Abstract
Background
Arteriosclerotic disorders increase the risk of dementia. As they have common causes and risk factors, coronary heart disease (CHD) could influence the course of dementia.
Aim
To determine whether CHD increases the speed of cognitive decline in Alzheimer’s disease, and to discuss the potential for secondary cardiovascular prevention to modify this decline.
Design and setting
Prospective multicentre cohort study in general practices in six cities in Germany.
Method
Participants were patients with probable mild-to-moderate Alzheimer’s dementia or mixed dementia (n = 118; mean age 85.6 [±3.4] years, range 80–96 years). The authors assessed the presence of CHD according to the family physicians’ diagnosis. Cognitive performance was measured during home visits for up to 3 years in intervals of 6 months, using Mini Mental State Examination (MMSE) and Clinical Dementia Rating Sum of Boxes (CDR-SoB). The authors also recorded whether patients died in the observation period.
Results
At baseline, 65 patients (55%) had CHD and/or a heart condition following a myocardial infarction. The presence of CHD accelerated cognitive decline (MMSE, P<0.05) by about 66%, and reduced cognitive-functional ability (CDR-SoB, P<0.05) by about 83%, but had no impact on survival.
Conclusion
The study shows that CHD has a significant influence on cognitive decline in older patients with late-onset dementia. The dementia process might therefore be positively influenced by cardiovascular prevention, and this possible effect should be further investigated.
Keywords: Alzheimer’s disease, cardiovascular diseases, cardiovascular risk, cognitive decline, dementia, family practice, primary health care
INTRODUCTION
Demographic changes will lead to an increase in cases of dementia, with 35.6 million people already suffering from dementia worldwide.1 This number is expected to double over the next 20 years. Alzheimer’s disease (AD) accounts for 60% of dementia cases, followed by vascular dementia. Mixed forms are frequently diagnosed.2
Arteriosclerotic disorders increase the risk of dementia and, even in the absence of a dementia process, lead to a decrease in cognitive function in old age.3 The most common atherosclerotic disease is coronary heart disease (CHD).4 The incidences of CHD and AD increase with age, and both diseases share common causes and risk factors,5 such as the ApoE4 allele,6 hypertension,7 hypercholesterolaemia,8 and smoking.9 In addition, both diseases mutually influence each other’s course and respective pathology. Patients with CHD tend to have higher amounts of senile plaques in the brain,10 and microangiopathy caused by arteriosclerosis may contribute to neurodegeneration.5 Further, the presence of the amyloid beta peptide may increase mortality in patients with CHD.11
Secondary prevention of CHD is known to be highly effective.12 Because of their similar pathological mechanisms, cardiovascular prevention could reduce the incidence and slow the progression of AD.13
How this fits in
The presence of atherosclerotic disease increases the risk for Alzheimer’s disease. The impact of atherosclerotic disease, such as coronary heart disease (CHD), on the cognitive decline of dementia has been little investigated. Alzheimer’s disease and CHD share many risk factors and aetiological similarities. In cases where an impact of CHD on cognitive decline has been detected, cardiovascular prevention is an opportunity to reduce the progression of dementia.
GPs play a key role in initiating cardiovascular prevention. The risk of myocardial infarction in a patient with existing CHD can primarily be reduced by a change in behaviour patterns, for example, smoking cessation, increased physical exercise, or weight control.14–17 Medication for blood pressure, blood-thinning agents, and the lowering of blood lipids, especially low-density lipoprotein (LDL) cholesterol, is recommended.
It is already known that the greater the severity of arteriosclerosis, the higher the risk of dementia, especially in patients at genetic risk of AD.18 In addition, arteriosclerotic disorders have a negative impact on cognition in vascular dementia.19 One can therefore expect that the co-occurrence of AD and CHD would lead to a more severe course of cognitive decline.
However, the impact of CHD on cognitive decline in existing AD has rarely been investigated. An influence on the progression of cognitive impairment and mortality would underline the importance of optimal treatment and primary prevention of CHD to possibly decelerate cognitive deterioration in dementia. For this reason, the authors examined the association between CHD and cognitive decline in cases of AD.
METHOD
Study design
Ageing, cognition, and dementia in primary care patients (AgeCoDe) is a multicentre longitudinal study on the early diagnosis of mild cognitive impairment and dementia in primary health care in Germany. Patients were recruited between 1 January 2003 and 30 November 2004, based on a random selection from the registries of 138 general practices in six German cities (Bonn, Düsseldorf, Hamburg, Leipzig, Mannheim, and Munich). Following recruitment, patients were examined at 18-month intervals, and patients with dementia at the third measurement wave (4.5 years after the first examination) had additional interim assessments every 6 months to closely track the course of cognitive decline.
Inclusion criteria for the AgeCoDe study were: age ≥75 years, absence of dementia according to a GP’s judgement, and at least one contact with a GP within the previous 12 months. Exclusion criteria were: GP consultations by home visits only, living in a nursing home, severe illness with an anticipated fatal outcome within 3 months, language barrier, deafness or blindness, and an inability to provide informed consent. A total of 3327 patients initially free of dementia took part in the AgeCoDe study.
For the current analysis, only those patients who fulfilled the criteria of probable AD or mixed dementia were examined at the third measurement wave of the AgeCoDe study (see ‘definition of dementia’ section). Patients who did not fulfil these criteria were not selected because the aim of this study was to examine the interplay between CHD and AD. Of 1995 patients taking part in the third measurement wave of the AgeCoDe study, 167 were classified as having dementia. Of these, 118 were diagnosed as having AD or mixed dementia and were thus included in this study.
Assessment procedures
All patients included in the study were examined every 6 months over a period of 3 years in their home environment. These examinations included assessment with both the Mini Mental State Examination (MMSE)20 and the Clinical Dementia Rating Scale Sum of Boxes (CDR-SoB).21 The MMSE is a 30-point neuropsychological test that is used to estimate a patient’s global cognitive capability. Higher scores on MMSE indicate a better performance. The CDR-SoB is a clinical rating scale based on the sum of six items used to quantify a patient’s cognitive and functional disability. A higher CDR-SoB rating suggests a worse performance. In addition, the authors assessed marital status, living conditions, and whether patients died during followup, and determined the date of death by informant report.
At every measurement wave, patients’ GPs reported the current diagnoses recorded in the practice in a questionnaire. The authors considered CHD to be present if CHD or a myocardial infarction were indicated at least once in the period between the first examination and the third measurement wave of the AgeCoDe study (that is, the baseline of the current study). For the analysis, patients with a documented myocardial infarction were, if not already specified by the GP, assigned to CHD. The authors assessed the presence of hypertension, diabetes, hypercholesterolaemia/hyperlipidaemia, stroke, and cancer in the same way as for CHD. Patients’ educational levels were classified as high, medium, or low in accordance with the Comparative Analysis of Social Mobility in Industrial Nations classification system (CASMIN). The authors measured patients’ depressive symptoms using the 15-item version of the Geriatric Depression Scale.22
Definition of dementia
A dementia diagnosis was made by trained research physicians or psychologists using a structured interview for the diagnosis of AD, vascular dementia, and types of dementia with other aetiology (SIDAM) on the basis of the criteria of DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th edition).23 SIDAM incorporates a battery of neuropsychological tests, including the MMSE and the SIDAM-ADL (activities of daily living) scale, which were used to determine cognitive impairment and impairment of activities of daily living (defined as a value of >1 on the SIDAM-ADL scale), respectively. The aetiological diagnosis of probable AD was made using the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the AD and Related Disorders Association (NINCDS-ADRDA).24 Mixed dementia was diagnosed in cases with a history of cerebrovascular incidents without temporal relationship to cognitive decline. The authors did not classify patients with other specific causes of dementia (for example, Parkinson’s disease, alcohol consumption, brain tumour, or head injury) as AD or mixed dementia. All final diagnoses were made in a consensus conference involving the interviewer and an experienced geriatrician or psychiatrist.
Statistical analysis
The association of prevalent CHD at baseline with patients’ cognitive capacity was examined by latent variable growth curve modelling (LGCM),25 using the software Mplus (version 7.11). Because the intervals between measurement points were equally spaced, time was included using fixed time scores. The maximum likelihood method was used for parameter estimation.
The authors modelled base level of cognitive performance (intercept) and the linear decline (slope) in MMSE and CDR-SoB. CHD status (present/absent) was entered as a predictor of intercept and slope in the model while controlling for age, sex, education level, and time since dementia diagnosis.
Full information maximum likelihood (FIML) estimation was used to handle missing data. FIML does not estimate specific values for missing observations, but solely uses the available information from every observation to estimate model parameters. Consequently, cases with missing values do not have to be excluded from the analysis but contribute to the model estimation with their recorded observations.26 FIML is currently recommended as the state-of-the-art method for treating missing data, and may be useful to enhance power and reduce bias arising from complete cases analysis.27
Model fit was assessed using root mean square error of approximation (RMSEA) and comparative fit index (CFI), where a CFI >0.95 and RMSEA <0.05 represent a very good model fit.28 Multivariate Cox proportional hazard regression analysis was performed to assess the impact of CHD on survival, using SPSS (version 22.0). The authors adjusted for age, sex, education, and time since dementia diagnosis.
RESULTS
Table 1 contains the baseline data of the study sample. On average, subjects were cognitively assessed for 26 months. Of 118 patients with AD, 65 (55%) could be assessed over an 18-month period, and 38 (33%) over a 36-month follow-up period. In the first 18 months, 33 (28%) of the patients died. At the end of the study, 59 (50%) of the patients were deceased.
Table 1.
Baseline data of study participants (n = 118)
| Mean | SD | |
|---|---|---|
| Age, years | 85.57 | 3.32 |
|
| ||
| Time since dementia diagnosis | 1.56 | 1.35 |
|
| ||
| MMSEa at baseline | 20.04 | 5.00 |
|
| ||
| CDR-SoB at baseline | 6.99 | 4.14 |
|
| ||
| Female sex | n | % |
|
| ||
| 88 | 74.6 | |
|
| ||
| Education | ||
| Low | 75 | 63.5 |
| Middle | 29 | 24.6 |
| High | 14 | 11.9 |
|
| ||
| Marital status | ||
| Single | 11 | 9.3 |
| Married | 51 | 43.2 |
| Divorced | 7 | 5.9 |
| Widowed | 49 | 41.5 |
| Living alone | 60 | 50.8 |
| Depressive symptoms (Geriatric Depression Scale ≥6) | 16 | 13.6 |
| History of smoking | 47 | 39.8 |
| Hypertension | 103 | 87.3 |
| Diabetes | 45 | 38.1 |
| Hypercholesterolaemia/hyperlipidaemia | 86 | 72.9 |
| Stroke | 12 | 10.2 |
| Cancer | 16 | 13.5 |
| Coronary heart disease | 65 | 55.1 |
The Mini Mental State Examination (MMSE) was not available at any measurement time point for five patients, so an analysis of this cognitive variable was only possible for 113 patients. CDR-SoB = Clinical Dementia Rating Sum of Boxes. SD = standard deviation.
The MMSE was not available at any measurement time point for five patients, so an analysis of this cognitive variable was only possible for 113 patients.
Cognitive performance and cognitive decline
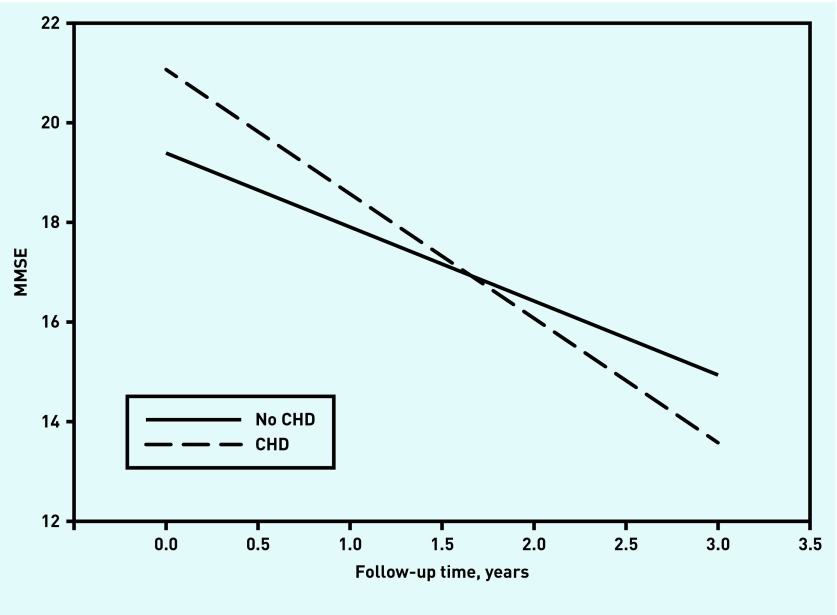
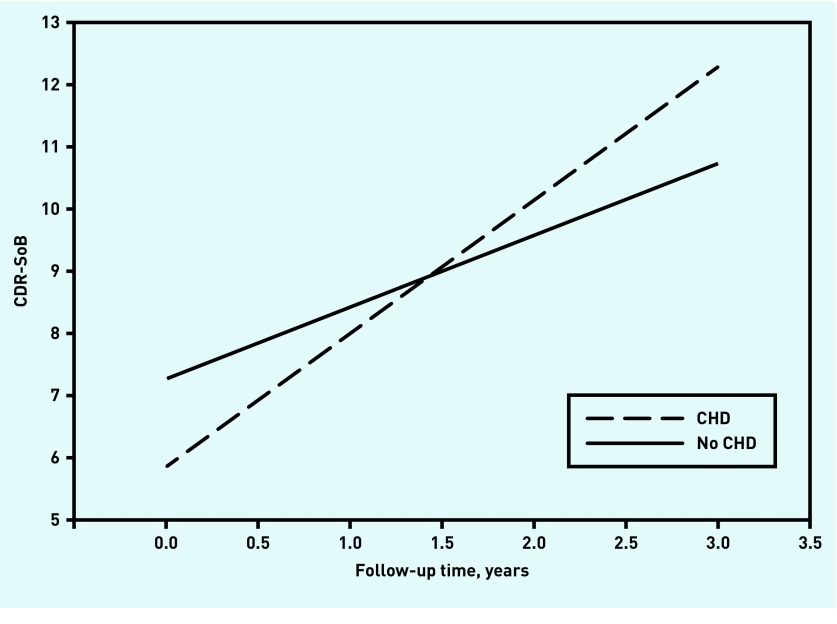
The models fitted the data well. Fit indices and estimates of the effect of CHD on intercept and slope of cognition are presented in Table 2. The estimated cognitive decline is depicted in Figures 1 and 2.
Table 2.
Results of the latent variable growth curve model
| MMSE (n= 113)a | CDR-SoB (n= 118)b | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Est | P-value | Est | P-value | ||
| Intercept | Time since dementia diagnosis | −1.54 | <0.001 | 1.30 | <0.001 |
| Age | 0.01 | 0.918 | 0.77 | 0.358 | |
| Sexc | −0.12 | 0.908 | −0.01 | 0.946 | |
| Medium educationd | −1.71 | 0.090 | 0.93 | 0.244 | |
| High educationd | 0.31 | 0.827 | 1.39 | 0.214 | |
| CHDe | 1.73 | 0.051 | −1.45 | 0.036 | |
|
| |||||
| Slope | Time since dementia diagnosis | −0.23 | 0.385 | 0.05 | 0.797 |
| Age | −0.07 | 0.383 | 0.03 | 0.581 | |
| Sexc | −1.02 | 0.131 | −0.46 | 0.366 | |
| Medium educationd | −0.04 | 0.951 | 0.30 | 0.503 | |
| High educationd | −0.35 | 0.687 | 0.72 | 0.283 | |
| CHDe | −1.03 | 0.039 | 1.01 | 0.008 | |
The fit for the MMSE model was good (χ2 [53] = 67.795, P = 0.08, root mean square error of approximation [RMSEA] = 0.050, comparative fit index [CFI] = 0.958).
The fit for the CDR-SoB model was good (χ2 [53] = 69.14, P = 0.07, RMSEA = 0.051, CFI = 0.964).
Female sex was used as reference.
Low educational level was used as reference.
Absence of CHD was used as reference. CDR-SoB = Clinical Dementia Rating Sum of Boxes. CHD = coronary heart disease. Est = estimate of the effect on intercept and slope of cognition. MMSE = Mini Mental State Examination.
Figure 1.
Cognitive decline measured by MMSE. Range = 0–30. Lower scores indicate worse cognitive performance. CHD = coronary heart disease. MMSE = Mini Mental State Examination.
Figure 2.
The course of cognitive and functional performance measured by CDR-SoB. Range = 0–18. Higher scores indicate worse cognitive and functional performance. CDR-SoB = Clinical Dementia Rating Sum-of-Boxes. CHD = coronary heart disease.
As expected, time since incident dementia diagnosis was related to the intercept of MMSE and CDR-SoB, a more recent diagnosis being associated with fewer deficits. No other covariate showed significant effects on intercept or slope.
At baseline, patients with CHD were significantly less impaired in the CDR-SoB, and a similar trend (P<0.10) was found in the MMSE. However, the presence of CHD at baseline was associated with significantly stronger cognitive decline both in the MMSE and in the CDR-SoB.
In patients without CHD, the estimated annual MMSE was 1.5 points, and this decline accelerated by about 66% to 2.5 MMSE points per year for patients with CHD. The decline in the CDR-SoB was even more pronounced: the estimated annual decline increased from 1.2 to 2.2 points (83%).
Effects of CHD on survival
The authors found no significant effect of CHD on survival (hazard ratio [HR] = 0.98, P = 0.941). However, males had a higher risk of death within the observation period (HR = 2.17, P = 0.012).
DISCUSSION
Summary
The main finding of this study is that, in older patients with a recent diagnosis of dementia, the course of cognitive decline is influenced by the presence of CHD. As measured by the MMSE, this decline accelerated by about 66% per year for patients with CHD. At 83%, the decline in the CDR-SoB was even more pronounced.
No effect of CHD on survival was observed in this study. Thus, the authors could not find evidence that the coincidence of AD and CHD leads to more severe physical impairment. However, the sample size might have been too small to detect an effect.
Strengths and limitations
The strengths of the study include a GP-based sampling, a close monitoring of cognitive decline, and statistical consideration of drop-outs. However, the observational design of the AgeCoDe study does not allow causal inferences to be made. In addition, the sample size was limited, and confirmation in other samples and settings is desirable.
Comparison with existing literature
The prospective Rotterdam study showed that there is a connection between evidence of arteriosclerosis and the likelihood of incident AD or vascular dementia.18 Patients with severe arteriosclerosis (two or more indicators) carried a two- to threefold higher risk of developing either AD or all-cause dementia than those without arteriosclerosis, and the risk for incident vascular dementia was even higher.
The current study extends this finding and shows that CHD also has a deleterious effect on cognitive decline after patients receive a diagnosis of AD. This highlights the importance of cardiovascular prevention,29 and suggests a beneficial role for optimal cardiovascular prophylaxis on cognitive decline in dementia.
However, previous studies on cardiovascular prevention have shown a need for improvement. In primary care, only about half of high-risk patients received drug therapy according to guidelines.30 In addition, behavioural health measures, such as smoking cessation or nutritional counselling, are still too rarely part of primary care treatment.31
As yet, there is no conclusive evidence that antiplatelet or cholesterol-lowering drugs have a positive effect on dementia disorders or their progression.32 But cognitive impairment was not defined as the primary endpoint of these studies. Older people benefit from medical secondary prevention even without an existing CHD. In particular, the risk of myocardial infarction, stroke, and potentially mortality can be reduced by statins.33,34 The course of dementia has a significant impact on the need for care and the autonomy of the patient.35,36 Therefore, a positive effect of cardiovascular prevention on cognition could mean that patients need months’ or years’ less care.
Implications for research and practice
The results of this study show that CHD may accelerate cognitive decline in dementia. It is well established that cardiovascular prevention reduces the incidence of atherosclerotic diseases. It may also be effective at slowing progression of dementia, although evidence from randomised controlled trials is lacking.
Additional studies are required to examine the effects that preventing CHD by early treatment of its risk factors has on dementia and cognitive decline.37 A recent study showed that an excessive reduction in blood pressure in older patients with dementia or mild cognitive impairment can even promote the progression of cognitive decline.38 This demonstrates the importance of studies on the effects of cardiovascular prevention in older people.
As well as pharmacological treatment, physical exercise has a positive effect on dementia,39 possibly mediated by cardiovascular mechanisms. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER, a multidomain intervention) found that diet, exercise, cognitive training and vascular risk monitoring could improve or maintain cognitive functioning in older patients at risk of dementia.40 The transferability of the results to people with existing dementia should be examined in further prospective studies.
Due to the connection between the heart and the brain, efforts have been made for high-risk patients to be treated by neurologists and cardiovascular specialists.41 Multidisciplinary treatment of cardiovascular risk factors could influence not only the progression of dementia, but also the onset of AD. Multidisciplinary heart–brain clinics could be provided in the future for this purpose. Such clinics could also support physicians in risk stratification and medical decision-making for patients with multimorbidity. It would also allow the impact of lifestyle changes on CHD, cognitive decline, and dementia to be examined in one place. In an interdisciplinary approach, the family doctor should remain the main coordinator of the treatment and care of patients with dementia.
The results presented here should encourage GPs to ensure that, in particular, patients with dementia receive optimal and effective prophylactic treatment to prevent progression of arteriosclerosis. Further research on the causal relationship between CHD and cognitive decline in dementia is warranted.
Acknowledgments
The authors wish to thank members of the AgeCoDe Study Group, as well as all participating patients and their GPs for their collaboration.
Funding
This study is part of the German Research Network on Dementia (KND) and the German Research Network on Degenerative Dementia (KNDD), and was funded by the German Federal Ministry of Education and Research (KND grant reference numbers: 01GI0102, 01GI0420, 01GI0422, 01GI0423, 01GI0429, 01GI0431, 01GI0433, 01GI0434. KNDD grant reference numbers: 01GI0710, 01GI0711, 01GI0712, 01GI0713, 01GI0714, 01GI0715, 01GI0716). This study is moreover published in affiliation with the Health Service Research Initiative (Study on needs, health service use, costs and health-related quality of life in a large sample of oldest-old primary care patients [85+; AgeQualiDe]) that was also funded by the German Federal Ministry of Education and Research (grants: 01GY1322A, 01GY1322B, 01GY1322C, 01GY1322D, 01GY1322E, 01GY1322F, 01GY1322G).
Ethical approval
The Ageing, cognition, and dementia in primary care patients (AgeCoDe) study was completed in accordance with the Helsinki declaration and approved by the Ethics Committee of the Medical Association of Hamburg (file OB/8/02) and all other participating centres. All participants gave written informed consent.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Prince M, Bryce R, Albanese E, et al. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Kalaria RN, Maestre GE, Arizaga R, et al. Alzheimer’s disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7(9):812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verhaegen P, Borchelt M, Smith J. Relation between cardiovascular and metabolic disease and cognition in very old age: cross-sectional and longitudinal findings from the Berlin Aging Study. Health Psychol. 2003;22(6):559–569. doi: 10.1037/0278-6133.22.6.559. [DOI] [PubMed] [Google Scholar]
- 4.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics — 2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casserly I, Topol EJ. Convergence of atherosclerosis and Alzheimer’s disease: inflammation, cholesterol, and misfolded proteins. Lancet. 2004;363(9415):1139–1146. doi: 10.1016/S0140-6736(04)15900-X. [DOI] [PubMed] [Google Scholar]
- 6.Anoop S, Misra A, Meena K, Luthra K. Apolipoprotein E polymorphism in cerebrovascular and coronary heart diseases. Indian J Med Res. 2010;132:363–378. [PubMed] [Google Scholar]
- 7.Skoog I, Gustafson D. Hypertension and related factors in the etiology of Alzheimer’s disease. Ann N Y Acad Sci. 2002;977:29–36. doi: 10.1111/j.1749-6632.2002.tb04796.x. [DOI] [PubMed] [Google Scholar]
- 8.Martins IJ, Berger T, Sharman MJ, et al. Cholesterol metabolism and transport in the pathogenesis of Alzheimer’s disease. J Neurochem. 2009;111(6):1275–1308. doi: 10.1111/j.1471-4159.2009.06408.x. [DOI] [PubMed] [Google Scholar]
- 9.Rusanen M, Kivipelto M, Quesenberry CP, et al. Heavy smoking in midlife and long-term risk of Alzheimer disease and vascular dementia. Arch Intern Med. 2011;171(4):333–339. doi: 10.1001/archinternmed.2010.393. [DOI] [PubMed] [Google Scholar]
- 10.Sparks DL, Hunsaker JC, Scheff SW, et al. Cortical senile plaques in coronary artery disease, aging and Alzheimer’s disease. Neurobiol Aging. 1990;11(6):601–607. doi: 10.1016/0197-4580(90)90024-t. [DOI] [PubMed] [Google Scholar]
- 11.Stamatelopoulos K, Sibbing D, Rallidis LS, et al. Amyloid-beta (1–40) and the risk of death from cardiovascular causes in patients with coronary heart disease. J Am Coll Cardiol. 2015;65(9):904–916. doi: 10.1016/j.jacc.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 12.Murphy E, Vellinga A, Byrne M, et al. Primary care organisational interventions for secondary prevention of ischaemic heart disease: a systematic review and meta-analysis. Br J Gen Pract. 2015. DOI: https://doi.org/10.3399/bjgp15X685681. [DOI] [PMC free article] [PubMed]
- 13.O’Brien JT, Markus HS. Vascular risk factors and Alzheimer’s disease. BMC Med. 2014;12:218. doi: 10.1186/s12916-014-0218-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forman DE, Alexander K, Brindis RG, et al. Improved cardiovascular disease outcomes in older adults. F1000Res. 2016;5:112. doi: 10.12688/f1000research.7088.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergert FW, Braun M, Clarius H, et al. Guideline Group Hessen, editor. Practice guideline for cardiovascular prevention. [In German]. Hausärztliche Leitlinie kardiovaskuläre Prävention. 2011. pp. 1–106. [Primary care guideline for cardiovascular prevention). In German, no English abstract available). Version 1.00. http://www.pmvforschungsgruppe.de/pdf/03_publikationen/kardiopraev_ll.pdf (accessed 22 Nov 2016].
- 16.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34(28):2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 17.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Hofman A, Ott A, Breteler MM, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet. 1997;349(9046):151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- 19.Chandra M, Anand KS. Vascular disease burden in Indian subjects with vascular dementia. Australas Med J. 2015;8(7):227–234. doi: 10.4066/AMJ.2015.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 22.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 23.Zaudig M, Mittelhammer J, Hiller W, et al. SIDAM — a structured interview for the diagnosis of dementia of the Alzheimer type, multi-infarct dementia, and dementias of other aetiology according to ICD-10 and DSM-III-R. Psychol Med. 1991;21(1):225–236. doi: 10.1017/s0033291700014811. [DOI] [PubMed] [Google Scholar]
- 24.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of the Department of Health and Human Service Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 25.Duncan TE, Duncan SC, Strycker LA. An introduction to latent variable growth curve modeling: concepts, issues, and applications. 2nd edn. Mahwah, NJ: Erlbaum; 2006. [Google Scholar]
- 26.Enders CK. Analyzing longitudinal data with missing values. Rehabil Psychol. 2011;56(4):267–288. doi: 10.1037/a0025579. [DOI] [PubMed] [Google Scholar]
- 27.Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60:549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- 28.Kline RB. Principles and practice of structural equation modeling. 4th edn. New York, NY: Guilford Press; 2015. [Google Scholar]
- 29.Dalen JE, Alpert JS, Goldberg RJ, Weinstein RS. The epidemic of the 20th century: coronary heart disease. Am J Med. 2014;127(9):807–812. doi: 10.1016/j.amjmed.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Kasteleyn MJ, Wezendonk A, Vos RC, et al. Repeat prescriptions of guideline-based secondary prevention medication in patients with type 2 diabetes and previous myocardial infarction in Dutch primary care. Fam Pract. 2014;1(6):688–693. doi: 10.1093/fampra/cmu042. [DOI] [PubMed] [Google Scholar]
- 31.Wittchen HU, Pieper L, Eichler T, et al. Prevalence and treatment of diabetes mellitus and cardiovascular disease: DETECT — a nationwide supply study of over 55 000 GP patients in prevention and care research. (In German). In: Kirch W, Badura B, Pfaff H, editors. Prevention and care research: selected contributions from the 2nd National Prefecture Congress and the 6th German Congress for Health Care Research, Dresden, 24 to 27 October 2007. Berlin: Springer; 2008. pp. 315–328. [Google Scholar]
- 32.Ligthart SA, Moll van Charante EP, Van Gool WA, Richard E. Treatment of cardiovascular risk factors to prevent cognitive decline and dementia: a systematic review. Vasc Health Risk Manag. 2010;6:775–785. doi: 10.2147/vhrm.s7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Afilalo J, Duque G, Steele R, et al. Statins for secondary prevention in elderly patients: a hierarchical bayesian meta-analysis. J Am Coll Cardiol. 2008;51(1):37–45. doi: 10.1016/j.jacc.2007.06.063. [DOI] [PubMed] [Google Scholar]
- 34.Savarese G, Gotto AM, Paolillo S, et al. Benefits of statins in elderly subjects without established cardiovascular disease: a meta-analysis. J Am Coll Cardiol. 2013;62(22):2090–2099. doi: 10.1016/j.jacc.2013.07.069. [DOI] [PubMed] [Google Scholar]
- 35.Schäufele M, Köhler L, Hendlmeier I, et al. Prevalence of dementia and medical care in German nursing homes: a nationally representative survey. (In German) Psychiatr Prax. 2013;40(4):200–206. doi: 10.1055/s-0033-1343141. [DOI] [PubMed] [Google Scholar]
- 36.Hajek A, Brettschneider C, Ernst A, et al. Longitudinal predictors of informal and formal caregiving time in community-dwelling dementia patients. Soc Psychiatry Psychiatr Epidemiol. 2016;51(4):607–616. doi: 10.1007/s00127-015-1138-7. [DOI] [PubMed] [Google Scholar]
- 37.Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56(1):42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 38.Mossello E, Pieraccioli M, Nesti N, et al. Effects of low blood pressure in cognitively impaired elderly patients treated with antihypertensive drugs. JAMA Intern Med. 2015;175(4):578–585. doi: 10.1001/jamainternmed.2014.8164. [DOI] [PubMed] [Google Scholar]
- 39.Yaffe K, Barnes D, Nevitt M, et al. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161(14):1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 40.Ngandu T, Lehtisalo J, Solomon A, et al. A 2-year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 41.de la Torre JC. In-house heart-brain clinics to reduce Alzheimer’s disease incidence. J Alzheimers Dis. 2014;42(Suppl 4):S431–S442. doi: 10.3233/JAD-141560. [DOI] [PubMed] [Google Scholar]