Abstract
Background:
Vancomycin-resistant enterococci (VRE) are a major and emerging hospital-acquired pathogen associated with high mortality, particularly among the critically ill and Intensive Care Units (ICUs) patients. This study aimed to determine the prevalence and demographic and clinical characteristics of VRE among patients admitted to a university hospital in Riyadh, Saudi Arabia.
Methods:
A study was conducted during the period from September 2014 to November 2015 at King Khalid University Hospital, a tertiary care hospital in Riyadh, Saudi Arabia, including in-patients with VRE infection. Data were collected using laboratory results and the medical records of admitted patients and were analyzed using SPSS version 19.0 statistical software.
Results:
In a one-year period, 231 enterococci were isolated from blood, urine, exudates, sputum, stool, and body fluid. There were 191 (82.7%) vancomycin-sensitive enterococci (VSE) and 40 (17.3%) isolates were VRE. The Enterococcus species included E. faecalis 168 (72.7%), E. faecium, 53 (22.8%) E. gallinarum 5 (2.2%), and E. avium 5 (2.2%). VRE were more significant from blood specimens (P < 0.0001) while VSE were significantly more predominant from urine specimens (P < 0.0001). VRE were more commonly isolated from patients in ICUs and oncology unit (P = 0.0151 and P < 0.001, respectively) while VSE were more predominant in the medical and surgical areas (P = 0.0178 and P = 0.0178, respectively).
Conclusions:
This study highlights the high prevalence of VRE in the hospital and the association of enterococcal infections with high-risk areas and oncology units, which warrant more studies looking for better management of these infections.
Keywords: Epidemiology, Hospital Infection, Prevalence, Vancomycin-resistant Enterococci
Introduction
Enterococci are one of the major causes of nosocomial infections and bacteremia.[1,2] They were identified as a cause of community-acquired infections, including pelvic infections, neonatal infections, and urinary tract infections (UTIs). They also cause surgical bacteremia, endocarditis, wound infections, and rarely meningitis.[3] Most of the clinically significant enterococcal infections are caused by two species, Enterococcus faecalis and Enterococcus faecium. Recently, the emergence of antimicrobial resistance in enterococci, particularly to the glycopeptides, has caused great challenges for clinicians.[4,5] Antimicrobial therapy of serious enterococcal infection is becoming more complex due to the inherent resistance shown by enterococci to several commonly used antibiotics, such as cephalosporins, low-level aminoglycosides, and low-level clindamycin. With the increase in the prevalence of vancomycin-resistant enterococci (VRE), there were only a few effective antimicrobial agents left for the management of severe infections and results in the selection and spreading of multidrug-resistant (MDR) strains in hospitals.[4,5,6] Asymptomatic intestinal colonization by VRE has been associated with multiple risk factors and often precedes true infection.[7,8] Many countries in Europe, Asia, Australia, South America, and some in Africa reported the increasement in the prevalence of VRE.[9,10,11] However, there are no enough data available on the prevalence, epidemiology, and risk factors of VRE in Saudi Arabia. The aim of this study was to investigate the risk factors and clinical and epidemiological characteristics of infection with VRE among patients admitted to a university hospital.
Methods
Patients
This study was carried out on patients with enterococcal infection admitted to King Khalid University Hospital, Riyadh, Saudi Arabia, a tertiary teaching hospital with 850 beds that serves a population of <1.5 million inhabitants. The study was conducted from September 2014 to November 2015. Prospective investigations were carried out on patients with clinically significant enterococcal infection attending hospital clinics, emergency rooms, medical and surgical wards, and Intensive Care Units (ICUs). For some patients, data were collected retrospectively. Patients might acquire VRE colonization and subsequent infection during current or prior hospitalization. It was not always possible to determine in each single case the source of colonization preceding infection. However, all cases enrolled in this study had a clinically significant infection. All admitted patients were followed prospectively. However, for some patients, retrospective reviews of their files were done to obtain some information on previous admission, underlying diseases, prior VRE bacteremia, and ICUs stay. Patients were followed until discharge or death. Microbiological and clinical information were gathered including age, gender, hospitalization ward (medical wards, surgical wards, or ICU), source of the infection, clinical diagnosis, history of vancomycin intake, surgical interventions, invasive procedure and devices, and current medication profile. This study was approved by the Institutional Ethics Review Board of King Khalid University Hospital.
Bacterial culture: Identification and susceptibility testing
Clinical specimens included urine, pus, tissue, blood, and body fluids. Surveillance cultures (taken within 24 h after admission) for VRE screening received usually from ICUs such as rectal swabs were excluded. Culture and antibiotic susceptibility of the isolates were performed as per the standard recommendations. The clinical significance of the Enterococcus isolates was assessed retrospectively by analyzing the clinical criteria such as catheterization in UTIs, signs of sepsis, and other laboratory tests such as leukocytosis. Colonies isolated from routine clinical specimens resembling enterococci were initially identified by Gram-staining, growth in 6.5% NaCl broth, and bile esculin hydrolysis. In vitro susceptibilities of the isolates to ampicillin, ciprofloxacin, vancomycin, gentamicin, teicoplanin, and linezolid were determined by the disk diffusion method and interpreted according to the Clinical and Laboratory Standards Institute (CLSI) guidelines, 2007. Care was taken to view the vancomycin zone of inhibition in transmitted light after 24 h of incubation at 37°C.[12] The minimum inhibitory concentration (MIC) of vancomycin was determined by the E-test for all the enterococci isolates, which showed intermediate sensitivity by the Kirby-Bauer disc diffusion method. A lawn culture of enterococci, 0.5 McFarland's standard, was made on 5% Mueller Hinton blood agar. The E-strip, which was obtained from Himedia®, was applied with a MIC scale, facing up, using sterile forceps, with the higher concentration facing the edge of the plate. The plates were examined after 24 h of incubation at 37°C. The zone of inhibition was observed in the form of an ellipse. The antibiotic susceptibility pattern was interpreted as per the CLSI Guidelines, 2007.[12]
Statistical analysis
Statistical analyses were performed using SPSS version 19.0 statistical software (SPSS Inc. Wacker Drive, Chicago, IL, USA). Continuous variables were presented as means ± standard deviations (SDs) and were compared using the Student's t-test. Categorical variables were compared using Chi-squared test or Fisher's exact test if the expected values were <5. A value of P < 0.05 was considered statistically significant.
Results
In a 1-year period, 231 enterococci were isolated from blood, urine, exudates, sputum, stool, and body fluid (peritoneal, ascetic, bile, and drains) [Table 1]. Urine in 95 (41.4%) isolates followed by blood 63 (27.2%) and wound swab 45 (19.4) were the most commonly involved specimens. VRE were more significant from blood specimens (P < 0.0001) while VSE were significantly more predominant from urine specimens (P < 0.0001). In all other specimens (body fluid, cerebrospinal fluid, pus, tissue, and wound swab), there was no significant difference in the frequency of isolated VSE and VRE [Table 2]. The most common isolated Enterococcus species was E. faecalis 169 (73.2%) followed by E. faecium 53 (22.9%). E. gallinarum and E. avium represented 5 (2.2%) each [Figure 1]. There were 191 (82.7%) VSE, and 40 (17.3%) isolates were VRE. Thirty-three (62.3%) of E. faecium isolates were vancomycin resistant while only two (1.2%) of E. faecalis isolates were vancomycin resistant. The susceptibility pattern of E. faecalis and E. faecium to other clinically important antibiotics was also analyzed [Table 3]. E. faecalis showed high susceptibility to most tested antimicrobial agents. A high level of gentamicin resistance was seen in 40 (23.7%) of the E. faecalis isolates and 36 (67.9%) of E. faecium isolates. Linezolid was the most active antibiotic against E. faecium followed by teicoplanin and tigecycline. In comparison to vancomycin-susceptible enterococci (VSE), VRE were more commonly isolated from patients in ICUs and oncology unit (P = 0.0151 and P < 0.001, respectively) whereas VSE were more commonly isolated from patients in medical and surgical wards (P = 0.0178, Figure 2). The detailed clinical and microbiological data for patients who acquired VRE while in ICU, oncology, and surgical and medical wards are shown in Tables 4–6, respectively. Overall, 40 VRE patients developed serious or persistent infection including bacteremia (24), wound infection (9), UTI (6), and peritonitis (2). One patient had peritonitis postcolectomy complicated with bacteremia. Intestinal VRE-carriage was identified in three patients admitted to the ICU. The mean age of VRE-infected patients was 55.4 ± 22.0 years. Blood was the most common specimens received from VRE-infected patients in the ICU, oncology, and surgical and medical wards (11/16, 7/12, and 5/12, respectively). Five patients had concomitant infection with organisms other than VRE: Multi-resistant Acinetobacter baumannii recovered from the blood of an AIDS patient with Kaposi sarcoma and septic shock, Enterobacter cloacae from ascetic fluid of a patient with terminal stage cancer of the colon and peritonitis, multi-resistant Pseudomonas aeruginosa from a patient with diabetic foot infection, nonidentified Gram-negative Bacilli from the blood of a patient with adenocarcinoma of the gallbladder, and extended-spectrum beta-lactamases producing Escherichia coli from ascetic fluid of a patient with liver disease. Thirteen of the VRE-infected patients (32.5%) for whom the treatment information was available received vancomycin prior or during the course of infection.
Table 1.
Type of specimens from which enterococci were isolated
| Type of specimen | All enterococcal species (n = 231) |
|---|---|
| Blood | 64 (27.7) |
| Body fluid | 18 (7.8) |
| Cerebrospinal fluid | 1 (0.4) |
| Pus | 1 (0.4) |
| Tissue | 6 (2.6) |
| Urine | 95 (41.4) |
| Wound swab | 46 (19.9) |
Data are presented as n (%).
Table 2.
Distribution of specimens among VRE and VSE
| Type of specimen | VSE (n = 191) | VRE (n = 40) | χ2 | P |
|---|---|---|---|---|
| Blood | 41 (21.5) | 23 (57.5) | 21.44 | <0.0001 |
| Body fluid | 16 (8.4) | 2 (5.0) | 0.525 | 0.469 |
| CSF | 1 (0.5) | 0 | 0.210 | 0.647 |
| Pus | 1 (0.5) | 0 | 0.210 | 0.647 |
| Tissue | 6 (3.1) | 0 | 1.290 | 0.256 |
| Urine | 89 (46.6) | 6 (15.0) | 13.637 | <0.0001 |
| Wound swab | 37 (19.4) | 9 (22.5) | 0.203 | 0.652 |
Data are presented as n (%). CSF: Cerebral spinal fluid; VRE: Vancomycin-resistant enterococci; VSE: Vancomycin-sensitive enterococci.
Figure 1.
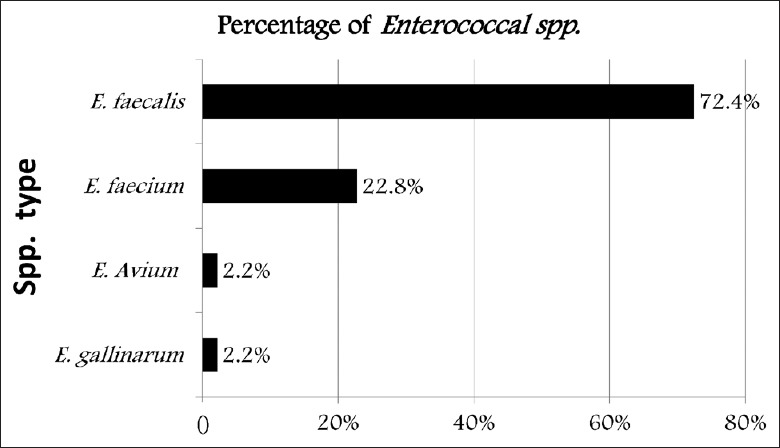
Frequency of different enterococci isolated from hospitalized patients.
Table 3.
Susceptibility pattern of E. faecalis and E. faecium to clinically important antibiotics
| Species (number tested) | Antimicrobial | GM | TP | VAN | AMP | CIP | TEG | LZD | NIT |
|---|---|---|---|---|---|---|---|---|---|
| E. faecium (53) | R | 36 (67.9) | 15 (28.3) | 33 (62.3) | 51 (96.2) | 40 (75.5) | 16 (30.2) | 6 (11.3) | 29 (54.7) |
| S | 17 (32.1) | 38 (71.7) | 20 (37.7) | 2 (3.8) | 13 (24.5) | 37 (69.8) | 47 (88.7) | 24 (45.3) | |
| E. faecalis (168) | R | 40 (23.7) | 0 | 2 (1.2) | 6 (3.6) | 38 (23.1) | 10 (6.5) | 11 (7.1) | 22 (13.0) |
| S | 128 (76.3) | 168 (100) | 166 (98.8) | 162 (96.4) | 130 (76.9) | 158 (93.5) | 157 (92.9) | 147 (87.0) |
Data are presented as n (%). GM: Gentamycin; TP: Teicoplanin; VAN: Vancomycin; AMP: Ampicillin; CIP: Ciprofloxacin; TEG: Tigecycline; LZD: Linezolid; NIT: Nitrofurantoin; E. faecalis: Enterococcus faecalis; E. faecium: Enterococcus faecium; R: Resistant; S: Sensitive.
Figure 2.
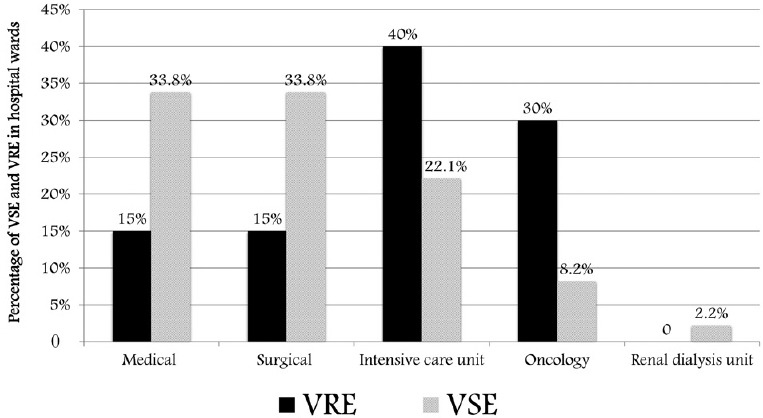
Distribution of VSE and VRE in hospital wards. VRE: Vancomycin-resistant enterococci; VSE: Vancomycin-sensitive enterococci.
Table 4.
Clinical characteristics and microbiological details of VRE infected patients admitted to the ICU
| Age (years) | Gender | Specimen | Clinical data | Enterococcus spp. | Resistance phenotype | VAN (μg/ml) | TP (μg/ml) | LZD | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| 3 | Female | Blood | Short bowel syndrome-pleural effusion | E. faecium | VAN A | >256 | >256.00 | S | NA |
| 59 | Male | Blood | IHD-CAP-fever-DM-HF | E. faecium | NA | >16 | 1.00 | S | TAZ-CRO-AZT |
| 34 | Male | Blood | AIDS-PCP-Kaposi sarcoma, septic shock | E. faecium | VAN A | >256 | 16.00 | S | NA |
| 35 | Male | Blood | CML, fever | E. faecium | VAN B | 32 | 0.25 | S | COL, VAN |
| 65 | Male | Urine | P-CABG | E. faecium | VAN B | 16 | 1.00 | R | VAN + MER |
| 34 | Female | Blood | AML-central line | E. faecium | VAN A | >256 | >256.00 | S | AK-CAZ-VAN |
| 67 | Female | Wound swab | Postlaparotomy | E. faecium | NA | 16 | 1.00 | S | MER-LZD |
| 62 | Female | Blood | Postcardiac surgery, tracheostomy | E. faecium | VAN A | >256 | >256.00 | S | NA |
| 82 | Male | Blood | Postlaparotomy | E. faecium | VAN B | >32 | >16.00 | S | NA |
| 72 | Female | Blood | P-CABG | E. faecium | >16/24 | 0.75 | S | MER-VAN | |
| 44 | Male | Wound swab | DM, HTN, sacral infected wound | E. faecium | VAN B | >16 | 0.25 | S | MER-VAN |
| 75 | Female | Blood | Brachial artery thrombosis | E. faecium | VAN B | >16 | >0.25 | S | VAN |
| 1 | Female | Blood | Brain tumor, neutropenia, fever, chemotherapy | E. faecium | NA | >32 | >32.00 | S | NA |
| 2* | Female | Blood | Short bowel syndrome-fever | E. gallinarum | NA | 6 | 1.00 | S | VAN + MTZ |
| 77 | Male | Wound swab | Septic arthritis, fever | E. gallinarum | NA | 16 | 1.00 | S | NA |
| 33 | Female | Wound swab | Polymyositis | E. gallinarum | NA | 4 | 0.25 | S | NA |
*2 months old. P-CABG: Postcoronary artery bypass grafting; DM: Diabetes mellitus; HTN: Hypertension; IHD: Ischemic heart disease; HF: Heart failure; CAP: Community acquired pneumonia; AML: Acute myeloid leukemia; CML: Chronic myeloid leukemia, AIDS: Acquired immune deficiency syndrome; PCP: Pneumocystis carinii pneumonia; NA: Not available; TAZ: Tazocin (piperacillin and tazobactam); CRO: Ceftriaxone; AZT: Aztreonam; VAN: Vancomycin; MTZ: Metronidazole; MER: Meropenem; LZD: Linezolid; AK: Amikacin; CAZ: Ceftazidime; COL: Colistin; R: Resistant; S: Sensitive; VRE: Vancomycin-resistant enterococci; E. faecium: Enterococcus faecium; E. gallinarum: Enterococcus gallinarum; TP: Teicoplanin; ICU: Intensive Care Unit.
Table 6.
Clinical characteristics and microbiological details of VRE patients admitted to medical and surgical wards
| Age (years) | Gender | Specimen | Clinical data | Enterococcus spp. | Resistance phenotype | VAN (mg/ml) | TP (mg/ml) | LZD | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| 69 | Female | Blood | Massive pulmonary embolism | E. faecium | VAN B | 16 | 0.25 | S | NA |
| 73 | Female | Blood | IHD-DM, HTN | E. faecalis | VAN B | 4 | 0.38 | S | NA |
| 54 | Female | Wound swab | Postlaparotomy | E. faecium | VAN B | >16 | 1.00 | S | MER-VAN |
| 50 | Male | Wound swab | DM, infected diabetic wound | E. faecium | VAN B | >24 | 0.75 | S | MER-VAN |
| 58 | Male | Blood | Postsplenectomy | E. gallinarum | VAN B | 6 | 1.00 | S | AN |
| 68 | Female | Body fluid | Ascites+liver disease | E. faecium | VAN B | 12 | 0.25 | S | NA |
| 73 | Male | Wound swab | Postsurgery | E. faecium | VAN A | >256 | 16.00 | S | VAN+TAZ |
| 66 | Female | Urine | Vomiting, vaginal bleeding | E. faecium | VAN B | >16 | 0.25 | S | NA |
| 71 | Male | Urine | Postcraniotomy | E. faecium | VAN B | 4 | 0.25 | S | NA |
| 57 | Male | Blood | Fever | E. faecalis | VAN B | 4 | 0.25 | S | NA |
| 77 | Male | Blood | IHD, CVA, encephalopathy | E. faecium | VAN B | >16 | 0.38 | S | NA |
| 65 | Male | Wound swab | Postsurgery | E. faecium | VAN B | >16 | 1.00 | S | NA |
IHD: Ischemic heart disease; DM: Diabetes mellitus; HTN: Hypertension; CVA: Cerebrovascular accidents; VAN: Vancomycin, MER: Meropenem; TAZ: Tazocin (piperacillin and tazobactam); TP: Teicoplanin; LZD: Linezolid; R: Resistant; S: Sensitive; NA: Not available; E. faecium: Enterococcus faecium; E. gallinarum: Enterococcus gallinarum; E. faecalis: Enterococcus faecalis; VRE: Vancomycin-resistant enterococci.
Table 5.
Clinical characteristics and microbiological details of VRE patients admitted to oncology wards
| Age (years) | Gender | Specimen | Clinical data | Enterococcus Spp. | Resistance phenotype | VAN (mg/ml) | TP (mg/ml) | LZD | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| 59 | Male | Blood | CA-breast, chemotherapy, peritoneal metastasis | E. faecium | VAN B | >16–24 | 0.25 | S | VAN + TAZ |
| 75 | Female | Ascetic fluid + blood | Terminal CA-colon, colectomy, stoma infection, fever | E. faecium | VAN B | 32 | 0.57 | S | CIP-AMP TP |
| 63 | Female | Blood | CA-pancreas, DM, postoperative, fever | E. faecium | VAN B | 16 | 1.00 | R | CXM-MTZ |
| 64 | Female | Blood | CA-breast-postmastectomy, chemotherapy, fever -neutropenia | E. faecium | VAN A | >256 | 24.00 | S | NA |
| 60 | Female | Blood | Cholangiocarcinoma, fever, postcholecystectomy, metastasis-peritonitis | E. faecium | VAN B | 16 | 1.00 | S | MER-LZD-CRO |
| 40 | Male | Blood | Multiple myeloma | E. faecium | VAN B | 16 | >0.38 | S | AN |
| 30 | Male | Wound swab | CA-stomach, postgastrectomy, wound infection | E. gallinarum | VAN B | >32 | 0.75 | S | VAN + CIP |
| 60 | Female | Urine | CA-esophagus | E. faecium | VAN A | >256 | >16.00 | S | VAN |
| 80 | Male | Urine | Multiple myeloma | E. faecium | VAN B | >16 | 0.38 | S | NA |
| 25 | Female | Blood | AML | E. faecium | VAN B | >16 | 1.00 | S | NA |
| 43 | Female | Blood | Lymphoma | E. faecium | VAN B | >16 | 1.00 | S | NA |
| 91 | Male | Urine | CA-prostate-bone metastasis | E. faecium | VAN B | >16 | 0.25 | S | NA |
CA: Cancer; AML: Acute myeloid leukemia; CXM: Cefuroxime; MER: Meropenem; TAZ: Tazocin (piperacillin and tazobactam); CRO: Ceftriaxone; VAN: Vancomycin; MTZ: Metronidazole; LZD: Linezolid; AMP: Ampicillin; CIP: Ciprofloxacin; TP: Teicoplanin; R: Resistant; S: Sensitive; NA: Not available; VRE: Vancomycin-resistant enterococci; E. faecium: Enterococcus faecium; E. gallinarum: Enterococcus gallinarum.
Discussion
In the present study, the overall prevalence of enterococci was 231 isolates. In a study from Riyadh, a total of 206 enterococci were isolated from 240 patients with nosocomial infections, with specimens collected from in-patients and out-patients at King Khalid University Hospital (140 specimens) and King Saud Medical City Hospital (100 specimens).[13] The prevalence of VRE in this study is 17.3%, consistent with a report from a hospital in Brazil (15.8%).[14] A higher isolation rate of VRE was reported from Iran (23.7%) and from pediatric hematology/oncology patients in Egypt (75.0%).[15,16] These differences might be due to the study design and study population (pediatric age) in the previous study. A study from a University Hospital in Belgium revealed even a higher incidence of VRE 586/1260 (46.5%).[17] In a recent national surveillance of antimicrobial resistance among Gram-positive Bacteria in Saudi Arabia involving 13750 isolates, enterococci account only for 3·1%.[18] In Europe, surveillance data showed variable VRE rates among different countries ranging from <2.0% (Finland, Holland) to >20% (Ireland, Greece, Portugal). Italy is a country with a low rate level (4.2%).[19] Rates of VRE in the USA hospitals reached 33.0%.[20] Similar to other studies, we found that the maximum number of isolates were obtained from urine followed by blood and wound swab.[13,14,15] However, in one study, the maximum number of isolates were obtained from pus (43.0%), followed by urine (31.0%).[21] More interestingly, we found that blood was the most common specimens received from VRE-infected patients in the ICU, oncology, and surgical and medical wards. Most of those patients were critically ill. Several studies have shown that infections caused by VRE, particularly bloodstream infections, are more serious and associated with higher mortality rate compared to those caused by vancomycin-susceptible enterococci (VSE).[22,23,24] Moreover, we found that 30.0% (12/40) of our VRE-infected patients were oncology patients and 40.0% (16/40) were ICU patients. Screening for and identifying VRE colonization prior to ICU and oncology wards will provide an opportunity to initiate strict infection control measures and prevent nosocomial transmission. In a retrospective review of 2115 cancer and transplant recipient patients, VRE fecal colonization was documented in 5.4% of patients with leukemia, 4.9% of hematopoietic stem cell transplantation recipients, and 2.2% of patients with lymphoma. Twenty-nine (29.3%) of those developed bacteremia.[25] The Enterococcus isolates obtained in the study were mostly E. faecalis (72.4%) followed by E. faecium (22.8%). This species distribution is similar to that reported from a regional study in which among 206 of enterococcal isolates, 166 (69.2%) were identified as E. faecalis and 27 (11.3%) as E. faecium.[13] Sreeja et al.[21] reported a similar result in their study that E. faecalis (76.0%) formed the major isolate, followed by E. faecium (24.0%). Similarly, in a study from Iran, 106 (57.0%) isolates were identified as E. faecalis and 80 (43.0%) of the isolates were identified as E. faecium.[15] An increase in the isolation rate of E. faecium and other non-faecalis species of Enterococcus was found in one study.[26] It has been well documented that ICUs are major reservoirs of VRE in the health care setting.[27] VRE colonization on admission to the ICU is a major determinant of VRE infection. In a meta-analysis of published studies to assess trends of VRE colonization in the ICU, 7.1–10.6% of patients admitted to the ICU were colonized with VRE on admission.[28] The risk of VRE infection in the ICU among colonized patients (0–45.0%) was higher than the risk of VRE infection among noncolonized cases (<2.0%). Susceptible populations, such as solid organ transplant recipients,[29] cancer patients,[25] and allogeneic hematopoietic stem cell transplant recipients,[30] are at greatest risk of colonization and subsequent infection and bacteremia. In addition, VRE colonization was identified in 184 ICU patients (17.6%) for whom routine perianal swab cultures were obtained on ICU admission to a tertiary hospital in Korea.[31] Of these, 11.9% developed VRE infection. Interestingly, in the study, there was a greater prevalence of VRE among patients admitted in the ICU and oncology areas. These results are in agreement with that reported in a Saudi study which recovered 85.0% of the total VRE isolates from the ICU; 112 (46.7%) from the surgical ward, 67 (27.9%) and 27 (11.3%) from the internal medicine ward.[13] The same finding was reported from Brazil, in which the maximum prevalence of VRE was found in patients admitted in ICUs (38.2%), followed by the emergency ward (23.5%).[14] More important, nearly 69.0% of ICU patients in the study developed septicemia. Almost all of these patients were critically ill with complex underlying pathology. The presence of severe comorbidity and combined infection with organisms other than VRE are possible risk factors for the development of VRE bacteremia in our ICU patients. Four of the VRE isolates recovered from the ICU patients were highly resistant to vancomycin and all were of the E. faecium species. Varying levels of resistance was seen to various antibiotics. Most of the E. faecium isolates were resistant to ampicillin (96.0%) with the least resistance level to linezolid (11.3%). On the other hand, compared to E. faecium, E. faecalis showed a more susceptible antimicrobial profile to all tested antibiotics. Our VRE-infected patients have limited therapeutic options to other clinically useful antibiotics, including teicoplanin, ciprofloxacin, and high-level gentamicin, leading to potentially more serious outcome and high fatality.
This study had several limitations. First, we had a relatively small number of studied patients, thus reducing the statistical power of the results. Second, we had difficulty in determining VRE infection versus colonization from current or prior hospitalization for each individual case. Third, there was a lack of molecular studies that might have led to a better understanding of the epidemiology and source of infection of VRE-colonized or infected patients.
In conclusion, the presence of high prevalence of VRE in this study signals the emergence of more VRE-infected patients, particularly in critical areas such as ICUs and oncology units. Therefore, the rational use of antibiotics and a more detailed study using phenotypic and genotypic methods are needed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
References
- 1.Emori TG, Gaynes RP. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev. 1993;6:428–42. doi: 10.1128/cmr.6.4.428. doi: 10.1128/CMR.6.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moellering RC., Jr Emergence of Enterococcus as a significant pathogen. Clin Infect Dis. 1992;14:1173–6. doi: 10.1093/clinids/14.6.1173. doi: 10.1093/clinids/14.6.1173. [DOI] [PubMed] [Google Scholar]
- 3.O’Driscoll T, Crank CW. Vancomycin-resistant enterococcal infections: Epidemiology, clinical manifestations, and optimal management. Infect Drug Resist. 2015;8:217–30. doi: 10.2147/IDR.S54125. doi: 10.2147/IDR.S54125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cetinkaya Y, Falk P, Mayhall CG. Vancomycin-resistant enterococci. Clin Microbiol Rev. 2000;13:686–707. doi: 10.1128/cmr.13.4.686-707.2000. doi: 10.1128/CMR.13.4.686-707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray BE. Vancomycin-resistant enterococcal infections. N Engl J Med. 2000;342:710–21. doi: 10.1056/NEJM200003093421007. doi: 10.1056/NEJM200003093421007. [DOI] [PubMed] [Google Scholar]
- 6.Mundy LM, Sahm DF, Gilmore M. Relationships between enterococcal virulence and antimicrobial resistance. Clin Microbiol Rev. 2000;13:513–22. doi: 10.1128/cmr.13.4.513-522.2000. doi: 10.1128/CMR.13.4.513-522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zirakzadeh A, Patel R. Vancomycin-resistant enterococci: Colonization, infection, detection, and treatment. Mayo Clin Proc. 2006;81:529–36. doi: 10.4065/81.4.529. doi: 10.4065/81.4.529. [DOI] [PubMed] [Google Scholar]
- 8.Shenoy ES, Paras ML, Noubary F, Walensky RP, Hooper DC. Natural history of colonization with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE): a systematic review. BMC infectious diseases. 2014;14:177. doi: 10.1186/1471-2334-14-177. doi: 10.1186/1471-2334-14-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christidou A, Gikas A, Scoulica E, Pediaditis J, Roumbelaki M, Georgiladakis A, et al. Emergence of vancomycin-resistant enterococci in a tertiary hospital in Crete, Greece: A cluster of cases and prevalence study on intestinal colonisation. Clin Microbiol Infect. 2004;10:999–1005. doi: 10.1111/j.1469-0691.2004.00992.x. doi: 10.1111/j.1469-0691.2004.00992.x. [DOI] [PubMed] [Google Scholar]
- 10.Willems RJ, Top J, van Santen M, Robinson DA, Coque TM, Baquero F, et al. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg Infect Dis. 2005;11:821–8. doi: 10.3201/eid1106.041204. doi: 10.3201/eid1106.041204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin P, Williams V, Bush K, Dyck M, Hirji Z, Larios OE, et al. Martin, Philippe. “The Prevalence of Methicillin-Resistant Staphylococcus aureus, Vancomycin-Resistant Enterococcus, Extended-Spectrum Beta-lactamase-producing Enterobacteriaceae, Carbapenem-Resistant Enterobacteriaceae and Clostridium difficile Infection in Canadian Hospitals. A Comparison of Survey Results in 2010 2012 and 2016.”. Open Forum Infect Dis. 2016;3(suppl 1) doi: 10.1093/ofid/ofw172.1193. [Google Scholar]
- 12.Performance Standards for Antimicrobial Susceptibility Testing, 17th The Informational Supplement, M100-S17. Wayne, PA: Clinical and Laboratory Standards Institute; 2007. [Last accessed 2016 Dec 27]. The Clinical and Laboratory Standards Institute. Available from: https://www.science.report/pub/15127014 . [Google Scholar]
- 13.Salem-Bekhit MM, Moussa IM, Muharram MM, Alanazy FK, Hefni HM. Prevalence and antimicrobial resistance pattern of multidrug-resistant enterococci isolated from clinical specimens. Indian J Med Microbiol. 2012;30:44–51. doi: 10.4103/0255-0857.93032. doi: 10.4103/0255-0857.93032. [DOI] [PubMed] [Google Scholar]
- 14.Furtado GH, Martins ST, Coutinho AP, Soares GM, Wey SB, Medeiros EA. Incidence of vancomycin-resistant Enterococcus at a university hospital in Brazil. Rev Saude Publica. 2005;39:41–6. doi: 10.1590/s0034-89102005000100006. doi: 10.1590/S0034-89102005000100006. [DOI] [PubMed] [Google Scholar]
- 15.Kafil HS, Asgharzadeh M. Vancomycin-resistant Enterococcus faecium and Enterococcus faecalis isolated from education hospital of Iran. Maedica (Buchar) 2014;9:323–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Tonbary YA, Soliman OE, Sarhan MM, Hegazi MA, El-Ashry RA, El-Sharkawy AA, et al. Nosocomial infections and fever of unknown origin in pediatric hematology/oncology unit: A retrospective annual study. World J Pediatr. 2011;7:60–4. doi: 10.1007/s12519-010-0212-1. doi: 10.1007/s12519-010-0212-1. [DOI] [PubMed] [Google Scholar]
- 17.Vandamme P, Vercauteren E, Lammens C, Pensart N, Ieven M, Pot B, et al. Survey of enterococcal susceptibility patterns in Belgium. J Clin Microbiol. 1996;34:2572–6. doi: 10.1128/jcm.34.10.2572-2576.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibl AM, Memish ZA, Kambal AM, Ohaly YA, Ishaq A, Senok AC, et al. National surveillance of antimicrobial resistance among Gram-positive bacteria in Saudi Arabia. J Chemother. 2014;26:13–8. doi: 10.1179/1973947813Y.0000000084. doi: 10.1179/1973947813Y.0000000084. [DOI] [PubMed] [Google Scholar]
- 19.European Antimicrobial Resistance Surveillance System (EARSS). Annual Report. 2011. [Last accessed on 2013 Oct 09]. Available from: http://www.rivm.nl/earss .
- 20.Ramsey AM, Zilberberg MD. Secular trends of hospitalization with vancomycin-resistant Enterococcus infection in the United States 2000-2006. Infect Control Hosp Epidemiol. 2009;30:184–6. doi: 10.1086/593956. doi: 10.1086/593956. [DOI] [PubMed] [Google Scholar]
- 21.Sreeja S, Babu PR S, Prathab AG. The prevalence and the characterization of the Enterococcus species from various clinical samples in a tertiary care hospital. J Clin Diagn Res. 2012;6:1486–8. doi: 10.7860/JCDR/2012/4560.2539. doi: 10.7860/JCDR/2012/4560.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmeli Y, Eliopoulos G, Mozaffari E, Samore M. Health and economic outcomes of vancomycin-resistant enterococci. Arch Intern Med. 2002;162:2223–8. doi: 10.1001/archinte.162.19.2223. doi: 10.1001/archinte.162.19.2223. [DOI] [PubMed] [Google Scholar]
- 23.Hautemanière A, Hunter PR, Diguio N, Albuisson E, Hartemann P. A prospective study of the impact of colonization following hospital admission by glycopeptide-resistant enterococci on mortality during a hospital outbreak. Am J Infect Control. 2009;37:746–52. doi: 10.1016/j.ajic.2009.02.007. doi: 10.1016/j.ajic.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Sakka V, Tsiodras S, Galani L, Antoniadou A, Souli M, Galani I, et al. Risk-factors and predictors of mortality in patients colonised with vancomycin-resistant enterococci. Clin Microbiol Infect. 2008;14:14–21. doi: 10.1111/j.1469-0691.2007.01840.x. doi: 10.1111/j.1469-0691.2007.01840.x. [DOI] [PubMed] [Google Scholar]
- 25.Matar MJ, Tarrand J, Raad I, Rolston KV. Colonization and infection with vancomycin-resistant Enterococcus among patients with cancer. Am J Infect Control. 2006;34:534–6. doi: 10.1016/j.ajic.2006.04.205. doi: 10.1016/j.ajic.2006.04.205. [DOI] [PubMed] [Google Scholar]
- 26.Low DE, Keller N, Barth A, Jones RN. Clinical prevalence, antimicrobial susceptibility, and geographic resistance patterns of enterococci: Results from the SENTRY Antimicrobial Surveillance Program 1997–1999. Clinical Infectious Diseases. 2001;(Supplement 2):S133–S45. doi: 10.1086/320185. doi: 10.1086/320185. [DOI] [PubMed] [Google Scholar]
- 27.Lin MY, Hayden MK. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: Recognition and prevention in Intensive Care Units. Crit Care Med. 2010;38(8 Suppl):S335–44. doi: 10.1097/CCM.0b013e3181e6ab12. doi: 10.1097/CCM.0b013e3181e6ab12. [DOI] [PubMed] [Google Scholar]
- 28.Ziakas PD, Thapa R, Rice LB, Mylonakis E. Trends and significance of VRE colonization in the ICU: A meta-analysis of published studies. PLoS One. 2013;8:e75658. doi: 10.1371/journal.pone.0075658. doi: 10.1371/journal.pone.0075658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel R, Allen SL, Manahan JM, Wright AJ, Krom RA, Wiesner RH, et al. Natural history of vancomycin-resistant enterococcal colonization in liver and kidney transplant recipients. Liver Transpl. 2001;7:27–31. doi: 10.1053/jlts.2001.20784. doi: 10.1053/jlts.2001.20784. [DOI] [PubMed] [Google Scholar]
- 30.Weinstock DM, Conlon M, Iovino C, Aubrey T, Gudiol C, Riedel E, et al. Colonization, bloodstream infection, and mortality caused by vancomycin-resistant Enterococcus early after allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2007;13:615–21. doi: 10.1016/j.bbmt.2007.01.078. doi: 10.1016/j.bbmt.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 31.Kim YJ, Kim SI, Kim YR, Lee JY, Park YJ, Kang MW. Risk factors for vancomycin-resistant enterococci infection and mortality in colonized patients on Intensive Care Unit admission. Am J Infect Control. 2012;40:1018–9. doi: 10.1016/j.ajic.2012.01.009. doi: 10.1016/j.ajic.2012.01.009. [DOI] [PubMed] [Google Scholar]


