Summary
The prognostic value of interim positron emission tomography (PET) was evaluated after 2 cycles of doxorubicin, bleomycin, vinblastin and dacarbazine in classical Hodgkin lymphoma patients (n = 229), based on Deauville criteria. In early stage non-bulky disease, bulky stage II disease, advanced stage low International Prognostic Score (IPS ≤2) and advanced stage (IPS ≥3), 3-year progression-free survival rates in PET2-negative vs. PET2-positive groups were 95·9% vs. 76·9% (P < 0·0018), 83·3% vs. 20·0% (P = 0·017), 77·0% vs. 30·0% (P < 0·001) and 71·0% vs. 44·4% (P = 0·155), respectively. The outcome after positive PET2 was better than previously reported. The results from non-randomized studies of PET2-guided therapy would be valuable with careful interpretation.
Keywords: Hodgkin lymphoma, positron emission tomography, prognostic
The interim positron emission tomography (PET) scan after 2 cycles (PET2) has prognostic value in patients with classical Hodgkin lymphoma (cHL) (Hutchings et al, 2006; Matloub et al, 2006; Gallamini et al, 2007; Terasawa et al, 2009; Cerci et al, 2010; Le Roux et al, 2011; Zinzani et al, 2012; Biggi et al, 2013). In previous studies, the outcome of patients with positive PET2 was dismal regardless of pre-treatment clinical characteristics. These studies were essentially based on the criteria proposed by Juweid et al (2007) for interpretation of PET.
More recently, the Deauville criteria (Meignan et al, 2009) has been broadly used for interpretation of the PET scan in cHL. As only a few small studies have applyied this criteria for assessing the prognostic value of PET2 in cHL, we performed a review of interim PET scans based on Deauville criteria and analysed the prognostic role of the interim PET scan for predicting progression-free survival (PFS) for cHL treated with standard ABVD (doxorubicin, bleomycin, vinblastin, dacarbazine). Of note, three nuclear medicine physicians independently evaluated the PET2 images, blinded for the clinical information including outcome and the originally issued report of PET scan.
Patients and methods
This study was approved by institutional review board. Medical record of patients with cHL diagnosed between January 2001 and May 2011 and treated with ABVD were reviewed. None received BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone). Patients who received additional treatment as a part of initial treatment other than radiation therapy, such as brentuximab vedotin or rituximab, were excluded. Patients who had an interim PET scan (after 2 cycles, immediately before the next treatment [PET2] or after 3 cycles [PET3] of ABVD) in our institution were included and were reviewed for outcome. Three nuclear medicine physicians were given the medical record number and the date of the initial (if available) and interim PET scan. They were blinded for the clinical outcome and the report of PET scan, and independently interpreted the interim PET scan based on the Deauville scoring system (scores of 1–5): 1. No uptake; 2. uptake ≤mediastinum; 3. Uptake >mediastinum and ≤liver; 4. uptake moderately more than liver; 5. markedly increased uptake than liver or new sites. By the Deauville criteria (Meignan et al, 2009), a positive interim PET is defined by a score ≥4; by Juweid criteria (Juweid et al, 2007), a positive interim PET is ≥3. Consensus on the score was determined if 2 readers agreed on scores. If consensus was not obtained by this method, the case was reviewed as a group to obtain a consensus score.
Patients fasted for at least 6 h prior to PET scan, and blood glucose level of 4·4–6·7 mmol/l (80–120 mg/dL) was confirmed prior to radiopharmaceuticals infusion. Until 2007, the infused dose of radiopharmaceutical was 555–740 MBq for the two dimensional (2D) mode, and after that the dose was 296–555 MBq for the three-dimensional (3D) mode. The scans were performed between 60 and 90 min after the infusion of radiopharmaceuticals.
Bulky disease was defined by disease size >10 cm or >one third of internal transverse thoracic diameter. In early stage disease, favourable disease was defined by the absence of an elevated (≥50) erythrocyte sedimentation rate, ≥3 sites of disease involvement, extranodal disease or bulky disease. Early stage unfavourable disease was defined as the presence of any of these characteristics.
PFS was defined as the time from diagnosis to disease progression, relapse or death from any cause. Kaplan–Meier plots were used to depict the PFS, and difference in the survival in two groups was compared by log-rank test using GraphPad Prism Version 5 (La Jolla, CA, USA). Hazard ratio by Cox proportional hazard model and kappa agreement index were calculated using stata Version 9 (College station, TX, USA). P < 0·05 were considered statistically significant.
Result and discussion
A total of 325 patients were eligible for analysis and had PET2 (n = 229) or PET3 (n = 96). Potentially due to the limited number of patients, PET3 had little prognostic value for PFS for patients with early stage or advanced stage diseases. Therefore, we focused on PET2 for the further analysis (n = 229). All scans were PET/computerized tomography (CT) except for 10 patients who had PET without CT scan. This group included 127 patients with early stage non-bulky disease, 11 patients with stage II bulky disease, 56 patients with advanced stage low International Prognostic Score (IPS, ≤2), and 35 patients with advanced stage with high IPS (≥3). The median ages (years) of patients in each groups were 32 (range 18–77), 36 (20–60), 30 (19–79) and 49 (19–84). The median follow up duration of surviving patients was 45 months. Treatment regimens were not changed based on PET2 result. The hazard ratio of each characteristic for advanced stage disease is summarized in Table S1. None of the IPS determinants was found significantly prognostic by univariate analysis. The 3-year PFS rate for advanced stage IPS 0–2 vs. >2 was 73% vs. 64% (P = 0·478).
Out of 229 cases of PET2, consensus on Deauville score was obtained by the initial review (at least 2 readers agreed on score) in 215 cases. Among them, all three interpreters provided the same score for 62 cases (27%). Overall, the agreement on Deauville score was considered fair, determined by kappa agreement index of 0·13, 0·29 and 0·38 for each pair. In 14 cases, three readers provided three different scores and thus, discussion was required to produce a consensus score. No PET2 had a final score of 5. We also analysed agreement on “positive (score ≥4)” or “negative (score ≤3)” in each case. In 217 cases (95%), the interpretation was unanimous. Agreement on this interpretation was considered good to excellent as evidenced by kappa agreement index of 0·81, 0·78 and 0·91 for each pair.
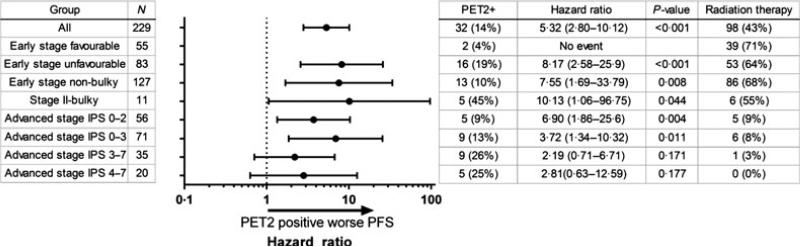
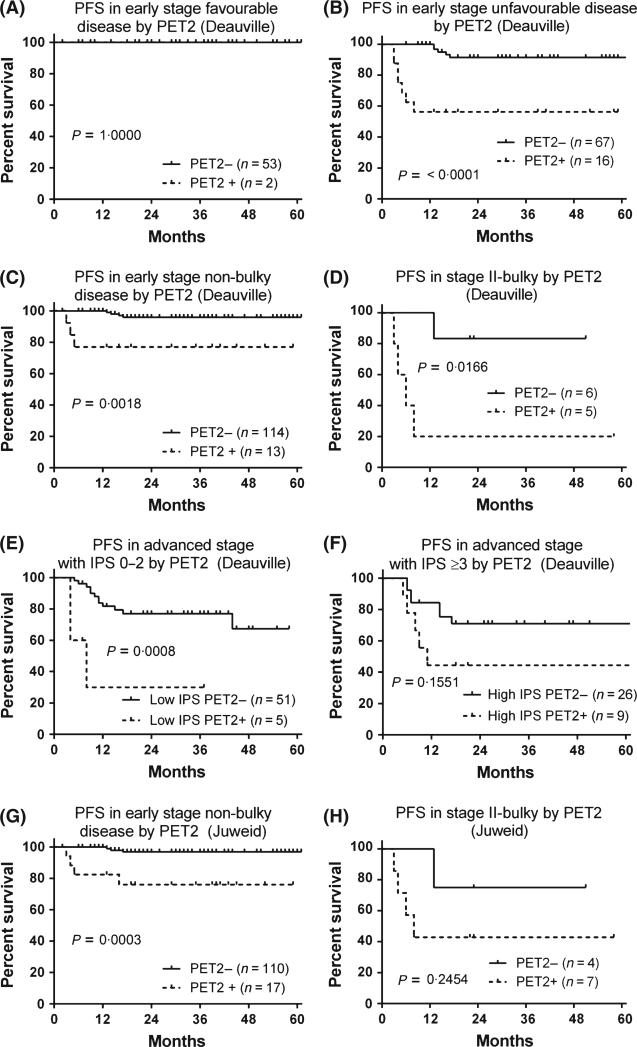
PET2 was positive in 32 cases (14·0%, Fig 1). In early stage favourable disease, early stage unfavourable disease, early stage non-bulky disease and bulky stage II disease, the 3-year PFS rates in PET2-negative vs. -positive patient groups were 100% vs. 100% (Fig 2A), 91·5% vs. 56·3% (P < 0·0001, Fig 2B), 95·9% vs. 76·9% (P = 0·0018, Fig 2C) and 83·3% vs. 20·0% (P = 0·017, Fig 2D), respectively. In advanced stage with IPS ≤2 and advanced stage IPS ≥3, the 3-year PFS rates in PET2-negative vs. -positive patient groups were 77·0% vs. 30·0% (P < 0·001, Fig 2E) and 71·0% vs. 44·4% (P = 0·155, Fig 2F), respectively.
Fig 1.
Hazard ratio of PET2 positivity in different disease subgroups. PET2, positron emission tomography scan after 2 cycles of chemotherapy; IPS, international Prognostic score.
Fig 2.
Kaplan–Meier Curves of A. Progression-free survival in patients with early stage favourable disease by PET2 (Deauville criteria; Meignan et al, 2009), B. Progression-free survival in patients with early stage unfavourable disease by PET2 (Deauville criteria), C. Progression-free survival in patients with early stage non-bulky disease by PET2 (Deauville criteria), D. Progression-free survival in patients with stage II bulky disease by PET2 (Deauville criteria), E. Progression-free survival in patients with advanced stage with IPS score ≤2 by PET2 (Deauville criteria), F. Progression-free survival in patients with advanced stage with IPS score ≥3 by PET2 (Deauville criteria), G. Progression-free survival in patients with early stage non-bulky disease by PET2 (Juweid criteria; Juweid et al, 2007), H. Progression-free survival in patients with stage II bulky disease by PET2 (Juweid criteria). PFS, progression-free survival; PET2, positron emission tomography scan after 2 cycles of chemotherapy; IPS, International Prognostic score.
In our study, no patient with advanced stage received a score of 3, and thus, the results based on Juweid criteria were the same as the results above for advanced stage disease. On the other hand, 4 patients with early stage non-bulky disease and 2 patients with stage II bulky disease received a PET2 score of 3. Thus, we re-analysed the PFS in early stage disease using the Juweid criteria. For early stage non-bulky disease, outcome was not much different from the analysis based on the Deauville criteria, with 3-year PFS rates for PET2-negative vs. -PET2 positive patients of 96 8% and 76·0% respectively (Fig 2G, P < 0·001). For bulky stage II disease, 3-year PFS rates were 75·0% and 42·9% respectively (P = 0·245, Fig 2H).
Multiple studies have applied the interim PET scan result to determine the subsequent treatment modality, such as radiation therapy or more intensive chemotherapy. For example, the US Intergroup trial S0816 was a phase II trial to test this approach in advanced stage Hodgkin lymphoma. In this study, patients with positive PET2 were switched to escalated BEACOPP, and had a 1-year PFS of 72%, which was considered promising (Press et al, 2013). However, the study did not include a control arm and the actual benefit of switching treatment remains unknown.
The strength of the present study is that the nuclear medicine physicians were blinded for clinical information except for the initial PET scan. The Deauville score provided was unanimous only in 27% of cases, suggesting difficulty in applying the Deauville scoring system even by an experienced radiologist. Nevertheless, it should be noted that unanimous agreement of the final interpretation based on Deauville criteria (negative for ≤3 vs. positive for ≥ 4) was obtained in 95% of cases.
The frequency of PET2 positivity varied depending on the stage at diagnosis. Though the number of patients with stage II-bulky disease was small, a positive PET2 was seen in up to 45% of cases, and was associated with very poor outcome. On the other hand, a positive PET2 was observed in 10% of patients with early stage non-bulky diseases, and such cases still had a 3-year PFS > 75%. Therefore, the impact of PET2 result on the outcome should be estimated in the context of initial disease status. It should also be noted that the present study is limited in number and lacks power among each subgroup.
In conclusion, this study confirmed that the PET2 result is a strong prognostic indicator for both early stage and advanced stage cHL. However, the prognostic impact may potentially be less in early stage non-bulky disease. It should be noted that PET2-positive patients may still have a 20–40% (bulky or advanced disease) or 75% (early stage non-bulky disease) chance of achieving durable complete response just by ABVD. There are multiple prospective studies applying PET2-guided planning for cHL. The results from such studies would be helpful in estimating the benefit of changing treatment based on PET2, with careful interpretation.
Supplementary Material
Acknowledgements
None.
Footnotes
Authorship contributions
Y.O. provided patient care, collected and analysed data and wrote the paper. H.C., B.C., A.J. and T.P. provided radio-graphic interpretation. M.F., B.D., N.F., J.R., L.F., F.H., M.A.R., S.N., F.S., L.W.K. provided patient care and edited the paper. A.Y. designed and supervised the study, provided patient care, analysed data and wrote the paper. All authors had access to the database.
Conflict of interest disclosure
No conflict of interest to disclose for the study.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Prognostic impact of each characteristic in advanced disease on progression free survival by univariate analysis using Cox proportional hazard model.
References
- Biggi A, Gallamini A, Chauvie S, Hutchings M, Kostakoglu L, Gregianin M, Meignan M, Malkowski B, Hofman MS, Barrington SF. International validation study for interim PET in ABVD-treated, advanced-stage hodgkin lymphoma: interpretation criteria and concordance rate among reviewers. Journal of Nuclear Medicine. 2013;54:683–690. doi: 10.2967/jnumed.112.110890. [DOI] [PubMed] [Google Scholar]
- Cerci JJ, Pracchia LF, Linardi CC, Pitella FA, Delbeke D, Izaki M, Trindade E, Soares J, Jr, Buccheri V, Meneghetti JC. 18F-FDG PET after 2 cycles of ABVD predicts event-free survival in early and advanced Hodgkin lymphoma. Journal of Nuclear Medicine. 2010;51:1337–1343. doi: 10.2967/jnumed.109.073197. [DOI] [PubMed] [Google Scholar]
- Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, Patti C, Loft A, Di Raimondo F, D'Amore F, Biggi A, Vitolo U, Stelitano C, Sancetta R, Trentin L, Luminari S, Iannitto E, Viviani S, Pierri I, Levis A. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin's lymphoma: a report from a joint Italian-Danish study. Journal of Clinical Oncology. 2007;25:3746–3752. doi: 10.1200/JCO.2007.11.6525. [DOI] [PubMed] [Google Scholar]
- Hutchings M, Loft A, Hansen M, Pedersen LM, Buhl T, Jurlander J, Buus S, Keiding S, D'Amore F, Boesen AM, Berthelsen AK, Specht L. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107:52–59. doi: 10.1182/blood-2005-06-2252. [DOI] [PubMed] [Google Scholar]
- Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, Wiseman GA, Kostakoglu L, Scheidhauer K, Buck A, Naumann R, Spaepen K, Hicks RJ, Weber WA, Reske SN, Schwaiger M, Schwartz LH, Zijlstra JM, Siegel BA, Cheson BD, Imaging Subcommittee of International Harmonization Project in, L. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. Journal of Clinical Oncology. 2007;25:571–578. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- Le Roux PY, Gastinne T, Le Gouill S, Nowak E, Bodet-Milin C, Querellou S, Mahe B, Dubruille V, Blin N, Salaun PY, Bodere-Kraeber F. Prognostic value of interim FDG PET/CT in Hodgkin's lymphoma patients treated with interim response-adapted strategy: comparison of International Harmonization Project (IHP), Gallamini and London criteria. European Journal of Nuclear Medicine and Molecular Imaging. 2011;38:1064–1071. doi: 10.1007/s00259-011-1741-0. [DOI] [PubMed] [Google Scholar]
- Matloub Y, Lindemulder S, Gaynon PS, Sather H, La M, Broxson E, Yanofsky R, Hutchinson R, Heerema NA, Nachman J, Blake M, Wells LM, Sorrell AD, Master-son M, Kelleher JF, Stork LC. Intrathecal triple therapy decreases central nervous system relapse but fails to improve event-free survival when compared with intrathecal methotrexate: results of the Children's Cancer Group (CCG) 1952 study for standard-risk acute lymphoblastic leukemia, reported by the Children's Oncology Group. Blood. 2006;108:1165–1173. doi: 10.1182/blood-2005-12-011809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meignan M, Gallamini A, Meignan M, Galla-mini A, Haioun C. Report on the First International Workshop on Interim-PET-Scan in Lymphoma. Leukaemia & Lymphoma. 2009;50:1257–1260. doi: 10.1080/10428190903040048. [DOI] [PubMed] [Google Scholar]
- Press OW, LeBlanc M, Rimsza LM, Schoder H, Friedberg JW, Evens AM, Li H, Bartlett NL, LaCasce AS, Sweetenham JW, Straus DJ, Noy A, Kostakoglul L, Grewal RK, Hsi ED, Gascoyne RD, Cheson BD, Kahl BS, Miller TP, Fisher RI. A phase II trial of response-adapted therapy of stages III-IV Hodgkin lymphoma using early interim FDGPET imaging: US Intergroup S0816. Hematological Oncology. 2013;31(Suppl 1) abstr 124. [Google Scholar]
- Terasawa T, Lau J, Bardet S, Couturier O, Hotta T, Hutchings M, Nihashi T, Nagai H. Fluorine-18-fluorodeoxyglucose positron emission tomography for interim response assessment of advanced-stage Hodgkin's lymphoma and diffuse large B-cell lymphoma: a systematic review. Journal of Clinical Oncology. 2009;27:1906–1914. doi: 10.1200/JCO.2008.16.0861. [DOI] [PubMed] [Google Scholar]
- Zinzani PL, Rigacci L, Stefoni V, Broccoli A, Puccini B, Castagnoli A, Vaggelli L, Zanoni L, Argnani L, Baccarani M, Fanti S. Early interim 18F-FDG PET in Hodgkin's lymphoma: evaluation on 304 patients. European Journal of Nuclear Medicine and Molecular Imaging. 2012;39:4–12. doi: 10.1007/s00259-011-1916-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.