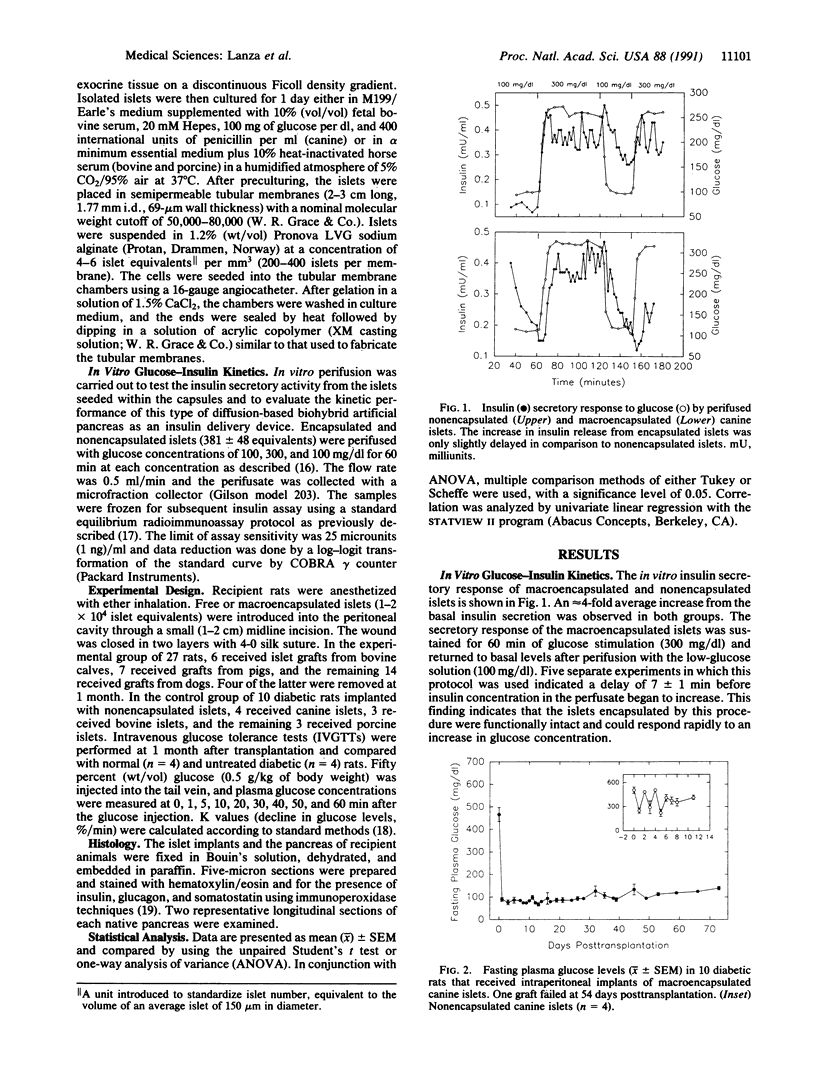
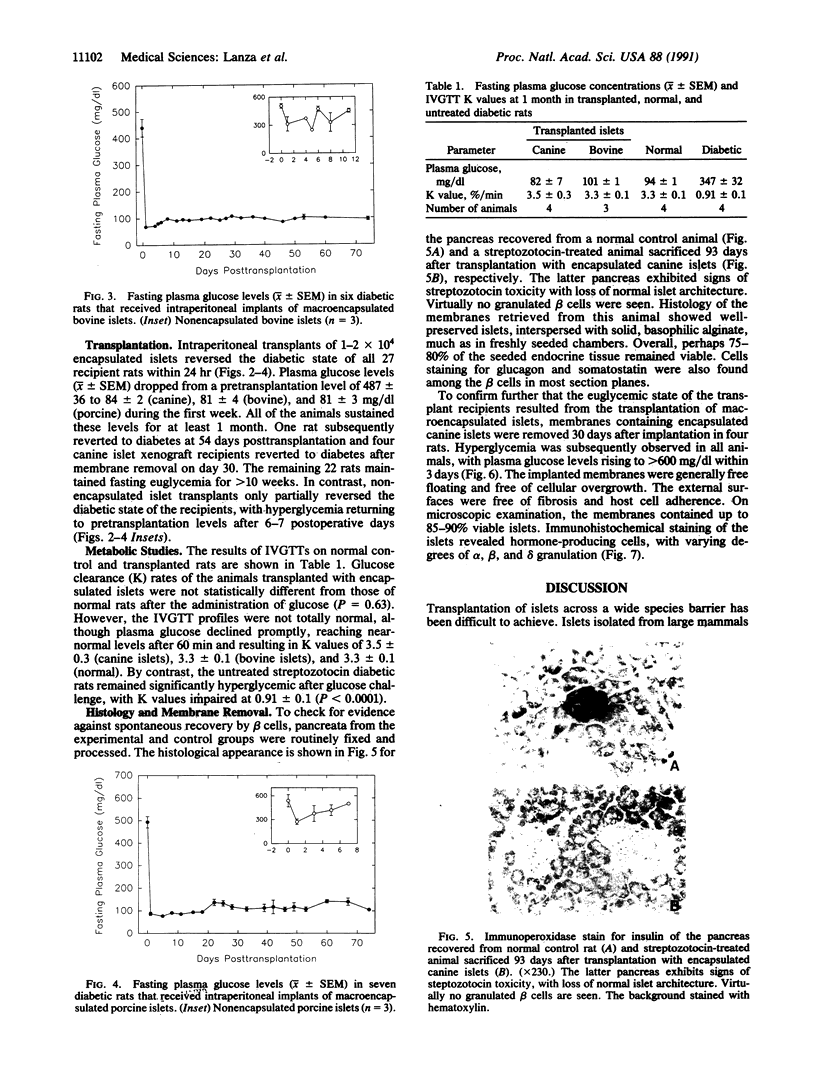
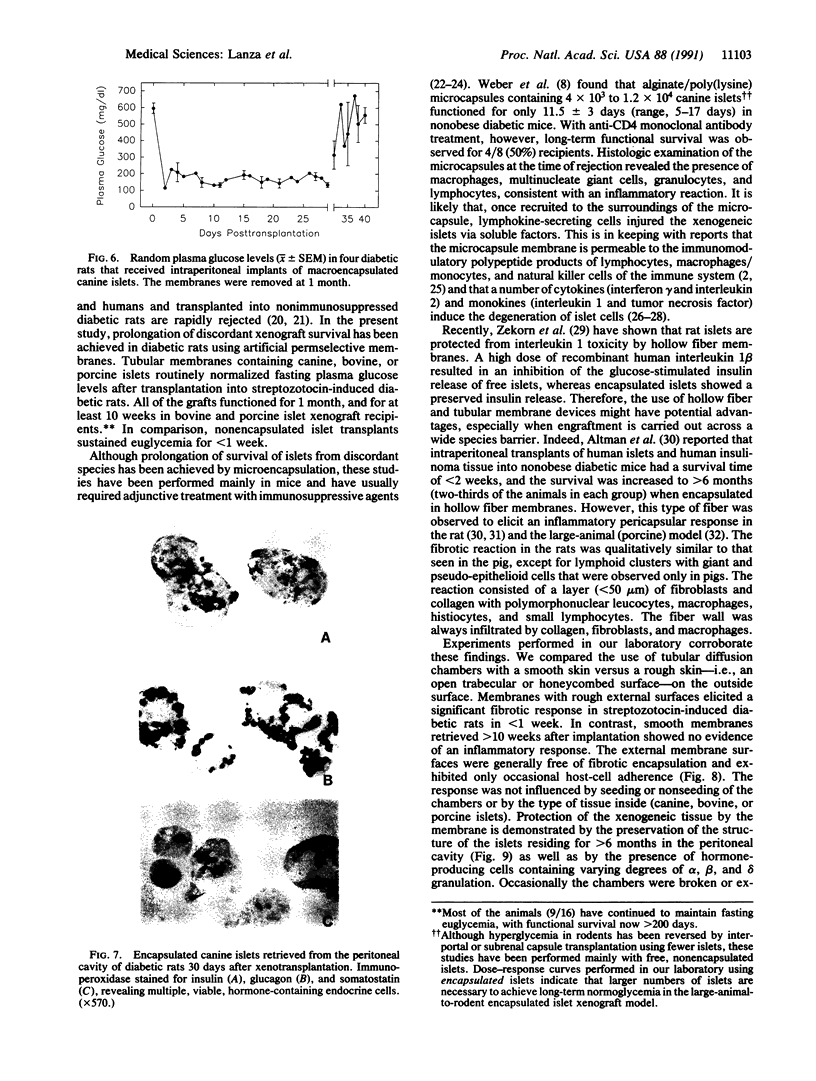
Abstract
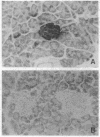
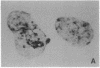
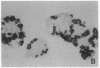
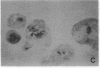
Permselective acrylic membranes were employed to prevent immune rejection of discordant islet xenografts isolated from various large animals. Canine, porcine, and bovine islets were seeded into tubular diffusion chambers and transplanted into the peritoneum of 27 nonimmunosuppressed streptozotocin-induced diabetic Lewis rats. Six recipients received islet grafts from bovine calves, 7 received grafts from pigs, and 14 received grafts from dogs. Four of the latter were removed at 1 month. In the control group of 10 diabetic rats, 4 received nonencapsulated canine islets, 3 received nonencapsulated bovine islets, and 3 received nonencapsulated porcine islets. Recipients of encapsulated islets promptly dropped from a pretransplantation plasma glucose level of 487 +/- 36 (mean +/- SEM) to 84 +/- 2 (canine), 81 +/- 4 (bovine), and 81 +/- 3 mg/dl (porcine) during the first week. All of the animals sustained these levels for at least 1 month. One rat spontaneously reverted to diabetes at 54 days posttransplantation; 4 other rats became hyperglycemic (glucose, greater than 600 mg/dl) after membrane removal on day 30. The remaining 22 rats maintained fasting euglycemia for greater than 10 weeks. In contrast, rats that received nonencapsulated islets became hyperglycemic in less than 7 days. Intravenous glucose tolerance test K values (decline in glucose levels, %/min) at 1 month for the canine and bovine encapsulated islet transplant group were 3.5 +/- 0.3 and 3.3 +/- 0.1 compared with 3.3 +/- 0.1 (P = 0.63) and 0.91 +/- 0.1 (P less than 0.0001) for normal (n = 4) and diabetic (n = 4) control groups. Morphologic studies of long-term functioning grafts (30-130 days) revealed well-preserved alpha, beta, and delta cells, with varying degrees of granulation. These results demonstrate that immune isolation of islet tissue using permselective artificial membranes can protect discordant islet xenografts from immune rejection in the absence of any immunosuppressive drugs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J. J., Houlbert D., Callard P., McMillan P., Solomon B. A., Rosen J., Galletti P. M. Long-term plasma glucose normalization in experimental diabetic rats with macroencapsulated implants of benign human insulinomas. Diabetes. 1986 Jun;35(6):625–633. doi: 10.2337/diab.35.6.625. [DOI] [PubMed] [Google Scholar]
- Altman J. J., Penfornis A., Boillot J., Maletti M. Bioartificial pancreas in autoimmune nonobese diabetic mice. ASAIO Trans. 1988 Jul-Sep;34(3):247–249. [PubMed] [Google Scholar]
- Archer J., Kaye R., Mutter G. Control of streptozotocin diabetes in Chinese hamsters by cultured mouse islet cells without immunosuppression: a preliminary report. J Surg Res. 1980 Jan;28(1):77–85. doi: 10.1016/0022-4804(80)90086-4. [DOI] [PubMed] [Google Scholar]
- Auchincloss H., Jr Xenogeneic transplantation. A review. Transplantation. 1988 Jul;46(1):1–20. doi: 10.1097/00007890-198807000-00001. [DOI] [PubMed] [Google Scholar]
- Chicheportiche D., Darquy S., Lepeintre J., Capron F., Halban P. A., Reach G. High-performance liquid chromatography analysis of circulating insulins distinguishes between endogenous insulin production (a potential pitfall with streptozotocin diabetic rats) and islet xenograft function. Diabetologia. 1990 Aug;33(8):457–461. doi: 10.1007/BF00405105. [DOI] [PubMed] [Google Scholar]
- Comens P. G., Wolf B. A., Unanue E. R., Lacy P. E., McDaniel M. L. Interleukin 1 is potent modulator of insulin secretion from isolated rat islets of Langerhans. Diabetes. 1987 Aug;36(8):963–970. doi: 10.2337/diab.36.8.963. [DOI] [PubMed] [Google Scholar]
- Darquy S., Reach G. Immunoisolation of pancreatic B cells by microencapsulation. An in vitro study. Diabetologia. 1985 Oct;28(10):776–780. doi: 10.1007/BF00265027. [DOI] [PubMed] [Google Scholar]
- Icard P., Penfornis F., Gotheil C., Boillot J., Cornec C., Barrat F., Altman J. J. Tissue reaction to implanted bioartificial pancreas in pigs. Transplant Proc. 1990 Apr;22(2):724–726. [PubMed] [Google Scholar]
- Kraegen E. W., Chisholm D. J., McNamara M. E. Timing of insulin delivery with meals. Horm Metab Res. 1981 Jul;13(7):365–367. doi: 10.1055/s-2007-1019271. [DOI] [PubMed] [Google Scholar]
- MOORHOUSE J. A., GRAHAME G. R., ROSEN N. J. RELATIONSHIP BETWEEN INTRAVENOUS GLUCOSE TOLERANCE AND THE FASTING BLOOD GLUCOSE LEVEL IN HEALTHY AND IN DIABETIC SUBJECTS. J Clin Endocrinol Metab. 1964 Feb;24:145–159. doi: 10.1210/jcem-24-2-145. [DOI] [PubMed] [Google Scholar]
- Pukel C., Baquerizo H., Rabinovitch A. Destruction of rat islet cell monolayers by cytokines. Synergistic interactions of interferon-gamma, tumor necrosis factor, lymphotoxin, and interleukin 1. Diabetes. 1988 Jan;37(1):133–136. doi: 10.2337/diab.37.1.133. [DOI] [PubMed] [Google Scholar]
- Ricordi C., Socci C., Davalli A. M., Staudacher C., Vertova A., Baro P., Sassi I., Braghi S., Guizzi N., Pozza G. Application of the automated method to islet isolation in swine. Transplant Proc. 1990 Apr;22(2):784–785. [PubMed] [Google Scholar]
- Scharp D. W., Lacy P. E., Finke E., Olack B. Low-temperature culture of human islets isolated by the distention method and purified with Ficoll or Percoll gradients. Surgery. 1987 Nov;102(5):869–879. [PubMed] [Google Scholar]
- Soon-Shiong P., Lu Z. N., Grewal I., Lanza R. P., Clark W. An in vitro method of assessing the immunoprotective properties of microcapsule membranes using pancreatic and tumor cell targets. Transplant Proc. 1990 Apr;22(2):754–755. [PubMed] [Google Scholar]
- Sorensen J. T., Colton C. K., Hillman R. S., Soeldner J. S. Use of a physiologic pharmacokinetic model of glucose homeostasis for assessment of performance requirements for improved insulin therapies. Diabetes Care. 1982 May-Jun;5(3):148–157. doi: 10.2337/diacare.5.3.148. [DOI] [PubMed] [Google Scholar]
- Sullivan S. J., Maki T., Borland K. M., Mahoney M. D., Solomon B. A., Muller T. E., Monaco A. P., Chick W. L. Biohybrid artificial pancreas: long-term implantation studies in diabetic, pancreatectomized dogs. Science. 1991 May 3;252(5006):718–721. doi: 10.1126/science.2024124. [DOI] [PubMed] [Google Scholar]
- TERASAKI P. I., ESAIL M. L., CANNON J. A., LONGMIRE W. P., Jr Destruction of lymphocytes in vitro by normal serum from common laboratory animals. J Immunol. 1961 Oct;87:383–395. [PubMed] [Google Scholar]
- Tze W. J., Tai J., Cheung S. Human islet xenograft survival in diabetic rats. A functional and immunohistochemical study. Transplantation. 1990 Mar;49(3):502–505. doi: 10.1097/00007890-199003000-00005. [DOI] [PubMed] [Google Scholar]
- Tze W. J., Tai J., Cheung S. Xenotransplantation of human islets in diabetic rats. Transplant Proc. 1989 Feb;21(1 Pt 3):2741–2743. [PubMed] [Google Scholar]
- Wang Y., Hao L., Gill R. G., Lafferty K. J. Autoimmune diabetes in NOD mouse is L3T4 T-lymphocyte dependent. Diabetes. 1987 Apr;36(4):535–538. doi: 10.2337/diab.36.4.535. [DOI] [PubMed] [Google Scholar]
- Warnke R., Levy R. Detection of T and B cell antigens hybridoma monoclonal antibodies: a biotin-avidin-horseradish peroxidase method. J Histochem Cytochem. 1980 Aug;28(8):771–776. doi: 10.1177/28.8.7003003. [DOI] [PubMed] [Google Scholar]
- Warnock G. L., Rajotte R. V. Critical mass of purified islets that induce normoglycemia after implantation into dogs. Diabetes. 1988 Apr;37(4):467–470. doi: 10.2337/diab.37.4.467. [DOI] [PubMed] [Google Scholar]
- Weber C. J., Zabinski S., Koschitzky T., Wicker L., Rajotte R., D'Agati V., Peterson L., Norton J., Reemtsma K. The role of CD4+ helper T cells in the destruction of microencapsulated islet xenografts in nod mice. Transplantation. 1990 Feb;49(2):396–404. doi: 10.1097/00007890-199002000-00034. [DOI] [PubMed] [Google Scholar]
- Zekorn T., Siebers U., Bretzel R. G., Renardy M., Planck H., Zschocke P., Federlin K. Protection of islets of Langerhans from interleukin-1 toxicity by artificial membranes. Transplantation. 1990 Sep;50(3):391–394. doi: 10.1097/00007890-199009000-00007. [DOI] [PubMed] [Google Scholar]
- Zekorn T., Siebers U., Filip L., Mauer K., Schmitt U., Bretzel R. G., Federlin K. Bioartificial pancreas: the use of different hollow fibers as a diffusion chamber. Transplant Proc. 1989 Feb;21(1 Pt 3):2748–2750. [PubMed] [Google Scholar]