Abstract
Background:
Few studies have focused on peripheral nerve conduction during exposure to microgravity. The −6° head-down tilt (HDT) comprises an experimental model used to simulate the space flight environment. This study investigated nerve conduction characteristics of rhesus monkeys before and after prolonged exposure to HDT.
Methods:
Six rhesus monkeys (3–4 years old) were tilted backward 6° from the horizontal. Nerve conduction studies (NCSs) were performed on the median, ulnar, tibial, and fibular motor nerves. Analysis of variance with a randomized block design was conducted to compare the differences in the NCS before and 7, 21, and 42 days after the −6° HDT.
Results:
The proximal amplitude of the CMAP of the median nerve was significantly decreased at 21 and 42 days of HDT compared with the amplitude before HDT (4.38 ± 2.83 vs. 8.40 ± 2.66 mV, F = 4.85, P = 0.013 and 3.30 ± 2.70 vs. 8.40 ± 2.66 mV, F = 5.93, P = 0.004, respectively). The distal amplitude of the CMAP of the median nerve was significantly decreased at 7, 21, and 42 days of HDT compared with the amplitude before HDT (7.28 ± 1.27 vs. 10.25 ± 3.40 mV, F = 4.03, P = 0.039; 5.05 ± 2.01 vs. 10.25 ± 3.40 mV, F = 6.25, P = 0.04; and 3.95 ± 2.79 vs. 10.25 ± 3.40 mV, F = 7.35, P = 0.01; respectively). The proximal amplitude of the CMAP of the tibial nerve was significantly decreased at 42 days of HDT compared with the amplitude before HDT (6.14 ± 1.94 vs. 11.87 ± 3.19 mV, F = 5.02, P = 0.039).
Conclusions:
This study demonstrates that the compound muscle action potential amplitudes of nerves are decreased under simulated microgravity in rhesus monkeys. Moreover, rhesus monkeys exposed to HDT might be served as an experimental model for the study of NCS under microgravity.
Keywords: Compound Muscle Action Potential, Head-down Tilt, Microgravity, Nerve Conduction, Nerve Conduction Velocity
Introduction
Prolonged exposure to microgravity has significant effects on physiological conditions.[1,2,3,4,5,6] Many studies have investigated the effects of microgravity on the neuromuscular system.[3,7,8,9,10,11,12,13,14] Most of these studies have focused on skeletal muscle changes under microgravity.[10,12,14] However, to the best of our knowledge, there is limited research regarding the effects of microgravity on the peripheral nervous system.[3] One study examined the conduction velocity (CV) in branching axon terminals following a space mission or bed rest and identified a decreased CV;[7] however, it did not measure the velocity in the main trunk of nerve fibers. Furthermore, studies have investigated the effects of hypokinesia on the peripheral nervous system. For example, one study investigated the manifestation of somatosensory-evoked potentials under hypokinesia,[8] which included both the peripheral and central sensory nervous systems. However, the conditions under hypokinesia/bed rest are different compared with microgravity; therefore, the effect of microgravity on the peripheral nervous system should be further evaluated.
Muscle atrophy and decreased muscle power[10,15,16,17] are prominent after a space mission. These changes may be reproduced through space environment simulations on the ground.[9] Ground-based models are a suitable alternative to space flights and induce similar modifications in the neuromuscular system.[9,18,19] The hindlimb unloading (HU) model is characterized by the absence of weightbearing and reduced motor activity,[1,3,8] and it is typically applied in rats. Moreover, the −6° head-down tilt (HDT) is another experimental model used to simulate the space flight environment of humans.[20]
To determine changes in nerve conduction under simulated microgravity, the current study aimed to investigate the nerve conduction characteristics of rhesus monkeys before and after prolonged exposure to HDT.
Methods
Ethics statement
All experimental procedures conducted with rhesus monkeys were approved by the Ethical Committee of the China Astronaut Research and Training Center, which is in accordance with the principles of the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC), approved by Institutional Animal Care and Use Committee (IACUC).
Animals
The HDT model in rhesus monkeys was selected as the model because the peripheral nervous system of primates is the most similar to humans compared with other species. Six rhesus monkeys at the age of 3–4 years (three males and three females) were laid on beds, which were tilted backward 6° from the horizontal. The head-down animals wore a special cloth, which enabled them to be fixed to the bed; however, their arms and legs were free to move, and they were provided access to food and water. The HDT model functions to reduce mechanical loading to the hindlimbs, which provides a similar condition as microgravity. The rhesus monkeys were provided with a regular dietary program, which included primate biscuits, fresh fruits, and vitamin syrup. The animals were housed one per cage or bed in rooms with air temperature maintained at 23 ± 2°C and a standard 12:12 h dark–light cycle (lights were turned on at 8:00 a.m. and off at 8:00 p.m.). The general health condition of the animals was carefully monitored. The monkeys were provided with toys (for example, the drum-shaped rattle, a Chinese traditional toy) during housing throughout the duration of the study, and the toys were available all the time except during experimental procedures. The animal keepers accompanied the monkeys during the day time to help relieve anxiety. The researchers spent time interacting with them for about 2–3 h every day.
Nerve conduction studies
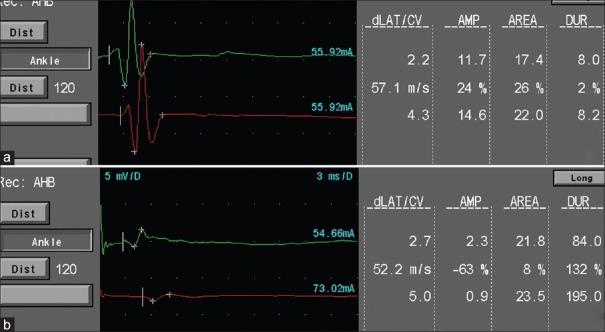
Skin temperature was maintained at 32°C with a heating pad during the examination. Nerve conduction studies (NCSs) were performed using surface electrodes on the median, ulnar, tibial, and fibular nerves on the right side to assess motor function in the upper and lower limbs using the key point electromyography system (31A06, Medoc Ltd., Israel).[21] The motor conduction velocities, the proximal and distal amplitudes of the compound muscle action potentials (CMAPs), and the proximal and distal latencies of the median, ulnar, tibial, and fibular nerves were recorded (representative images of the NCS of the tibial nerve are shown in Figure 1). The NCS was performed in the order of the median, ulnar, tibial, and fibular nerves before HDT and on 7, 21, and 42 days of HDT. During the procedure, toys were provided, and the researchers accompanied the monkeys to reduce pain and suffering. All animal housing and experiments occurred in the Animal Facility at China Astronaut Research and Training Center.
Figure 1.
NCS of tibial nerve before HDT and 42 days after HDT in rhesus monkeys. (a) Before HDT. (b) Forty-two days after HDT. NCS: Nerve conduction studies; HDT: Head-down tilt.
Statistical analysis
Statistical analysis was performed using SPSS software version 19.0 (IBM, Chicago, IL, USA). Descriptive statistics of the NCS were obtained before HDT. The data are represented as mean ± standard deviation (SD). Analysis of variance (ANOVA) using a randomized block design was conducted to compare the differences in the NCS before HDT and at 7, 21, and 42 days of HDT. ANOVA was conducted if no differences were identified between the animals. Post hoc analyses, including least significant difference and Student–Newman–Keuls, were performed if significant differences were identified in the ANOVA.
One rhesus monkey died on the day 21 of HDT because of inadaptation to the simulated microgravity, and one monkey injured its upper limbs as a result of rigorous movement. Thus, the ANOVA with a randomized block design and the ANOVA of the median and ulnar nerve parameters were performed using data collected from the remaining four rhesus monkeys. Data from the tibial and fibular nerves were obtained from the five remaining rhesus monkeys.
Results
The parameters of NCS before HDT (under normal conditions) and 7, 21, and 42 days of HDT are presented in Table 1. The proximal amplitude of the CMAP of the median nerve was significantly decreased at 21 and 42 days of HDT compared with the amplitude before HDT (4.38 ± 2.83 vs. 8.40 ± 2.66 mV, F = 4.85, P = 0.013 and 3.30 ± 2.70 vs. 8.40 ± 2.66 mV, F = 5.93, P = 0.004, respectively). The proximal amplitude of the CMAP of the median nerve at 42 days of HDT was significantly decreased compared with that at 7 days of HDT (3.30 ± 2.70 vs. 7.70 ± 1.50 mV, F = 4.40, P = 0.022).
Table 1.
NCS before and at 7, 21, and 42 days of HDT in rhesus monkeys (n = 6)
| Time points | Parameters | Median | Ulnar | Tibial | Fibular |
|---|---|---|---|---|---|
| Before HDT | MCV (m/s) | 59.55 ± 6.20 | 62.67 ± 9.02 | 57.45 ± 4.50 | 60.32 ± 4.94 |
| Proximal CMAP (mV) | 8.40 ± 2.66 | 4.33 ± 1.87 | 11.87 ± 3.19 | 7.72 ± 4.60 | |
| Distal CMAP (mV) | 10.25 ± 3.40 | 5.58 ± 2.05 | 11.17 ± 2.68 | 7.68 ± 4.15 | |
| 7 days | MCV (m/s) | 53.15 ± 4.76 | 56.53 ± 14.41 | 52.66 ± 5.61 | 55.50 ± 8.16 |
| Proximal CMAP (mV) | 7.70 ± 1.50 | 5.25 ± 3.36 | 12.98 ± 4.99 | 6.48 ± 4.91 | |
| Distal CMAP (mV) | 7.28 ± 1.27† | 6.58 ± 3.60 | 12.86 ± 5.03 | 7.00 ± 3.26 | |
| 21 days | MCV (m/s) | 58.45 ± 6.86 | 63.03 ± 8.00 | 51.98 ± 6.74 | 56.42 ± 5.15 |
| Proximal CMAP (mV) | 4.38 ± 2.83* | 2.95 ± 1.40 | 8.48 ± 3.52 | 5.28 ± 2.84 | |
| Distal CMAP (mV) | 5.05 ± 2.01† | 4.13 ± 1.53 | 10.84 ± 3.00 | 5.74 ± 2.34 | |
| 42 days | MCV (m/s) | 57.45 ± 3.16 | 61.8 ± 1.82 | 52.36 ± 8.38 | 57.00 ± 6.53 |
| Proximal CMAP (mV) | 3.30 ± 2.70*,§ | 2.50 ± 2.43 | 6.14 ± 1.94‡,¶ | 5.38 ± 4.05 | |
| Distal CMAP (mV) | 3.95 ± 2.79†,|| | 4.03 ± 4.08 | 8.04 ± 2.86 | 5.06 ± 4.32 |
Data represent means ± SDs. *For the median nerve, statistically different versus the proximal CMAP before HDT; †For the median nerve, statistically different versus distal CMAP before HDT; ‡For the tibial nerve, statistically different versus the proximal CMAP before HDT; §For the median nerve, statistically different versus the proximal CMAP 7 days of HDT; ||For the median nerves, statistically different versus distal CMAP 7 days of HDT; ¶For the tibial nerve, statistically different versus the proximal CMAP 7 days of HDT. NCS: Nerve conduction study; HDT: Head-down tilt; MCV: Motor conduction velocity; CMAP: Compound muscle action potential; SDs: Standard deviations.
The distal amplitude of the CMAP of the median nerve was significantly decreased at 7, 21, and 42 days of HDT compared with the amplitude before HDT (7.28 ± 1.27 vs. 10.25 ± 3.40 mV, F = 4.03, P = 0.039; 5.05 ± 2.01 vs. 10.25 ± 3.40 mV, F = 6.25, P = 0.04; and 3.95 ± 2.79 vs. 10.25 ± 3.40 mV, F = 7.35, P = 0.01, respectively). The distal amplitude of the CMAP of the median nerve at 42 days of HDT was significantly decreased compared with that at 7 days of HDT (3.95 ± 2.79 vs. 7.28 ± 1.27 mV, F = 3.33, P = 0.08).
The proximal amplitude of the CMAP of the tibial nerve was significantly decreased at 42 days of HDT compared with the amplitude before HDT (6.14 ± 1.94 vs. 11.87 ± 3.19 mV, F = 5.02, P = 0.039). The proximal amplitude of the CMAP of the tibial nerve at 42 days of HDT was significantly decreased compared with that at 7 days of HDT (6.14 ± 1.94 vs. 12.98 ± 4.99 mV, F = 6.84, P = 0.008).
The proximal and distal latencies of the nerves and the nerve CVs were prolonged and decreased slightly compared with the parameters before HDT but were not significantly different.
Discussion
Previous studies regarding the effects of microgravity on the neuromuscular system have mainly focused on muscle atrophy induced by muscle disuse.[7] Few studies have investigated the electrophysiological characteristics of the peripheral nervous system after prolonged microgravity. The current investigation focused on the electrophysiological characteristics of the peripheral nervous system and identified a significant decrease in the proximal and distal amplitudes of the CMAP of the median and tibial nerves as early as day 7 of HDT. Previous research identified a longer duration and a slower falling rate of the action potentials of muscle fibers, thereby reflecting a change in the motor fiber impulses of its nerve,[22] which may contribute to the decreased amplitude. Furthermore, atypical combinations of myosin heavy chain mRNA isoforms have been demonstrated to be transiently increased in the soleus fibers of rats that experienced 7 days of HU, which suggests a disruption of transcriptional and translational activity.[23,24,25] These changes were present at a very early stage and may explain the CMAP amplitude decrease in the NCS identified in the current study. Furthermore, as the recording surface electrodes were attached at the muscle belly, a decrease in muscle power and muscle atrophy may contribute to the CMAP amplitude decrease of the median and tibial nerves. However, there were no significant differences in the proximal and distal amplitudes of the CMAP of the ulnar and fibular nerves at 42 days of HDT. The reason for this finding may be that 42 days of HDT is not sufficient to produce dramatic differences. Studies of rats under microgravity have typically lasted for 14 days or less, and significant changes in multiple parameters such as the nerve CV and axon diameter have been identified.[3,8,22] In humans, studies are typically sustained for 6 months, with a minimum of 2 months.[9] Primates most closely resemble humans; however, 42 days of HDT was likely not sufficient to induce similar changes. Moreover, one study demonstrated that the equilibrium state in rats shifted to a different level at 14 days of HU. The transitional period between these two equilibrium states may have been marked by a transitory disorganization.[8] We infer that the electrophysiological characteristics of peripheral nerves were under a transitory disorganization on day 42 of HDT. Thus, the amplitudes of the CMAP of the ulnar and peroneal nerves did not exhibit significant decreases. Further studies are necessary to better understand the mechanisms during this transitional period.
A significant decrease in the CV of muscle fibers[7,9,26] and a reduction in the CV in branching axon terminals[9] were previously identified. In this study, the latencies of the nerves and the nerve CVs were not significantly different after HDT. The mechanisms that modulate the CV in nerve fibers are not clear, and changes in the membrane properties at the nodes of Ranvier may represent one factor that plays a role in this modulation.[9] Changes in the membrane properties may be induced through modifications in the synthesis of voltage-gated membrane channels in excitable muscle membranes. Wittwer et al.[27] demonstrated gene expression alterations following prolonged unloading of rat soleus muscles. There was an increase in the mRNAs that coded for ion channels, which may elucidate the increased shortening velocity of the soleus muscle following spaceflight.[27] In our study, the most likely explanations for the lack of change in the latencies of the nerves and nerve CVs following HDT were that the 42 days of exposure to HDT was not sufficient to generate significant changes, and the electrophysiological characteristics of the peripheral nerve fibers were under a transitory disorganization.[8] Furthermore, to the best of our knowledge, the nerve CV depends on the axonal diameter, myelin sheath thickness, and internodal distance.[28,29] Previous research indicates that although myelin was thinner following HU, the mean axonal diameter for the small fibers was slightly decreased in HU rats; in contrast, it was not significantly different for the large fibers, which were in charge of motor function, and the distances between the nodes of Ranvier and the global structure of the nerve fibers were not significantly different.[3] Thus, the nerve CVs may not substantially change based on nerve morphology. Moreover, the current findings are consistent with previous research regarding fatigue, in which the CV along nerve fibers was not substantially reduced.[30]
In this study, rhesus monkeys were subjected to HDT to resemble the space flight environment, which is a model of substantial value in the investigation of microgravity effects on astronauts. Experiments that involve astronauts, including ground-based experiments, are expensive, and the impact of microgravity on the motor function of astronauts is often minimized through countermeasures.[31] Furthermore, the pretest conditions of astronauts are challenging to control; thus, the potential heterogeneity may impact the reliability of the findings.[9] Moreover, although studies have evaluated changes in muscle fibers and axon terminals under microgravity, few studies have investigated these variables in nerve trunks. The current findings provide novel evidence related to the electrophysiological characteristics of nerve trunks under microgravity.
Despite the importance of the experimental model and findings identified in this study, several study limitations must be considered. First, the sample size is relatively small. Second, 42 days of HDT may represent a transitory period between two equilibriums. Thus, future studies with a larger sample size and a longer duration of HDT are necessary to confirm these findings.
Conclusively, this study demonstrates that the CMAP amplitudes of nerves are decreased under HDT in rhesus monkeys. Moreover, results of the present study suggest that rhesus monkeys exposed to HDT might be used as an experimental model for the study of NCS under microgravity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
References
- 1.Adams GR, Caiozzo VJ, Baldwin KM. Skeletal muscle unweighting: Spaceflight and ground-based models. J Appl Physiol (1985) 2003;95:2185–201. doi: 10.1152/japplphysiol.00346.2003. doi: 10.1152/japplphysiol.00346.2003. [DOI] [PubMed] [Google Scholar]
- 2.Aubert AE, Beckers F, Verheyden B. Cardiovascular function and basics of physiology in microgravity. Acta Cardiol. 2005;60:129–51. doi: 10.2143/AC.60.2.2005024. doi: 10.2143/AC.60.2.2005024. [DOI] [PubMed] [Google Scholar]
- 3.Canu MH, Carnaud M, Picquet F, Goutebroze L. Activity-dependent regulation of myelin maintenance in the adult rat. Brain Res. 2009;1252:45–51. doi: 10.1016/j.brainres.2008.10.079. doi: 10.1016/j.brainres.2008.10.079. [DOI] [PubMed] [Google Scholar]
- 4.Prakash M, Fried R, Götze O, May F, Frings-Meuthen P, Mulder E, et al. Microgravity simulated by the 6°head-down tilt bed rest test increases intestinal motility but fails to induce gastrointestinal symptoms of space motion sickness. Dig Dis Sci. 2015;60:3053–61. doi: 10.1007/s10620-015-3738-1. doi: 10.1007/s10620-015-3738-1. [DOI] [PubMed] [Google Scholar]
- 5.Hargens AR, Vico L. Long-duration bed rest as an analog to microgravity. J Appl Physiol (1985) 2016;120:891–903. doi: 10.1152/japplphysiol.00935.2015. doi: 10.1152/japplphysiol.00935.2015. [DOI] [PubMed] [Google Scholar]
- 6.Li K, Guo X, Jin Z, Ouyang X, Zeng Y, Feng J, et al. Effect of simulated microgravity on human brain gray matter and white matter – Evidence from MRI. PLoS One. 2015;10:e0135835. doi: 10.1371/journal.pone.0135835. doi: 10.1371/journal.pone.0135835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cescon C, Gazzoni M. Short term bed-rest reduces conduction velocity of individual motor units in leg muscles. J Electromyogr Kinesiol. 2010;20:860–7. doi: 10.1016/j.jelekin.2010.03.008. doi: 10.1016/j.jelekin.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Canu MH, Langlet C, Dupont E, Falempin M. Effects of hypodynamia-hypokinesia on somatosensory evoked potentials in the rat. Brain Res. 2003;978:162–8. doi: 10.1016/s0006-8993(03)02804-x. doi: 10.1016/S0006-8993(03)02804-X. [DOI] [PubMed] [Google Scholar]
- 9.Ruegg DG, Kakebeeke TH, Gabriel JP, Bennefeld M. Conduction velocity of nerve and muscle fiber action potentials after a space mission or a bed rest. Clin Neurophysiol. 2003;114:86–93. doi: 10.1016/s1388-2457(02)00329-2. doi: 10.1016/S1388-2457(02)00329-2. [DOI] [PubMed] [Google Scholar]
- 10.Kozlovskaia I, Kreidich I, Rakhmanov A. Mechanisms of the effects of weightlessness on the motor system of man. Physiologist. 1981;24:37–44. doi: 10.1016/j.pmrj.1981.24.611. [Google Scholar]
- 11.Bock O, Fowler B, Comfort D. Human sensorimotor coordination during spaceflight: An analysis of pointing and tracking responses during the “Neurolab” space shuttle mission. Aviat Space Environ Med. 2001;72:877–83. doi: 10.1007/BF0209727. [PubMed] [Google Scholar]
- 12.Newman DJ, Lathan CE. Memory processes and motor control in extreme environments. IEEE Trans Syst Man Cybern C Appl Rev. 1999;29:387–94. doi: 10.1109/5326.777074. doi: 10.1109/5326.777074. [DOI] [PubMed] [Google Scholar]
- 13.Manzey D, Lorenz B, Schiewe A, Finell G, Thiele G. Behavioral aspects of human adaptation to space: Analyses of cognitive and psychomotor performance in space during an 8-day space mission. Clin Investig. 1993;71:725–31. doi: 10.1007/BF00209727. doi: 10.1007/BF00209727. [DOI] [PubMed] [Google Scholar]
- 14.Berger M, Mescheriakov S, Molokanova E, Lechner-Steinleitner S, Seguer N, Kozlovskaya I. Pointing arm movements in short- and long-term spaceflights. Aviat Space Environ Med. 1997;68:781–7. doi: 10.1136/jnnp-1997-302457. [PubMed] [Google Scholar]
- 15.Kozlovskaya IB, Kreidich YuV, Oganov VS, Koserenko OP. Pathophysiology of motor functions in prolonged manned space flights. Acta Astronaut. 1981;8:1059–72. doi: 10.1016/0094-5765(81)90079-5. doi: 10.1016/0094-5765(81)90079-5. [DOI] [PubMed] [Google Scholar]
- 16.Jaweed M. Orthopaedic Physical Therapy Secrets. Philadelphia: Lippincott, Williams & Wilkins; 1994. Muscle structure and function. Space Physiology and Medicine; pp. 317–26. [Google Scholar]
- 17.Edgerton VR, Roy RR. Neuromuscular adaptations to actual and simulated spaceflight. Compr Physiol. 1996;4:33–67. doi: 10.1016/s1569-2574(08)60134-3. doi: 10.1002/cphy.cp040132. [DOI] [PubMed] [Google Scholar]
- 18.Trappe SW, Trappe TA, Lee GA, Widrick JJ, Costill DL, Fitts RH. Comparison of a space shuttle flight (STS-78) and bed rest on human muscle function. J Appl Physiol (1985) 2001;91:57–64. doi: 10.1152/jappl.2001.91.1.57. doi: 10.1385/NMM: 91: 1: 175. [DOI] [PubMed] [Google Scholar]
- 19.Widrick JJ, Trappe SW, Romatowski JG, Riley DA, Costill DL, Fitts RH. Unilateral lower limb suspension does not mimic bed rest or spaceflight effects on human muscle fiber function. J Appl Physiol (1985) 2002;93:354–60. doi: 10.1152/japplphysiol.01245.2001. doi: 10.1152/japplphysiol. 01245.2001. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XY, Ma HS, Li L, Dai ZQ, Du J, Zheng W, et al. Longitudinal metabolic changes in the thalamus of macaque brain during 42-day head down tilt. Pak J Zool. 2016;48:575–81. [Google Scholar]
- 21.Ogata K, Shimon S, Owen J, Manske PR. Effects of compression and devascularisation on ulnar nerve function. A quantitative study of regional blood flow and nerve conduction in monkeys. J Hand Surg Br. 1991;16:104–8. doi: 10.1016/0266-7681(91)90143-c. doi: 10.1016/0266-7681(91)90143-C. [DOI] [PubMed] [Google Scholar]
- 22.De-Doncker L, Kasri M, Picquet F, Falempin M. Physiologically adaptive changes of the L5 afferent neurogram and of the rat soleus EMG activity during 14 days of hindlimb unloading and recovery. J Exp Biol. 2005;208:4585–92. doi: 10.1242/jeb.01931. doi: 10.1242/jeb.01931. [DOI] [PubMed] [Google Scholar]
- 23.Stevens L, Gohlsch B, Mounier Y, Pette D. Changes in myosin heavy chain mRNA and protein isoforms in single fibers of unloaded rat soleus muscle. FEBS Lett. 1999;463:15–8. doi: 10.1016/s0014-5793(99)01596-3. doi: 10.1016/S0014-5793(99)01596-3. [DOI] [PubMed] [Google Scholar]
- 24.Andersen JL, Schiaffino S. Mismatch between myosin heavy chain mRNA and protein distribution in human skeletal muscle fibers. Am J Physiol. 1997;272:C1881–9. doi: 10.1152/ajpcell.1997.272.6.C1881. doi: 10.1186/sC1881-014-0199-0. [DOI] [PubMed] [Google Scholar]
- 25.Andersen JL, Gruschy-Knudsen T, Sandri C, Larsson L, Schiaffino S. Bed rest increases the amount of mismatched fibers in human skeletal muscle. J Appl Physiol (1985) 1999;86:455–60. doi: 10.1152/jappl.1999.86.2.455. doi: 10.1999/S1474-442270025-4. [DOI] [PubMed] [Google Scholar]
- 26.Gazzoni M, Farina D, Merletti R. Motor unit recruitment during constant low force and long duration muscle contractions investigated with surface electromyography. Acta Physiol Pharmacol Bulg. 2001;26:67–71. doi: 101.4103/0366-6999.180511AE. [PubMed] [Google Scholar]
- 27.Wittwer M, Flück M, Hoppeler H, Müller S, Desplanches D, Billeter R. Prolonged unloading of rat soleus muscle causes distinct adaptations of the gene profile. FASEB J. 2002;16:884–6. doi: 10.1096/fj.01-0792fje. doi: 10.1096/fj.01-0792fje. [DOI] [PubMed] [Google Scholar]
- 28.Brill MH, Waxman SG, Moore JW, Joyner RW. Conduction velocity and spike configuration in myelinated fibres: Computed dependence on internode distance. J Neurol Neurosurg Psychiatry. 1977;40:769–74. doi: 10.1136/jnnp.40.8.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waxman SG. Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve. 1980;3:141–50. doi: 10.1002/mus.880030207. doi: 10.1002/mus.880030207. [DOI] [PubMed] [Google Scholar]
- 30.Rabischong E, Doutrelot PL, Ohanna F. Compound motor action potentials and mechanical failure during sustained contractions by electrical stimulation in paraplegic patients. Paraplegia. 1995;33:707–14. doi: 10.1038/sc.1995.149. doi: 10.1038/sc.1995.149. [DOI] [PubMed] [Google Scholar]
- 31.Kozlovskaya IB, Barmin VA, Stepantsov VI, Kharitonov NM. Results of studies of motor functions in long-term space flights. Physiologist. 1990;33(1 Suppl):S1–3. [PubMed] [Google Scholar]