Abstract
Background:
It is well documented that sevoflurane postconditioning (SP) has a significant myocardial protection effect. However, the mechanisms underlying SP are still unclear. In the present study, we investigated the hypothesis that the Pim-1 kinase played a key role in SP-induced cardioprotection by regulating dynamics-related protein 1 (Drp1).
Methods:
A Langendorff model was used in this study. Seventy-two rats were randomly assigned into six groups as follows: CON group, ischemia reperfusion (I/R) group, SP group, SP+proto-oncogene serine/threonine-protein kinase 1 (Pim-1) inhibitor II group, SP+dimethylsufoxide group, and Pim-1 inhibitor II group (n = 12, each). Hemodynamic parameters and infarct size were measured to reflect the extent of myocardial I/R injury. The expressions of Pim-1, B-cell leukemia/lymphoma 2 (Bcl-2) and cytochrome C (Cyt C) in cytoplasm and mitochondria, the Drp1 in mitochondria, and the total Drp1 and p-Drp1ser637 were measured by Western blotting. In addition, transmission electron microscope was used to observe mitochondrial morphology. The experiment began in October 2014 and continued until July 2016.
Results:
SP improved myocardial I/R injury-induced hemodynamic parametric changes, cardiac function, and preserved mitochondrial phenotype and decreased myocardial infarct size (24.49 ± 1.72% in Sev group compared with 41.98 ± 4.37% in I/R group; P < 0.05). However, Pim-1 inhibitor II significantly (P < 0.05) abolished the protective effect of SP. Western blotting analysis demonstrated that, compared with I/R group, the expression of Pim-1 and Bcl-2 in cytoplasm and mitochondria as well as the total p-Drp1ser637 in Sev group (P < 0.05) were upregulated. Meanwhile, SP inhibited Drp1 compartmentalization to the mitochondria followed by a reduction in the release of Cyt C. Pretreatment with Pim-1 inhibitor II significantly (P < 0.05) abolished SP-induced Pim-1/p-Drp1ser637 signaling activation.
Conclusions:
These findings suggested that SP could attenuate myocardial ischemia-reperfusion injury by increasing the expression of the Pim-1 kinase. Upregulation of Pim-1 might phosphorylate Drp1 and prevent extensive mitochondrial fission through Drp1 cytosolic sequestration.
Keywords: Cardioprotection, Ischemia-reperfusion Injury, Mitochondria, Pim-1, Postconditioning, Sevoflurane
Introduction
Myocardial ischemia and reperfusion injury (MIRI) is a kind of phenomenon that the myocardial structural injury is aggravated after the recovery of blood flow which is associated with calcium overload, production of a large number of reactive oxygen species (ROS),[1,2] the opening of mitochondrial membrane permeability transition pore (MPTP),[3] and cell apoptosis.[4] Numerous evidences have indicated that the I/R injury is implicated in coronary heart disease, myocardial infarction, cardiac arrest, and other cardiovascular diseases; therefore, it is of great significance to clarify the mechanisms of this process.
Mitochondria are highly dynamic organelles which are in a fine balance of continuous fusion and fission.[5] Recently, a multitude of studies have documented that mitochondrial dynamics plays a crucial role in MIRI. Indeed, mitochondrial morphological changes represent as from fusion to fission following exposure to I/R injury, while inhibiting the extensive mitochondrial fission (or fragmentation) contributes to alleviating this damage.[6,7,8] Dynamics-related protein 1 (Drp1) is a major protein that promotes the division in cells. It is expressed in the cytoplasm of most tissues, especially the brain and heart.[9] Mitochondrial fission occurs when Drp1 transfers from the cytoplasm to the outer mitochondrial membrane. Ong et al.[10] first discovered that silencing of Drp1 in HL-1 cells could reduce cell apoptosis, and pretreatment with Mdivi-1 (the Drp1 inhibitor) would decrease the myocardial infarct size under hypoxic condition.
Pim-1 kinase is a downstream signal molecule of Jun N-terminal kinase/signal transducers and activators of transcription (JNK/STAT) and protein kinase B (Akt) signaling pathway, which is relevant to cell survival, proliferation, differentiation, and apoptosis.[11] The latest research showed that Pim-1 kinase could increase phosphorylation of Drp1ser637 and inhibit Drp1 translating to the mitochondria so as to reduce the I/R injury through Drp1 cytoplasmic sequestration.[12]
A multitude of experimental and clinical studies have reported that sevoflurane can reduce myocardium injury when administrated following the I/R, which is well known as sevoflurane postconditioning (SP).[13,14] However, the specific mechanisms underlying SP remain unknown and further studies are warranted.
Here, we hypothesized that the Pim-1 kinase-regulating Drp1 played an important role in myocardial protection afforded by SP.
Methods
Animals
Male, healthy Sprague-Dawley rats (10–12 weeks old) weighing 250–300 g were provided by the PengYue animal feed distribution centers of Jinan city, Shandong Province (No. SCXK [Lu] 2014-0007, Shandong, China) and housed on a 12 h light/dark cycle with free access to food and water. The environment temperature and humidity were maintained between 22°C–24°C and 40–60%. All animal care and experimental protocols complied with the guidelines of the Animal Care and Use Committee of Xuzhou Medical University.
Isolated, perfused heart preparation
Sprague-Dawley rats were anesthetized with 3% sodium pentobarbital (30 mg/kg) and anticoagulated with heparin (500 IU/kg) through intraperitoneal injection. The hearts were rapidly removed by bilateral thoracotomy, placed in ice-cold buffer, and the aorta was cannulated with a 50-ml syringe needle. The isolated hearts were retrogradely perfused through the aorta at a constant perfusion pressure of 100 cmH2O (1 cmH2O = 0.098 kPa) with Krebs-Henseleit (K-H) buffer containing 118.5 mmol/L NaCl, 24.8 mmol/L NaHCO3, 11 mmol/L D-glucose, 4.7 mmol/L KCl, 1.2 mmol/L MgSO4·7H2O, 1.2 mmol/L KH2PO4, and 2.25 mmol/L CaCl2·2H2O, with pH of 7.4. Before it can be used, the perfusate buffer was saturated with a 95% O2 and 5% CO2 mixed gas at 37°C.[15] A small opening in the left atrial appendage was cut to make a latex balloon which was connected to a pressure sensor (TaiMeng Technology, Chengdu, China) to the computer into the left heart chamber through the left atrium followed by mitral valve. The balloon was inflated with water to adjust the left ventricular end-diastolic pressure (LVEDP) to 4–10 mmHg (1 mmHg = 0.133 kPa) at the beginning of the experiment and was continuously kept during the study. Cardiac functional indexes including LVEDP, left ventricular developed pressure (LVDP), positive and negative LVDP/dt (+dp/dtmax, −dp/dtmax), and heart rate (HR) were monitored with a computer-based system at the end of equilibration (T0), 30 min (T1), 60 min (T2), 90 min (T3), and 120 min (T4) of reperfusion, respectively.
Experimental protocol of ischemia/reperfusion injury
The whole procedure lasted for 180 min. All animals (except for the rats in the Control group [Group CON]) were subjected to 30 min of ischemia followed by 120 min of reperfusion.[16] Rats were randomly divided into six groups as follows (n = 72, 12 per group): (1) Group CON, (2) ischemia reperfusion group (Group I/R), (3) SP group (Group Sev), (4) SP+Pim-1 inhibitor II group (Group Sev+PIM-Inh), (5) SP+dimethylsufoxide group (Group Sev+DMSO), and (6) Pim-1 inhibitor II group (Group PIM-Inh). The hearts were continuously perfused for 180 min in Group CON. After 30 min of equilibration followed by 30 min of ischemia, the isolated hearts were subjected to perfused with K-H buffer for 120 min in Group I/R; Groups Sev, Sev+PIM-Inh, Sev+DMSO, and PIM-Inh received 15 min of perfusion with K-H solution containing 3% sevoflurane, 3% sevoflurane + 1 μmol/L Pim-1 inhibitor II (provided by Millipore, USA), 3% sevoflurane + 0.02% DMSO, and 1 μmol/L Pim-1 inhibitor II, respectively, at the beginning of reperfusion and then received K-H buffer continuously for 105 min.
Measurements of hemodynamics
Hemodynamic parameters were recorded at the end of equilibration (T0), 30 min (T1), 60 min (T2), 90 min (T3), and 120 min (T4) of reperfusion as functional indexes of the left ventricular. The measured hemodynamic parameters were HR, LVEDP, LVDP, maximum increase in the rate of LVDP (+dP/dt), and maximum decrease in the rate of LVDP (−dP/dt).
Western blotting analysis
Proteins were extracted from cardiomyocytes that were harvested and placed in lysis buffer to measure the total Drp1 and p-Drp1ser637 expressions, as described previously.[17] In other experiments, the expressions of Pim-1, Bcl-2, and cytochrome C (Cyt C) in cytoplasm and mitochondria, and the Drp1 in mitochondria were measured with Tissue Mitochondria Isolation Kit (Beyotime, USA). The purity of the isolated mitochondria was about 90%. The protein concentration was determined using the Bicinchoninic Acid (BCA) Protein Assay Kit according to the manufacturer's protocol (Beyotime, USA). The supernatant was mixed with 5X loading buffer and heated for 15 min at 100°C. The extracts were injected into each sample hole and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After electrophoresis, proteins were electrophoretically transferred to a polyvinylidene difluoride filter (PVDF) membrane (0.45 mm, Millipore, USA). The membrane was blocked in washing buffer with 5% nonfat milk for 2 h and incubated overnight with the corresponding primary antibodies at 4°C. The membrane was placed at room temperature for 0.5 h and then incubated with secondary antibody. The signals of the detected proteins were visualized by a BCIP/NBT Alkaline Phosphatase Chromogenic Color Kit (Beyotime, USA). The staining was quantified by scanning the films, and the band density was determined with Image-J software (National Institutes of Health, USA, http://imagej.nih.gov/ij/download/).
Myocardial infarct size
At the end of reperfusion period, hearts were frozen at −80°C for 30 min. The frozen hearts were cut transversely into 5 pieces, each 1 mm thick, and stained with 1% triphenyl tetrazolium chloride (TTC) for 30 min in a 37°C water bath.[18] Then, the slices were fixed in 4% formaldehyde solution for 15 min. At last, each slice was photographed by Epson Perfection V300 Photo Scanner (Epson, Japan). The viable myocardium presented brick red, and infarct tissues appeared pale white. Infarct size and left ventricular area were measured using Image-J software (National Institutes of Health, USA), with the infarct size expressed as a percentage of the total left ventricular area (×100%).
Transmission electron microscope
At the end of reperfusion, the anterior left ventricular tissues were cut into ultra-thin sections (1 mm × 1 mm × 1 mm). As previously described, they underwent through fixation, rinsing, dehydration, paraffin embedding, slicing, and double staining by uranyl acetate as well as lead citrate.[19] Then, mitochondrial morphology was observed by a Tecnai G2 T12 transmission electron microscope (TEM, FEI, USA).
Statistical analysis
Data were analyzed using SPSS software version 13.0 (BECKMAN, SanDiego, CA, USA). Data were expressed as mean ± standard deviation (SD). The groups were compared using repeated measures analysis of variance (ANOVA), and comparisons between groups were performed by one-way analysis of variance (ANOVA) followed by post hoc test (Newman-Keuls test). Differences with P < 0.05 were considered statistically significant.
Results
Sevoflurane postconditioning improves hemodynamic parameters
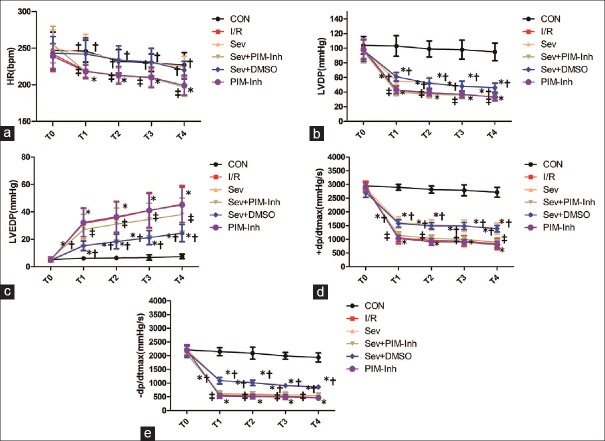
Figure 1 shows that there were no differences in baseline hemodynamics among the experimental groups (P > 0.05). At T1–T4, compared with CON group, HR, LVDP, and ±dp/dtmax decreased while LVEDP increased in I/R and Sev groups (P < 0.05); compared with I/R group, HR, LVDP, and ±dp/dtmax increased while LVEDP decreased in Sev group (P < 0.05), and no significant changes were found in each index in PIM-Inh group (P > 0.05); compared with Sev group, HR, LVDP, and ±dp/dtmax decreased while LVEDP increased in Sev+PIM-Inh group (P < 0.05), and no significant changes were found in each index in Sev+DMSO group (P > 0.05).
Figure 1.
Hemodynamic changes at different points in rats subjected to myocardial ischemia-reperfusion injury. (a) The changes of HR at T0–T4; (b) the changes of LVDP at T0–T4; (c) the changes of LVEDP at T0–T4; (d) the changes of + dp/dtmax at T0–T4; (e) and the changes of −dp/dtmax at T0–T4. Data were reported as mean ± SD (n = 6 for each group). *P < 0.05 versus CON; †P < 0.05 versus I/R; ‡P < 0.05 versus Sev (repeated measures ANOVA). CON: Control group; I/R: Ischemia/reperfusion; Sev: Sevoflurane postconditioning; Sev+PIM-Inh: Sevoflurane postconditioning + Pim-1 inhibitor II; Sev–DMSO: Sevoflurane postconditioning + dimethyl sulfoxide; PIM-Inh: Pim-1 inhibitor II; T0: The end of equilibration; T1: 30 min of reperfusion; T2: 60 min of reperfusion; T3: 90 min of reperfusion; T4: 120 min of reperfusion; HR: Heart rate; LVDP: Left ventricular developed pressure; LVEDP: Left ventricular end-diastolic pressure; +dp/dtmax: Maximum rate of left ventricular development pressure; −dp/dtmax: Maximum rate of left ventricular fall pressure; SD: Standard deviation; ANOVA: Analysis of variance.
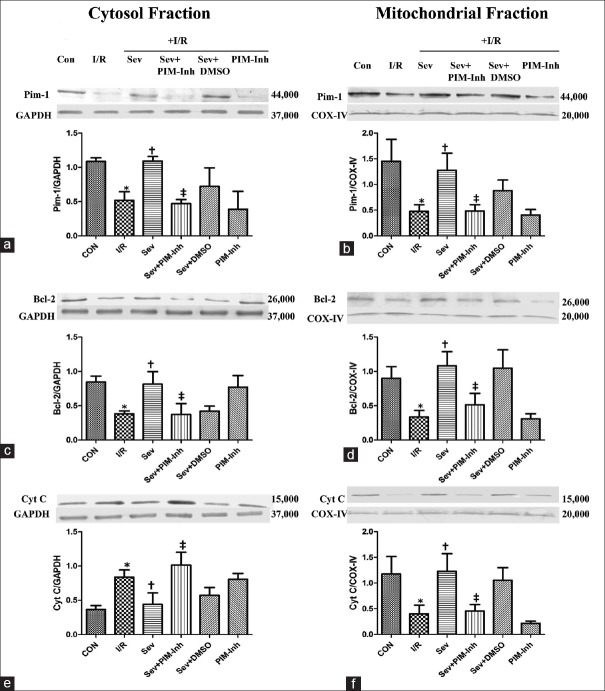
Sevoflurane postconditioning activates the Pim-1 kinase in the isolated rat hearts
Compared with CON group, the expression of the Pim-1 [Figure 2a and 2b] and Bcl-2 [Figure 2c and 2d] in cytoplasm and mitochondria was downregulated; the expression of Cyt C increased in cytoplasm [Figure 2e] but decreased in mitochondria [Figure 2f] in I/R group (P < 0.05); SP significantly increased the expression of the Pim-1 and Bcl-2 in cytoplasm and mitochondria, reducing the release of Cyt C from mitochondria to the cytoplasm compared with I/R group (P < 0.05); and pretreatment with 1 μmol/L Pim-1 inhibitor II abolished SP-induced increase of the Pim-1 and Bcl-2 in cytoplasm and mitochondria as well as reduction of Cyt C release compared with Sev group (P < 0.05), and there were no differences of each index in Sev+DMSO group (P > 0.05).
Figure 2.
Western blotting analysis of cytosolic and mitochondrial fraction of Pim-1, Bcl-2, and Cyt C in myocardium. The Pim-1 (44,000 Da) (a and b) was analyzed by Western blotting with specific Pim-1 antibody at 1 h of reperfusion; the Bcl-2 (26,000 Da) (c and d) was analyzed by Western blotting with specific Bcl-2 antibody at 1 h of reperfusion; and the Cyt C (15,000 Da) (e and f) was analyzed by Western blotting with specific Cyt C antibody at 1 h of reperfusion. (n = 3 for each group). Data were reported as mean ± SD. *P < 0.05 versus CON; †P < 0.05 versus I/R; ‡P < 0.05 versus Sev (repeated measures ANOVA). CON: Control group; I/R: Ischemia/reperfusion; Sev: Sevoflurane postconditioning; Sev+PIM-Inh: Sevoflurane postconditioning + Pim-1 inhibitor II; Sev+DMSO: Sevoflurane postconditioning + dimethyl sulfoxide; PIM-Inh: Pim-1 inhibitor II; Pim-1: Proto-oncogene serine/threonine-protein kinase 1; Bcl-2: B-cell leukemia/lymphoma 2; Cyt C: Cytochrome C; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; COX-IV: Cytochrome C oxidase IV; SD: Standard deviation; ANOVA: Analysis of variance.
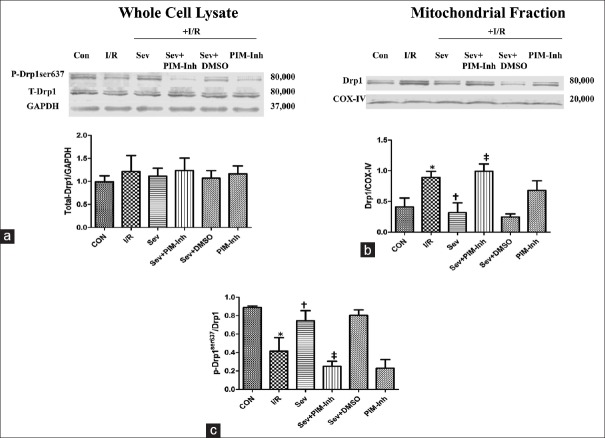
Pim-1 kinase upregulates p-Drp1ser637 and inhibits dynamics-related protein 1 translating to the mitochondria
To investigate the possible mechanism of Pim-1 involved in SP-induced cardioprotection, we further examined the expression of total Drp1 and p-Drp1ser637 in myocardium as well as Drp1 translation to the mitochondria. There were no obvious changes in total Drp1 expression in each group (P > 0.05) [Figure 3a]. Compared with CON group, the p-Drp1ser637 expression level was downregulated [Figure 3b] and the content of Drp1 in mitochondria increased [Figure 3c] in I/R group (P < 0.05); SP significantly increased the p-Drp1ser637 expression level and inhibited Drp1 translating to the mitochondria compared with I/R group (P < 0.05), and no significant changes were found in each index in PIM-Inh group (P > 0.05); pretreatment with 1 μmol/L Pim-1 inhibitor II abolished SP-induced increase of p-Drp1ser637 and inhibition of Drp1 compartmentalization to the mitochondria compared with Sev group (P < 0.05), and no significant differences were found in each index in Sev+DMSO group (P > 0.05).
Figure 3.
Western blotting analysis of total Drp1 and p-Drp1ser637 in myocardium as well as Drp1 translation to the mitochondria. The total Drp1 (80,000 Da) (a) and the Drp1 in mitochondria (c) were analyzed by Western blotting with specific Drp1 antibody at 1 h of reperfusion; The p-Drp1ser637 (80,000 Da) (b) was analyzed by Western blotting with specific p-Drp1ser637 antibody at 1 h of reperfusion (n = 3 for each group). Data were reported as mean ± SD. *P < 0.05 versus CON; †P < 0.05 versus I/R; ‡P < 0.05 versus Sev (one-way ANOVA). CON: Control group; I/R: Ischemia/reperfusion; Sev: Sevoflurane postconditioning; Sev+PIM-Inh: Sevoflurane postconditioning + Pim-1 inhibitor II; Sev+DMSO: Sevoflurane postconditioning + dimethyl sulfoxide; PIM-Inh: Pim-1 inhibitor II; Drp1: Dynamics-related protein 1; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; COX-IV: Cytochrome C oxidase IV; SD: Standard deviation; ANOVA: Analysis of variance.
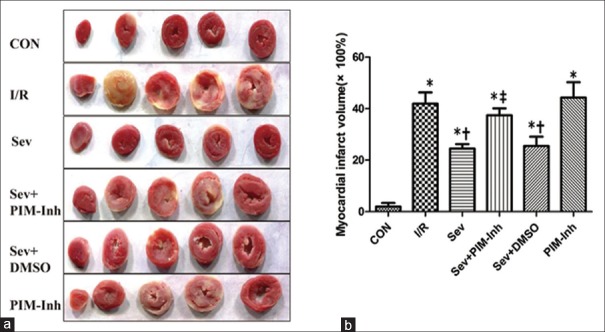
Sevoflurane postconditioning decreases myocardial infarct size
As shown in Figure 4, infarct sizes were significantly (P < 0.05) increased at the end of reperfusion. Consistent with the improved functional recovery, infarct size in the Sev group was significantly smaller (24% ± 2%) than that (42% ± 4%) in I/R group (P < 0.05). Pretreatment with 1 μmol/L Pim-1 inhibitor II significantly increased the infarct sizes (37% ± 3%) compared with Sev group (P < 0.05), and the Sev+DMSO group showed no difference (P > 0.05).
Figure 4.
Myocardial infarct size of isolated hearts in six groups. (a) Representative cross sections of rat hearts from six groups after I/R and staining with TTC to visualize the infarcted area; (b) infarct size expressed as percentage of the left ventricular area (×100%) for each group. Values are represented as mean ± SD (n = 6 for each group), *P < 0.05 versus CON; †P < 0.05 versus I/R; ‡P < 0.05 versus Sev (one-way ANOVA). CON: Control group; I/R: Ischemia/reperfusion; Sev: Sevoflurane postconditioning; Sev+PIM-Inh: Sevoflurane postconditioning + Pim-1 inhibitor II; Sev+DMSO: Sevoflurane postconditioning + dimethyl sulfoxide; PIM-Inh: Pim-1 inhibitor II; SD: Standard deviation; ANOVA: Analysis of variance.
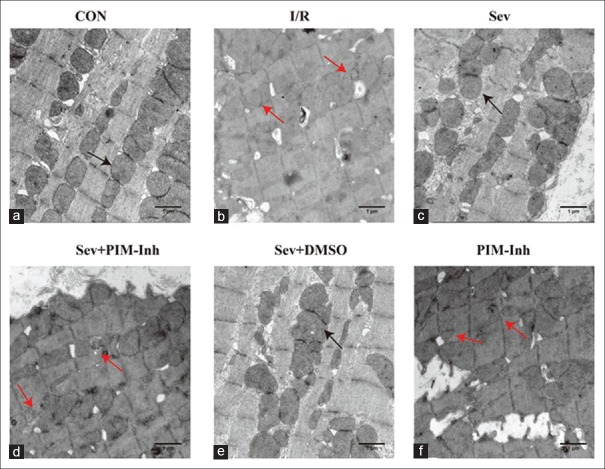
Sevoflurane postconditioning preserves mitochondrial morphological integrity
To further prove the reliability of the Pim-1 kinase regulating Drp1 involved in the preservation of mitochondrial integrity, we then observed mitochondrial morphology by TEM. As shown in Figure 5, mitochondria from I/R group appeared smaller, irregular, and had less dense cristae compared with CON group; SP greatly restored mitochondrial morphology and organization. Pim-1 inhibitor II significantly attenuated the effect of maintaining mitochondrial phenotype by SP [Figure 5a–5f].
Figure 5.
Transmission electron microscope (uranyl acetate-lead citrate stained, original magnification ×30,000) performance of the myocardial mitochondria in six groups. (a) In the CON group, the mitochondria were normal (b) in the I/R group, the mitochondria appeared small, irregular, and less dense cristae (c) in the Sev group, the severity of mitochondrial injury decreased compared with the I/R group (d) in the Sev+PIM-Inh group, the severity of mitochondrial injury increased compared with the Sev group (e) in the Sev+DMSO group, no significant changes were found compared with the Sev group (f) in the PIM-Inh group, no significant changes were found compared with the I/R group (n = 3 for each group, scale bar = 1 μm). The black arrow shows normal mitochondria, and the red arrow shows fragmented mitochondria. CON: Control group; I/R: Ischemia/reperfusion; Sev: Sevoflurane postconditioning; Sev+PIM-Inh: Sevoflurane postconditioning + Pim-1 inhibitor II; Sev+DMSO: Sevoflurane postconditioning + dimethyl sulfoxide; PIM-Inh: Pim-1 inhibitor II.
Discussion
The present study reveals a novel mechanism underlying sevoflurane-induced cardioprotection. In addition to decreasing the infarct size and improving cardiac functional recovery, SP also significantly upregulated the Pim-1 kinase and its subsequent increase of p-Drp1ser637 inhibited the activity of Drp1. Interestingly, phosphorylation of Drp1 was sequestered to the cytoplasm so as to preserve mitochondrial integrity in isolated rat hearts subjected to I/R injury. Pretreatment with Pim-1 inhibitor II significantly abolished SP-induced myocardial protection. Collectively, these results demonstrate that the Pim-1 kinase regulating Drp1 may mediate SP-induced cardioprotection.
Since 1990, sevoflurane has been the most widely used volatile anesthetic with the advantages of smooth induction, stable intraoperative hemodynamics, and rapid analepsia. Considerable evidences indicate that cardioprotective effects of SP are tightly in association with inhibition of the opening of the MPTP,[20] promoting the release of nitric oxide[21] and activating reperfusion injury salvage kinase,[22] but the particular mechanism has not been completely understood.
Mitochondria are essential to myocardial function based on their pivotal role in energy production. In response to ischemia or hypoxia, mitochondria undergo rapid swelling and fragmentation. Cyt C is a small molecular protein located in the inner mitochondrial membrane. With the disruption of mitochondrial structure, the Cyt C is released from mitochondria into the cytoplasm, subsequently triggering programed cell death apoptosis. Moreover, the reduction of Cyt C in mitochondria will in turn influence electron transfer chain for adenosine triphosphate production, ultimately leading to cell death.[23] Hence, the maintenance of the normal mitochondrial morphology is the prerequisite to ensure proper function.
Pim-1 is a proto-oncogene serine/threonine protein kinase which can reduce MIRI if upregulated.[24,25] It is documented that the cardioprotection of the Pim-1 kinase is linked to stabilization of the mitochondrial structure and upregulation of the anti-apoptotic protein Bcl-2, promoting cell survival and inhibition of cardiac hypertrophy and fibrosis.[26] Previous studies have indicated that Bcl-2 was found to interact with Bax to inhibit apoptosis, and consequently maintained the integrity of the mitochondrial membrane and reduced the release of apoptotic proteins including Cyt C.[27]
In this experimental study, we attempted to investigate the role of Pim-1 in SP-induced cardioprotection. The isolated rat heart I/R model is used in this study which has been widely used in studies about cardioprotection. The results suggested that I/R made Pim-1 expression downregulated compared with CON group, while SP significantly increased the expressions of Pim-1 and Bcl-2 in cytoplasm and mitochondria, thus keeping the mitochondrial integrity which was evidenced by a pronounced reduction of Cyt C release as well as the observations from TEM. However, when pretreated with the Pim-1 inhibitor II, myocardial protective effect of SP was blunted remarkably. These findings suggested that SP exerted cardioprotective properties through upregulation of the Pim-1 kinase which increased the expression of Bcl-2 and maintained mitochondrial integrity.
However, the specific mechanism of Pim-1 in preservation of the mitochondrial networks is not well recognized to date. Recent studies have shown that morphological changes of the mitochondria are closely involved in MIRI.[28,29,30] In the process of I/R, the mitochondria tend to divide and fragmented with the destruction of the dynamic balance. Mitochondrial fission is a result of a variety of factors that involve five proteins including Drp1, fission protein 1 (Fis1), optic atrophy 1 (Opa1), and mitofusins 1 and 2 (Mfn1 and Mfn2), in which the primary one is Drp1.[31,32,33] In the model of left anterior descending coronary artery ligation in rats for 30 min followed by 150 min of reperfusion, Zepeda et al.[34] discovered that I/R caused mitochondrial fragmentation and a significantly higher ratio of Drp1 in mitochondria to cytoplasm, which indicated that the I/R injury leads to the process of mitochondrial fission by Drp1. In fact, Drp1 has multiple sites for posttranslational modification, including phosphorylation, sumoylation, ubiquitination, and nitrosylation.[35,36] In general, Drp1 is mainly cytosolic, but upon activation, it translates to the mitochondrial surface, resulting in continuous fission events. Sharp et al.[37] discovered that in the model of I/R in rats, Drp1 was activated by dephosphorylation of Drp1 at serine 637 and accumulated on the mitochondrial surface which resulted in the disruption of reticular structure of the mitochondria, ultimately leading to the MIRI. More recently, it was also demonstrated that Pim-1 could increase the level of p-Drp1ser637 and inhibit Drp1 compartmentalization to the mitochondria, thus reducing mitochondrial fission and I/R injury.[12]
Therefore, we further investigated whether SP-induced cardioprotection by Pim-1 was associated with Drp1. We found that I/R leads to dephosphorylation of Drp1 at serine 637 and Drp1 compartmentalization to the mitochondria although the total Drp1 expression did not reach statistical significance. It is generally accepted that mitochondrial fragmentation will inevitably result in the disruption of membrane integrity, represented as the release of Cyt C from mitochondria to the cytosolic fraction. Compared with I/R group, SP increased the expression of p-Drp1ser637 and Drp1 cytoplasmic sequestration, which was mediated by the Pim-1 kinase. Nevertheless, the cardioprotective effect of SP was obviously blunted when Pim-1 inhibitor II was administrated. Collectively, the Pim-1 kinase mediated the myocardial protection of SP by regulating Drp1.
In the present study, we first propose that the Pim-1 kinase-regulating Drp1 exerts a crucial role in mediating SP-induced cardioprotection. Upregulated Pim-1 increases the expression of Bcl-2 which might promote cell survival. In addition, Pim-1 increases the p-Drp1ser637 expression to inhibit the activity of Drp1 and subsequently maintains mitochondrial integrity. Bax/bak of proapoptotic Bcl-2 family members mediate the release of DDP/TIMM8a, a mitochondrial intermembrane space protein from mitochondria into the cytoplasm, where it banded to the C-terminal portion of Drp1 and promoted mitochondrial fission.[38,39] Hence, it should be noted that the upregulation of Bcl-2 by Pim-1 contributes to the inhibition of Drp1-mediated mitochondrial fission in SP-induced cardioprotection. Further studies about the characteristics and mechanisms of Pim-1 and Drp1 in cardioprotection are particularly needed and it might provide promising strategies into clinical cardiovascular disease treatment for humans in the future.
The results of the current study should be interpreted within the constraint of several potential limitations. Although Drp1-ser637 is a phosphorylation site for Pim-1, it cannot be excluded that the other residues on Drp1 might also be phosphorylated. In addition, due to the restriction of the experimental settings, we were unable to directly calculate mitochondrial morphology and organization, which might be more convincing. Moreover, we used an isolated Langendorff-perfused heart model to mimic the process of I/R injury, which could not determine whether the relationship of Pim-1 and Drp1 existed in humans who have suffered from MIRI. Finally, it is widely accepted that the mitochondrial structure is closely associated with its function. However, we only demonstrated the changes of mitochondrial morphology in the process of phosphorylation of Drp1 by the Pim-1 kinase, but failed to assess the role of healthy and preserved mitochondria in the regulation of Ca2+, ROS, and oxidative phosphorylation. These, however, are recognized as key mediators of I/R injury.
Taken together, the results demonstrate that the Pim-1 kinase might mediate SP-induced cardioprotection by regulating Drp1. Phosphorylation of Drp1 prevents extensive mitochondrial fission through Drp1 cytosolic sequestration.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
References
- 1.Gong KZ, Zhang ZG, Li AH, Huang YF, Bu P, Dong F, et al. ROS-mediated ERK activation in delayed protection from anoxic preconditioning in neonatal rat cardiomyocytes. Chin Med J. 2004;117:395–400. [PubMed] [Google Scholar]
- 2.Aldakkak M, Stowe DF, Chen Q, Lesnefsky EJ, Camara AK. Inhibited mitochondrial respiration by amobarbital during cardiac ischaemia improves redox state and reduces matrix Ca2+ overload and ROS release. Cardiovasc Res. 2008;77:406–15. doi: 10.1016/j.cardiores.2007.08.008. doi: 10.1016/j.cardiores.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Gateau-Roesch O, Argaud L, Ovize M. Mitochondrial permeability transition pore and postconditioning. Cardiovasc Res. 2006;70:264–73. doi: 10.1016/j.cardiores.2006.02.024. doi: 10.1016/j.cardiores.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Xu FF, Liu XH. Calreticulin translocation aggravates endoplasmic reticulum stress-associated apoptosis during cardiomyocyte hypoxia/reoxygenation. Chin Med J. 2015;128:353–60. doi: 10.4103/0366-6999.150103. doi: 10.4103/0366-6999.150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liesa M, Palacín M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845. doi: 10.1152/physrev.00030.2008. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 6.Hall AR, Burke N, Dongworth RK, Hausenloy DJ. Mitochondrial fusion and fission proteins: Novel therapeutic targets for combating cardiovascular disease. Br J Pharmacol. 2014;171:1890–906. doi: 10.1111/bph.12516. doi: 10.1111/bph.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Disatnik MH, Ferreira JC, Campos JC, Gomes KS, Dourado PM, Qi X, et al. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J Am Heart Assoc. 2013;2:e000461. doi: 10.1161/JAHA.113.000461. doi: 10.1161/JAHA.113.000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamga Pride C, Mo L, Quesnelle K, Dagda RK, Murillo D, Geary L, et al. Nitrite activates protein kinase A in normoxia to mediate mitochondrial fusion and tolerance to ischaemia/reperfusion. Cardiovasc Res. 2014;101:57–68. doi: 10.1093/cvr/cvt224. doi: 10.1093/cvr/cvt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharp WW. Dynamin-related protein 1 as a therapeutic target in cardiac arrest. J Mol Med (Berl) 2015;93:243–52. doi: 10.1007/s00109-015-1257-3. doi: 10.1007/s00109-015-1257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–22. doi: 10.1161/CIRCULATIONAHA.109.906610. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y, Li T, Wu C, Bittle GJ, Chen S, Wu ZJ, et al. Pim-1 mediated signaling during the process of cardiac remodeling following myocardial infarction in ovine hearts. J Mol Cell Cardiol. 2013;63:89–97. doi: 10.1016/j.yjmcc.2013.07.012. doi: 10.1016/j.yjmcc.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Din S, Mason M, Völkers M, Johnson B, Cottage CT, Wang Z, et al. Pim-1 preserves mitochondrial morphology by inhibiting dynamin-related protein 1 translocation. Proc Natl Acad Sci U S A. 2013;110:5969–74. doi: 10.1073/pnas.1213294110. doi: 10.1073/pnas.1213294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen HT, Yang CX, Li H, Zhang CJ, Wen XJ, Zhou J, et al. Cardioprotection of sevoflurane postconditioning by activating extracellular signal-regulated kinase 1/2 in isolated rat hearts. Acta Pharmacol Sin. 2008;29:931–41. doi: 10.1111/j.1745-7254.2008.00824.x. doi: 10.1111/j.1745-7254.2008.00824.x. [DOI] [PubMed] [Google Scholar]
- 14.Gong JS, Yao YT, Fang NX, Huang J, Li LH. Sevoflurane postconditioning alleviates action potential duration shortening and L-type calcium current suppression induced by ischemia/reperfusion injury in rat epicardial myocytes. Chin Med J. 2012;125:3485–91. doi: 10.3760/cma.j.issn.0366-6999.2012.19.022. [PubMed] [Google Scholar]
- 15.Liu JD, Deng Q, Tian HH, Pang YT, Deng GL. Wnt/Glycogen synthase kinase 3ß/ß-catenin signaling activation mediated sevoflurane preconditioning-induced cardioprotection. Chin Med J. 2015;128:2346–53. doi: 10.4103/0366-6999.163375. doi: 10.4103/0366-6999.163375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apaijai N, Chinda K, Palee S, Chattipakorn S, Chattipakorn N. Combined vildagliptin and metformin exert better cardioprotection than monotherapy against ischemia-reperfusion injury in obese-insulin resistant rats. PLoS One. 2014;9:e102374. doi: 10.1371/journal.pone.0102374. doi: 10.1371/journal.pone.0102374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shao ZH, Sharp WW, Wojcik KR, Li CQ, Han M, Chang WT, et al. Therapeutic hypothermia cardioprotection via Akt- and nitric oxide-mediated attenuation of mitochondrial oxidants. Am J Physiol Heart Circ Physiol. 2010;298:H2164–73. doi: 10.1152/ajpheart.00994.2009. doi: 10.1152/ajpheart.00994.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allahyari S, Delazar A, Najafi M. Evaluation of general toxicity, anti-oxidant activity and effects of Ficus carica leaves extract on ischemia/reperfusion injuries in isolated heart of rat. Adv Pharm Bull. 2014;4(Suppl 2):577–82. doi: 10.5681/apb.2014.085. doi: 10.5681/apb.2014.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang YH, et al. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res. 2012;110:1484–97. doi: 10.1161/CIRCRESAHA.111.263848. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huhn R, Heinen A, Weber NC, Hollmann MW, Schlack W, Preckel B. Hyperglycaemia blocks sevoflurane-induced postconditioning in the rat heart in vivo: Cardioprotection can be restored by blocking the mitochondrial permeability transition pore. Br J Anaesth. 2008;100:465–71. doi: 10.1093/bja/aen022. doi: 10.1093/bja/aen022. [DOI] [PubMed] [Google Scholar]
- 21.Inamura Y, Miyamae M, Sugioka S, Kaneda K, Okusa C, Onishi A, et al. Aprotinin abolishes sevoflurane postconditioning by inhibiting nitric oxide production and phosphorylation of protein kinase C-delta and glycogen synthase kinase 3beta. Anesthesiology. 2009;111:1036–43. doi: 10.1097/ALN.0b013e3181bbbf9b. doi: 10.1097/ALN.0b013e3181bbbf9b. [DOI] [PubMed] [Google Scholar]
- 22.Yao YY, Zhu MH, Zhang FJ, Wen CY, Ma LL, Wang WN, et al. Activation of Akt and cardioprotection against reperfusion injury are maximal with only five minutes of sevoflurane postconditioning in isolated rat hearts. J Zhejiang Univ Sci B. 2013;14:511–7. doi: 10.1631/jzus.B1200195. doi: 10.1631/jzus.B1200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welchen E, Gonzalez DH. Cytochrome c, a hub linking energy, redox, stress and signaling pathways in mitochondria and other cell compartments. Physiol Plant. 2016;157:310–21. doi: 10.1111/ppl.12449. doi: 10.1111/ppl.12449. [DOI] [PubMed] [Google Scholar]
- 24.Stumpner J, Redel A, Kellermann A, Lotz CA, Blomeyer CA, Smul TM, et al. Differential role of Pim-1 kinase in anesthetic-induced and ischemic preconditioning against myocardial infarction. Anesthesiology. 2009;111:1257–64. doi: 10.1097/ALN.0b013e3181bdf9f4. doi: 10.1097/ALN.0b013e3181bdf9f4. [DOI] [PubMed] [Google Scholar]
- 25.Stumpner J, Smul TM, Redel A, Hilz T, Tischer-Zeitz T, Eisenbarth H, et al. Desflurane-induced and ischaemic postconditioning against myocardial infarction are mediated by Pim-1 kinase. Acta Anaesthesiol Scand. 2012;56:904–13. doi: 10.1111/j.1399-6576.2012.02657.x. doi: 10.1111/j.1399-6576.2012.02657.x. [DOI] [PubMed] [Google Scholar]
- 26.Fischer KM, Cottage CT, Konstandin MH, Völkers M, Khan M, Sussman MA. Pim-1 kinase inhibits pathological injury by promoting cardioprotective signaling. J Mol Cell Cardiol. 2011;51:554–8. doi: 10.1016/j.yjmcc.2011.01.004. doi: 10.1016/j.yjmcc.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu X, Yao Y, Cheng R, Zhang Y, Dai Z, Wan G, et al. Plasminogen K5 activates mitochondrial apoptosis pathway in endothelial cells by regulating Bak and Bcl-x(L) subcellular distribution. Apoptosis. 2011;16:846–55. doi: 10.1007/s10495-011-0618-9. doi: 10.1007/s10495-011-0618-9. [DOI] [PubMed] [Google Scholar]
- 28.Aon MA, Cortassa S, Akar FG, O’Rourke B. Mitochondrial criticality: A new concept at the turning point of life or death. Biochim Biophys Acta. 2006;1762:232–40. doi: 10.1016/j.bbadis.2005.06.008. doi: 10.1016/j.bbadis.2005.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang SJ, Kim W. Mitochondrial dynamics in the heart as a novel therapeutic target for cardioprotection. Chonnam Med J. 2013;49:101–7. doi: 10.4068/cmj.2013.49.3.101. doi: 10.4068/cmj.2013.49.3.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cellier L, Tamareille S, Kalakech H, Guillou S, Lenaers G, Prunier F, et al. Remote ischemic conditioning influences mitochondrial dynamics. Shock. 2016;45:192–7. doi: 10.1097/SHK.0000000000000500. doi: 10.1097/SHK.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 31.Losón OC, Song Z, Chen H, Chan DC. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell. 2013;24:659–67. doi: 10.1091/mbc.E12-10-0721. doi: 10.1091/mbc.E12-10-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer CS, Elgass KD, Parton RG, Osellame LD, Stojanovski D, Ryan MT. Adaptor proteins MiD49 and MiD51 can act independently of Mff and Fis1 in Drp1 recruitment and are specific for mitochondrial fission. J Biol Chem. 2013;288:27584–93. doi: 10.1074/jbc.M113.479873. doi: 10.1074/jbc.M113.479873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otera H, Miyata N, Kuge O, Mihara K. Drp1-dependent mitochondrial fission via MiD49/51 is essential for apoptotic cristae remodeling. J Cell Biol. 2016;212:531–44. doi: 10.1083/jcb.201508099. doi: 10.1083/jcb.201508099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zepeda R, Kuzmicic J, Parra V, Troncoso R, Pennanen C, Riquelme JA, et al. Drp1 loss-of-function reduces cardiomyocyte oxygen dependence protecting the heart from ischemia-reperfusion injury. J Cardiovasc Pharmacol. 2014;63:477–87. doi: 10.1097/FJC.0000000000000071. doi: 10.1097/FJC.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 35.Santel A, Frank S. Shaping mitochondria: The complex posttranslational regulation of the mitochondrial fission protein DRP1. IUBMB Life. 2008;60:448–55. doi: 10.1002/iub.71. doi: 10.1002/iub.71. [DOI] [PubMed] [Google Scholar]
- 36.Chang CR, Blackstone C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann N Y Acad Sci. 2010;1201:34–9. doi: 10.1111/j.1749-6632.2010.05629.x. doi: 10.1111/j.1749-6632.2010.05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, et al. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: Therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J. 2014;28:316–26. doi: 10.1096/fj.12-226225. doi: 10.1096/fj.12-226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, et al. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol. 2002;159:931–8. doi: 10.1083/jcb.200209124. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arnoult D, Rismanchi N, Grodet A, Roberts RG, Seeburg DP, Estaquier J, et al. Bax/Bak-dependent release of DDP/TIMM8a promotes Drp1-mediated mitochondrial fission and mitoptosis during programmed cell death. Curr Biol. 2005;15:2112–8. doi: 10.1016/j.cub.2005.10.041. doi: 10.1016/j.cub.2005.10.041. [DOI] [PubMed] [Google Scholar]