Abstract
Background:
Limited studies have reported on radiation risks of increased ionizing radiation exposure to medical personnel in the urologic community. Fluoroscopy is readily used in many urologic surgical procedures. The aim of this study was to determine radiation exposure to all operating room personnel during percutaneous nephrolithotomy (PNL), commonly performed for large renal or complex stones.
Materials and Methods:
We prospectively collected personnel exposure data for all PNL cases at two academic institutions. This was collected using the Instadose™ dosimeter and reported both continuously and categorically as high and low dose using a 10 mrem dose threshold, the approximate amount of radiation received from one single chest X-ray. Predictors of increased radiation exposure were determined using multivariate analysis.
Results:
A total of 91 PNL cases in 66 patients were reviewed. Median surgery duration and fluoroscopy time were 142 (38–368) min and 263 (19–1809) sec, respectively. Median attending urologist, urology resident, anesthesia, and nurse radiation exposure per case was 4 (0–111), 4 (0–21), 0 (0–5), and 0 (0–5) mrem, respectively. On univariate analysis, stone area, partial or staghorn calculi, surgery duration, and fluoroscopy time were associated with high attending urologist and resident radiation exposure. Preexisting access that was utilized was negatively associated with resident radiation exposure. However, on multivariate analysis, only fluoroscopy duration remained significant for attending urologist radiation exposure.
Conclusion:
Increased stone burden, partial or staghorn calculi, surgery and fluoroscopy duration, and absence of preexisting access were associated with high provider radiation exposure. Radiation safety awareness is essential to minimize exposure and to protect the patient and all providers from potential radiation injury.
Keywords: Fluoroscopy, kidney calculi/surgery, percutaneous nephrostomy, radiation dosage
INTRODUCTION
Percutaneous nephrolithotomy (PNL) is commonly performed for large or complex renal stones. Fluoroscopy remains the mainstay of intraoperative imaging worldwide.[1] Unfortunately, fluoroscopy predisposes both patients and providers to ionizing radiation. Extensive literature has evaluated the risk of radiation exposure to patients and surgeons during fluoroscopic procedures.[2,3] Continued efforts have been made to improve the efficient use of fluoroscopy and decrease radiation exposure in the operating room.[1,4,5]
Limited studies, however, have reported on the risks of increased radiation exposure to medical personnel in the urologic community. Throughout their career, providers may perform hundreds to thousands of procedures requiring radiation, subjecting them to increasing cumulative radiation dose and increasing the chances of stochastic effects. A previous retrospective study by Cohen et al. demonstrated that the 9-month total radiation exposure of an experienced academic urologist was well below the annual accepted limits (50 mSv/year whole body).[6] Although these results provide some reassurance, there is technically no consensus on a negligent dose of radiation to the operator and patient. Even minor amounts of radiation exposure can potentially result in adverse effects.[7] It is becoming increasingly important for the urologist to understand the principles of radiation safety to provide protection to the health-care staff and patient, as well as the urologist. California state law now requires all surgeons performing cases utilizing fluoroscopy to be in possession of a Fluoroscopy Supervisor and Operator Permit issued by the state Department of Public Health, to ensure appropriate training regarding radiation exposure (26 California Code of Regulations § 17-30463).
In this study, we prospectively examine covariates that predict the radiation exposure to urologists and other operating room personnel during PNL procedures.
MATERIALS AND METHODS
Between May 2013 and May 2014, fluoroscopy duration and radiation exposure of various operating room personnel during PNL were prospectively collected from 2 academic institutions: University of California, San Diego (UCSD) and University of Pittsburgh Medical Center (UPMC). All personnel wore lead aprons and thyroid shields during each procedure, as are standard of care. During each case, four different providers (urology attending physician, urology resident physician, anesthesia provider, and circulating nurse) wore Instadose™ radiation dosimeters (Mirion Technologies, Smyrna, GA, USA) on the outside of the providers' thyroid shields. The Instadose™ devices function similar to a thermoluminescent detector (TLD) but instead permits immediate readings after each case with a lower limit of detection of 1 mrem (0.01 mSv) and a minimum reportable dose of 3 mrem (0.03 mSv). A USB compatible detector enables device readings via personal computer. Accumulated dose stored on Instadose™ is processed through a proprietary algorithm. No control badge is required because background exposure is accounted for and removed through this algorithm.
PNL was carried out using previously described techniques.[8] Following each case, the device was read and the amount of radiation recorded. This reading erased the previous exposure, resetting the dosimeter, and allowing it to be reused for each subsequent case.
The study was conducted as a quality improvement study. Patient demographic data including age, body mass index (BMI), and gender were recorded. Preoperative radiographic imaging was utilized to determine stone location, stone type, stone burden (defined as the sum of the greatest axial cross-sectional areas of the stones as viewed on preoperative excretory urogram or noncontrast computed tomography), and presence of hydronephrosis. Perioperative data including presence of access preoperatively, utilized preoperative access, obtainment of additional access despite the presence of access preoperatively, a number of access obtained, surgery duration, fluoroscopy time, and intraoperative surgical complications were also collected.
Statistical tests were performed using Statistical Package for the Social Sciences (version 22.0, Chicago, IL, USA). Descriptive data are presented as total numbers (percentages) and medians (range) unless otherwise noted. Patient demographics and perioperative characteristics were compared between the two sites using independent t-tests, Mann–Whitney U, or Chi-squared tests as appropriate. Radiation exposure was transformed into a binary variable with ≥10 mrem (or the amount of radiation received from one single chest X-ray) as significant for high exposure; radiation exposure <10 mrem was considered low exposure.[9] Univariate analyses using Mann–Whitney U or Chi-squared tests were performed as appropriate to determine the candidate predictors of radiation exposure (P < 0.05). Multivariate analysis using binary logistic regression models was then performed to determine associations between radiation exposure and the candidate predictors. Significance was set at two-tailed P-value of 0.05.
RESULTS
A total of 91 cases from 66 unique patients were included in this study. Mean age was 51 years, median BMI was 29.7 kg/m2, and 54% of cases were female. Demographic information is shown in Table 1.
Table 1.
Patient demographics
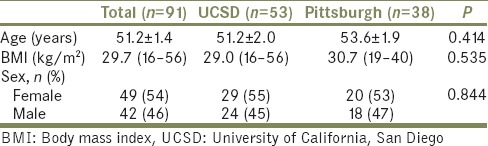
Perioperative characteristics are depicted in Table 2. Ninety-five percent of stones were located in the kidney, with 48% being either partial or full staghorn calculi. Axial stone cross-sectional area was 311 (12–2470) mm2. Ureteroscopy was also performed in addition to PNL in 41% of the procedures. Fifty-one percent of cases (n = 46) had previous access in the form of a preexisting nephrostomy tube, 85% of these cases (n = 39) utilized the preexisting access, whereas 15% (n = 7) had a new access. Moreover, in 7 patients with preexisting access, we utilized a new access in addition to the preexisting one. Sixteen percent of cases were repeat or second-look procedures. Thirty-seven percent of the patients presented with hydronephrosis on preoperative imaging. Median surgery duration and fluoroscopy time were 142 (38–368) min and 263 (19–1809) sec, respectively. All accesses performed intraoperative were conducted under fluoroscopic guidance by the surgical team.
Table 2.
Perioperative characteristics
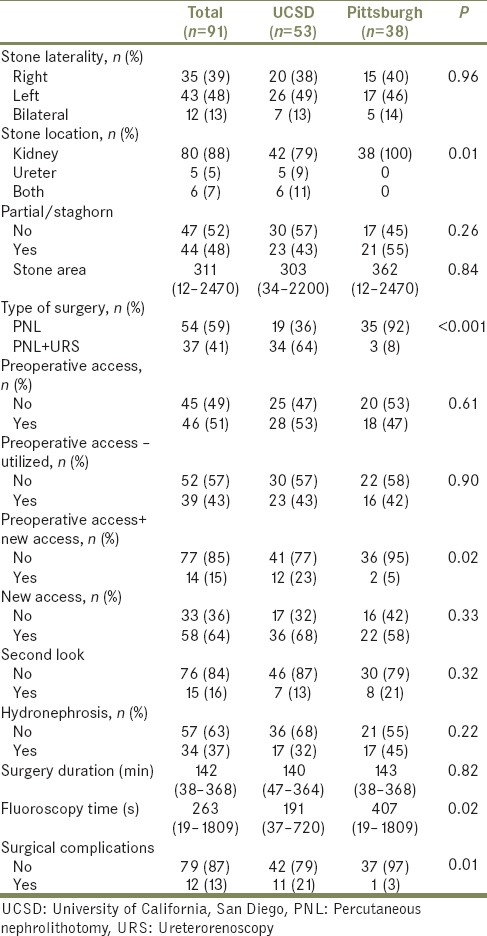
Twelve patients (13%) experienced complications. Five cases (5%) had a renal or ureteral perforation, one of which required termination. Seven additional cases, for a total of 9%, were terminated prematurely due to complications. Two of these were due to inability to obtain access, two due to difficult ventilation, one due to hypothermia, one due to ongoing bleeding, and one due to pneumothorax during attempted upper pole access.
Median radiation exposure for the attending urologist, resident, anesthesia, and circulating nurse was 4 (0–111), 4 (0–21), 0 (0–5), and 0 (0–5) mrem, respectively, as shown in Table 3. Mean radiation exposure for the attending urologist, resident, anesthesia, and circulating nurse was 8.13 ± 1.62, 4.07 ± 0.48, 0.28 ± 0.11, and 0.31 ± 0.12 mrem, respectively. The attending urologist and resident experienced high radiation (≥10 mrem) in 25.3% cases (n = 23; UCSD = 10; UPMC = 13) and 11.0% cases (n = 10; UCSD = 4; UPMC = 6), respectively. Total attending urologist radiation exposure from all PCNL during the year was 265 mrem at UCSD and 475 mrem at UPMC. Total resident radiation exposure from all PCNL during the year was 195 mrem at UCSD and 175 mrem at UPMC.
Table 3.
Provider radiation exposure
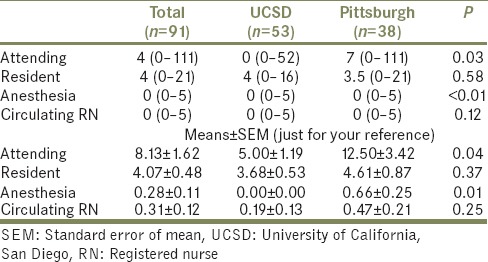
Univariate analysis identified covariate predictors of high (≥10 mrem) and low (<10 mrem) radiation exposure [Tables 4a and b]. Stone area (attending urologist: P =0.02; resident: P <0.01), partial or staghorn calculi (attending urologist: P =0.02; resident: P =0.03), surgery duration (P < 0.001), and fluoroscopy time (P < 0.001) were associated with attending urologist and resident radiation exposure. Preexisting access that was utilized was negatively associated with resident radiation exposure (P = 0.03). All other covariates were not significant predictors of radiation exposure. There were no significant associations between any covariates and anesthesia or circulating nurse radiation exposure.
Table 4a.
Continuous variables: Univariate analysis
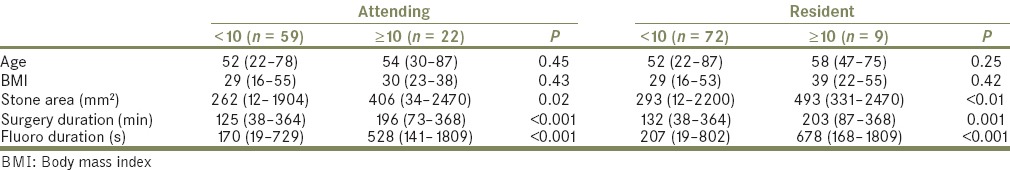
Table 4b.
Categorical variables: Univariate analysis
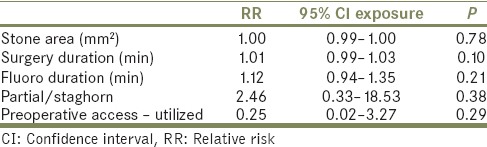
Multivariate binary logistic regression analyses using significant variables from univariate analysis were performed on attending urologist [Table 5a] and resident radiation exposure [Table 5b]. On multivariate analysis, only fluoroscopy duration remained significant for attending urologist radiation exposure with a 1-min increase in fluoroscopy time associated with a 22% increased risk of high attending urologist radiation exposure (relative risk 1.22, 95% confidence interval 1.03–1.44, P = 0.022). For resident radiation exposure, no covariates were significant on multivariate analysis.
Table 5a.
Multivariate regression for attending radiation exposure
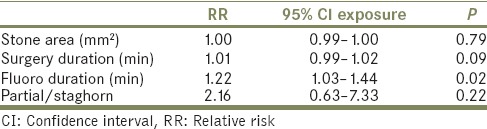
Table 5b.
Multivariate regression for resident radiation exposure
DISCUSSION
Although reliance on fluoroscopy in surgery is increasing, physician knowledge of radiation exposure and radiation safety is lacking.[10,11,12] The utilization of fluoroscopy introduces risks associated with ionizing radiation, which is potentially detrimental.[13] It is important for urologists to understand the factors that increase the risk of radiation exposure to protect the patient and all personnel in the operating room.
In this multicenter study, we prospectively measured and assessed predictors of radiation doses delivered to providers during PNL. Our median urology attending and resident radiation dose of 4 mrem fell within the range of previously published studies (0.17–5.6 mrem).[14] Both the anesthetist and the circulating nurse received minimal radiation exposure also in line with previous studies.[15,16] On univariate analysis, increased stone burden, partial or staghorn calculi, surgery duration, and fluoroscopy time were associated with high urology attending and resident radiation exposure; preexisting access that was utilized was associated with lower resident radiation exposure. Over the 12-month period, total radiation exposure from PNL was significantly below the maximum annual occupational dose limit of 50 mSv (5000 mrem) recommended by the 2007 International Commission on Radiological Protection and adopted by the Nuclear Regulatory Commission, which was in line with our previous study.[6,17]
Although studies have looked at various factors affecting fluoroscopy time[18] and patient radiation exposure,[4,19] no previous study to our knowledge has looked at factors associated with increased radiation doses to providers during PNL. Tepeler et al. found that fluoroscopy time was significantly increased with increased stone burden and multiple access sites but unaffected by patient BMI, stone configuration, degree of hydronephrosis, history of open renal surgery, or location of access.[18] In line with this study, we identified on univariate analysis that increased stone burden increased radiation exposure. Mancini et al. retrospectively reviewed the radiation exposures of 96 patients who underwent PNLs and found that increased effective radiation exposure was associated with increased body mass, higher stone burden, nonbranched stones, and multiple percutaneous access tracts but not stone site and composition, percutaneous access site, and estimated blood loss.[4] Torrecilla Ortiz et al. also found that patient BMI was associated with increased radiation exposure.[19]
A major strength of this study is the prospective measurement of radiation exposure; radiation dosages of each provider were collected immediately following each case. We also measured radiation doses compared to fluoroscopy duration, which has been demonstrated to correlate poorly with patient exposure dose as it does not take into consideration fluoroscopy dose rate.[20] Radiation doses of various operating room personnel including the anesthetist and circulating nurse were collected, which provide comparative measurements for radiation risk of personnel at different spatial locations of the operating room during a given procedure.
Our study is not without limitations. C-arm systems were not standardized across all cases. Furthermore, C-arm operator controls, such as collimation and pulse rate, were not standardized. Although the Instadose™ device allows for the prospective collection of radiation exposure, it is only able to collect data for a single case, and therefore cannot account for total annual radiation exposure resulting from PNL. The study also only utilized a single Instadose™ device placed on each of the providers' thyroid shields, the location typically used in estimating lens dose, to measure radiation exposure and may not be an accurate measure of effective dose as dose is not uniformly distributed throughout one's body.[20,21] Recorded doses would have been expectantly higher if worn at the waist due to typical radiation scatter pattern. The Instadose™ has a lower limit of detection of 1 mrem and is a relatively new device with potential limitations in its ability to accurately measure radiation doses; we did not validate our readings by correlating the dosages to the TLD readings. In addition, the Instadose™ device was placed on the outside of the lead apron, which raises questions regarding the clinical significance of our findings given that the lead apron should theoretically shield 95% of ionizing radiation and how much radiation actually makes it to the provider's skin. Although the study included multiple sites, different practices, caseload, equipment, each provider may stand at different distances from the X-ray source and the patient, and experience among urologists limit the generalizability of the data. This is reflected in the differences present in the two institutions. All providers in the UPMC cohort had increased exposure compared to the UCSD cohort with significant differences found in the exposures of the attending urologist (P = 0.025) and anesthesia (P = 0.003) providers [Table 3]. Compared to UPMC, the UCSD cohort had more cases with stones located in the ureter (P = 0.011) and underwent more combined PNL + URS procedures (P < 0.001) [Table 2]. Although surgical time was similar between the two sites, fluoroscopy time was significantly greater at UPMC than at UCSD (P = 0.022). There were also increased complications in the UCSD cohort (P = 0.012). Finally, on multivariate analysis, other than fluoroscopy duration, no other covariates were significant. This may have occurred due to our small sample size.
The study's goal was to identify characteristics that increase provider radiation exposure during percutaneous stone removal to cultivate our understanding and to ultimately lead to the development of methods to minimize radiation exposure. Radiation exposure has been previously decreased during PNLs by optimizing image positioning, the utilization of pulsed fluoroscopy, air instead of contrast during retrograde pyelogram, last image hold, and ultrasound to obtain access.[1] Further recommendations for exposure reduction include the following: Utilizing personal protective equipment (lead aprons, shields, gloves, and glasses), using fluoroscopy only during critical maneuvers, keeping body parts (e.g., hands) outside the radiation beam, using image collimation to narrow the focus of the X-ray beam, using a pulse rate setting as low as practical (e.g., 8 frames/s), minimizing the distance between the image intensifier (detector) and the patient, and maximizing the distance between personnel and the X-ray tube (source). Educational radiation awareness programs and feedback on fluoroscopy usage have also successfully decreased radiation exposure during endoscopic procedures.[22,23] Our study reinforces the importance of radiation safety awareness and the principle of As Low As Reasonably Achievable during fluoroscopy.
CONCLUSION
Increased stone burden, partial or staghorn calculi, surgery and fluoroscopy duration, and absence of preexisting access were associated with high provider radiation exposure. Radiation safety awareness is essential to minimize exposure and to protect the patient and all providers from radiation injury.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lipkin ME, Preminger GM. Imaging techniques for stone disease and methods for reducing radiation exposure. Urol Clin North Am. 2013;40:47–57. doi: 10.1016/j.ucl.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Ferrandino MN, Bagrodia A, Pierre SA, Scales CD, Jr, Rampersaud E, Pearle MS, et al. Radiation exposure in the acute and short-term management of urolithiasis at 2 academic centers. J Urol. 2009;181:668–72. doi: 10.1016/j.juro.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 3.John BS, Patel U, Anson K. What radiation exposure can a patient expect during a single stone episode? J Endourol. 2008;22:419–22. doi: 10.1089/end.2007.0268. [DOI] [PubMed] [Google Scholar]
- 4.Mancini JG, Raymundo EM, Lipkin M, Zilberman D, Yong D, Bañez LL, et al. Factors affecting patient radiation exposure during percutaneous nephrolithotomy. J Urol. 2010;184:2373–7. doi: 10.1016/j.juro.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 5.Lipkin ME, Preminger GM. Risk reduction strategy for radiation exposure during percutaneous nephrolithotomy. Curr Opin Urol. 2012;22:139–43. doi: 10.1097/MOU.0b013e32834fc36a. [DOI] [PubMed] [Google Scholar]
- 6.Cohen SA, Rangarajan SS, Chen T, Palazzi KL, Langford JS, Sur RL. Occupational hazard: Radiation exposure for the urologist – Developing a reference standard. Int Braz J Urol. 2013;39:209–13. doi: 10.1590/S1677-5538.IBJU.2013.02.09. [DOI] [PubMed] [Google Scholar]
- 7.Council NR. Health effects of exposure to low levels of ionizing radiation: BEIR V. Washington, DC: The National Academies Press; 1990. p. 436. [PubMed] [Google Scholar]
- 8.Smith AD, Reinke DB, Miller RP, Lange PH. Percutaneous nephrostomy in the management of ureteral and renal calculi. Radiology. 1979;133:49–54. doi: 10.1148/133.1.49. [DOI] [PubMed] [Google Scholar]
- 9.USNRC. Doses in Our Daily Lives. 2014. [Last accessed on 2014 Aug 29]. Available frtom: http://www.nrc.gov/about-nrc/radiation/around-us/doses-daily-lives.html. 1 .
- 10.Shiralkar S, Rennie A, Snow M, Galland RB, Lewis MH, Gower-Thomas K. Doctors' knowledge of radiation exposure: Questionnaire study. BMJ. 2003;327:371–2. doi: 10.1136/bmj.327.7411.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Söylemez H, Sancaktutar AA, Silay MS, Penbegül N, Bozkurt Y, Atar M, et al. Knowledge and attitude of European urology residents about ionizing radiation. Urology. 2013;81:30–5. doi: 10.1016/j.urology.2012.07.097. [DOI] [PubMed] [Google Scholar]
- 12.Friedman AA, Ghani KR, Peabody JO, Jackson A, Trinh QD, Elder JS. Radiation safety knowledge and practices among urology residents and fellows: Results of a nationwide survey. J Surg Educ. 2013;70:224–31. doi: 10.1016/j.jsurg.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Shrader-Frechette K. Trimming exposure data, putting radiation workers at risk: Improving disclosure and consent through a national radiation dose-registry. Am J Public Health. 2007;97:1782–6. doi: 10.2105/AJPH.2005.085027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim KP, Miller DL, Berrington de Gonzalez A, Balter S, Kleinerman RA, Ostroumova E, et al. Occupational radiation doses to operators performing fluoroscopically-guided procedures. Health Phys. 2012;103:80–99. doi: 10.1097/HP.0b013e31824dae76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumari G, Kumar P, Wadhwa P, Aron M, Gupta NP, Dogra PN. Radiation exposure to the patient and operating room personnel during percutaneous nephrolithotomy. Int Urol Nephrol. 2006;38:207–10. doi: 10.1007/s11255-005-4972-9. [DOI] [PubMed] [Google Scholar]
- 16.Bush WH, Jones D, Brannen GE. Radiation dose to personnel during percutaneous renal calculus removal. AJR Am J Roentgenol. 1985;145:1261–4. doi: 10.2214/ajr.145.6.1261. [DOI] [PubMed] [Google Scholar]
- 17.ICRP, 2007. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann. ICRP. 37(2-4) doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Tepeler A, Binbay M, Yuruk E, Sari E, Kaba M, Muslumanoglu AY, et al. Factors affecting the fluoroscopic screening time during percutaneous nephrolithotomy. J Endourol. 2009;23:1825–9. doi: 10.1089/end.2009.0256. [DOI] [PubMed] [Google Scholar]
- 19.Torrecilla Ortiz C, Meza Martínez AI, Vicens Morton AJ, Vila Reyes H, Colom Feixas S, Suarez Novo JF, et al. Obesity in percutaneous nephrolithotomy. Is body mass index really important? Urology. 2014;84:538–43. doi: 10.1016/j.urology.2014.03.062. [DOI] [PubMed] [Google Scholar]
- 20.Jaco JW, Miller DL. Measuring and monitoring radiation dose during fluoroscopically guided procedures. Tech Vasc Interv Radiol. 2010;13:188–93. doi: 10.1053/j.tvir.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Safak M, Olgar T, Bor D, Berkmen G, Gogus C. Radiation doses of patients and urologists during percutaneous nephrolithotomy. J Radiol Prot. 2009;29:409–15. doi: 10.1088/0952-4746/29/3/005. [DOI] [PubMed] [Google Scholar]
- 22.Weld LR, Nwoye UO, Knight RB, Baumgartner TS, Ebertowski JS, Stringer MT, et al. Safety, minimization, and awareness radiation training reduces fluoroscopy time during unilateral ureteroscopy. Urology. 2014;84:520–5. doi: 10.1016/j.urology.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 23.Ngo TC, Macleod LC, Rosenstein DI, Reese JH, Shinghal R. Tracking intraoperative fluoroscopy utilization reduces radiation exposure during ureteroscopy. J Endourol. 2011;25:763–7. doi: 10.1089/end.2010.0624. [DOI] [PubMed] [Google Scholar]


