Abstract
Background:
Vulvovaginal candidiasis is caused by Candida albicans. The vaginal epithelium, as the first site of the initial stage of infection by pathogens, plays an important role in resisting genital tract infections. Moreover, lactobacilli are predominant members of the vaginal microbiota that help to maintain a normal vaginal microenvironment. Therefore, Lactobacillus crispatus was explored for its capacity to intervene in the immune response of vaginal epithelial cells VK2/E6E7 to C. albicans.
Methods:
We examined the interleukin-2 (IL-2), 4, 6, 8, and 17 produced by VK2/E6E7 cells infected with C. albicans and treated with L. crispatus in vitro. The capacity of L. crispatus to adhere to VK2/E6E7 and inhibit C. albicans growth was also tested by scanning electron microscopy (SEM) and adhesion experiments.
Results:
Compared with group VK2/E6E7 with C. albicans, when treated with L. crispatus, the adhesion of C. albicans to VK2/E6E7 cells decreased significantly by 52.87 ± 1.22%, 47.03 ± 1.35%, and 42.20 ± 1.55% under competition, exclusion, and displacement conditions, respectively. SEM revealed that the invasion of C. albicans into VK2/E6E7 cells was caused by induced endocytosis and active penetration. L. crispatus could effectively protect the cells from the virulence of hyphae and spores of C. albicans and enhance the local immune function of the VK2/E6E7 cells. The concentrations of IL-2, 6, and 17 were upregulated significantly (P < 0.01) and that of IL-8 were downregulated significantly (P < 0.01) in infected VK2/E6E7 cells treated with L. crispatus. The concentration of IL-4 was similar to that of the group VK2/E6E7 with C. albicans (24.10 ± 0.97 vs. 23.12 ± 0.76 pg/ml, P = 0.221).
Conclusions:
L. crispatus can attenuate the virulence of C. albicans, modulate the secretion of cytokines and chemokines, and enhance the immune response of VK2/E6E7 cells in vitro. The vaginal mucosa has a potential function in the local immune responses against pathogens that can be promoted by L. crispatus.
Keywords: Candida albicans, Cytokines, Immunity, Lactobacillus crispatus, Scanning Electron Microscope, Vaginal Epithelial Cells
Introduction
Vulvovaginal candidiasis (VVC) – caused predominantly by Candida albicans – affects an estimated 70–75% of women at least once during their lives. In addition, 5–8% of women have recurrent vulvovaginal candidiasis (RVVC), which is defined as four or more episodes every year.[1] VVC and RVVC remain a serious problem in reproductive-age women.[2] C. albicans is an opportunistic pathogen with a significant ability to interact with the vaginal mucosa in terms of adherence, invasion, and induction of cell damage. The mucosal immunity may be stimulated in the absence of any sign or symptom of the disease.[3] However, relatively little is known about the host defense mechanisms against C. albicans infection.
Therefore, it is important to understand how immunity to C. albicans is regulated. To date, studies have investigated the anti-Candida immunity of myeloid or lymphoid cells by identifying the receptors and fungal moieties.[4] However, the mucosal epithelial surface is the first site of contact of C. albicans with its host, and local, rather than systemic immunity plays a decisive role in controlling the infection by initiating immune responses and even providing protection.
The significant role played by epithelial cells in the innate immune response to C. albicans has been demonstrated by several recent studies.[5,6] Epithelial cells from different sites of the human body[7,8,9] inhibited C. albicans growth by producing cytokines and antimicrobial peptides (AMPs).[9,10] However, the study of innate immune defense mechanisms of anti-C. albicans in the vagina has developed slowly.
Lactobacilli are predominant members of vaginal microbiome in healthy women and play an important role in maintaining a healthy vaginal flora, such as by competitive exclusion of pathogenic bacteria, competition for nutrients, production of antimicrobial substances, and activation of the immune system.[11] Co-aggregation of C. albicans and lactobacilli may be important in the vagina, especially to reduce the adhesion of the fungus to the vaginal mucosa and to restore the vaginal microbiome by the production of favorable metabolites[12] and the modulation of cytokine expression.[13]
Lactobacillus crispatus, an H2O2-producing strain, is the most common species among the healthy human vaginal microbiota and contributes to the normal bacterial composition of vaginal ecosystem.[14] In our study, we assessed the role of L. crispatus on the ability of C. albicans to adhere to the vaginal epithelial cells and the immunomodulatory effect of L. crispatus on the cytokine and chemokine production of epithelial cells induced by C. albicans.
Methods
Bacterial strains
L. crispatus cells (American Type Culture Collection [ATCC] 33820) cultured in de Man, Rogosa and Sharpe (MRS) broth (Difco, Becton, Dickinson and Company, USA) under anaerobic conditions (Gas-Pak system, BBL, Becton Dickinson Biosciences, Milan, Italy) at 37°C overnight were collected by centrifugation, washed twice, and suspended in phosphate-buffered saline (PBS) at 107 colony-forming units (CFU)/ml. The cells were counted by plating serial dilutions on MRS agar (Difco).[15]
C. albicans (ATCC 64548) was grown aerobically overnight on Sabouraud-Dextrose agar plates (Becton Dickinson, Cockeysville, MD, USA) at 30°C. One colony was used to inoculate 10 ml of Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, Life Technologies Corporation, CA, USA). Cells were suspended in RPMI 1640 and adjusted to a certain concentration after counting using a hemocytometer.
Culturing of VK2/E6E7
VK2/E6E7 is an epithelial cell line (ATCC CRL-2616) derived from a healthy premenopausal female undergoing vaginal repair surgery. VK2/E6E7 cells were immortalized with human papillomavirus16 E6E7 and were grown in 25 cm2 tissue culture flasks (Corning Costar, Milan, Italy) at 37°C (5% CO2) until confluence in Keratinocyte-Serum-Free medium (K-SFM; GIBCO-BRL San Giuliano Milanese, Milan, Italy) with 0.1 ng/ml human recombinant epidermal growth factor, 0.05 mg/ml bovine pituitary extract, 100 U of penicillin, and 100 μg of streptomycin/ml and additional calcium to a final concentration of 0.4 mmol/L. The medium was changed for every 2 days. Cells were counted using a hemocytometer and trypan blue dye exclusion test.
Competition with Candida albicans for adhesion to VK2/E6E7 by Lactobacillus crispatus
L. crispatus was grown in MRS broth at 37°C under anaerobic conditions. C. albicans was prepared by inoculating colonies isolated from Sabouraud agar (Oxoid, Milan, Italy) plates into RPMI 1640 (Gibco) at 107 CFU/ml. Cultures of L. crispatus were collected by centrifugation, washed three times, and suspended in RPMI 1640 at 107 CFU/ml. The concentration of L. crispatus suspensions was determined by sequential measurements of optical densities (OD) at 600 nm and quantification by colony counts. The relation between the OD600 and CFU was established.
The monolayers of VK2/E6E7 were seeded at 1 × 105 cells/ml in 6-well tissue culture plates and incubated at 37°C in 5% CO2. Overnight cultures of L. crispatus were used. The effect of L. crispatus on the adhesion of C. albicans to VK2/E6E7 was assessed using a L. crispatus/VK2/E6E7 ratio of 100:1.
Interference experiments with C. albicans were performed using procedures described by Osset et al.[16] with some modifications. For the exclusion tests, 1 × 107 lactobacilli and vaginal epithelial cells were co-cultured for 1 h at 37°C under microaerophilic conditions. Then, 1 × 107 C. albicans cells were added, and incubation was continued for 1 h. In the competition tests, the mixture of 1 × 107 lactobacilli and 1 × 107 C. albicans was added into VK2/E6E7 cell monolayers and then incubated for 1 h at 37°C. During the displacement tests, 1 × 107 C. albicans and epithelial cells were incubated together for 1 h at 37°C. Then, incubation was continued for 1 h after the addition of 1 × 107 lactobacilli. The adhesion to VK2/E6E7 cells was evaluated using microscopy (×100) after Gram's stain by counting the number of bacteria and C. albicans attached to 30 consecutive cells. The results of the three experiments described above showed the average number of microorganisms per VK2/E6E7 cell compared with adhesion without lactobacilli (control value). The control values were taken as 100% of adhesion, and inhibition of C. albicans adherence was calculated by subtracting each adhesion percentage from its corresponding control value. The adhesion experiments were performed three times with at least three replicates per group.
Determination of interleukin-2, interleukin-4, interleukin-6, interleukin-8, and interleukin-17 using an enzyme-linked immunosorbent assay
To examine the production of cytokines and chemokines, VK2/E6E7 cells (1 × 105 cells/ml) were co-cultured with C. albicans (1 × 105/ml) at a ratio of 1:1 in separate wells for 12 h in a total volume of 2 ml of K-SFM complete medium in 6-well tissue culture plates (Costar, Corning, NY, USA). After co-cultured for 12 h, the culture medium was aspirated, washed three times with PBS, and replaced with 1 ml 1 × 107 lactobacilli for an additional 24 h. There were four groups in this study, which included group VK2/E6E7 with medium alone, group VK2/E6E7 with C. albicans, group VK2/E6E7 with L. crispatus, and group VK2/E6E7 with suspensions of L. crispatus after infection with C. albicans. The control groups included VK2/E6E7 with medium alone (negative control), VK2/E6E7 with C. albicans, and VK2/E6E7 with L. crispatus over the same time period. The epithelial cell–C. albicans coculture supernatants were then collected and centrifuged at 12,000 ×g for 5 min, transferred to a new tube, and stored at −80°C for enzyme-linked immunosorbent assay (ELISA) analysis. Supernatants were assayed for the levels of proinflammatory (interleukin-6, IL-6), Th1-immunoregulatory (IL-2), Th2-immunoregulatory (IL-4), chemotactic (IL-8), and Th17-immunoregulatory (IL-17) cytokines according to the ELISA manufacturer's instructions. Standard curves were generated in every set of experiments. The absorbance values and concentrations of each cytokine were determined by using a Ceres 900 automated microplate reader (Bio-Tek Corp., Wisnooski, VT, USA) and Kineticalc software (Bio-Tek). Experiments were performed independently for three times.
Scanning electron microscopy observations
VK2/E6E7 treated with C. albicans or L. crispatus were fixed in 2.5% glutaraldehyde for 12 h, and then washed with 0.1 mol/L phosphoric acid buffer (pH = 7.4) three times for 10 min each. The cells were then dehydrated in 70% ethanol for 2 h and kept dehydrated in an ascending series of ethanol (75%, 80%, 85%, 90%, 95%, and 100%, 6 min each).[17] The cells were left to air-dry for a few seconds and mounted on scanning electron microscopy (SEM) stubs with double-sided carbon tape. The dried samples were sputtered with gold and observed under a Hitachi S-3400N electron microscope at high-vacuum mode.
Statistical analysis
The data were expressed as the mean ± standard deviation (SD). Statistical analyses were performed using SPSS statistical package 17.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 6.0 software (GraphPad, San Diego, CA, USA). One-way analysis of variance was used to compare the results of different experimental groups. Post hoc group comparisons were conducted using LSD method. Differences were considered statistically significant at P < 0.05.
Results
Inhibition of Candida albicans’ adhesion to VK2/E6E7 by Lactobacillus crispatus
To investigate the influence of L. crispatus on the adhesion of C. albicans to VK2/E6E7, the cells were treated with L. crispatus and infected with C. albicans during assays of exclusion, competition, and displacement. The results demonstrated that L. crispatus caused significantly decreased adhesion of C. albicans to VK2/E6E7, by 52.87 ± 1.22%, 47.03 ± 1.35%, and 42.20 ± 1.55% in the competition, exclusion, and displacement experiments, respectively [Figure 1].
Figure 1.
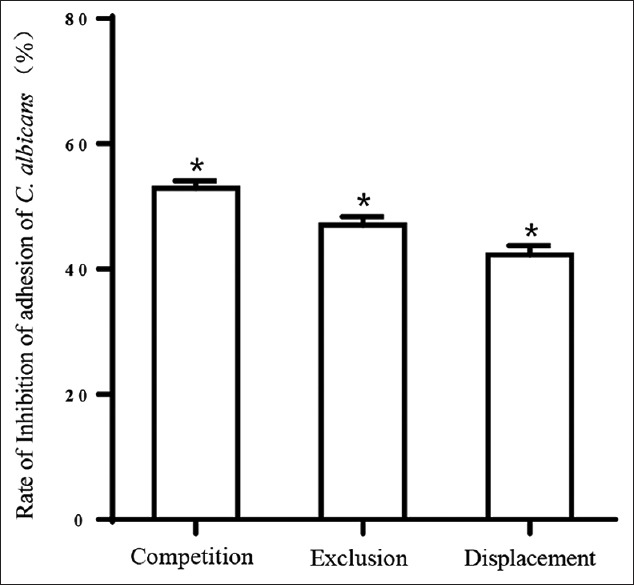
Inhibition of the adhesion of Candida albicans to vaginal epithelial cells. Treatment of 1 × 105 vaginal epithelial cells with 1 × 107 Lactobacillus crispatus or 1 × 107 Candida albicans was assessed using microscopy (×100) after Gram's staining, by counting the number of microorganisms attached to 30 consecutive cells. The results of the three conditions (i.e., exclusion, competition, and displacement) were expressed as the average number of Candida albicans per VK2/E6E7 cell and compared with adhesion without Lactobacilli (control value). The control values were taken as 100% of adhesion, and the inhibition of Candida albicans’ adherence was calculated by subtracting each adhesion percentage from its corresponding control value. The data are expressed as the mean percentage of adherence ± standard deviation from three independent experiments. *P < 0.01, between Candida albicans grown in the presence of Lactobacillus crispatus versus Candida albicans alone.
Scanning electron microscopy assessment
The SEM study further verified the interaction between VK2/E6E7, C. albicans, and L. crispatus. Typical SEM images of untreated VK2/E6E7 are shown in Figure 2a. As shown in Figure 2b, when VK2/E6E7 cells were treated with C. albicans for 6 h, no significant morphological changes to the cells were observed. The immune responses of the epithelial cells could prevent the invasion of C. albicans. However, we observed significant morphological changes of cells after 12 h of stimulation with C. albicans [Figure 2c], with the cell walls being penetrated by the hypha and the induction of cellular endocytosis by the C. albicans hypha. When VK2/E6E7 cells were treated with L. crispatus for 24 h after incubation with C. albicans for 12h, several probiotic bacteria were observed to be oriented toward VK2/E6E7 and appeared to protect the cells from the virulence of hypha and spores of C. albicans [Figure 2d]. L. crispatus inhibited the adhesion, hyphal formation, and proliferation of C. albicans, as well as maintaining the morphology and viability of vaginal epithelial cells notably.
Figure 2.
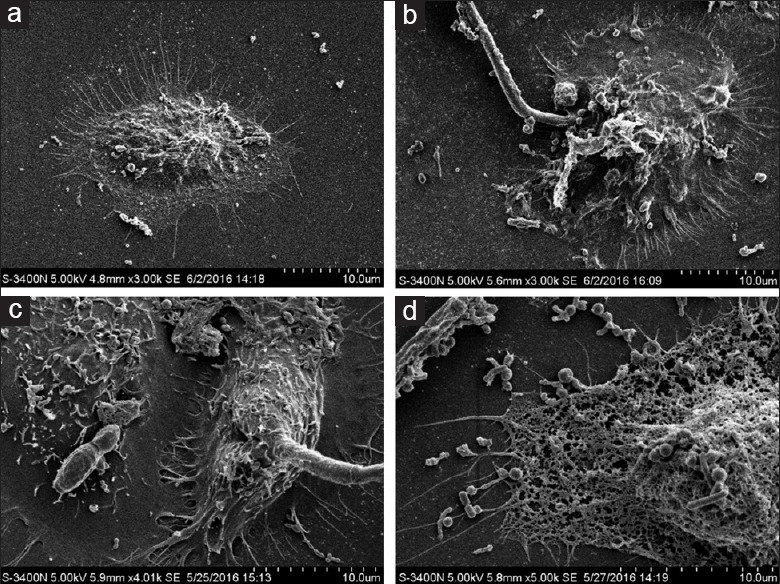
Scanning electron microscope images of vaginal epithelial cells treated with Candida albicans or Lactobacillus crispatus. (a) Untreated VK2/E6E7 cells; (b) VK2/E6E7 cells treated with Candida albicans for 6 h; (c) VK2/E6E7 cells treated with Candida albicans for 12 h; (d) VK2/E6E7 cell treated with Lactobacillus crispatus for 24 h after incubation with Candida albicans for 12 h; magnification ×10,000.
Lactobacillus crispatus modulates cytokines’ secretion by human vaginal epithelial cell
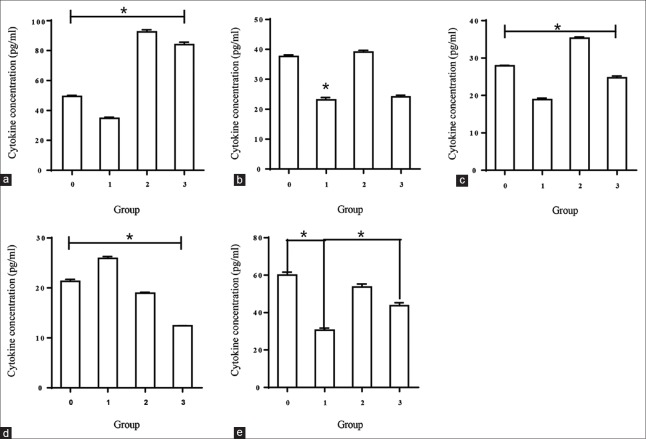
To determine whether L. crispatus could influence the immune response of VK2/E6E7 to C. albicans, a set of experiments were performed using four groups of cells, which were defined as group VK2/E6E7 with medium alone, group VK2/E6E7 with C. albicans, group VK2/E6E7 with L. crispatus, and group VK2/E6E7 with suspensions of L. crispatus after infection with C. albicans. A negative control was conducted by culturing C. albicans alone for cytokine production. The supernatants of the cultures were then collected and examined for pro-inflammatory (IL-6), Th1-immunoregulatory (IL-2), Th2-immunoregulatory (IL-4), chemotactic (IL-8) cytokines, and Th17 responses (IL-17).
The negative control (C. albicans alone) showed no significant presence of cytokines or chemokines. C. albicans-specific production of cytokines by epithelial cells is illustrated in Table 1 and Figure 3. ELISAs showed that the concentration of C. albicans-specific cytokines was greater in the supernatant induced by L. crispatus than that detected with C. albicans infection alone.
Table 1.
Constitutive and Candida-specific cytokine and chemokine production by vaginal epithelial cells
| Cytokines | Stimulus | Cytokine concentration (pg/ml, mean ± SD) |
|---|---|---|
| IL-2 | Medium alone | 49.48 ± 0.99 |
| Medium + Candida | 34.81 ± 1.22 | |
| Medium + L. crispatus | 92.50 ± 2.33 | |
| Medium + Candida + L. crispatus | 84.05 ± 2.75 | |
| IL-4 | Medium alone | 37.65 ± 0.85 |
| Medium + Candida | 23.12 ± 0.76 | |
| Medium + L. crispatus | 39.07 ± 0.98 | |
| Medium + Candida + L. crispatus | 24.10 ± 0.97 | |
| IL-6 | Medium alone | 27.97 ± 0.15 |
| Medium + Candida | 18.91 ± 0.65 | |
| Medium + L. crispatus | 35.33 ± 0.55 | |
| Medium + Candida + L. crispatus | 24.72 ± 0.83 | |
| IL-8 | Medium alone | 21.30 ± 0.72 |
| Medium + Candida | 25.93 ± 0.68 | |
| Medium + L. crispatus | 18.97 ± 0.23 | |
| Medium + Candida + L. crispatus | 12.41 ± 0.58 | |
| IL-17 | Medium alone | 60.03 ± 2.71 |
| Medium + Candida | 30.65 ± 1.87 | |
| Medium + L. crispatus | 53.72 ± 2.72 | |
| Medium + Candida + L. crispatus | 43.72 ± 2.72 |
L. crispatus: Lactobacillus crispatus; IL: Interleukin; SD: Standard deviation.
Figure 3.
The secretion of IL-2, IL-4, IL-6, IL-8, and IL-17 from VK2/E6E7 cells, as assessed by enzyme-linked immunosorbent assay. Groups were defined as 0 for VK2/E6E7 treated with medium alone, 1 for VK2/E6E7 treated with Candida albicans, 2 for VK2/E6E7 treated with Lactobacillus crispatus, and 3 for VK2/E6E7 treated with a suspension of Lactobacillus crispatus for 24 h after infection with Candida albicans. Data are presented as the mean ± SD. *P < 0.001. (a) Concentration of IL-2, (b) concentration of IL-4, (c) concentration of IL-6, (d) concentration of IL-8, (e) and concentration of IL-17. IL: Interleukin.
Table 1 shows that the IL-2 concentrations produced by VK2/E6E7 cultured with L. crispatus only were significantly higher than that in group VK2/E6E7 with medium alone (92.50 ± 2.33 vs. 49.48 ± 0.99 pg/ml, P < 0.001). When VK2/E6E7 cells were stimulated with C. albicans, IL-2 was released at an obviously lower level compared with that produced by group VK2/E6E7 with suspensions of L. crispatus (34.81 ± 1.22 vs. 84.05 ± 2.75 pg/ml, P < 0.001).
VK2/E6E7 produced lower concentrations of IL-4 in response to C. albicans compared with medium only (23.12 ± 0.76 vs. 37.65 ± 0.85 pg/ml, P < 0.001). When treated with L. crispatus after infection with C. albicans, the IL-4 concentrations showed no statistically significant differences compared with group VK2/E6E7 with C. albicans (24.10 ± 0.97 vs. 23.12 ± 0.76 pg/ml, P = 0.221).
In addition, VK2/E6E7 treated with L. crispatus and infected with C. albicans showed an increased release of IL-6 compared with cells stimulated with C. albicans alone (24.72 ± 0.83 vs. 18.91 ± 0.65 pg/ml, P < 0.001), but to a lesser degree than that induced by L. crispatus alone (35.33 ± 0.55 vs. 18.91 ± 0.65 pg/ml, P < 0.001). The concentrations of IL-6 decreased obviously in group VK2/E6E7 with C. albicans compared with that in group VK2/E6E7 with medium alone (18.91 ± 0.65 vs. 27.97 ± 0.15 pg/ml, P < 0.001).
In contrast, the concentrations of IL-8 increased dramatically in group VK2/E6E7 with C. albicans compared with that in group VK2/E6E7 with medium alone (25.93 ± 0.68 vs. 21.30 ± 0.72 pg/ml, P < 0.001) and group VK2/E6E7 with L. crispatus showed a marked reduction in the IL-8 level compared with group VK2/E6E7 with C. albicans alone (12.41 ± 0.06 vs. 25.93 ± 0.68 pg/ml, P < 0.001).
VK2/E6E7 with medium alone showed an approximately 2-fold increase in the concentration of IL-17 compared with group VK2/E6E7 with C. albicans alone (60.03 ± 2.71 vs. 30.65 ± 1.87 pg/ml, P < 0.001). L. crispatus increased the secretion of IL-17 as demonstrated in group VK2/E6E7 with L. crispatus (53.72 ± 2.72 vs. 30.65 ± 1.87 pg/ml, P < 0.001) and group VK2/E6E7 with suspensions of L. crispatus (43.72 ± 2.72 vs. 30.65 ± 1.87 pg/ml, P < 0.001).
Discussion
This study explored the regulatory role of L. crispatus on the interaction of C. albicans and vaginal epithelial cells. C. albicans is a commensal pathogen of the vaginal microbiota that can cause VVC in women if the balance of the normal vaginal flora is disrupted or the immune defense is compromised.[3] The pathogenicity of C. albicans depends on several properties, including invading tissues, evading the host immune system, and facilitating the infection.[18]
As the first barrier of the vagina, the mucosa prevents infections by different kinds of pathogens. Many investigations have demonstrated that the epithelial cells play vital roles in local immune responses to commensal and pathogenic microbes, including antigen presentation, antimicrobial activity, and cytokine and chemokine production.[19,20,21] In our study, we investigated the reaction of VK2/E6E7 epithelial cells when infected by C. albicans and treated with L. crispatus to identify the potential function of the mucosa in local immune responses against the pathogen in vitro.
To the best of our knowledge, lactobacilli are the predominant bacteria in a healthy vagina, which maintains the homeostasis between the microbiota and the vaginal microenvironment.[22] To investigate whether L. crispatus could influence immune responses of vaginal epithelial cells to C. albicans, experiments were performed after preinfection with C. albicans, followed by incubation with L. crispatus or treatment with C. albicans only.
The influence of L. crispatus on the adhesion of C. albicans to VK2/E6E7 and on the regulation of cytokine and chemokine release was examined. The results of interference experiments demonstrated that L. crispatus inhibited C. albicans growth and adherence to VK2/E6E7 cells. In the competition experiment, the rate of inhibition of adhesion by C. albicans to Vk2/E6E7 was the highest. Adhesion to the mucosa is the first step of infection. Therefore, L. crispatus could play a determinant role in the treatment of C. albicans infection. Various studies have demonstrated the inhibition of C. albicans growth by the Lactobacillus in vivo and in vitro,[23,24] and several mechanisms for Lactobacillus inhibition of C. albicans invasion have been proposed, including competing with pathogenic microorganisms for adhesion to epithelial cells, inhibiting the growth of other bacteria through antibacterial products, and modulating the mucosal immune response.
To determine the protective role played by L. crispatus further, SEM was performed. With increasing time, C. albicans spores were transformed to the hyphal form, which then perturbed the VK2/E6E7 cell wall. Hyphal formation and subsequent invasion are the predominant virulence properties of C. albicans infection.[25] The images showed that the epithelial cells responded to C. albicans when VK2/E6E7 cells were treated with this fungal pathogen for 6 h. We could infer that the innate immune response of VK2/E6E7 played an important role during the first stage of infection and commensal colonization. However, the cell line was invaded strongly when VK2/E6E7 cells were treated with C. albicans for 12 h. Our results suggested that C. albicans could invade VK2/E6E7 through two distinct mechanisms, resulting in cellular damage. The first is the induction of cellular endocytosis by C. albicans hypha and the other is through active penetration without endocytosis. The SEM images showed that L. crispatus significantly enhanced the capacity of VK2/E6E7 to resist virulent infection by C. albicans to maintain the viability of the vaginal epithelial cells.
Activation of the innate immune response by C. albicans induces the production of various cytokines and chemokines by VK2/E6E7 cells. It is generally accepted that an effective Th1 response is crucial for defense against C. albicans infections.[26] In contrast, a Th2-type response is considered nonprotective.[27] The cytokines and chemokines examined in our study were representative of members of pro-inflammatory, regulatory T-helper, and chemotactic cytokine classes.
Our study demonstrated a decrease in the IL-2 and IL-4 secretion after the interaction between C. albicans and vaginal epithelial cells. Infection with C. albicans followed by L. crispatus treatment caused a dramatic increase in IL-2 and a slight decrease in IL-4. Therefore, these results suggested that L. crispatus could promote the host defense and protect the epithelium from C. albicans-induced cell injury. Previous investigations demonstrated that the mucosa is involved in innate immunity against fungal infections by producing various cytokines such as IL-6, IL-8, and IL-17.[28,29] IL-6 is a pro-inflammatory cytokine, and we observed an increase in IL-6 production against C. albicans following treatment with L. crispatus, which suggested that L. crispatus promotes the host's defense against C. albicans. Chemokine production is critical at the sites of pathogenic invasion. Our results agreed with the previous observations of elevated IL-8 production during infection.[13] Incubation of VK2/E6E7 with L. crispatus resulted in decreased immune activation by C. albicans in terms of IL-8 production. L. crispatus diminished the activity of C. albicans, thereby indicating its potential antifungal properties. The investigation of anti-C. albicans activity demonstrated that the production of IL-17 was impaired in response to C. albicans infection, suggesting a local immune response to the infection in vaginal epithelial cells. L. crispatus induced the secretion of IL-17 and significantly reduced C. albicans viability and growth, which suggested that these probiotic bacteria could control C. albicans’ virulence. Extensive evidence has confirmed that when encountered with C. albicans, epithelial cells induce an inflammatory cascade leading to tissue irritation.[30]
In summary, we investigated comprehensively the local immune responses of vaginal epithelial cells induced by L. crispatus against C. albicans infection in vitro. Moreover, our results suggested that vaginal epithelial cells are important in the innate host defense against C. albicans infection. Our data showed that L. crispatus modulated the host pro-inflammatory responses against C. albicans in different ways. L. crispatus could enhance the immune function of a vaginal epithelial cell line and attenuate the virulence of C. albicans. Thus, the vaginal mucosa has the potential to function in local immune responses against the fungal pathogen, which can be promoted by L. crispatus.
In the case of localized C. albicans infections, however, further experiments are needed to decipher their contributions. In addition, we should identify additional cytokines secreted by the vaginal mucosa infection by C. albicans. The signaling pathway regulation mechanisms, including whether L. crispatus could induce the production of AMPs, for example β-defensins, and the roles of pattern recognition receptors and their downstream signal pathway in immune responses in vaginal epithelial cells induced by L. crispatus, should be investigated. Studies on the host-pathogen interaction, in which L. crispatus acts as an anti-inflammatory molecule, will also be conducted to reveal the detailed mechanism of the local immune response to mucosal fungal infections.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
References
- 1.Sobel JD. Pathogenesis of Candida vulvovaginitis. Curr Top Med Mycol. 1989;3:86–108. doi: 10.1007/978-1-4612-3624-5_5. doi: 10.1007/978-1-4612-3624-5_5. [DOI] [PubMed] [Google Scholar]
- 2.Rizzo A, Domenico MD, Carratelli CR, Paolillo R. The role of chlamydia and Chlamydophila infections in reactive arthritis. Intern Med. 2012;51:113–7. doi: 10.2169/internalmedicine.51.6228. doi: 10.1016/j.imlet.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961–71. doi: 10.1016/S0140-6736(07)60917-9. doi: 10.1016/s0140-6736(07)60917-9. [DOI] [PubMed] [Google Scholar]
- 4.Weindl G, Wagener J, Schaller M. Epithelial cells and innate antifungal defense. J Dent Res. 2010;89:666–75. doi: 10.1177/0022034510368784. doi: 10.1177/0022034510368784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semlali A, Leung KP, Curt S, Rouabhia M. Antimicrobial decapeptide KSL-W attenuates Candida albicans virulence by modulating its effects on toll-like receptor, human ß-defensin, and cytokine expression by engineered human oral mucosa. Peptides. 2011;32:859–67. doi: 10.1016/j.peptides.2011.01.020. doi: 10.1016/j.peptides.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Netea MG, Joosten LA, van der Meer JW, Kullberg BJ, van de Veerdonk FL. Immune defence against Candida fungal infections. Nat Rev Immunol. 2015;15:630–42. doi: 10.1038/nri3897. doi: 10.1038/nri3897. [DOI] [PubMed] [Google Scholar]
- 7.Saunus JM, Kazoullis A, Farah CS. Cellular and molecular mechanisms of resistance to oral Candida albicans infections. Front Biosci. 2008;13:5345–58. doi: 10.2741/3085. doi: 10.2741/3085. [DOI] [PubMed] [Google Scholar]
- 8.Décanis N, Savignac K, Rouabhia M. Farnesol promotes epithelial cell defense against Candida albicans through Toll-like receptor 2 expression, interleukin-6 and human beta-defensin 2 production. Cytokine. 2009;45:132–40. doi: 10.1016/j.cyto.2008.11.011. doi: 10.1016/j.cyto.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Rouabhia M, Ross G, Pagé N, Chakir J. Interleukin-18 and gamma interferon production by oral epithelial cells in response to exposure to Candida albicans or lipopolysaccharide stimulation. Infect Immun. 2002;70:7073–80. doi: 10.1128/IAI.70.12.7073-7080.2002. doi: 10.1128/IAI.70.12.7073-7080.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mostefaoui Y, Claveau I, Ross G, Rouabhia M. Tissue structure, and IL-1beta, IL-8, and TNF-alpha secretions after contact by engineered human oral mucosa with dentifrices. J Clin Periodontol. 2002;29:1035–41. doi: 10.1034/j.1600-051x.2002.291109.x. doi: 10.1034/j.1600-051X.2002.291109.x. [DOI] [PubMed] [Google Scholar]
- 11.van de Wijgert JH, Borgdorff H, Verhelst R, Crucitti T, Francis S, Verstraelen H, et al. The vaginal microbiota: What have we learned after a decade of molecular characterization? PLoS One. 2014;9:e105998. doi: 10.1371/journal.pone.0105998. doi: 10.1371/journal.pone.0105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnarumma G, Molinaro A, Cimini D, De Castro C, Valli V, De Gregorio V, et al. Lactobacillus crispatus L1: High cell density cultivation and exopolysaccharide structure characterization to highlight potentially beneficial effects against vaginal pathogens. BMC Microbiol. 2014;14:137. doi: 10.1186/1471-2180-14-137. doi: 10.1186/1471-2180-14-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizzo A, Losacco A, Carratelli CR. Lactobacillus crispatus modulates epithelial cell defense against Candida albicans through Toll-like receptors 2 and 4, interleukin 8 and human ß-defensins 2 and 3. Immunol Lett. 2013;156:102–9. doi: 10.1016/j.imlet.2013.08.013. doi: 10.1016/j.imlet.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Ma B, Forney LJ, Ravel J. Vaginal microbiome: Rethinking health and disease. Annu Rev Microbiol. 2012;66:371–89. doi: 10.1146/annurev-micro-092611-150157. doi: 10.1146/annurev-micro-092611-150157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marco ML, Kleerebezem M. Assessment of real-time RT-PCR for quantification of Lactobacillus plantarum gene expression during stationary phase and nutrient starvation. J Appl Microbiol. 2008;104:587–94. doi: 10.1111/j.1365-2672.2007.03578.x. doi: 10.1111/j.1365-2672.2007.03578.x. [DOI] [PubMed] [Google Scholar]
- 16.Osset J, Bartolomé RM, García E, Andreu A. Assessment of the capacity of Lactobacillus to inhibit the growth of uropathogens and block their adhesion to vaginal epithelial cells. J Infect Dis. 2001;183:485–91. doi: 10.1086/318070. doi: 10.1086/318070. [DOI] [PubMed] [Google Scholar]
- 17.Wu S, Gao Y, Zhang Y, Wang E, Xu X, Lei Z. An entomopathogenic strain of Beauveria bassiana against Frankliniella occidentalis with no detrimental effect on the predatory mite Neoseiulus barkeri: Evidence from laboratory bioassay and scanning electron microscopic observation. PLoS One. 2014;9:e84732. doi: 10.1371/journal.pone.0084732. doi: 10.1371/journal.pone.0084732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harriott MM, Noverr MC. Importance of Candida-bacterial polymicrobial biofilms in disease. Trends Microbiol. 2011;19:557–63. doi: 10.1016/j.tim.2011.07.004. doi: 10.1016/j.tim.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naglik JR, Richardson JP, Moyes DL. Candida albicans pathogenicity and epithelial immunity. PLoS Pathog. 2014;10:e1004257. doi: 10.1371/journal.ppat.1004257. doi: 10.1371/journal.ppat.1004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moyes DL, Naglik JR. Mucosal immunity and Candida albicans infection. Clin Dev Immunol 2011. 2011 doi: 10.1155/2011/346307. 346307. doi: 10.1155/2011/346307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szabo EK, Maccallum DM. A novel renal epithelial cell in vitro assay to assess Candida albicans virulence. Virulence. 2014;5:286–96. doi: 10.4161/viru.27046. doi: 10.4161/viru.27046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vásquez A, Jakobsson T, Ahrné S, Forsum U, Molin G. Vaginal lactobacillus flora of healthy Swedish women. J Clin Microbiol. 2002;40:2746–9. doi: 10.1128/JCM.40.8.2746-2749.2002. doi: 10.1128/JCM.40.8.2746-2749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chassot F, Camacho DP, Patussi EV, Donatti L, Svidzinski TI, Consolaro ME. Can Lactobacillus acidophilus influence the adhesion capacity of Candida albicans on the combined contraceptive vaginal ring? Contraception. 2010;81:331–5. doi: 10.1016/j.contraception.2009.12.011. doi: 10.1016/j.contraception.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Martín R, Sánchez B, Suárez JE, Urdaci MC. Characterization of the adherence properties of human Lactobacilli strains to be used as vaginal probiotics. FEMS Microbiol Lett. 2012;328:166–73. doi: 10.1111/j.1574-6968.2011.02495.x. doi: 10.1111/j.1574-6968.2011.02495.x. [DOI] [PubMed] [Google Scholar]
- 25.Tang SX, Moyes DL, Richardson JP, Blagojevic M, Naglik JR. Epithelial discrimination of commensal and pathogenic Candida albicans. Oral Dis. 2016;22(Suppl 1):114–9. doi: 10.1111/odi.12395. doi: 10.1111/odi.12395. [DOI] [PubMed] [Google Scholar]
- 26.Schaller M, Boeld U, Oberbauer S, Hamm G, Hube B, Korting HC. Polymorphonuclear leukocytes (PMNs) induce protective Th1-type cytokine epithelial responses in an in vitro model of oral candidosis. Microbiology. 2004;150(Pt 9):2807–13. doi: 10.1099/mic.0.27169-0. doi: 10.1099/mic.0.27169-0. [DOI] [PubMed] [Google Scholar]
- 27.Clemons KV, Stevens DA. Overview of host defense mechanisms in systemic mycoses and the basis for immunotherapy. Semin Respir Infect. 2001;16:60–6. doi: 10.1053/srin.2001.22729. doi: 10.1053/srin.2001.22729. [DOI] [PubMed] [Google Scholar]
- 28.Rizzetto L, Kuka M, De Filippo C, Cambi A, Netea MG, Beltrame L, et al. Differential IL-17 production and mannan recognition contribute to fungal pathogenicity and commensalism. J Immunol. 2010;184:4258–68. doi: 10.4049/jimmunol.0902972. doi: 10.4049/jimmunol.0902972. [DOI] [PubMed] [Google Scholar]
- 29.Steele C, Fidel PL., Jr Cytokine and chemokine production by human oral and vaginal epithelial cells in response to Candida albicans. Infect Immun. 2002;70:577–83. doi: 10.1128/IAI.70.2.577-583.2002. doi: 10.1128/iai.70.2.577-583.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rast TJ, Kullas AL, Southern PJ, Davis DA. Human epithelial cells discriminate between commensal and pathogenic interactions with Candida albicans. PLoS One. 2016;11:e0153165. doi: 10.1371/journal.pone.0153165. doi: 10.1371/journal.pone.0153165. [DOI] [PMC free article] [PubMed] [Google Scholar]