Abstract
Introduction:
Steinstrasse (SS) is a known complication of shock wave lithotripsy (SWL). Although the majority of SS clears spontaneously, about 6% require intervention. This study was carried out to identify the factors that determine the need for intervention in SS.
Materials and Methods:
This was a retrospective study of all patients who developed steinstrasse following SWL at our center. They were divided into two groups: a) Those cleared spontaneously and b) Those required intervention. The two groups were compared with regard to demographic profile, stone factors and factors related to steinstrasse.
Results:
Out of 2436 cases of SWL, 89 (3%) formed steinstrasse. The majority of the patients (35%) who required intervention had stone sizes of 10-14 mm. Coptcoat type III steinstrasse required significantly more interventions for clearance (P = 0.001). The site and the size of the SS was not a predictor of intervention for SS.
Conclusions:
Early intervention is warranted in patients with steinstrasse where the lead fragment is >5 mm (Coptcoat type III).
Keywords: Interventions, shock wave lithotripsy, steinstrasse
INTRODUCTION
The shock wave lithotripsy (SWL) began after the pioneering work of Dornier, at the Urology University at Munich and was accepted by the US Food and Drug Administration in 1984.[1] It is still effective in treating renal calculi with lesser hospital stay and duration of treatment.[2] Steinstrasse (SS) or “stone street,” is an aggregation of particles in the ureter formed following extracorporeal SWL. This is a well-recognized, transient, and usually asymptomatic complication of SWL that occurs in 4%–7%.[3] It is seen in 15% of routine radiographic images taken 24 and 48 h after lithotripsy.[4] Although the majority of SS clears spontaneously, about 6% require intervention.[5] Patients with SS are initially treated conservatively, but in the case of complications such as obstruction, infection, pain or failed passage of the fragments, further treatment is recommended. Prompt intervention can reduce morbidity. In this study, we tried to determine the factors that predict the need for intervention in a patient with SS.
MATERIALS AND METHODS
This was a retrospective study spanning 6 years from June 2005 to June 2011. All patients who had developed SS following SWL for renal/ureteric calculi were eligible for inclusion. A Dornier compact Delta II Lithotripter was used for SWL in all cases. Sedoanalgesia (5 mg morphine and 25 mg pethidine) was used with electrocardiogram and pulse oximeter monitoring. Shock sequence was performed using gradual ramping-starting at 8 kV and gradually increased to 14 kV for renal calculi and 16 kV for ureteric calculi. It was increased one level after each set of 25 shocks at a frequency of 70/min for renal and 80/min for ureter. Renal stones were given 1500 shocks/session and ureteric stones 2000 shocks/session. Fragmentation was assessed by fluoroscopy during the procedure and with plain X-rays at 7 days following SWL. All patients received alpha blockers (tamsulosin 0.4 mg) daily from the first session of SWL. SS was defined as an aggregation of stone particles in the ureter seen on a plain X-ray following SWL. It has been classified by Coptcoat et al.[4] into three types - Type I is made up of particles 2 mm in diameter or smaller. Type II has a leading large fragment of 4–5 mm in diameter with a tail of 2-mm particles. Type III is composed of large fragments >5 mm.[4]
The indications for intervention in SS are - rising creatinine levels, urosepsis, and failure to pass fragments within a reasonable time. Treatment is required in the presence of symptoms (pain and sepsis) or a silent obstruction over a 30-day period.[6] Treatment options include placement of a percutaneous nephrostomy (PCN) to allow fragments to pass, ureteroscopy and transurethrallithotripsy, SWL of a lead fragment or open ureterolithotomy.[7] At our center, we observe patients with SS for 2 weeks if there is gravelluria and are otherwise asymptomatic. However, if there is no passage of fragments for 1 week and X-ray kidney, ureter, and bladder (KUB) shows the persistence of the stone then intervention is planned as our patients come from far areas. In case of features of suspected sepsis-like high-grade fever with chills, severe loin pain, vomiting, or debris in the (pelvicalyceal system) on ultrasound a PCN is carried out.
Patients with SS were divided into two groups. Group A (n = 42) where there was a spontaneous clearance of SS and Group B (n = 47) where intervention was required. The two groups were compared with regard to demographic profile (age, sex, and comorbidities), stone factors (original size, side involved, site of calculi, obstruction) and SS factors (Coptcoat type, length of SS, site of SS) to determine any predictive factors for intervention. We performed a univariate analysis of potential factors associated with the need for intervention. We used IBM SPSS Version 16, SPSS Inc, Chicago, USA. P value was determined using paired t-test.
RESULTS AND OBSERVATIONS
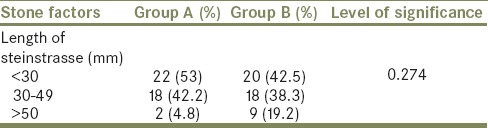
The flowchart for our study is shown in Table 1. Of 2436 patients who received SWL, only 89 (3%) formed SS. Of the 89 patients with SS 42 (47%) had spontaneous clearance and 47 (53%) required interventions. Thirty-seven required SWL to the lead fragment, six required PCN insertion and four required ureteroscopic lithotripsy and Double J (DJ) stenting. Both groups were comparable with regard to their demographic profile [Table 2]. Most of the patients who formed SS and required intervention had stone sizes of 10–14 mm but this was not statistically significant [Table 3]. Although larger stones were more liable to form SS, the initial stone size did not correlate with need for intervention [Table 3]. The grade of hydronephrosis had no association with the clearance of SS (P= 0.2). Coptcoat type III SS required significantly more interventions for clearance (P = 0.001) [Table 4]. The majority of the SS were located in the distal ureter (77%), followed by the proximal ureter (17%), and the mid ureter (6%). The site or the lengths of SS were not significantly associated with the requirement for intervention [Table 5].
Table 1.
Flowchart
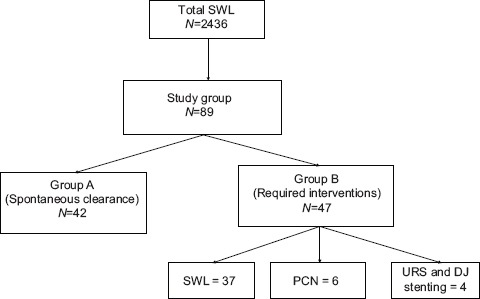
Table 2.
Demographic profile
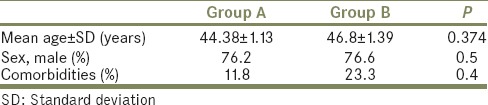
Table 3.
Size of the stone and formation of steinstrasse
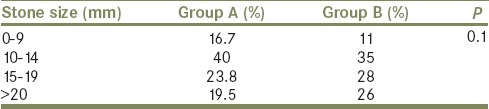
Table 4.
Type of steinstrasse (Coptcoat) and intervention
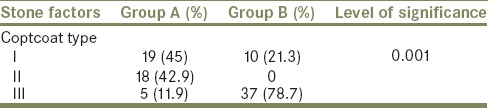
Table 5.
Length of the steinstrasse and intervention
DISCUSSION
With the advent of better lithotripters and the increasing popularity of endoscopic procedures such as mini-perc and RIRS, the incidence of SS has dramatically fallen.[8,9] In the early studies of SWL, SS was common, occurring in up to 20% of patients.[10,11] However after refining the technique like gradual ramping, gradual increase in the number of shocks (kV), low energy shock wave for disintegration of the stones and also better lithotripters, the incidence of SS decreased. With comprehensive understanding of the pathophysiology of calculi and shock waves there is a much better result and encourage SWL as the first line therapy at present.[12] The incidence of SS varies from 4% to 7%.[3,5,13] Most of the SS were located in the distal ureter (77%), followed by the proximal ureter (17%) and the mid ureter (6%) as in other series.[10,14]
Contrary to the findings by several groups that larger stones formed larger SS[4,10,13] we found that most of the SS were formed by the calculus sized between 10 mm and 14 mm.
It was shown that the use of DJ stents preoperatively lowers the incidence of SS in stones with size more than 1.5 cm[15] whereas recent report did not support stenting.[16] However, none of the patients in our study were stented before the SWL. As per the department policy, we place a stent in conjunction with SWL only for solitary kidneys with stone size >15 mm. The use of tamsulosin, a α1A, and α1D receptor antagonist concurrently with SWL improves the outcome of SS.[17,18] Stone size and the site have been shown to be significant predictive factors determining SS formation.[5] In the same series, it was also shown that the chance of SS formation was 3.7 times less when stone size was <2 cm. It was also shown that the incidence of SS was 2.7 times less for lumbar ureteral stones compared to renal stones. Many studies have shown that high renal intrapelvic pressure is associated with reduced or absent renal pelvic motility and thereby inhibiting pelvic and ureteral peristalsis.[19] Hence, radiologically dilated systems have less propulsive power and decreased antegrade fluid pressure with more probability of failure of lithotripsy. However, in this study, the grade of hydronephrosis did not have any effect on the clearance of SS (P = 0.2).
In our series, the need for intervention was not shown to be significantly associated with the length of the SS (P = 0.274). A similar observation was made in a study of 885 patients with urinary stones (650 renal and 235 ureteric).[14]
The only factor which we found to be significantly associated with the prediction for intervention was the type of SS particularly Coptcoat type III (P = 0.001). In 1988 Coptcoat et al.[4] concluded that type III will always require intervention and there is little to be gained by waiting for spontaneous passage. Another series of 1647 patients found that 12 (0.73%) and 17 (1.03%) patients formed Coptcoat type II and III SS, respectively. Most cleared with SWL to the leading fragment. Only two in the type III SS required additional interventions like DJ stenting and PCN.[20]
Being a retrospective study, this series has its inherent limitations. There was no solitary kidney in the study. The length of the SS was measured using the PACS software available in the institution, which may not be accurate. Noncontrast computerized tomography KUB was not done as the risk of radiation outweighs the benefit.
CONCLUSION
Early intervention is warranted in patients with SS where the lead fragment is >5 mm (Coptcoat type III).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Chaussy C, Schmiedt E, Jocham D, Brendel W, Forssmann B, Walther V. First clinical experience with extracorporeally induced destruction of kidney stones by shock waves. J Urol. 1982;127:417–20. doi: 10.1016/s0022-5347(17)53841-0. [DOI] [PubMed] [Google Scholar]
- 2.Srisubat A, Potisat S, Lojanapiwat B, Setthawong V, Laopaiboon M. Extracorporeal shock wave lithotripsy (ESWL) versus percutaneous nephrolithotomy (PCNL) or retrograde intrarenal surgery (RIRS) for kidney stones. Cochrane Database Syst Rev. 2014;11:CD007044. doi: 10.1002/14651858.CD007044.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Ather MH, Shrestha B, Mehmood A. Does ureteral stenting prior to shock wave lithotripsy influence the need for intervention in steinstrasse and related complications? Urol Int. 2009;83:222–5. doi: 10.1159/000230028. [DOI] [PubMed] [Google Scholar]
- 4.Coptcoat MJ, Webb DR, Kellet MJ, Whitfield HN, Wickham JE. The steinstrasse: A legacy of extracorporeal lithotripsy? Eur Urol. 1988;14:93–5. doi: 10.1159/000472910. [DOI] [PubMed] [Google Scholar]
- 5.Madbouly K, Sheir KZ, Elsobky E, Eraky I, Kenawy M. Risk factors for the formation of a steinstrasse after extracorporeal shock wave lithotripsy: A statistical model. J Urol. 2002;167:1239–42. [PubMed] [Google Scholar]
- 6.Satar N, Doran S, Ozkeceli R, Turkyilmaz R. Treatment of the multiple small stone particles (steinstrasse) in the lover ureter after the extracorporeal shockwave lithotripsy (ESWL) treatment. Turkish Journal of Medical Sciences. 1998;28:269–71. [Google Scholar]
- 7.Mandhani A, Ansari MS, Srivastava A, Kapoor R, Kumar A, Goyal R, et al. Does the type of steinstrasse predict the outcome of expectant therapy? Indian J Urol. 2006;22:135. [Google Scholar]
- 8.Weaver J, Monga M. Extracorporeal shockwave lithotripsy for upper tract urolithiasis. Curr Opin Urol. 2014;24:168–72. doi: 10.1097/MOU.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 9.Rassweiler J, Rassweiler MC, Frede T, Alken P. Extracorporeal shock wave lithotripsy: An opinion on its future. Indian J Urol. 2014;30:73–9. doi: 10.4103/0970-1591.124211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fedullo LM, Pollack HM, Banner MP, Amendola MA, Van Arsdalen KN. The development of steinstrassen after ESWL: Frequency, natural history, and radiologic management. AJR Am J Roentgenol. 1988;151:1145–7. doi: 10.2214/ajr.151.6.1145. [DOI] [PubMed] [Google Scholar]
- 11.Schnabel MJ, Brummeisl W, Burger M, Rassweiler JJ, Knoll T, Neisius A, et al. Shock wave lithotripsy in Germany: Results of a nationwide survey. Urologe A. 2015;54:1277–82. doi: 10.1007/s00120-015-3920-2. [DOI] [PubMed] [Google Scholar]
- 12.Neisius A, Lipkin ME, Rassweiler JJ, Zhong P, Preminger GM, Knoll T. Shock wave lithotripsy: The new phoenix? World J Urol. 2015;33:213–21. doi: 10.1007/s00345-014-1369-3. [DOI] [PubMed] [Google Scholar]
- 13.Kim SC, Oh CH, Moon YT, Kim KD. Treatment of steinstrasse with repeat extracorporeal shock wave lithotripsy: Experience with piezoelectric lithotriptor. J Urol. 1991;145:489–91. doi: 10.1016/s0022-5347(17)38376-3. [DOI] [PubMed] [Google Scholar]
- 14.Sayed MA, el-Taher AM, Aboul-Ella HA, Shaker SE. Steinstrasse after extracorporeal shockwave lithotripsy: Aetiology, prevention and management. BJU Int. 2001;88:675–8. doi: 10.1046/j.1464-4096.2001.02435.x. [DOI] [PubMed] [Google Scholar]
- 15.Al-Awadi KA, Abdul Halim H, Kehinde EO, Al-Tawheed A. Steinstrasse: A comparison of incidence with and without J stenting and the effect of J stenting on subsequent management. BJU Int. 1999;84:618–21. doi: 10.1046/j.1464-410x.1999.00280.x. [DOI] [PubMed] [Google Scholar]
- 16.Ozkan B, Dogan C, Can GE, Tansu N, Erozenci A, Onal B. Does ureteral stenting matter for stone size. A retrospective analyses of 1361 extracorporeal shock wave lithotripsy patients? Cent European J Urol. 2015;68:358–64. doi: 10.5173/ceju.2015.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhagat SK, Chacko NK, Kekre NS, Gopalakrishnan G, Antonisamy B, Devasia A. Is there a role for tamsulosin in shock wave lithotripsy for renal and ureteral calculi? J Urol. 2007;177:2185–8. doi: 10.1016/j.juro.2007.01.160. [DOI] [PubMed] [Google Scholar]
- 18.Mohamed HI. The efficacy of tamsulosin therapy after extracorporeal shock-wave lithotripsy for ureteric calculi: A prospective randomised, controlled study. Arab J Urol. 2013;11:398–404. doi: 10.1016/j.aju.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan PC, Lennon GM, McLean PA, Fitzpatrick JM. The effects of acute and chronic JJ stent placement on upper urinary tract motility and calculus transit. Br J Urol. 1994;74:434–9. doi: 10.1111/j.1464-410x.1994.tb00418.x. [DOI] [PubMed] [Google Scholar]
- 20.Tayib A, Mosli H, Farsi H, Atwa M, Saada H. Five years experience in the management of steinstrasse post shock wave lithotripsy. J King Abdulaziz Univ Med Sci. 2008;15:71–9. [Google Scholar]


