Abstract
Purpose:
The purpose of this in vitro research project was to evaluate and compare the wear behavior of human tooth enamel opposing monolithic zirconia and other different ceramic restorative materials and also to observe the tetragonal to monoclinic phase transformation in zirconia-based ceramics that may occur while simulating wear occurring at room temperature in a wet environment.
Materials and Methods:
A total of sixty samples were prepared for this study. Fifteen discs of glazed zirconia, 15 discs of polished zirconia without glaze, 15 discs of metal ceramic, and 15 discs of lithium disilicate were fabricated. Sixty extracted premolars were collected and randomly divided into four groups of 15 each. The discs and extracted human premolars were placed onto holders on a two-body wear machine under a constant load of 5 kg to simulate the oral wear cycle. A diffractometer was used to analyze phase transformation. One-way analysis of variance and Tukey's post hoc tests was used.
Results:
The mean loss of height of tooth samples and its standard deviation for Group I (monolithic zirconia with glaze), Group II (mechanically polished monolithic zirconia without glaze), Group III (porcelain fused to metal), and Group IV (glazed monolithic lithium disilicate) was obtained as 0.2716 ± 0.1409, 0.1240 ± 0.0625, 0.1567 ± 0.0996, and 0.2377 ± 0.1350, respectively. The highest mean loss in height was observed in Group I and the least was observed in Group II.
Conclusion:
Mechanically polished zirconia showed the least amount of enamel wear followed by porcelain fused to metal and glazed monolithic lithium disilicate, whereas glazed monolithic zirconia showed the highest enamel wear.
Keywords: Lithium disilicate, phase transformation, wear, zirconia
INTRODUCTION
Ceramics are key materials for dental restorations due to their high wear resistance, biocompatibility, and above all, esthetics.[1] Although dental ceramics have excellent properties that meet requirements of a prosthetic material, it has one major drawback: Irreversible wear of opposing tooth structure under certain conditions. Several investigators have demonstrated that, in general, ceramic substrates cause greater and hence destructive abrasive wear of human enamel.[2,3]
A ceramic restorative material that combines good strength without the disadvantage of increased enamel wear would be a significant addition to clinical dental practice. Although numerous in vitro wear studies have been conducted on dental ceramics, few studies have provided detailed information to characterize and predict the wear behavior of a range of dental ceramics used for all ceramic systems at present. These studies have reported the effect of individual ceramic systems on the wear of enamel.[4,5] Taking these studies into consideration, it is challenging to compare the effect of different ceramic systems on the wear of enamel because these studies have been conducted in different environmental and testing conditions as well as in the presence of altered variables. Therefore, this in vitro research project was undertaken to evaluate and compare the wear behavior of human tooth enamel opposing different ceramic restorative materials; monolith zirconia with glaze, mechanically polished monolith zirconia without glaze, porcelain fused to metal and lithium disilicate glass ceramic using a reciprocating sliding wear testing apparatus in the same controlled environment and testing conditions using same variables.
A second part of the study is to observe the phase transformation of zirconia structure due to wear in the presence of artificial saliva. Zirconia exists in three major phases: Monoclinic, tetragonal, and cubic. The tetragonal crystalline state is responsible for the high strength and fracture toughness of yttrium oxide stabilized tetragonal zirconium dioxide polycrystals (Y-TZP) materials. Once a crack propagates within a Y-TZP material, the energy supplied by the crack can trigger the tetragonal to monoclinic phase transformation in the surrounding grains. This phase transformation leads to a local compressive stress field that hinders further crack propagation.[6,7] This so-called transformation toughening is what makes Y-TZP the strongest ceramic material class used in the dentistry. However, it is well known that on Y-TZP material surfaces, the tetragonal to monoclinic phase transformation can slowly occur by contact with water.[8,9,10] This is called low-temperature degradation (LTD). Zirconia has the propensity to undergo LTD, which is a kinetic phenomenon in which the polycrystalline tetragonal material slowly transforms into monoclinic zirconia over a rather narrow but important temperature range, typically between room temperature and approximately 400°C in the presence of water, depending on the stabilizer used, its concentration, and the grain size (GS) of the ceramic. This time dependant phase transformation is followed by microcracking and loss of strength which starts at the surface and then proceeds into the bulk.[11,12,13]
Therefore, a strict control of the phase transformation is thus imperative to retain those properties which make the ceramic material so desirable. Hence, a part of the study included observing the tetragonal to monoclinic phase transformation that may occur while simulating wear occurring at room temperature in a wet environment.
MATERIALS AND METHODS
Fabrication of zirconia discs [Box 1]
Box 1.
Material description

A disc of dimension 10 mm in diameter and 2 mm in height was fabricated in wax using the precision milling. Zirconia blanks were fed into the milling machine and thirty discs of the required dimensions were obtained. The discs were finished using a rubber point and later sintered. The thirty discs were randomly divided into two groups of 15 each. The discs in the first group were dried with tissue paper and glaze liquid was applied and fired. The discs of the second group were cleaned in an ultrasonic bath for 10 min and polished using Zircon-Brite polishing paste.
Fabrication of metal ceramic discs [Box 1]
A steel template with five standardized circular molds, each of diameter 10 ± 0.2 mm and depth 0.5 ± 0.1 mm was precision milled. Using this template, 15 wax patterns of standard dimensions for metal discs were fabricated and sprued. The wax pattern fabricated discs was carefully removed from the mold using a U-shaped stapler pin[14] following the same technique used to retrieve Class II inlay wax patterns. They were invested in phosphate-bonded investment material and standardized casting procedure was carried out. The metal discs were then divested and sandblasted. The sandblasted metal discs were then secured within the molds of the refractory template and degassing was carried out. A steel template with five molds of diameter 10 ± 0.2 mm and depth 2 ± 0.1 mm was precision-milled to accommodate veneering ceramic. All the discs were layered according to manufacturer's instruction with opaque, dentin, and enamel porcelain to the desired thickness of 1.5 mm. The samples were finished and polished.
Fabrication of lithium disilicate discs [Box 1]
A steel template with five standardized circular molds, each of the diameter 10 mm and depth 2 mm, was precision-milled. Using this template, 15 wax patterns of standard dimensions were fabricated, sprued and the standardized ceramic pressing procedure was carried out. The discs were then divested. After fine divestment, the reaction layer formed during the press procedure was removed using IPS e.max Press Invex Liquid. All 15 samples were first finished using diamond burs. Zircon-Brite polishing paste was used to polish each specimen. All discs were then cleaned in an ultrasonic bath for 10 min. Discs were dried with tissue paper and glaze liquid was applied evenly and fired according to manufacturer's instructions.
All ceramic samples were then thermocycled 500 times between 5°C and 55°C.
Modified Fusayama artificial saliva was made and was stored at 37 ± 1°C.
Sixty freshly extracted human unrestored, caries-free, nonattrited maxillary first and second premolars of young adolescent patients undergoing orthodontic extractions were collected. They were disinfected in formalin and debrided of calculus using an ultrasonic scaler and preserved in saline. The sixty extracted premolars were randomly divided into four groups of 15 each. The teeth were then mounted in autopolymerizing acrylic resin. The teeth samples were then randomly divided into four groups of 15 each:
Group I: To abrade against glazed zirconia [Figure 1]
Group II: To abrade against polished zirconia without glaze [Figure 1]
Group III: To abrade against porcelain fused to metal [Figure 1]
Group IV: To abrade against lithium disilicate [Figure 1].
Figure 1.
The ceramic discs Groups I, II, III, and IV
The teeth samples were placed on the worktable of the profilometry machine and the X, Y, and Z axes were adjusted. The profile of each sample of the tooth was traced and transferred to tracing paper. A perpendicular was dropped from the height of the cusp tip to the base of the tooth where it is embedded into the acrylic. This height was measured as the baseline height of that particular tooth.
The discs and extracted human premolars were placed onto holders on a two-body wear machine in which tooth sample was attached to the upper member, and the disc was attached to the lower member (rotating wheel). The disc was additionally secured with the help of condensation silicone putty. The cusp tips and ceramic discs were positioned under a constant load of 5 kg. Artificial saliva was sprayed between the tooth sample and ceramic surface at intervals during testing so as to further simulate the oral conditions. The specimens were made to rub against one another in a rotating motion to simulate the oral wear cycle. The test was run for a total of 10,000 cycles on the wear machine, for each sample.
The teeth samples were then placed onto the work table of the profilometer in the same orientation as that of the first measurement. The three axes were adjusted and the height was measured again up to the base of the tooth, where it is embedded into the acrylic. A second tracing of the reduced profile of the tooth after wear was made on the previous tracing paper which contained the profile of that tooth before wear and the reduction in height was calculated.
The zirconia sample, before wear, was powdered to a fine grain and tightly packed onto the random mount present on the stage of the machine. The diffractometer was used. The knife edge attached above was first zeroed and then raised by 1.200 mm so as to contact the sample and the X-ray diffraction (XRD) was carried out. The data so obtained were assessed and plotted as a graph using OriginPro 8 (OriginLab Corporation, Northampton, Massachusetts). The graph was then compared with those in the database-PCPDF. The same protocol was carried out for the sample obtained after wear.
RESULTS
In all study groups, the height of tooth significantly reduced after the 10,000 cycles of enamel wear when compared to its initial height.
The mean loss of height of tooth samples and its standard deviation for Group I (monolithic zirconia with glaze), Group II (mechanically polished monolithic zirconia without glaze), Group III (porcelain fused to metal), and Group IV (lithium disilicate) was obtained as 0.2716 ± 0.1409, 0.1240 ± 0.0625, 0.1567 ± 0.0996, and 0.2377 ± 0.1350, respectively. The highest mean loss in height was observed in Group I and the least was observed in Group II.
Table 1 shows the comparison of the mean loss of height in the study groups using one-way analysis of variance. There was statistically significant difference in loss of tooth height among the study groups.
Table 1.
Comparison of the mean loss of height in the study groups using one-way ANOVA test
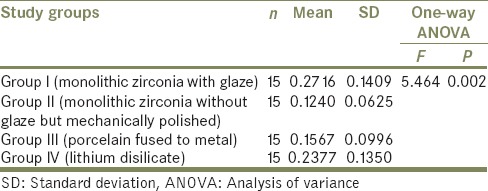
Table 2 shows the pair-wise comparison of mean loss of height in the study groups using Tukey's post hoc test. The mean difference between Group I and Group II was observed as 0.1476 with P = 0.004. The mean difference between Group I and Group III was observed as 0.1149 with P = 0.038. Both these are statistically significant. The mean difference between Group I and Group IV was observed as 0.0339 and was not statistically significant.
Table 2.
Pair-wise comparison of mean loss of height in the study groups using Tukey's post hoc test
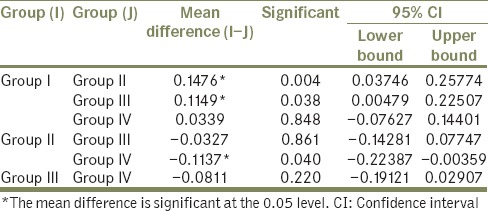
The mean difference between Group II and Group III was not statistically significant at −0.0327. The mean difference between Group II and Group IV was, however, statistically significant at −0.1137.
The mean difference between Group III and Group IV was −0.0811, with P = 0.220, hence not statistically significant.
Graph 1 compares the loss of height of all four groups. It is observed that Group II shows the least amount of wear while Group I shows the significantly higher wear of opposing tooth. However, Group IV shows a greater loss of height than Groups II and III but still lesser than Group I.
Graph 1.
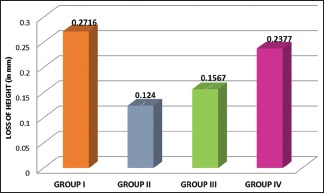
Mean loss of height in all the study groups
Zirconia specimens were analyzed on an X-ray diffractometer to observe the presence of tetragonal or monoclinic phase before as well as after wear.
[Graph 2] shows the XRD spectrum for zirconia specimen before wear, predominantly tetragonal phase was found in the sample.
Graph 2.
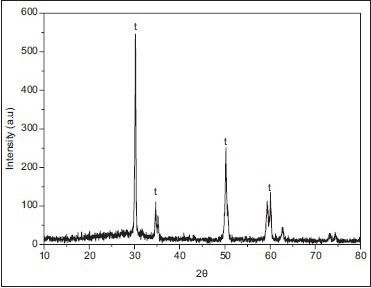
X-ray diffraction spectrum of zirconia ceramic specimen from Group I before wear
[Graph 3] shows the XRD spectrum for zirconia specimen after wear; this sample too showed predominantly tetragonal phase of zirconia; hence, no significant change in the phase from tetragonal to monoclinic.
Graph 3.
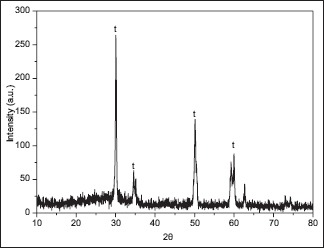
X-ray diffraction spectrum of zirconia ceramic specimen from Group I after wear
DISCUSSION
Physiological wear is surface degradation that results in progressive, but the very slow loss of convexity of the cusps, which manifests as; a flattening of cusp tips on the posterior teeth and incisal edges of mammelons on the anterior teeth. However, the rate of wear may be disturbed by the introduction of restorations with wear properties differing from those of the replaced tooth structure. The severity of this problem may be accentuated by an improper occlusal scheme; for example, group function with a porcelain occlusion can cause more wear than canine-guided mutually protected occlusion.[2] Therefore, there is a need to investigate the wear properties of restorative materials and their potential abrasive effects on the opposing natural teeth.
In this in vitro study, wear of enamel when opposed to different ceramic systems was simulated to the oral environment using a wear machine. Two-body wear that was brought about by this machine provided a combined action of impact, followed by sliding that matches the inherent action of closure during mastication of the mandibular teeth onto the maxillary teeth for a total of 10,000 cycles.[15] A weight of 5 kg (49 N), which is comparable to normal chewing force, was exerted onto the specimens.[16]
Enamel varies in its properties depending on its position in the tooth and its histological structure.[17] Cuspal enamel is stronger and can withstand forces in a direction parallel to the enamel rods than perpendicular to the rods. Hence, in this study, freshly extracted nonattrited, noncarious premolars of young adolescent patients undergoing orthodontic extractions were used. Since this study was to observe the wear behavior of enamel in a clinical environment, only the cuspal tips of the dental specimens were held in contact with the ceramic specimens.
Li and Zhou[18] investigated the influence lubrication on the wear behavior of human enamel, using a reciprocating wear test apparatus. It was found that the depth and severity of the wear scars were much smaller with artificial saliva lubrication than in dry conditions and therefore, concluded that saliva plays an important lubricant effect during the wear process of enamel in the oral environment. Because the surface hardness of glass or ceramic decreases in an aqueous environment, the two mating materials can easily adhere at the microscopic level of sharp asperities in the presence of saliva.[19] Consequently, a more intimate approximation between the contacting surfaces results in a higher coefficient of friction associated with exposure to aqueous media.[20] Hence, artificial saliva was used in this study to simulate the oral environment.
When two materials in contact slide over each other, one or both of the materials will suffer wear on the surface, generally the softer of the two, i.e., tooth enamel.[21] According to this hypothesis, it was expected that being hardest of the two other ceramics under consideration, glazed zirconia as well as polished unglazed zirconia would produce the highest wear of the enamel. Our results corroborated with this hypothesis that showed that Group I (glazed monolithic zirconia) caused statistically significant wear of the opposing enamel as in the study by Heintze et al. where it was reported that flat glazed surfaces show more antagonistic wear than polished surfaces[22] but Group II (mechanically polished zirconia without glaze), however, showed least amount of wear of the opposing tooth enamel which was a point to ponder.
Recent studies show that a glazed zirconia surface brought about more wear than its polished surface.[23] Possible explanation is that the glazed surface is quickly worn away to reveal the rough surface of zirconia ceramic beneath. This may occur due to chairside adjustments that are made before cementation of the prosthesis or may also occur within a short period of function. The surfaces of all materials are rough at a microscopic level with sharp, rugged projections called asperities, which have a surface profile of peaks and valleys.[21] The zirconia underneath the glaze has high asperities, and owing to its high hardness value (1378–1354 Hv), tends to abrade the comparatively softer enamel opposite to it.[23]
Mechanically polished monolith zirconia (Group II) in our study showed the least amount of tooth wear among all four groups, even though it was the hardest material of the lot. Most ceramics have comparatively higher hardness values than human enamel, and the hardness of a ceramic has been used as a predictor of its potential to abrade opposing teeth.[1] However, scientific studies have demonstrated a poor correlation between material's hardness and the abrasive potential of ceramic materials on human enamel.[24,25] Hence, polished zirconia demonstrated less enamel wear than glazed zirconia, a finding that is in agreement with studies conducted by Preis et al.[26] and Sabrah et al.[27]
It was originally thought that glazed ceramics would afford the enamel a certain degree of protection against wear. However, in this study, glazed lithium disilicate (Group IV) as well as glazed porcelain fused to metal (Group III) caused greater wear of the antagonistic tooth enamel than polished unglazed zirconia. In a study conducted by Wang et al.,[28] scanning electron microscopy images of the worn surface of enamel after wear showed particles sticking on the surface of lithium disilicate antagonist as ascribed to the accumulation of chipped off enamel and glass-ceramic particles. As the lithium disilicate specimen slides over the enamel surface, sliding contact generates a frictional force which results in tensile, compressive, and shear stresses on the enamel surface. Microcracks develop within the subsurface and these subsurface cracks propagate to eventually form a particle which becomes displaced according to Reid et al.[29]
As stated by Seghi et al.,[24] Dicor glass ceramic was found to be significantly harder than other glass ceramics but had lower relative abrasiveness. Lack of significant crystalline phase may account for this lower abrasiveness despite its high hardness value. However, pressable lithium disilicate material consists of higher crystalline content (70% volume), which may be a contributing factor for increased abrasiveness of antagonistic tooth enamel, as seen in this study.
As evidenced by the literature and the results of this study, it may be prudent to conclude that polishing the surface of the ceramic is the wisest choice of surface finish when opposing natural dentition.
Powder XRD analysis was done on the zirconia specimens before and after wear simulation on Bruker D8 Advance X-ray diffractometer using 2θ–θ method, where θ is the angle of reflection. The samples were observed for tetragonal and monoclinic phases of zirconia and the data so collected were interpreted to achieve the zirconia spectra graph. In the spectra obtained by the 2θ–θ method in the first zirconia sample before wear, strong peaks representing the tetragonal phase were observed, while peaks assigned to the monoclinic phase were not distinct. In the XRD of the sample after wear in the presence of artificial saliva, peaks of tetragonal phase were observed only, with no presence of transformation-produced monoclinic phase. Irrespective of the mechanism, it is evident that the presence of moisture as well as increased temperatures can induce this t → m transformation.
The XRD results of the present study are in agreement with the results of the study carried out by Eástková et al.[30] in 2004 in which absolutely no layer of transformed zirconia was recorded when specimen tested at 140°C/15 h. The transformed layer was found at a temperature of 180°C and higher only, the extent of t → m transformation increasing with the rise in temperature. Numerous researchers have reported that reducing the average GS in zirconia-based ceramics has a beneficial effect on the stability of the tetragonal phase[31,32] and therefore on LTD. The absence of transformation of phase in the specimen used in this study may be due to the reduced GS of the material, 0.5 µm which is well within the limits of standard GS allowed for zirconia intended for dental use.
Thus, taking all these factors into consideration, the operator must be capable of making an informed decision when selecting a restorative material which will restore form, function, and esthetics as well as protect the present dentition from additional damage.
CONCLUSION
Mechanically polished zirconia showed the least amount of wear of enamel as compared to glazed monolithic zirconia thus suggesting that polishing the monolithic zirconia surface might be the best treatment to reduce surface roughness and antagonistic wear of enamel. This is beneficial in high-load bearing areas, making polish a superior choice over an over-glaze
Lithium disilicate showed statistically significant amount of antagonistic enamel wear thus making it a poor choice of prosthesis in patients with parafunctional habits
Glazed porcelain fused to metal caused greater wear of the antagonistic tooth enamel than polished unglazed zirconia
The zirconia showed almost no t → m transformation after 10,000 cycles of wear in a wet environment thus preventing any detrimental changes in its mechanical properties when used in the oral cavity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kelly J. Ceramics in restorative and prosthetic dentistry. Annu Rev Mater Sci. 1997;27:443–68. [Google Scholar]
- 2.Wiley MG. Effects of porcelain on occluding surfaces of restored teeth. J Prosthet Dent. 1989;61:133–7. doi: 10.1016/0022-3913(89)90360-0. [DOI] [PubMed] [Google Scholar]
- 3.Mahalick JA, Knap FJ, Weiter EJ. Occusal wear in prosthodontics. J Am Dent Assoc. 1971;82:154–9. doi: 10.14219/jada.archive.1971.0018. [DOI] [PubMed] [Google Scholar]
- 4.Mulay G, Dugal R, Buhranpurwala M. An evaluation of wear of human enamel opposed by ceramics of different surface finishes. J Indian Prosthodont Soc. 2015;15:111–8. doi: 10.4103/0972-4052.155031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khandelwal M, Jain D. A comparative evaluation of wear of enamel caused by ceramics with different fusion temperatures. J Indian Prosthodont Soc. 2013;13:513–9. doi: 10.1007/s13191-012-0197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly JR, Denry I. Stabilized zirconia as a structural ceramic: An overview. Dent Mater. 2008;24:289–98. doi: 10.1016/j.dental.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Lughi V, Sergo V. Low temperature degradation aging-of zirconia: A critical review of the relevant aspects in dentistry. Dent Mater. 2010;26:807–20. doi: 10.1016/j.dental.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Chevalier J, Gremillard L, Deville S. Low-temperature degradation of zirconia and implications for biomedical implants. Annu Rev Mater Res. 2007;37:1–32. [Google Scholar]
- 9.Chevalier J, Gremillard L, Virkar AV, Clarke DR. The tetragonal-monoclinic transformation in zirconia: Lessons learned and future trends. J Am Ceram Soc. 2009;92:1901–20. [Google Scholar]
- 10.Chevalier J. What future for zirconia as a biomaterial? Biomaterials. 2006;27:535–43. doi: 10.1016/j.biomaterials.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 11.Ban S, Suehiro Y, Nakanishi H, Nawa H. Fracture toughness of dental zirconia before and after autoclaving. J Ceram Soc Jpn. 2010;118:406–9. [Google Scholar]
- 12.Eichler J, Rodel J, Ulrich E, Mark H. Effect of grain size on mechanical properties of submicrometer 3Y-TZP: Fracture strength and hydrothermal degradation. J Am Ceram Soc. 2007;90:2830–6. [Google Scholar]
- 13.Lin JD, Duh JG. Crystallite size and microstrain of thermally aged low-ceria-and low-yttria-doped zirconia. J Am Ceram Soc. 1998;81:853–60. [Google Scholar]
- 14.Soratur SH. Essentials of Prosthodontics. New Delhi: Jaypee Brothers Publishers; 2009. p. 159. [Google Scholar]
- 15.Elmaria A, Goldstein G, Vijayaraghavan T, Legeros RZ, Hittelman EL. An evaluation of wear when enamel is opposed by various ceramic materials and gold. J Prosthet Dent. 2006;96:345–53. doi: 10.1016/j.prosdent.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Jung YS, Lee JW, Choi YJ, Ahn JS, Shin SW, Huh JB. A study on the in-vitro wear of the natural tooth structure by opposing zirconia or dental porcelain. J Adv Prosthodont. 2010;2:111–5. doi: 10.4047/jap.2010.2.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips RW. Skinners Science of Dental Materials. 8th ed. Philadelphia: W.B Saunders Co., Harcourt Brace and Co; 1982. [Google Scholar]
- 18.Li H, Zhou Z. Wear behaviour of human teeth in dry and artificial saliva conditions. Wear. 2002;249:980–4. [Google Scholar]
- 19.Callister WD., Jr . Materials Science and Engineering: An Introduction. 4th ed. New York: John Wiley & Sons; 1997. pp. 179–95. [Google Scholar]
- 20.Koran A, Craig RG, Tillitson EW. Coefficient of friction of prosthetic tooth materials. J Prosthet Dent. 1972;27:269–74. doi: 10.1016/0022-3913(72)90034-0. [DOI] [PubMed] [Google Scholar]
- 21.Hutchings I. Tribology: Friction and Wear of Engineering Materials. London: Butterworth-Heinemann Ltd; 1992. [Google Scholar]
- 22.Heintze SD, Cavalleri A, Forjanic M, Zellweger G, Rousson V. Wear of ceramic and antagonist – A systematic evaluation of influencing factors in vitro . Dent Mater. 2008;24:433–49. doi: 10.1016/j.dental.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Janyavula S, Lawson N, Cakir D, Beck P, Ramp LC, Burgess JO. The wear of polished and glazed zirconia against enamel. J Prosthet Dent. 2013;109:22–9. doi: 10.1016/S0022-3913(13)60005-0. [DOI] [PubMed] [Google Scholar]
- 24.Seghi RR, Rosenstiel SF, Bauer P. Abrasion of human enamel by different dental ceramics in vitro . J Dent Res. 1991;70:221–5. doi: 10.1177/00220345910700031301. [DOI] [PubMed] [Google Scholar]
- 25.Won-Suck O, Delong R, Anusavice KJ. Effect of restorative materials on wear of human enamel. J Prosthet Dent. 2002;64:194–203. [Google Scholar]
- 26.Preis V, Behr M, Kolbeck C, Hahnel S, Handel G, Rosentritt M. Wear performance of substructure ceramics and veneering porcelains. Dent Mater. 2011;27:796–804. doi: 10.1016/j.dental.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Sabrah AH, Cook NB, Luangruangrong P, Hara AT, Bottino MC. Full-contour Y-TZP ceramic surface roughness effect on synthetic hydroxyapatite wear. Dent Mater. 2013;29:666–73. doi: 10.1016/j.dental.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Liu Y, Wenjie S, Feng H, Tao Y, Ma Z. Friction and wear behaviors of dental ceramics against natural tooth enamel. J Eur Ceram Soc. 2012;32:2599–606. [Google Scholar]
- 29.Reid CN, Fisher J, Jacobsen PH. Fatigue and wear of dental materials. J Dent. 1990;18:209–15. doi: 10.1016/0300-5712(90)90114-t. [DOI] [PubMed] [Google Scholar]
- 30.Eástková K, Hadraba H, Cihlá J. Hydrothermal ageing of tetragonal zirconia ceramics. Ceram Silikáty. 2004;48:85–92. [Google Scholar]
- 31.Garvie RC. Stabilization of the tetragonal structure in zirconia microcrystals. J Phys Chem. 1978;82:218–24. [Google Scholar]
- 32.Tsukuma K, Kubota Y, Tsukidate T. In: Thermal and mechanical properties of Y2O3-stabilized tetragonal zirconia polycrystals. Science and Technology of Zirconia II. Claussen N, Ruehle M, Heuer AH, editors. Columbus, OH: The American Ceramic Society; 1984. pp. 382–90. [Google Scholar]


