Abstract
Purpose:
The aim of this study was to investigate the cytotoxicity in human gingival fibroblast by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and corrosion behavior by potentiodynamic polarization technique of commercially pure titanium (Ti 12) and its alloy Ti-6Al-4V (Ti 31).
Materials and Methods:
In the present in vitro study, cytotoxicity of Ti 12 and Ti 31 in human gingival fibroblast by MTT assay and the corrosion behavior by potentiodynamic polarization technique in aqueous solutions of 0.1 N NaCl, 0.1 N KCl, and artificial saliva with and without NaF were studied.
Results:
The independent t-test within materials and paired t-test with time interval showed higher cell viability for Ti 12 compared to Ti 31. Over a period, cell viability found to stabilize in both Ti 12 and Ti 31. The effects of ions of Ti and alloying elements aluminum and vanadium on the cell viability were found with incubation period of cells on samples to 72 h. The electrochemical behavior of Ti 12 and Ti 31 in different experimental solutions showed a general tendency for the immersion potential to shift steadily toward nobler values indicated formation of TiO2 and additional metal oxides. The multiphase alloy Ti-6Al-4V showed more surface pitting.
Conclusion:
The commercially pure Ti showed better cell viability compared to Ti 31. Less cell viability in Ti 31 is because of the presence of aluminum and vanadium. A significant decrease in cytotoxicity due to the formation of TiO2 over a period of time was observed both in Ti 12 and Ti 31. The electrochemical behavior of Ti 12 and Ti 31 in different experimental solutions showed a general tendency for the immersion potential to shift steadily toward nobler values indicated formation of TiO2 and additional metal oxides. Ti 31 alloy showed surface pitting because of its multiphase structure.
Keywords: Artificial saliva, corrosion, cytotoxicity, gingival fibroblast, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay, scanning electron microscopy, Ti-6Al-4V, titanium
INTRODUCTION
There is a continuous increase in the use of titanium (Ti) and its alloys for applications in orthodontics, endodontics, prosthodontics, and implantology due to their low density, excellent corrosion resistance, and unique biocompatibility.[1,2,3,4,5] The chemistry of Ti is a key factor in determining implant-tissue interactions. When Ti and its alloys are present in the oral environment, they are subjected to biodegradation by dissolution in saliva, chemical/physical destruction, wear and erosion caused by food, bacterial activity, and pH. The metallic ions released into the oral cavity will be in contact with adjacent tissues and as a consequence adverse reactions such as toxicity, allergy, and mutagenicity can appear.[6]
Biocompatibility is generally related with the corrosion property of the materials since metal ions often release into the adjacent environment, during the corrosion process and affect the tissues around it.[1,7] Therefore, the evaluation of cytotoxicity along with its corrosive potential becomes important, which in turn has a considerable influence on clinical longevity of a material in service, contributing to the success of the dental implant. The relationship between polarization resistance and the type of tissue reaction for various pure metals and alloys have been reported in several studies.[7,8,9,10,11,12]
The aim of this study was to investigate the cytotoxicity in human gingival fibroblast by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and corrosion behavior by potentiodynamic polarization technique of commercially available Ti and its alloy Ti-6Al-4V obtained from an industry.
METHODOLOGY
Materials
Materials studied were 8 mm rods of commercially pure Ti, hereafter will be known as Ti 12 (ASTM B 348 Grade 1) and its alloy Ti-6Al-4V, hereafter will be known as Ti 31 (ASTM B 348 Grade 5) procured from Mishra Dhatu Nigam Ltd., Hyderabad, India.
Cytotoxicity test
A fresh biopsy specimen of human gingival tissue of patients undergoing orthodontic treatment was collected after obtaining informed consent according to the Institutional Ethics Committee regulations. It was then cultured for fibroblast in Dulbecco's Modified Eagle medium. Cultures were incubated at 37°C in a humidified atmosphere of 5% CO2 in air. Cells were passaged and frozen till treatment. In the present study, 7 cut samples from the rods of Ti 12 and Ti 31 received of dimension 5 mm thickness and 8 mm diameter were used for cytotoxicity evaluation with fibroblast cells. Cells derived from cell lines, after attaining confluence, were treated with different doses of Ti 12 and Ti 31 for different time point. Then, these cells were observed for their proliferation and viability by MTT colorimetric assay as described by Wang et al. 2013.[1] Percentage viability was calculated as follows:
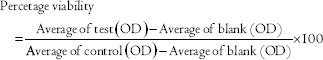
Evaluation of corrosion behavior
The potentiodynamic polarization test was conducted using instrument CH1604D Electrochemical Analyzer Beta at room temperature on specimens of surface area 5026 mm2 (diameter 8 mm) for corrosion rate in the following solutions:
0.1 N NaCl
0.1 N KCl
Artificial saliva with (0.1 g) and without NaF having composition 0.1 g NaCl, 1.21 g KCl, 0.7 g NaH2 PO4.2H2O, and CO (NH2)2 with pH adjusted to 6.75 ± 0.75.
The corroded samples were also subjected to scanning electron microscopy (SEM) study. The rates of corrosion in different experimental solutions were determined.
RESULTS AND DISCUSSION
The results of cytotoxicity of Ti 12 and Ti 31 at different time intervals are shown in Figures 1–4. Independent t-test within materials and paired t-test with time intervals showed higher cell viability for Ti 12 [Figures 1 and 3] compared to Ti 31 [Figures 2 and 4] at 48 and 72 h [Figures 1–4] with the exception for Ti 12 at 72 h for 5 µl dose. Approximately 13%–17% cell death was observed at higher concentration of 100 µl dose in the case of Ti 31. A cell viability of above 90% for both Ti 12 and Ti 31 was seen for dose of 5, 10, 25, and 50 µl. Results showed a marginal influence of fibroblast viability on material composition. The effect of ions of Ti and alloying elements aluminum and vanadium on the viability is evident with incubation period of the cells on the samples to 72 h.
Figure 1.
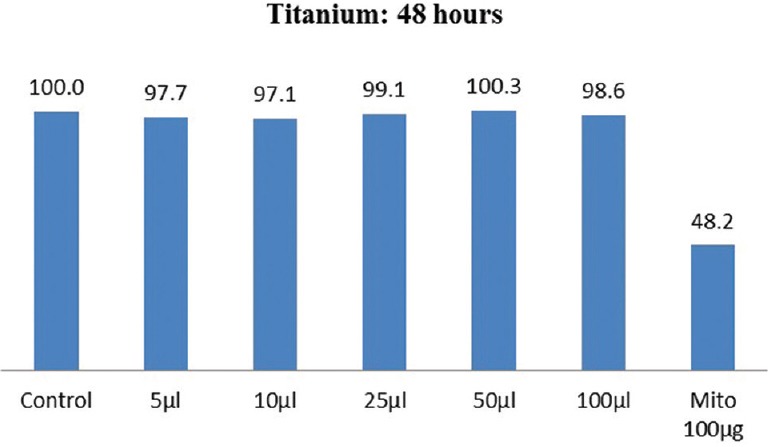
Cell viability of commercially pure titanium (Ti 12) after 48 h
Figure 4.
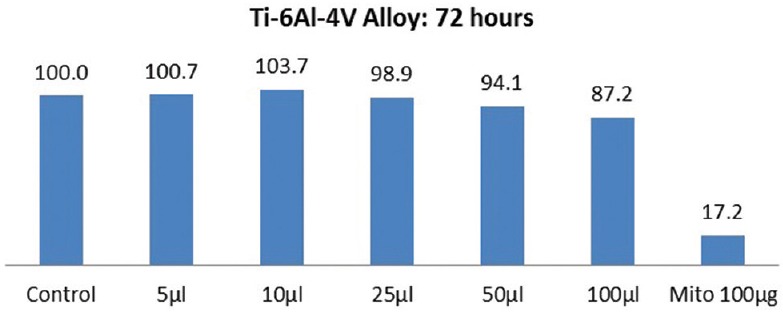
Cell viability of Ti-6Al-4V (Ti 31) after 72 h
Figure 3.
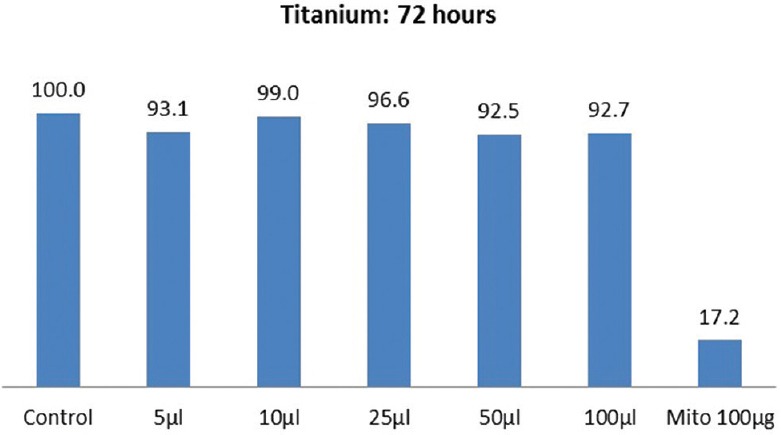
Cell viability of commercially pure titanium (Ti 12) after 72 h
Figure 2.
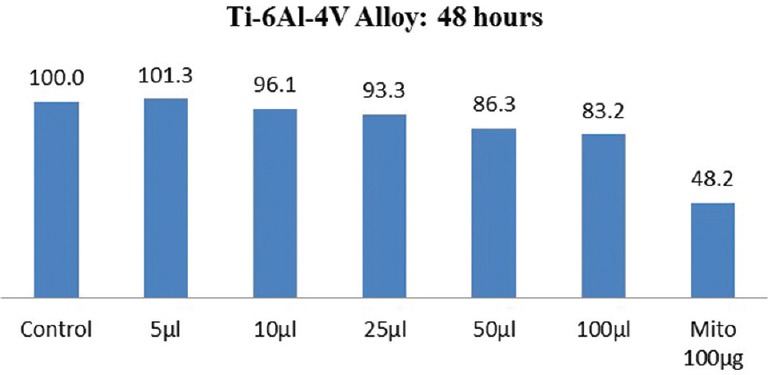
Cell viability of Ti-6Al-4V (Ti 31) after 48 h
Medical and dental devices have been widely used in various disciplines. As these devices have direct contact with the tissues and cells of the body, they not only require good physical and chemical properties but also have good compatibility. The cytotoxicity test, one of the biological evaluation and screening tests, uses tissue cells in vitro to observe cell growth, reproduction, and morphological effects by medical and dental devices.[13] Fibroblasts are the most fundamental cell type of mesenchymal origin; hence, it can be taken as a model to study the behavior of any cells of the same origin. Of course, the exact behavior of the osteoblast-like cells cannot be predicted by the behavior of fibroblasts, but can be taken as the basis for the future research and development because of common initial fundamental properties for growth and differentiation. In vitro conditions cannot replicate in vivo situations due to large number of factors that would have to be taken into consideration. It is, however, possible to determine the basic cell reaction for a single-type cell, which could be representative of the cells of common origin. The cell viability was determined using MTT test that is based on mitochondrial succinate dehydrogenase ability to reduce the yellow MTT dye (the solvent tetrazolium salt) into insoluble Formazan. The amount of formazan is directly proportional to the number of viable cells. After adding solution, optical density of the resulting solutions was measured with a microplate reader at 570 nm. Optical density was converted to a percentage of controls for each cell culture plate.[1,14]
The cytotoxic effect is slightly more in Ti 31 compared to Ti 12 over a period. This can be attributed to the presence of aluminum and vanadium.[4,15,16] However, the effect of toxicity was found to stabilize and decrease with time. It appears that the increase in TiO2 passivating layer over a period of time that formed when come in contact with tissue cells moderated the toxicity by curtailing the release of Ti, Al, and V ions from test materials into the tissue cells.[17]
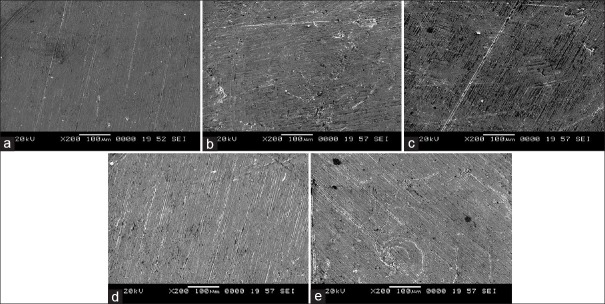
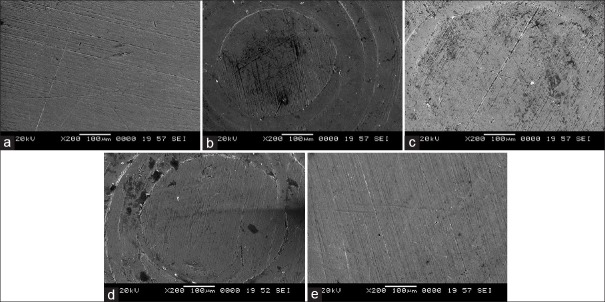
Potentiodynamic polarization measurements were used to determine (CH1604D Electrochemical Analyzer Beta) the active–passive characteristics of Ti 12 and Ti 31 in solutions of 0.1 N NaCl, 0.1 NKCl, and artificial saliva with and without NaF. Linear polarization, corrosion rate, and corrosion density (I cor) in experimental solutions are shown in Table 1. The cyclic polarization curves of Ti 12 and Ti 31 are shown in Figures 5 and 6, respectively. The degradation mechanism of metal after being corroded in different solutions was observed under a SEM (JOEL JED-2300). Figures 7 and 8 illustrate the surface morphology of Ti 12 and Ti 31 specimen after photentiodynamic cyclic polarization treatment in experimental solutions.
Table 1.
The corrosion parameters of Ti 12 and Ti 31 electrode in different solutions
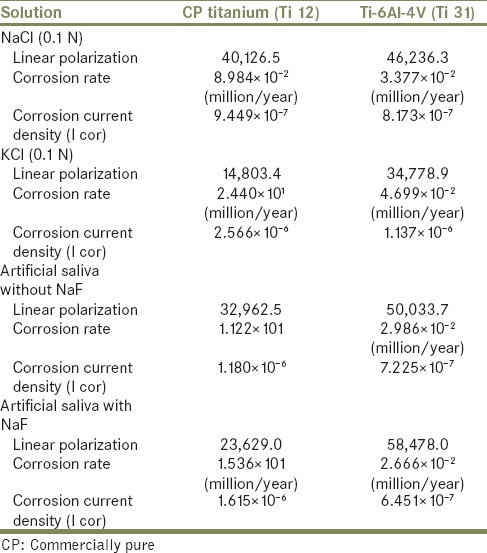
Figure 5.
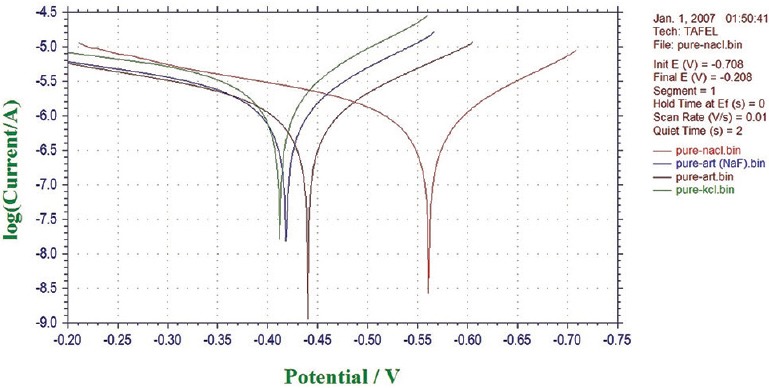
Cyclic potentiodynamic polarization curves of Ti 12 electrode in different solutions
Figure 6.
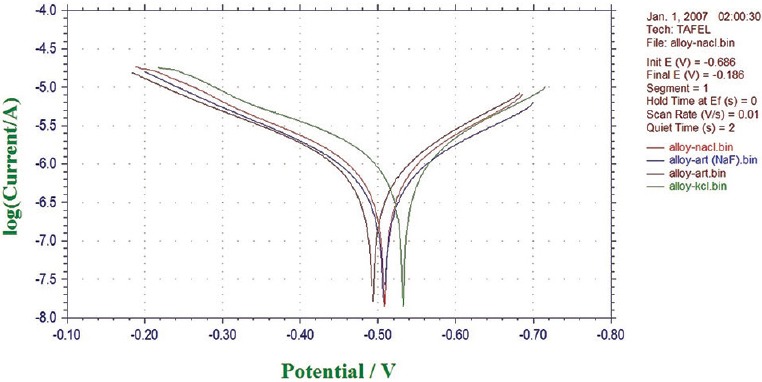
Cyclic potentiodynamic polarization curves of Ti 31 electrode in different solutions
Figure 7.
Surface morphology of Ti 12 before and after potentiodynamic cyclic anode polarization treatment. (a) Precorrosion. (b) 0.1 NaCl. (c) 0.1 N KCl. (d) Artificial saliva. (e) Artificial saliva with NaF
Figure 8.
Surface morphology of Ti 31 before and after potentiodynamic cyclic anode polarization treatment. (a) Precorrosion. (b) 0.1 NaCl. (c) 0.1 N KCl. (d) Artificial saliva. (e) Artificial saliva with NaF
Titanium is a reactive metal. In air and aqueous electrolytes, it forms spontaneously dense oxide film at its surface. This unwanted reaction product becomes potential barrier against dissolution of metal, and therefore Ti exhibits excellent corrosion barrier.[18] However, Ti oxide is classified as possible carcinogenic to humans (Group 2B) by the International Agency for Research and Cancer.[19]
The present study of cyclic polarization technique provides qualitative view of pitting corrosion mechanism, and it is highly useful method for determining the susceptibility of metals or alloys to pitting when placed in a specific corrosive environment. The cyclic polarization curves of Ti 12 and Ti 31 electrode in 0.1 N NaCl, 0.1 N KCl, and artificial saliva with and without NaF are shown in Figures 5 and 6. It is clear from these figures that the polarization curves have the same features and are characterized by the appearance of active, passive, and transpassive regions. Materials characterized by this type of cyclic polarization curve are known to resist localized corrosion but are susceptible to pitting as well as crevice corrosion in the experimental solutions. In general, the pitting and crevice corrosion can be evaluated based on the formation of a loop, and the evaluation of Ti 12 and Ti 31 can be made on the area of loops that form in the cyclic polarization curves. In general, higher the loop area, greater is the tendency toward pitting and crevice corrosion.[20] It is clear from the Figures 5 and 6 that the area of loop is nearly same in all solutions. It is concluded from the hysteresis loop observed during the reverse anodic scan that there exists the possibility of pitting. Pitting on the passive surface has been explained by a competitive adsorption mechanism in which chloride and fluoride ions migrate to the metal/oxide film interface. The corrosion parameters of Ti 12 and Ti 31 electrodes in different solutions are given in Table 1. The main characteristic common to all metals and environments is the localized character of corrosion nucleation. Preferred sites for such nucleation generally depend on the metal structure, which is associated with the presence of different phases, or to environmental pollutant deposition at discrete points, which result in the formation of a greater number of small product nuclei that spread with exposure time until the surface is completely covered.[4,21] To confirm the degradation mechanism of Ti 12 and Ti 31 before and after being corroded in the experimental solutions, the electrodes were observed under SEM. Figures 7 and 8 illustrate the surface morphology of Ti 12 and Ti 31 specimen, respectively. It is evident from the SEM analysis that a large number of pits were formed on the surface of the specimen when corroded. Therefore, it is concluded that the metals degrade due to pitting corrosion. In the case of Ti 12 material, less surface pitting was observed compared to Ti 31. This can be attributed to the fact that commercially Ti 12 is a single phase metal, and therefore extent of attack by corrosive media is less compared to Ti-6Al-4V (Ti 31), which is a multiphase alloy containing α and β structures.
CONCLUSION
Taking into consideration the limitations related to the present study, it can be concluded that both Ti 12 and Ti 31 are suitable for dental and medical applications. The commercially pure Ti showed better cell viability compared to Ti 31. Less cell viability in Ti 31 is because of the presence of aluminum and vanadium. A significant decrease in cytotoxicity due to the formation of TiO2 over a period of time was observed both in Ti 12 and Ti 31.
The electrochemical behavior of Ti 12 and Ti 31 in different experimental solutions showed a general tendency for the immersion potential to shift steadily toward nobler values indicated formation of TiO2 and additional metal oxides. Variation in I cor and corrosion rates were found in different solutions. Ti 31 alloy showed surface pitting because of its multiphase structure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wang QY, Wang YB, Lin JP, Zheng YF. Development and properties of Ti-In binary alloys as dental biomaterials. Mater Sci Eng C Mater Biol Appl. 2013;33:1601–6. doi: 10.1016/j.msec.2012.12.070. [DOI] [PubMed] [Google Scholar]
- 2.Lautenschlager EP, Monaghan P. Titanium and titanium alloys as dental materials. Int Dent J. 1993;43:245–53. [PubMed] [Google Scholar]
- 3.Wang RR, Fenton A. Titanium for prosthodontic applications: A review of the literature. Quintessence Int. 1996;27:401–8. [PubMed] [Google Scholar]
- 4.Faria AC, Rosa AL, Rodrigues RC, Ribeiro RF. In vitro cytotoxicity of dental alloys and cpTi obtained by casting. J Biomed Mater Res B Appl Biomater. 2008;85:504–8. doi: 10.1002/jbm.b.30972. [DOI] [PubMed] [Google Scholar]
- 5.Bhasin SS, Singh V, Ahmed T, Singh BP. In: Studies on titanium-based dental implant material. 27th Annual Cocoa Beach Conference on Advanced Ceramics and Composites-A: Ceramic Engineering and Science Proceedings. Kriven WM, Hua-Tay L, editors. Vol. 24. Hoboken, New Jersey: John Wiley and Sons; 2008. pp. 245–54. [Google Scholar]
- 6.Faria AC, Rodrigues RC, Antunes RP, de Mattos Mda G, Rosa AL, Ribeiro RF. Effect of temperature variation on the cytotoxicity of cast dental alloys and commercially pure titanium. J Appl Oral Sci. 2009;17:421–6. doi: 10.1590/S1678-77572009000500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okazaki Y, Rao S, Ito Y, Tateishi T. Corrosion resistance, mechanical properties, corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials. 1998;19:1197–215. doi: 10.1016/s0142-9612(97)00235-4. [DOI] [PubMed] [Google Scholar]
- 8.Paschoal AL, Vanâncio EC, Canale Lde C, da Silva OL, Huerta-Vilca D, Motheo Ade J. Metallic biomaterials TiN-coated: Corrosion analysis and biocompatibility. Artif Organs. 2003;27:461–4. doi: 10.1046/j.1525-1594.2003.07241.x. [DOI] [PubMed] [Google Scholar]
- 9.Jorge JR, Barão VA, Delben JA, Faverani LP, Queiroz TP, Assunção WG. Titanium in dentistry: Historical development, state of the art and future perspectives. J Indian Prosthodont Soc. 2013;13:71–7. doi: 10.1007/s13191-012-0190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMahon RE, Ma J, Verkhoturov SV, Munoz-Pinto D, Karaman I, Rubitschek F, et al. A comparative study of the cytotoxicity and corrosion resistance of nickel-titanium and titanium-niobium shape memory alloys. Acta Biomater. 2012;8:2863–70. doi: 10.1016/j.actbio.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 11.Khan MA, Williams RL, Williams DF. In-vitro corrosion and wear of titanium alloys in the biological environment. Biomaterials. 1996;17:2117–26. doi: 10.1016/0142-9612(96)00029-4. [DOI] [PubMed] [Google Scholar]
- 12.Savadi RC, Goyal C. Study of biomechanics of porous coated root form implant using overdenture attachment: A 3D FEA. J Indian Prosthodont Soc. 2010;10:168–75. doi: 10.1007/s13191-010-0035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soenen SJ, Manshian B, Montenegro JM, Amin F, Meermann B, Thiron T, et al. Cytotoxic effects of gold nanoparticles: A multiparametric study. ACS Nano. 2012;6:5767–83. doi: 10.1021/nn301714n. [DOI] [PubMed] [Google Scholar]
- 14.Wataha JC, Craig RG, Hanks CT. Precision of and new methods for testing in vitro alloy cytotoxicity. Dent Mater. 1992;8:65–70. doi: 10.1016/0109-5641(92)90056-i. [DOI] [PubMed] [Google Scholar]
- 15.Velasco-Ortega E, Jos A, Cameán AM, Pato-Mourelo J, Segura-Egea JJ. In vitro evaluation of cytotoxicity and genotoxicity of a commercial titanium alloy for dental implantology. Mutat Res. 2010;702:17–23. doi: 10.1016/j.mrgentox.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Salsbury RL, Bernecki TF. Quality Control of Hydroxyapatite Coating: Purity and Crystalline Determinations. National Thermal Spray Conference. ASM International; 1991. pp. 471–3. [Google Scholar]
- 17.Hsiao IL, Huang YJ. Titanium oxide shell coatings decrease the cytotoxicity of ZnO nanoparticles. Chem Res Toxicol. 2011;24:303–13. doi: 10.1021/tx1001892. [DOI] [PubMed] [Google Scholar]
- 18.Steinemann SG. Titanium – the material of choice? Periodontol 2000. 1998;17:7–21. doi: 10.1111/j.1600-0757.1998.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 19.IARC Monographs. No. 2 Titanium Dioxide. 2006. [Last accessed on 2016 Mar 05]. Available from: http://www.monographs.iarc.fr/ENG/Meetings/93-titaniumdioxie.pdf .
- 20.Gurrappa I. Characterization of titanium alloy Ti-6Al-4V for chemical, marine and industrial applications. Mater Charact. 2003;51:131. [Google Scholar]
- 21.Shahba RM, Ghannem WA, El-Shenawy AE, Ahmed AS, Tantawy SM. Corrosion and inhibition of Ti-Al-4V alloy in NaCl solution. Int J Electrochem Sci. 2011;6:5499–509. [Google Scholar]