Abstract
With widespread availability and the use of antiretroviral therapy, patients with human immunodeficiency virus (HIV) in the United States are living long enough to experience non-AIDS–defining illnesses. HIV is associated with an increased risk for cardiovascular disease (CVD) because of traditional CVD risk factors, residual virally mediated inflammation despite HIV treatment, and side effects of antiretroviral therapy. No United States population-wide studies have evaluated patterns of CVD mortality for HIV-infected subjects. Our central hypothesis was that the proportionate mortality from CVD (CVD mortality/total mortality) in the HIV-infected population increased from 1999 to 2013. We used the Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research online database of the United States public health data to assess proportionate CVD mortality from 1999 to 2013 in the HIV-infected, general, and inflammatory polyarthropathy populations; the inflammatory polyarthropathy population was included as a positive control group. Total mortality in the HIV-infected population decreased from 15,739 in 1999 to 8,660 in 2013; however, CVD mortality increased from 307 to 400 during the same period. Thus, proportionate CVD mortality for the HIV-infected population increased significantly from 1999 to 2013 (p <0.0001); this pattern was consistent across races, particularly for men. In contrast, proportionate CVD mortality decreased for the general and inflammatory polyarthropathy populations from 1999 to 2013. In conclusion, CVD has become an increasingly common cause of death in HIV-infected subjects since 1999; understanding evolving mortality risks in the HIV-infected population is essential to inform routine clinical care of HIV-infected subjects as well as CVD prevention and treatment.
Antiretroviral therapy (ART) has improved longevity in the human immunodeficiency virus (HIV)–infected population over the past 2 decades, resulting in a growing prevalence of HIV-infected subjects at risk for chronic non-AIDS illnesses.1,2 Cardiovascular disease (CVD) is the most common cause of mortality among adults in the United States3 and occurs earlier and more commonly in HIV-infected subjects, who have an estimated 50% greater risk for myocardial infarction (MI) compared with uninfected subjects and an estimated 4.5-fold greater risk for sudden cardiac death.2,4–9 Although traditional CVD risk factors are common in the HIV-infected population, this increased risk for CVD is independent of traditional risk factors and related to a complex interplay of chronic inflammation and side effects of ART, particularly protease inhibitors.10–13 Despite this elevated risk for CVD, HIV-infected patients receiving care for acute MI are significantly less likely than uninfected patients to receive standard of care procedures for acute MI, such as coronary angiography.14 Although some cohort studies have shown increases in non-AIDS–defining illnesses in recent years, there is a dearth of population-based, large-scale data on CVD mortality patterns in the HIV-infected population.1,15,16 In light of this data gap, the purpose of this study was to analyze temporal patterns in proportionate CVD mortality for HIV-infected adults in the United States.
Methods
We conducted the analysis using the publicly available detailed mortality database from the Centers for Disease Control and Prevention (CDC) Wide-Ranging Online Data for Epidemiologic Research (WONDER). The CDC WONDER database has been described previously and used for large epidemiologic studies published in recent years.17–19 The detailed mortality database within CDC WONDER consists of county-level national mortality and population data on the basis of death certificates of US residents, which include underlying causes of death, up to 20 additional multiple causes, and demographic and geographic data. Death certificates of all in-hospital and out-of-hospital deaths of US residents are captured.
The primary outcome for this study was change in proportionate CVD mortality from 1999 to 2013. We chose proportionate CVD mortality rather than absolute rates of CVD mortality as the primary outcome for a number of reasons. First, the only denominator available within the database for crude and age-adjusted mortality rates was the overall (rather than HIV-infected) US population; because the number of HIV-infected subjects dying from CVD represents an exceedingly small portion of the entire living US population, these mortality rates were too low to differ meaningfully from year to year. Furthermore, we believed that assessing temporal changes in proportionate CVD mortality specifically in HIV-infected subjects would help clinicians caring for patients with HIV understand their evolving risks for CVD mortality.
Only adults aged 25 years and older at the time of death were included for analysis; persons aged <25 years or for whom age at the time of death was unknown were excluded from all analyses. Twenty-five was used as the age cutoff rather than a younger age (such as 18 or 21) because CDC WONDER data are sortable by decade and inclusion of a population younger than 25 would have necessitated inclusion of subjects aged as young as 15. To calculate proportionate CVD mortality, we selected “diseases of the circulatory system” (International Classification of Diseases (ICD)10 codes I00 to I99) as the underlying cause of death, then selected all causes of death (all ICD10 codes) as the underlying cause of death, and subsequently divided the number of CVD deaths by all causes of death to determine proportionate mortality. We assessed the entire category of “diseases of the circulatory system” – which includes mortality from differing mechanisms including stroke, MI, arrhythmias, and heart failure – for the primary outcome because we sought to identify broadly the full spectrum of CVD deaths for this analysis. Assessment of subtypes of CVD deaths (e.g., MI alone) was performed in a secondary analysis.
The calculation of proportionate CVD mortality was done separately for each year from 1999 to 2013 and stratified by gender for the following race and/or ethnic groups: non-Hispanic whites, non-Hispanic blacks, and Hispanics. First, these sets of calculations were performed in HIV-infected subjects (defined as those with a diagnosis of HIV (ICD10 codes B20 to B24) on the death certificate as a multiple cause of death). Subsequently, calculations of proportionate CVD mortality were performed separately for the general population and those with inflammatory polyarthropathies (ICD10 codes M05 to M13 listed as multiple cause of death). We 2017 performed the separate analysis for the inflammatory polyarthropathy population because the population of HIV-infected subjects on ART is undergoing an epidemiologic transition to a chronic inflammatory disease population; we anticipated that it would be informative to evaluate CVD mortality patterns in other chronic inflammatory disease populations for which natural history is better understood. Rheumatoid arthritis, for instance, has been associated with increased risks for CVD because of a number of proposed mechanisms.20–24 Linear regression models were used to assess within-group trends in proportionate CVD mortality from 1999 to 2013; slope (β) was used to assess trends over time (change in proportionate mortality per year), with a p value <0.05 indicating statistical significance.
To address potential systematic differences between in-hospital and out-of-hospital deaths, we performed a set of sensitivity analyses including only in-hospital deaths. Additionally, we performed sensitivity analyses assessing temporal trends in proportionate mortality from specific CVD subtypes (MI, stroke, heart failure, and arrhythmia, separately), rather than all circulatory diseases. We also performed an exploratory analysis to assess cause-specific CVD mortality from 1999 to 2013 by race–gender groups. Specific causes of mortality were determined by ICD10 codes of underlying causes of death: hypertensive diseases (I10 to I15), ischemic heart disease (I20 to I25), pulmonary heart disease (I26 to I28), cardiomyopathy and heart failure (I42 and I50), cerebrovascular disease (I60 to I69), or other CVD death (all I00 to I99 diagnoses other than I10 to 15, I20 to 28, I42, I50, and I60 to I69). We stratified by white and/or black race only (not Hispanic origin) for this analysis because of low numbers of subtypes of CVD events in certain subgroups. Because the analyses for this study were conducted using only deidentified, population-level (not individual-level) data that are publicly available, this study was exempt in the institutional review board review process, and a waiver of informed consent was applied.
Results
Characteristics of the HIV-infected and general populations of persons aged 25 years and older at the time of death are provided in Table 1. Compared with the general population, HIV-infected subjects dying from CVD were more likely to be men, black, younger, urban-dwelling, and to have died in a medical facility. Among HIV-infected persons dying from any cause, those dying from CVD tended to be older.
Table 1. Selected characteristics of subjects with CVD mortality and all-cause mortality in the HIV-infected population and general population, aged 25 years and older, 1999 to 2013.
| Variable | HIV-Infected Dying from CVD (n = 5313) | HIV-Infected Dying from any Cause (n = 189,703) | General Population Dying from CVD (n = 12,729,594) | General Population Dying from any cause (n = 35,782,848) |
|---|---|---|---|---|
| Male Gender (% of total) | 4104 (77.2%) | 140661 (74.1%) | 6091218 (47.9%) | 18215748 (50.9%) |
| Age at death (years) | ||||
| 25-34 | 167 (3.1%) | 21177 (11.2%) | 62048 (4.9%) | 635010 (1.8%) |
| 35-44 | 966 (18.1%) | 61762 (32.6%) | 231748 (1.8%) | 1213821 (3.4%) |
| 45-54 | 1902 (35.7%) | 65035 (34.3%) | 685564 (5.4%) | 2659241 (7.4%) |
| 55-64 | 1448 (27.3%) | 30064 (15.8%) | 1230262 (9.7%) | 4249673 (11.9%) |
| 65-74 | 607 (11.4%) | 9124 (4.8%) | 1953662 (15.3%) | 6250197 (17.5%) |
| 75-84 | 184 (3.5%) | 2203 (1.2%) | 3696042 (29.0%) | 9981161 (27.9%) |
| 85+ | 39 (0.7%) | 338 (0.2%) | 4870268 (38.3%) | 10793745 (30.2%) |
| Race | ||||
| American Indian or Alaska Native | 28 (0.5%) | 1134 (0.6%) | 52080 (4.1%) | 192925 (0.5%) |
| Asian or Pacific Islander | 31 (0.6%) | 1375 (0.7%) | 231737 (1.8%) | 645825 (1.8%) |
| Black | 2757 (51.9%) | 102534 (54.0%) | 1479489 (11.6%) | 4082424 (11.4%) |
| White | 2497 (47.0%) | 84660 (44.6%) | 10966288 (86.1%) | 30861674 (86.2%) |
| Infected with Hepatitis C Virus* | 255 (4.8%) | 7174 (3.8%) | 16624 (1.3%) | 204413 (0.6%) |
| Urbanization | ||||
| Large Central Metro | 3085 (58.1%) | 104001 (54.8%) | 3524056 (27.7%) | 9670862 (27.0%) |
| Large Fringe Metro | 821 (15.5%) | 31906 (16.8%) | 2798096 (22.0%) | 7967461 (22.2%) |
| Medium Metro | 847 (15.9%) | 30803 (16.2%) | 2611823 (20.5%) | 7544111 (21.1%) |
| Small Metro | 235 (4.4%) | 9310 (4.9%) | 1267329 (10.0%) | 3619806 (10.1%) |
| Non-Metro | 325 (6.1%) | 13683 (7.2%) | 2528290 (19.9%) | 6980608 (19.5%) |
| Place of Death | ||||
| Medical Facility - Inpatient | 2217 (41.7%) | 106608 (56.2%) | 4376590 (34.4%) | 12785944 (35.7%) |
| Medical Facility – Outpatient or ER | 714 (13.4%) | 8394 (4.4%) | 1436586 (11.3%) | 2462305 (6.9%) |
| Medical Facility – Dead on Arrival | 77 (1.4%) | 973 (0.5%) | 163754 (1.3%) | 307843 (0.9%) |
| Medical Facility – Status Unknown | 7 (0.1%) | 483 (0.3%) | 17031 (0.1%) | 45375 (0.1%) |
| Decedent's Home | 1513 (28.5%) | 32937 (17.4%) | 2926588 (23.0%) | 9143039 (25.6%) |
| Hospice Facility | 86 (1.6%) | 6712 (3.5%) | 201914 (1.6%) | 902284 (2.5%) |
| Nursing home / long term care | 414 (7.8%) | 22035 (11.6%) | 3059260 (24.0%) | 7940069 (22.2%) |
| Other | 250 (4.7%) | 10230 (5.4%) | 496262 (3.9%) | 2037984 (5.7%) |
| Unknown | 35 (0.7%) | 1331 (0.7%) | 51609 (0.4%) | 158005 (0.4%) |
Hepatitis C virus based on listing of B17.1 (acute hepatitis C) or B18.2 (chronic viral hepatitis C) as a multiple cause of death on death certificate.
For the overall HIV-infected population, CVD mortality increased from 1999 to 2013 but total mortality decreased; proportionate CVD mortality increased from 1.95% in 1999 to 4.62% in Supplementary Table 1; β = 0.0019, p <0.0001). Analyses stratified by race and/or ethnic subgroups are shown in Figure 1 (men) and Figure 2 (women) and in Supplementary Table 1. The pattern of increasing proportionate CVD mortality from 1999 to 2013 was highly significant for men (β = 0.0022, p <0.0001) and consistent across race–gender groups; for women, this trend was also statistically significant (β = 0.0011, p = 0.0028) although the yearly increase in proportionate mortality was roughly half that for men. CVD mortality increased significantly over this period for non-Hispanic white men (beta = 0.0025, p <0.0001), non-Hispanic black men (β = 0.0021, p <0.0001), and Hispanic men (β = 0.0022, p <0.0001). For women, the pattern of increasing proportionate CVD mortality from 1999 to 2013 was statistically significant for non-Hispanic blacks (β = 0.00054, p = 0.01) but not for non-Hispanic whites (β = 0.0015, p = 0.08) or Hispanics (β = 0.0005, p = 0.74).
Figure 1.
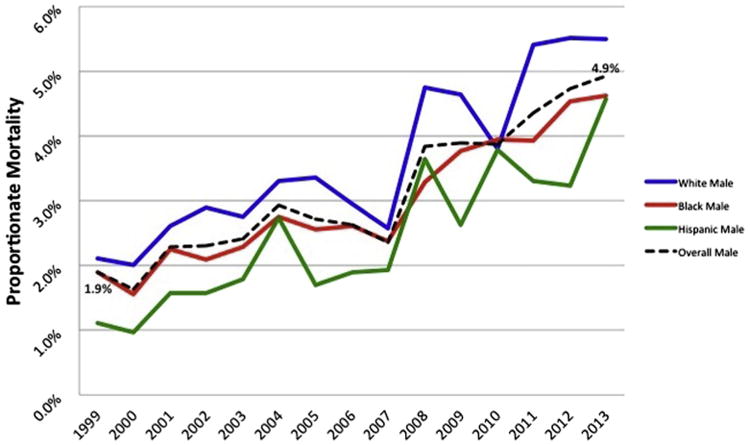
Proportionate circulatory mortality in HIV-infected men aged 25 years and older, 1999 to 2013.
Figure 2.
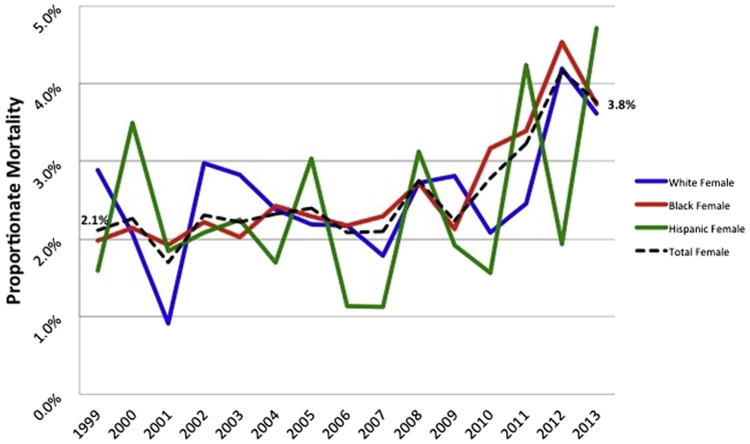
Proportionate circulatory mortality in HIV-infected women aged years 25 and older, 1999 to 2013.
Proportionate CVD Mortality from 1999 to 2013 for the overall HIV-infected population, the general US population, and the US population with inflammatory polyarthropathies is shown in Figure 3. Although proportionate CVD mortality increased more than twofold for HIV-infected persons, proportionate CVD mortality decreased in the general and inflammatory polyarthropathy populations during the same period. As expected, CVD remains responsible for a greater overall proportion of deaths in the general and inflammatory polyarthropathy populations than in the HIV-infected population given the decreasing but still-substantial burden of AIDS-related deaths among HIV-infected subjects.1,15,16 In a sensitivity analysis in which only in-hospital deaths were included, there remained a substantial increase in proportionate CVD mortality from 1999 to 2013 for the HIV-infected population and a decrease in proportionate CVD mortality during the same period in the general population (Supplementary Table 2). In another sensitivity analysis assessing CVD subtypes, proportionate mortality due to ischemic heart disease (mortality due to ischemic heart disease/overall mortality) increased threefold in the HIV-infected population, from 0.8% in 1999 to 2.5% in 2013 but decreased in the general population, from 22.8% in 1999 to 14.6% in 2013 (Supplementary Table 3).
Figure 3.
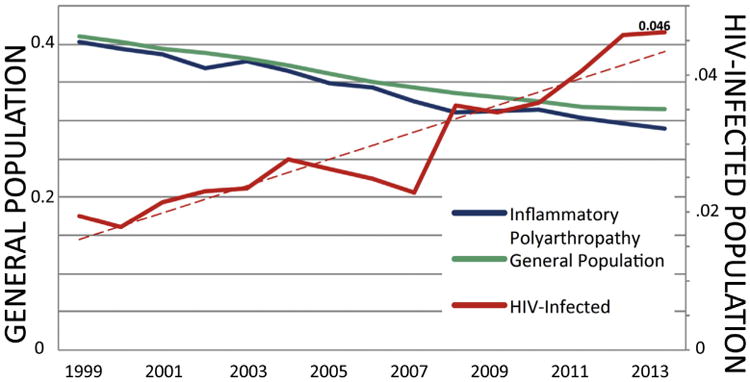
Proportionate mortality for CVD of all deaths within the general population, inflammatory polyarthropathy population, and HIV-infected population.
When we assessed proportionate mortality due to specific CVD subtypes among separate race–gender groups for HIV-infected subjects and the general population, a few patterns persisted across groups (Tables 2 and 3; Figure 4). Hypertensive heart and renal diseases (ICD10 codes I10 to I15) and pulmonary circulatory diseases (such as pulmonary embolism and pulmonary hypertension; ICD10 codes I26 to I28) were more likely to be designated as the causes of CVD-related death for HIV-infected subjects than for the general population. Conversely, CVD mortality was more likely to be because of heart failure and/or cardiomyopathy (ICD10 codes I42 and I50) or cerebrovascular diseases including stroke (ICD10 codes I60 to I69) in the general population than in the HIV-infected population.
Table 2. Proportionate mortality due to cardiovascular disease subtype as a percent of overall cardiovascular disease mortality by race–gender group, HIV-infected adults aged 25 years and older, 1999 to 2013.
| Race-Sex Groups | Total mortality | Circulatory Mortality | Ischemic Heart Disease (I20-25) | Hypertensive Diseases (I10-15) | Pulmonary Heart Disease and Diseases of Pulmonary Circulation (I26-28) | Cerebrovascular Disease (I60-69) | Cardiomyopathy (I42) and Heart Failure (I50) | |
|---|---|---|---|---|---|---|---|---|
| Black | Male | 68,799 | 1914 | 902 (47.1%) | 564 (29.5%) | 82 (4.3%) | 143 (7.5%) | 98 (5.1%) |
| Female | 33,735 | 843 | 304 (36.1%) | 244 (28.9%) | 83 (9.8%) | 100 (11.9%) | 36 (4.3%) | |
| White | Male | 69,901 | 2142 | 1367 (63.8%) | 271 (12.6%) | 141 (6.6%) | 132 (6.2%) | 70 (3.3%) |
| Female | 14,759 | 355 | 160 (45.1%) | 48 (13.5%) | 62 (17.5%) | 34 (9.6%) | 14 (3.9%) |
Table 3. Proportionate mortality due to cardiovascular disease subtype as a percent of overall cardiovascular disease mortality by race–gender group, all adults aged 25 years and older, 1999 to 2013.
| Race-Sex Groups | Total mortality | Circulatory Mortality | Ischemic Heart Disease (I20-25) | Hypertensive Diseases (I10-15) | Pulmonary Heart Disease and Diseases of Pulmonary Circulation (I26-28) | Cerebrovascular Disease (I60-69) | Cardiomyopathy (I42) and Heart Failure (I50) | |
|---|---|---|---|---|---|---|---|---|
| Black | Male | 2,042,792 | 707,549 | 338,809 (47.9%) | 89,439 (12.6%) | 12,300 (1.7%) | 111,263 (15.7%) | 70,977 (10.0%) |
| Female | 2,039,632 | 771,940 | 334,868 (43.4%) | 98,793 (12.8%) | 19,999 (2.6%) | 148,098 (19.2%) | 72,851 (9.4%) | |
| White | Male | 15,085,089 | 5,234,944 | 3,021,629 (57.7%) | 268,954 (5.1%) | 64,739 (1.2%) | 713,625 (13.6%) | 484,282 (9.3%) |
| Female | 15,776,585 | 5,731,344 | 2,700,021 (47.1%) | 373,653 (6.5%) | 98,719 (1.7%) | 1,121,834 (19.6%) | 589,017 (10.3%) |
Figure 4.
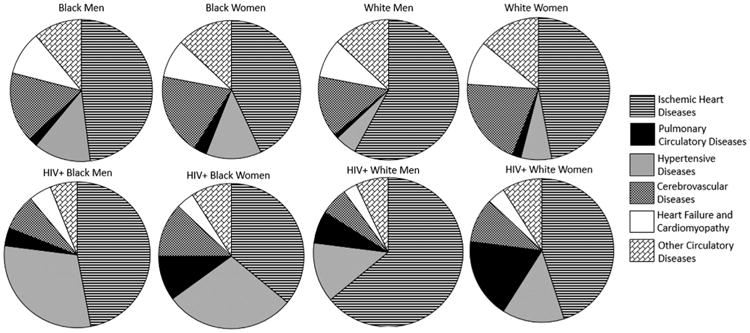
Proportionate CVD mortality by subtype of CVD: HIV-infected and general population by race–gender groups.
Discussion
In this study, we used a national US database of mortality data and found that the proportion of deaths related to CVD increased more than twofold in HIV-infected subjects between 1999 and 2013. This pattern was particularly apparent in men and contrasts sharply against the decrease in proportionate CVD-related mortality for the general US population and patients with inflammatory polyarthropathies during the same period. The increase in proportionate CVD mortality in HIV-infected persons is not surprising given their decreasing competing risks for AIDS-related mortality in recent years, their elevated risks for CVD, and the overall aging of the US. HIV population.
The results of this study underscore the emerging need for enhanced CVD risk prediction and prevention in the HIV-infected population. Traditional coronary heart disease risk prediction models, such as the Framingham Risk Score, may provide inadequate discrimination and be poorly calibrated in the HIV-infected population in light of the elevated HIV-related MI risks that may differ from traditional risk factors.25,26 Age and demographic disparities between the general population and HIV-infected population may also limit the applicability of traditional cardiovascular risk prediction models in the HIV-infected population. Accordingly, studies evaluating newer atherosclerotic CVD (ASCVD) risk prediction models (such as the American College of Cardiology/American Heart Association ASCVD risk estimator) with potential incorporation of HIV-specific variables are warranted.
To improve the cardiovascular health of the HIV-infected population, more large-scale randomized data on preventive cardiovascular interventions are needed. Although a number of small, randomized statin trials in the HIV-infected population have demonstrated significant improvements in lipids and subclinical CVD, there is a dearth of trial data assessing hard clinical end points.27 TheRandomized Trial to Prevent Vascular Events in HIV (REPRIEVE: AIDS Clinical Trials Group study A5332) has recently been initiated and represents the first large-scale randomized clinical trial testing a pharmacologic strategy (pitavastatin) for heart disease prevention in the HIV-infected population.
A number of limitations of this study must be acknowledged. Because death certificates were our sole source of data for this population-level analysis, our ability to assess disease-specific variables was limited, and we could not validate ICD10-coded diagnoses with expert chart review or other methods for adjudication. However, previous studies of large administrative databases have demonstrated ample agreement between individual-level ICD10 diagnoses assigned by providers in routine clinical care and the relative “gold standard” of diagnoses determined by blinded expert chart reviewers and/or auditors.28,29 We performed sensitivity analysis that included only in-hospital deaths due to concerns regarding systematic differences in ICD10-coded causes of death on in-hospital versus out-of-hospital deaths and still found a substantial increase in proportionate CVD mortality for the HIV-infected population and a decrease for the general population from 1999 to 2013.
It is also possible that there were systematic differences between the HIV-infected and general populations in how causes of death were classified. For instance, if an underlying cause of death was unclear for 2 subjects, it is possible that the HIV-infected individual's death was attributed to the most common cause in the HIV-infected population, AIDS-related illness, whereas the uninfected individual's death was attributed to CVD, the most common cause of death in the general adult population. If this were the case, it would have led to systematic underreporting of CVD deaths and resulting underestimation of the burden of CVD-related mortality in the HIV-infected population. Furthermore, it is possible that this theoretical underreporting of CVD deaths in the HIV-infected population could have changed from 1999 to 2013 with increased recognition of CVD risk in HIV, thus, partially contributing to the temporal increase in proportionate CVD mortality in the HIV population observed in this study. Another factor limiting our ability to compare HIV and general populations is the unique and significant aging of the HIV population from 1999 to 2013. Although this may account for some of the increase in CVD mortality in HIV-infected subjects during this period, the mean age of HIV-infected subjects dying from any cause also increased from 1999 to 2013, thus limiting the degree to which aging alone would impact proportionate mortality. Despite these limitations, the population-wide and 15-year scope of this study provides sufficient and varied hard clinical end points to evaluate temporal and demographic patterns to an extent not entirely replicable by longitudinal cohort studies. Furthermore, we believe that the population-level nature of this study, derived from the entire US population aged 25 years or older at the time of death from 1999 to 2013, represents a substantial and complementary addition to existing HIV mortality literature.
Supplementary Material
Acknowledgments
The authors thank the Centers for Disease Control and Prevention (CDC) for the publicly available data from the CDC WONDER online database (http://wonder.cdc.gov/).
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
Supplementary Data: Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.amjcard.2015.10.030.
References
- 1.Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD HIV Outpatient Study Investigators. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 2.Rodger AJ, Lodwick R, Schechter M, Deeks S, Amin J, Gilson R, Paredes R, Bakowska E, Engsig FN, Phillips A INSIGHT SMART ESPRIT Study Groups. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS. 2013;27:973–979. doi: 10.1097/QAD.0b013e32835cae9c. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 4.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012;60:351–358. doi: 10.1097/QAI.0b013e31825c7f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51:268–273. doi: 10.1097/QAI.0b013e3181a9992c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butt AA, Chang CC, Kuller L, Goetz MB, Leaf D, Rimland D, Gibert CL, Oursler KK, Rodriguez-Barradas MC, Lim J, Kazis LE, Gottlieb S, Justice AC, Freiberg MS. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011;171:737–743. doi: 10.1001/archinternmed.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tseng ZH, Secemsky EA, Dowdy D, Vittinghoff E, Moyers B, Wong JK, Havlir DV, Hsue PY. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59:1891–1896. doi: 10.1016/j.jacc.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paisible AL, Chang CC, So-Armah KA, Butt AA, Leaf DA, Budoff M, Rimland D, Bedimo R, Goetz MB, Rodriguez-Barradas MC, Crane HM, Gibert CL, Brown ST, Tindle HA, Warner AL, Alcorn C, Skanderson M, Justice AC, Freiberg MS. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr. 2015;68:209–216. doi: 10.1097/QAI.0000000000000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S, van Sighem A, Kesselring A, Gras L, Smit C, Prins JM, Kauffmann R, Richter C, de Wolf F, Reiss P. Episodes of HIV viremia and the risk of non-AIDS diseases in patients on suppressive antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;60:265–272. doi: 10.1097/QAI.0b013e318258c651. [DOI] [PubMed] [Google Scholar]
- 10.Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr. 2010;55:615–619. doi: 10.1097/QAI.0b013e3181f4b752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein JH, Hsue PY. Inflammation, immune activation, and CVD risk in individuals with HIV infection. JAMA. 2012;308:405–406. doi: 10.1001/jama.2012.8488. [DOI] [PubMed] [Google Scholar]
- 12.Tien PC, Choi AI, Zolopa AR, Benson C, Tracy R, Scherzer R, Bacchetti P, Shlipak M, Grunfeld C. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr. 2010;55:316–322. doi: 10.1097/QAI.0b013e3181e66216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Currier JS, Lundgren JD, Carr A, Klein D, Sabin CA, Sax PE, Schouten JT, Smieja M Working Group 2. Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008;118:e29–e35. doi: 10.1161/CIRCULATIONAHA.107.189624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearce D, Ani C, Espinosa-Silva Y, Clark R, Fatima K, Rahman M, Diebolt E, Ovbiagele B. Comparison of in-hospital mortality from acute myocardial infarction in HIV sero-positive versus sero-negative individuals. Am J Cardiol. 2012;110:1078–1084. doi: 10.1016/j.amjcard.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 15.Smit C, Geskus R, Walker S, Sabin C, Coutinho R, Porter K, Prins M CASCADE Collaboration. Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. AIDS. 2006;20:741–749. doi: 10.1097/01.aids.0000216375.99560.a2. [DOI] [PubMed] [Google Scholar]
- 16.Ingle SM, May MT, Gill MJ, Mugavero MJ, Lewden C, Abgrall S, Fatkenheuer G, Reiss P, Saag MS, Manzardo C, Grabar S, Bruyand M, Moore D, Mocroft A, Sterling TR, D'Arminio Monforte A, Hernando V, Teira R, Guest J, Cavassini M, Crane HM, Sterne JA Antiretroviral Therapy Cohort Collaboration. Impact of risk factors for specific causes of death in the first and subsequent years of antiretroviral therapy among HIV-infected patients. Clin Infect Dis. 2014;59:287–297. doi: 10.1093/cid/ciu261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999-2009) Pediatr Blood Cancer. 2013;60:1482–1486. doi: 10.1002/pbc.24557. [DOI] [PubMed] [Google Scholar]
- 18.Lott JP, Gross CP. Mortality from nonneoplastic skin disease in the United States. J Am Acad Dermatol. 2014;70:47–54. e41. doi: 10.1016/j.jaad.2013.09.039. [DOI] [PubMed] [Google Scholar]
- 19.Davis FG, Dolecek TA, McCarthy BJ, Villano JL. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol. 2012;14:1171–1177. doi: 10.1093/neuonc/nos152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Halm VP, Peters MJ, Voskuyl AE, Boers M, Lems WF, Visser M, Stehouwer CD, Spijkerman AM, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Smulders YM, Dijkmans BA, Nurmohamed MT. Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: a cross-sectional study, the CARRE investigation. Ann Rheum Dis. 2009;68:1395–1400. doi: 10.1136/ard.2008.094151. [DOI] [PubMed] [Google Scholar]
- 21.de Groot L, Posthumus MD, Kallenberg CG, Bijl M. Risk factors and early detection of atherosclerosis in rheumatoid arthritis. Eur J Clin Invest. 2010;40:835–842. doi: 10.1111/j.1365-2362.2010.02333.x. [DOI] [PubMed] [Google Scholar]
- 22.de Groot L, Jager NA, Westra J, Smit AJ, Kallenberg CG, Posthumus MD, Bijl M. Does reduction of disease activity improve early markers of cardiovascular disease in newly diagnosed rheumatoid arthritis patients? Rheumatology. 2015;54:1257–1261. doi: 10.1093/rheumatology/keu459. [DOI] [PubMed] [Google Scholar]
- 23.Chauhan K, Ackerman MJ, Crowson CS, Matteson EL, Gabriel SE. Population-based study of QT interval prolongation in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2015;33:84–89. [PMC free article] [PubMed] [Google Scholar]
- 24.Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, Siu S, Kraft J, Lynde C, Pope J, Gulliver W, Keeling S, Dutz J, Bessette L, Bissonnette R, Haraoui B. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:480–489. doi: 10.1136/annrheumdis-2014-206624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aboud M, Elgalib A, Pomeroy L, Panayiotakopoulos G, Skopelitis E, Kulasegaram R, Dimian C, CL F, Duncan A, Wierzbicki AS, Peters BS. Cardiovascular risk evaluation and antiretroviral therapy effects in an HIV cohort: implications for clinical management: the CREATE 1 study. Int J Clin Pract. 2010;64:1252–1259. doi: 10.1111/j.1742-1241.2010.02424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friis-Moller N, Thiebaut R, Reiss P, Weber R, Monforte AD, De Wit S, El-Sadr W, Fontas E, Worm S, Kirk O, Phillips A, Sabin CA, Lundgren JD, Law MG DAD Study Group. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17:491–501. doi: 10.1097/HJR.0b013e328336a150. [DOI] [PubMed] [Google Scholar]
- 27.Feinstein MJ, Achenbach CJ, Stone NJ, Lloyd-Jones DM. A systematic review of the usefulness of statin therapy in HIV-infected patients. Am J Cardiol. 2015;115:1760–1766. doi: 10.1016/j.amjcard.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 28.Henderson T, Shepheard J, Sundararajan V. Quality of diagnosis and procedure coding in ICD-10 administrative data. Med Care. 2006;44:1011–1019. doi: 10.1097/01.mlr.0000228018.48783.34. [DOI] [PubMed] [Google Scholar]
- 29.Quan H, Li B, Saunders LD, Parsons GA, Nilsson CI, Alibhai A, Ghali WA IMECCHI Investigators. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43:1424–1441. doi: 10.1111/j.1475-6773.2007.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


