Abstract
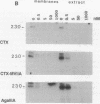
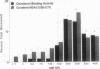
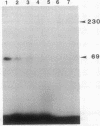
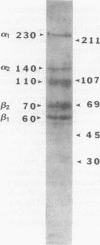
The omega-conotoxin GVIA (CTX) receptor has been purified 1900-fold to apparent homogeneity by monitoring both reversible binding of 125I-labeled CTX (125I-CTX) and photoincorporation of N-hydroxysuccinimidyl-4-azidobenzoate-125I-CTX (HSA-125I-CTX). Photoincorporation of HSA-125I-CTX into a 230-kDa protein exhibits a pharmacologic and chromatographic profile indicating that the 230-kDa protein is the CTX-binding subunit of the receptor. The pharmacologic specificity of 125I-CTX binding to the purified CTX receptor closely resembles that of the native membrane-bound form with respect to sensitivity towards CTX (Kd = 32 pM) and other peptide toxin antagonists. The purified CTX receptor comprises the 230-kDa protein (alpha 1) and four additional proteins with apparent molecular masses of 140 (alpha 2), 110, 70 (beta 2), and 60 (beta 1) kDa. This subunit structure closely resembles that of the 1,4-dihydropyridine-sensitive L-type calcium channel.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Saisu H. Identification of the receptor for omega-conotoxin in brain. Probable components of the calcium channel. J Biol Chem. 1987 Jul 15;262(20):9877–9882. [PubMed] [Google Scholar]
- Ahlijanian M. K., Westenbroek R. E., Catterall W. A. Subunit structure and localization of dihydropyridine-sensitive calcium channels in mammalian brain, spinal cord, and retina. Neuron. 1990 Jun;4(6):819–832. doi: 10.1016/0896-6273(90)90135-3. [DOI] [PubMed] [Google Scholar]
- BRADLEY L. B., JACOB M., JACOBS E. E., SANADI D. R. Uncoupling of oxidative phosphorylation by cadmium ion. J Biol Chem. 1956 Nov;223(1):147–156. [PubMed] [Google Scholar]
- Barhanin J., Schmid A., Lazdunski M. Properties of structure and interaction of the receptor for omega-conotoxin, a polypeptide active on Ca2+ channels. Biochem Biophys Res Commun. 1988 Feb 15;150(3):1051–1062. doi: 10.1016/0006-291x(88)90736-x. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Curtis B. M., Catterall W. A. Purification of the calcium antagonist receptor of the voltage-sensitive calcium channel from skeletal muscle transverse tubules. Biochemistry. 1984 May 8;23(10):2113–2118. doi: 10.1021/bi00305a001. [DOI] [PubMed] [Google Scholar]
- De Jongh K. S., Merrick D. K., Catterall W. A. Subunits of purified calcium channels: a 212-kDa form of alpha 1 and partial amino acid sequence of a phosphorylation site of an independent beta subunit. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8585–8589. doi: 10.1073/pnas.86.21.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S. B., Williams M. E., Ways N. R., Brenner R., Sharp A. H., Leung A. T., Campbell K. P., McKenna E., Koch W. J., Hui A. Sequence and expression of mRNAs encoding the alpha 1 and alpha 2 subunits of a DHP-sensitive calcium channel. Science. 1988 Sep 23;241(4873):1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- Fosset M., Jaimovich E., Delpont E., Lazdunski M. [3H]nitrendipine receptors in skeletal muscle. J Biol Chem. 1983 May 25;258(10):6086–6092. [PubMed] [Google Scholar]
- Glossmann H., Ferry D. R., Boschek C. B. Purification of the putative calcium channel from skeletal muscle with the aid of [3H]-nimodipine binding. Naunyn Schmiedebergs Arch Pharmacol. 1983 Jun;323(1):1–11. doi: 10.1007/BF00498821. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Striessnig J. Molecular properties of calcium channels. Rev Physiol Biochem Pharmacol. 1990;114:1–105. doi: 10.1007/BFb0031018. [DOI] [PubMed] [Google Scholar]
- Haase H., Striessnig J., Holtzhauer M., Vetter R., Glossmann H. A rapid procedure for the purification of cardiac 1,4-dihydropyridine receptors from porcine heart. Eur J Pharmacol. 1991 May 25;207(1):51–59. doi: 10.1016/s0922-4106(05)80037-9. [DOI] [PubMed] [Google Scholar]
- Hess P. Calcium channels in vertebrate cells. Annu Rev Neurosci. 1990;13:337–356. doi: 10.1146/annurev.ne.13.030190.002005. [DOI] [PubMed] [Google Scholar]
- Hirning L. D., Fox A. P., McCleskey E. W., Olivera B. M., Thayer S. A., Miller R. J., Tsien R. W. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988 Jan 1;239(4835):57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- Hui A., Ellinor P. T., Krizanova O., Wang J. J., Diebold R. J., Schwartz A. Molecular cloning of multiple subtypes of a novel rat brain isoform of the alpha 1 subunit of the voltage-dependent calcium channel. Neuron. 1991 Jul;7(1):35–44. doi: 10.1016/0896-6273(91)90072-8. [DOI] [PubMed] [Google Scholar]
- Knaus H. G., Striessnig J., Koza A., Glossmann H. Neurotoxic aminoglycoside antibiotics are potent inhibitors of [125I]-Omega-Conotoxin GVIA binding to guinea-pig cerebral cortex membranes. Naunyn Schmiedebergs Arch Pharmacol. 1987 Nov;336(5):583–586. doi: 10.1007/BF00169318. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai Y., Seagar M. J., Takahashi M., Catterall W. A. Cyclic AMP-dependent phosphorylation of two size forms of alpha 1 subunits of L-type calcium channels in rat skeletal muscle cells. J Biol Chem. 1990 Dec 5;265(34):20839–20848. [PubMed] [Google Scholar]
- Marqueze B., Martin-Moutot N., Levêque C., Couraud F. Characterization of the omega-conotoxin-binding molecule in rat brain synaptosomes and cultured neurons. Mol Pharmacol. 1988 Aug;34(2):87–90. [PubMed] [Google Scholar]
- McCleskey E. W., Fox A. P., Feldman D. H., Cruz L. J., Olivera B. M., Tsien R. W., Yoshikami D. Omega-conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4327–4331. doi: 10.1073/pnas.84.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEnery M. W., Buhle E. L., Jr, Aebi U., Pedersen P. L. Proton ATPase of rat liver mitochondria. Preparation and visualization of a functional complex using the novel zwitterionic detergent 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate. J Biol Chem. 1984 Apr 10;259(7):4642–4651. [PubMed] [Google Scholar]
- McEnery M. W., Snowman A. M., Snyder S. H. Evidence for subtypes of the omega-conotoxin GVIA receptor. Identification of the properties intrinsic to the high-affinity receptor. Ann N Y Acad Sci. 1991;635:435–437. doi: 10.1111/j.1749-6632.1991.tb36519.x. [DOI] [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989 Jul 20;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Mintz I. M., Venema V. J., Adams M. E., Bean B. P. Inhibition of N- and L-type Ca2+ channels by the spider venom toxin omega-Aga-IIIA. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6628–6631. doi: 10.1073/pnas.88.15.6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y., Friedrich T., Kim M. S., Mikami A., Nakai J., Ruth P., Bosse E., Hofmann F., Flockerzi V., Furuichi T. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991 Apr 4;350(6317):398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Cruz L. J., de Santos V., LeCheminant G. W., Griffin D., Zeikus R., McIntosh J. M., Galyean R., Varga J., Gray W. R. Neuronal calcium channel antagonists. Discrimination between calcium channel subtypes using omega-conotoxin from Conus magus venom. Biochemistry. 1987 Apr 21;26(8):2086–2090. doi: 10.1021/bi00382a004. [DOI] [PubMed] [Google Scholar]
- Plummer M. R., Logothetis D. E., Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989 May;2(5):1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Snyder S. H. Calcium antagonist receptors. Ion Channels. 1988;1:213–249. doi: 10.1007/978-1-4615-7302-9_6. [DOI] [PubMed] [Google Scholar]
- Schneider T., Hofmann F. The bovine cardiac receptor for calcium channel blockers is a 195-kDa protein. Eur J Biochem. 1988 Jun 1;174(2):369–375. doi: 10.1111/j.1432-1033.1988.tb14107.x. [DOI] [PubMed] [Google Scholar]
- Sharp A. H., Imagawa T., Leung A. T., Campbell K. P. Identification and characterization of the dihydropyridine-binding subunit of the skeletal muscle dihydropyridine receptor. J Biol Chem. 1987 Sep 5;262(25):12309–12315. [PubMed] [Google Scholar]
- Sieber M., Nastainczyk W., Zubor V., Wernet W., Hofmann F. The 165-kDa peptide of the purified skeletal muscle dihydropyridine receptor contains the known regulatory sites of the calcium channel. Eur J Biochem. 1987 Aug 17;167(1):117–122. doi: 10.1111/j.1432-1033.1987.tb13311.x. [DOI] [PubMed] [Google Scholar]
- Snutch T. P., Leonard J. P., Gilbert M. M., Lester H. A., Davidson N. Rat brain expresses a heterogeneous family of calcium channels. Proc Natl Acad Sci U S A. 1990 May;87(9):3391–3395. doi: 10.1073/pnas.87.9.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snutch T. P., Tomlinson W. J., Leonard J. P., Gilbert M. M. Distinct calcium channels are generated by alternative splicing and are differentially expressed in the mammalian CNS. Neuron. 1991 Jul;7(1):45–57. doi: 10.1016/0896-6273(91)90073-9. [DOI] [PubMed] [Google Scholar]
- Starr T. V., Prystay W., Snutch T. P. Primary structure of a calcium channel that is highly expressed in the rat cerebellum. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5621–5625. doi: 10.1073/pnas.88.13.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striessnig J., Knaus H. G., Glossmann H. Photoaffinity-labelling of the calcium-channel-associated 1,4-dihydropyridine and phenylalkylamine receptor in guinea-pig hippocampus. A 195 kDa polypeptide carries both drug receptors and has similarities to the alpha 1 subunit of the purified skeletal-muscle calcium channel. Biochem J. 1988 Jul 1;253(1):39–47. doi: 10.1042/bj2530039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striessnig J., Knaus H. G., Grabner M., Moosburger K., Seitz W., Lietz H., Glossmann H. Photoaffinity labelling of the phenylalkylamine receptor of the skeletal muscle transverse-tubule calcium channel. FEBS Lett. 1987 Feb 23;212(2):247–253. doi: 10.1016/0014-5793(87)81354-6. [DOI] [PubMed] [Google Scholar]
- Stumpo R. J., Pullan L. M., Salama A. I. The inhibition of [125I]omega-conotoxin GVIA binding to neuronal membranes by neomycin may be mediated by a GTP-binding protein. Eur J Pharmacol. 1991 Feb 25;206(2):155–158. doi: 10.1016/0922-4106(91)90024-c. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Seagar M. J., Jones J. F., Reber B. F., Catterall W. A. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5478–5482. doi: 10.1073/pnas.84.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura M., Fukui H., Wada H. Different localization of receptors for omega-conotoxin and nitrendipine in rat brain. Biochem Biophys Res Commun. 1987 Dec 31;149(3):982–988. doi: 10.1016/0006-291x(87)90505-5. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Bean B. P., Hess P., Lansman J. B., Nilius B., Nowycky M. C. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986 Jul;18(7):691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- Wagner J. A., Snowman A. M., Biswas A., Olivera B. M., Snyder S. H. Omega-conotoxin GVIA binding to a high-affinity receptor in brain: characterization, calcium sensitivity, and solubilization. J Neurosci. 1988 Sep;8(9):3354–3359. doi: 10.1523/JNEUROSCI.08-09-03354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yeager R. E., Yoshikami D., Rivier J., Cruz L. J., Miljanich G. P. Transmitter release from presynaptic terminals of electric organ: inhibition by the calcium channel antagonist omega Conus toxin. J Neurosci. 1987 Aug;7(8):2390–2396. [PMC free article] [PubMed] [Google Scholar]