010383. [DOI] [PubMed] [Google Scholar]
- 38.Gioia LC, Kate M, Sivakumar L, Hussain D, Kalashyan H, Buck B, et al. Early Rivaroxaban Use After Cardioembolic Stroke May Not Result in Hemorrhagic Transformation: A Prospective Magnetic Resonance Imaging Study. Stroke. 2016;47:1917–1919. doi: 10.1161/STROKEAHA.116.013491. [DOI] [PubMed] [Google Scholar]
- 39.Baird AE, Dambrosia J, Janket S, Eichbaum Q, Chaves C, Silver B, et al. A three-item scale for the early prediction of stroke recovery. Lancet. 2001;357:2095–2099. doi: 10.1016/s0140-6736(00)05183-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Continued from the previous page
Baseline clinical and radiological characteristics of patients with and without follow up imaging
Factors associated with unfavourable outcome (mRS 3-6) at 3-months follow up (univariate analysis; follow-up imaging cohort)
Characteristics of Patients from top-recruiting centres versus standard recruiting centres


