Abstract
Background
Intravenous tranexamic acid (TXA) is an effective adjunct after hemorrhagic shock (HS) due to its antifibrinolytic properties. TXA is also a serine protease inhibitor and recent laboratory data demonstrated that intraluminal TXA into the small bowel inhibited digestive proteases and protected the gut. .ADAM-17 and TNFα are effective sheddases of intestinal syndecan-1 which when shed, exposes the underlying intestinal epithelium to digestive proteases and subsequent systemic insult. We therefore hypothesized that intraluminal TXA as a serine protease inhibitor would reduce intestinal sheddases and syndecan-1 shedding, mitigating gut and distant organ (lung) damage.
Methods
Mice underwent 90 minutes of hemorrhagic shock to a mean arterial pressure of 35±5 mm Hg following by the intraluminal administration of TXA or vehicle. After 3 hours, small intestine, lung, and blood were collected for analysis.
Results
Intraluminal TXA significantly reduced gut and lung histopathologic injury and inflammation compared to hemorrhagic shock alone. Gut, lung, and systemic ADAM-17 and TNFα were significantly increased by hemorrhagic shock but lessened by TXA. Additionally, gut and lung syndecan-1 immunostaining were preserved and systemic shedding lessened after TXA. TXA reduced ADAM-17 and TNFα, but not syndecan-1, in TXA-sham animals compared to sham vehicles.
Conclusions
Results of the present study demonstrate a beneficial effect of intraluminal TXA in the gut and lung after experimental hemorrhagic shock in part due to inhibition of the syndecan-1 shedding by ADAM-17 and TNFα. Further studies are needed to determine if orally administered TXA could provide similar intestinal protection and thus be of potential benefit to patients with survivable hemorrhage at risk for organ injury. This is particularly relevant in patients or soldiers who may not have access to timely medical care.
Level of evidence
NA
Keywords: hemorrhagic shock in mice, oral tranexamic acid, ADAM-17, TNFα, syndecan-1
Introduction
Tranexamic acid (TXA) is a synthetic derivative of the amino acid lysine that blocks the lysine binding sites on plasminogen, thereby inhibiting fibrinolysis and decreasing bleeding (1). It demonstrates an extensive scope of utility as an antifibrinolytic agent in hemorrhagic conditions, including injured patients and soldiers after hemorrhagic shock (2,3). It is marketed in the United States as Cyklokapron in its intravenous form, but the oral formulation is also FDA approved for the management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women (4). Oral TXA has significantly broadened bleeding indications in Europe and other countries where it has been shown to lessen postoperative blood loss and transfusion requirements and to reduce mortality rates in patients requiring urgent surgery (5). It has shown benefit after heart, orthopedic, and dental craniofacial surgery, as well in the treatment of menstrual bleeding, obstetrics, and hemophilia (6). Oral administration of TXA also takes advantage of its gut protease activity which has the potential to reduce gut barrier dysfunction and the subsequent development of multiple organ failure. One reason post-injury multiple organ failure remains an important cause of morbidity and mortality is the lack of early therapeutic interventions to prevent shock-induced gut dysfunction (7). In an elegant series of experiments in models of rodent peritonitis, endotoxemia, and hemorrhagic shock, DeLano et al. investigated whether enzymatic blockade of gut pancreatic enzymes by 6-amidino-2-naphtyl p-guanidinobenzoate dimethanesulfate, tranexamic acid, or aprotinin into the lumen of the small intestine could attenuate breakdown of the intestine and subsequent autodigestion with damage to the intestine, lung, and heart (8). The authors demonstrated a reduction in injury and mortality in animals receiving pancreatic enzyme blockade. Much of the pioneering work on pancreatic enzyme blockade has been done by Schmid-Schȍbein et al.(9-13) They have demonstrated in a number of studies that digestive enzymes normally present in the gut, escape into the gut wall and then are released systemically with breakdown of the intestinal barrier as occurs in shock. We sought to extend these findings to investigate the mechanisms by which inhibition of intestinal proteases specifically by TXA after hemorrhagic shock would protect the gut and distant organ (lung) injury.
Syndecans are transmembrane glycoproteins that modulate binding and signaling of cytokines, chemokines, and adhesion molecules on the surface of both endothelial and epithelial cells. We and others have shown that syndecan-1 is shed after hemorrhagic shock from both the lung and gut (14,15). Shedding of the cell surface ectodomain of syndecan-1 is regulated by multiple intracellular signaling pathways converging on a diverse group of proteases, referred to as sheddases. The metalloproteinases of the A Disintegrin And Metalloproteinase (ADAM) family is the largest group of sheddases. ADAM17 is one member of the ADAM family that is co-expressed with syndecan-1 in the gut and mediates syndecan-1 shedding in the lung (16,17). Also known as TNFα-converting enzyme (TACE), ADAM17 cleaves pro-TNFα to release active TNFα. TNFα is a potent cytokine involved in systemic inflammation, is elevated in injured patients and in the laboratory in rodents after hemorrhagic shock, and is a known syndecan-1 shedding agonist (18,19). We hypothesized that intraluminal TXA, as a serine gut protease inhibitor, would reduce ADAM-17 and TNFα, and therefore attenuate intestinal syndecan-1 shedding, mitigating gut and distant organ (lung) injury and inflammation.
Materials and Methods
Mouse model of trauma/hemorrhagic shock (T/HS)
All procedures performed were protocols approved by the University of Texas Houston Medical School Animal Welfare Committee. The experiments were conducted in compliance with the National Institutes of Health (NIH) guidelines on the use of laboratory animals. All animals were housed at constant room temperature with a 12:12-h light-dark cycle with access to food and water ad libitum until the evening before surgery when food was withheld. Male C57BL/6J mice, 8-10 weeks of age and weighing approximately 22 grams, were used for all experiments. An established coagulopathic mouse model of trauma-hemorrhagic shock was utilized (14). Under isoflurane anesthesia, a midline laparotomy incision was made, the organs inspected, and then the incision closed. Bilateral femoral arteries were cannulated for continuous hemodynamic monitoring and blood withdrawal or resuscitation. After a 10-minute period of equilibration, mice were bled to a mean arterial pressure (MAP) of 35±5 mmHg and maintained for 90 minutes. Shams underwent anesthesia and placement of catheters but were not subjected to hemorrhagic shock. At the completion of the shock period, TXA was delivered intraluminally (25mg/ml, 0.7 ml/animal diluted in water) into the duodenum, jejunum, and ileum and compared to animals that underwent shock alone or shams that received TXA or vehicle. Injections were performed with a 25-gauge needle and were spaced in intervals of about 5 cm for a total of six injections, as previously described by Delano et al. (8). Vascular catheters were removed, incisions closed, and the animals were then awoken from anesthesia. After three hours, animals were sacrificed, the jejunum and lungs harvested, and blood obtained via cardiac puncture. This time point was chosen based on our previous investigation in rodent models of hemorrhagic shock showing near complete dissolution of the glycocalyx and enhanced systemic shedding of syndecan-1 (20).
Gut analyses
To verify protease inhibition by TXA, the small intestinal wall was analyzed for protease activity using the Sigma-Aldrich Protease Fluorescent Detection Kit.
Gut histopathology
Small bowel samples that had been fixed in formalin and embedded in paraffin. Tissue sections were deparaffinized and rehydrated, and then stained with hematoxylin and eosin. Five random fields with 100-250 villi/mouse were analyzed in a blinded fashion at 100X magnification to quantify morphologic damage to the villi.(21) Damage was identified if any one of the following were present: a subepithelial space, moderate lifting of the epithelial layer from the lamina propria, massive epithelial lifting down the sides of the villi, denuded villi with lamina propria and dilated capillaries and/or digestion and disintegration of the lamina propria with hemorrhage, and ulceration. Results are expressed as expressed as the % of injured villi .
Gut inflammation
The EnzCheck Myeloperoxidase Activity Assay Kit (Enzo Life Sciences, ADI-907-029) was used to determine myeloperoxidase activity of gut lysates as an indicator of neutrophil infiltration. The fluorescence intensity of each sample was measured using excitation at 530 nm and emission at 590 nm. Samples were measured in duplicate with an average difference between measurements of 0.02 uU/mg.
Gut TNFα and A disintegrin and metalloproteinase (ADAM)-17
Levels of TNFα and ADAM-17, also known as TNFα converting enzyme (TACE), in small intestinal tissue were measured by mouse TNFα ELISA kit and mouse ADAM-17 ELISA kit (MyBioSource,San Diego, CA). The activity of ADAM-17 in the intestinal tissue was measured by SensoLyte 520 TACE (α-secretase) activity assay kit (AnaSpec, Fremont, CA) according to manufacturers’ instructions.
Gut Syndecan-1
Small intestine was harvested and sections fixed with 10% buffered formaldehyde, then embedded in paraffin and sectioned. At least 3 sections from separate animals in each group were immunostained at the same setting with 1:100 rat anti-mouse syndecan-1 monoclonal antibody (BD Pharmingen, San Jose, CA), then incubated with 1:500 Alexa Fluor 488 goat anti-rat IgG(Invitrogen, Carlsbad, Calif). Images were obtained using an Olympus 1X71 microscope with SimplePCI6 software. The relative fluorescence intensity was quantified at 100x using Image J software (NIH) and reported as relative fluorescence units (RFU). Syndecan-1 protein was measured in the same samples using the Mouse Syndecan-1 ELISA Kit (Biobyt, San Francisco, CA).
Lung
Histopathology
Lungs were harvested and sections fixed with 10% buffered formaldehyde, then embedded in paraffin and sectioned. Sections were stained with hematoxylin and eosin, and then scored using a 3-point scale based on alveolar thickness, capillary congestion, and cellularity. The overall lung injury score was calculated by averaging these three indices of injury (22).
Inflammation
Infiltration of neutrophils was assessed by MPO immunofluorescence staining. Paraffin-embedded tissue was cut into 5-μm-thick sections, then incubated with MPO primary antibody (1:100 MPO mouse monoclonal antibody; Abcam, Cambridge, Mass) followed by incubation with secondary antibody (goat anti-mouse; Alexa Fluor 568, Invitrogen, Carlsbad, Calif). Two random images were taken from each lung section with a fluorescent microscope (Nikon Eclipse Ti) at 200x and quantified using ImageJ software (National Institutes of Health). Results are reported as relative fluorescence units (RFU).
Syndecan-1, TNFα, and ADAM-17
To detect syndecan-1, lungs were sectioned and stained with rat anti-mouse syndecan-1 antibody (BD Biosciences, San Diego, Calif ) and Alexa Fluor 488 goat anti-rat IgG(Invitrogen, Carlsbad, Calif) following deparaffinization and antigen retrieval with citrate buffer. Two random images were taken from each lung section with a fluorescent microscope at 100x and quantified using ImageJ software (National Institutes of Health)(14).
Pulmonary TNFα and ADAM-17 were measured by mouse TNFα ELISA kit and mouse ADAM-17 ELISA kit (MyBioSource, San Diego, CA). The activity of ADAM-17 in lung tissue was measured by SensoLyte 520 TACE (α-secretase) activity assay kit (AnaSpec, Fremont, CA) according to manufacturers’ instructions. Lung MT-MMP-1 and MMP-9 were analyzed by Western blot.
Systemic Parameters
Blood was obtained at the time of sacrifice, and TNFα determined using the mouse TNFα ELISA kit (MyBioSource;San Diego, CA), ADAM17 using the Mouse A Disintegrin and Metalloproteinase 17 (ADAM17) ELISA Kit (MyBioSource, San Diego, CA) and syndecan-1 using the Mouse Syndecan-1 ELISA Kit (Biobyt, San Fancisco, CA)
Statistical analysis
Data were analyzed by one-way ANOVA with Bonferroni correction. Data are expressed as mean ± SEM with 8 mice/group.
RESULTS
Intraluminal TXA inhibits gut protease activity and mitigates gut and lung injury and inflammation
Intestinal protease activity was significantly reduced by TXA in sham treated animals (0.98±0.13 ng trypsin/mg protein) compared to sham vehicle animals (1.68±0.17 ng trypsin/mg protein). Hemorrhagic shock (3.60±0.38 ng trypsin/mg protein) led to a marked increase in gut protease activity that was reduced to sham levels by intraluminal TXA (1.39±0.30 ng trypsin/mg protein). Gut injury and inflammation (Figure 1) were markedly increased after hemorrhagic shock (38.9±3.2% injured villi and 1.37±0.19 mU/mg MPO) compared to shams (injury: 1.7± 0.6% injured villi sham vehicle and 1.4±0.4% injured villi sham TXA, p>0.05 and inflammation: 0.25±0.03 mU/mg MPO sham vehicle and 0.17±0.04 mU/mg MPO TXA shams, p>0.05) but was significantly reduced by intraluminal TXA (11.8±2.0 % injured villi and 0.66±0.09 mU/mg MPO).
Figure 1. Intraluminal tranexamic acid reduces gut injury and inflammation following hemorrhagic shock.
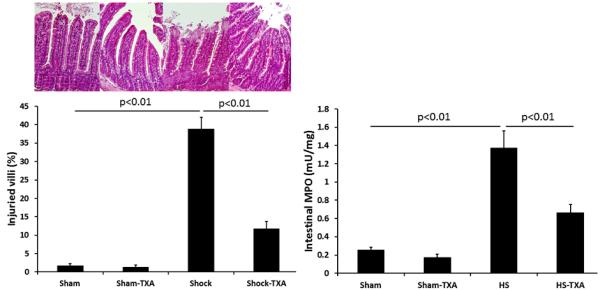
C57BL/6J mice underwent 90 minutes of hemorrhagic shock followed by the intraluminal injection of tranexamic acid into the small intestine. Three hours later, small intestine was analyzed for histopathologic injury expressed as the % of injured villi with representative images shown. Inflammation was assessed by measuring myeloperoxidase activity. Results are expressed as mean ± SE; n=8/group. Abbreviations: HS= hemorrhagic shock; TXA-tranexamic acid
In the lung (Figure 2), administration of intraluminal TXA was able to significantly lessen the lung histopathologic injury score (1.2±0.2) compared to HS (1.8±.0.2) but remained increased compared to shams (0.17±0.1 sham vehicle and 0.12±0.1 shams TXA, p>0.05). Hemorrhagic shock also led to a significant increase in lung inflammation (816,040±.61,658 RFUs) compared to shams (391356±.60,88 RFUs sham vehicle and 3384,646±34,159 RFUs sham TXA) but was significantly reduced by TXA (530,917±.71,632 RFUs).
Figure 2. Intraluminal tranexamic acid reduces pulmonary injury and inflammation following hemorrhagic shock.
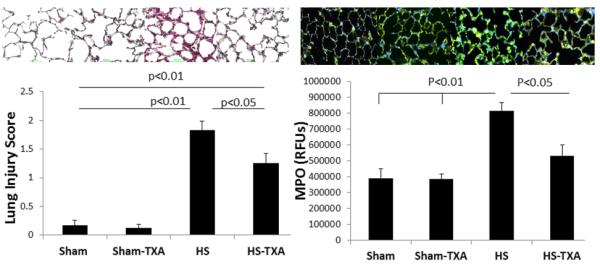
C57BL/6J mice underwent 90 minutes of hemorrhagic shock followed by the intraluminal injection of tranexamic acid into the small intestine. Three hours later, lungs were analyzed for histopathologic injury using a three point scoring system; representative photomicrographs are shown. Myeloperoxidase immunofluorescence staining was used to assess lung inflammation; representative images shown along with the corresponding images. Results are expressed as mean SE; n=8/group. Abbreviations: HS= hemorrhagic shock; TXA- tranexamic acid; MPO=myeloperoxidase; RFU= relative fluorescent units
Intraluminal TXA inhibits intestinal ADAM-17 and TNFα, and restores intestinal syndecan-1
Intestinal ADAM-17 protein (Fig. 3A) was reduced by TXA in sham treated animals (0.64±0.14 ng/mg protein) compared to sham vehicles (1.14±0.16 ng/mg protein). Hemorrhagic shock significantly increased ADAM-17 (2.24±0.24 ng/mg protein) but protein levels were reduced by TXA (1.42±0.12 ng/mg protein). To verify that protein levels reflected enzyme activity, ADAM-17 activity was also measured. As shown in Fig 3B, the trends were similar. ADAM-17 activity in sham animals treated with TXA was 49±5% vs sham vehicle at 100%. Hemorrhagic shock, however, resulted in an increase in activity to 446±20% while TXA administered after shock lessened activity to 237±13%. Active TNFα was measured as an end product of ADAM-17 cleavage. As with ADAM-17, there was a significant increase in TNFα after hemorrhagic shock (34.13±2.32 ng/mg protein) compared to shams (20.04±0.92 ng/mg protein vehicle). TXA significantly reduced intestinal TNFα (23.98±0.66 ng/mg protein) compared to HS animals. Similar to ADAM-17, TXA reduced TNFα in shams (20.04±0.92 ng/mg protein vehicle and 14.49±0.756 ng/mg protein sham TXA; p<0.01).
Figure 3. Intestinal ADAM-17 lessened by intraluminal tranexamic acid following hemorrhagic shock.
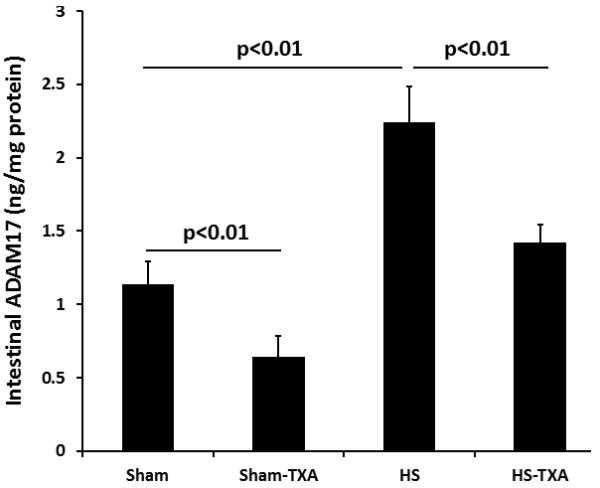
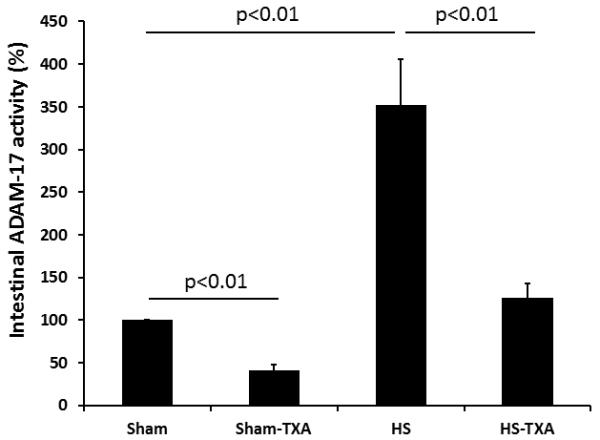
C57BL/6J mice underwent 90 minutes of hemorrhagic shock followed by the intraluminal injection of tranexamic acid into the small intestine. Three hours later, small intestine was analyzed for A. Intestinal ADAM-17 protein and B. Intestinal ADAM-17 activity. Results are expressed as mean ± SE; n=8/group. Abbreviations: HS= hemorrhagic shock; TXA= tranexamic acid; ADAM-17= A disintegrin and metalloproteinase
Lastly, we assessed the effect of TXA on intestinal syndecan-1 expression. As shown in Figure 4, syndecan-1 immunostaining was similar between shams (100±9 % sham vehicle vs 95±8 % sham TXA, p>0.05), significantly reduced by HS (39±5 %) but partially restored by TXA (65±8 %). Protein levels were also measured and confirmed the results of immunostaining. Hemorrhagic shock reduced intestinal syndecan-1 (109±10 pg/ml) compared to sham vehicle (240±22 pg/ml) and sham TXA (230±29 pg/ml) but was increased by TXA (179±18 pg/ml). Importantly, unlike with ADAM-17 and TNFα, TXA did not alter syndecan-1 immunostaining or protein in shams.
Figure 4. Intestinal syndecan-1 was preserved by intraluminal tranexamic acid following hemorrhagic shock.
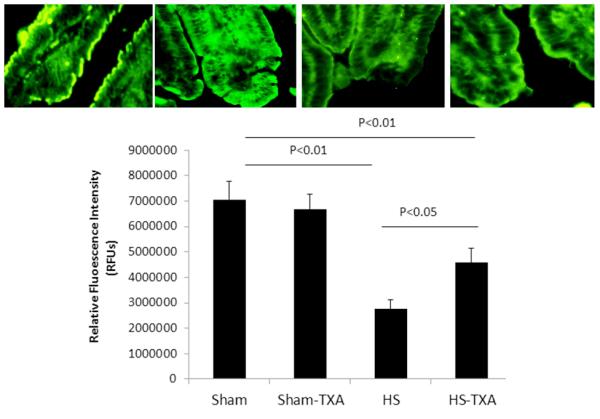
C57BL/6J mice underwent 90 minutes of hemorrhagic shock followed by the intraluminal injection of tranexamic acid into the small intestine. Three hours later, small intestine was immunostained for intestinal syndecan-1. Shown are immunostained images (100x magnification) and the corresponding quantitation. Results are expressed as mean ± SE; n=8/group. Abbreviations: HS= hemorrhagic shock; TXA= tranexamic acid; RFU= relative fluorescent units
Intraluminal TXA inhibits pulmonary ADAM-17 and TNFα and restores pulmonary syndecan-1
Similar to the intestine, pulmonary ADAM-17 protein was reduced by TXA in shams (1.04±0.08 ng/mg protein sham-TXA vs 1.54±0.06 ng/mg protein sham vehicle), significantly increased after hemorrhagic shock (2.41±0.10 ng/mg protein) but reduced after TXA administration (1.79±0.12 ng/mg protein)(Figure 5). Additionally, ADAM-17 activity (Figure 5) demonstrated comparable findings, with activity reduced by TXA in shams (59±4%) compared to sham vehicle (100%), elevated after hemorrhagic shock (395±19%), and lessened by TXA (243±13%). Although there was still a significant decrease by TXA, the reduction was not the same magnitude as in the gut.
Figure 5. Pulmonary ADAM-17 is reduced by intraluminal tranexamic acid following hemorrhagic shock.
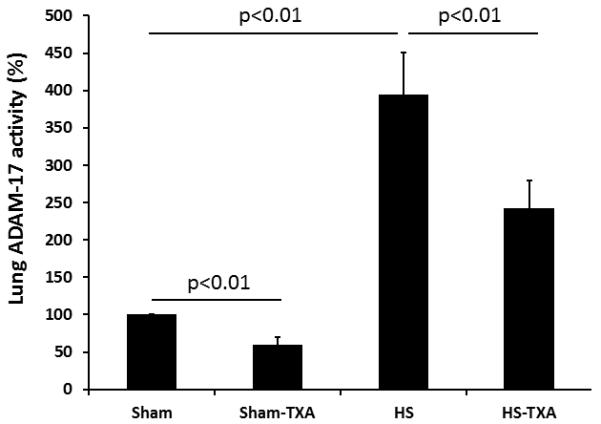
C57BL/6J mice underwent 90 minutes of hemorrhagic shock followed by the intraluminal injection of tranexamic acid into the small intestine. Three hours later, lungs were analyzed for A. Pulmonary ADAM-17 protein and B. Pulmonary ADAM-17 activity. Results are expressed as mean ± SE; n=8/group. Abbreviations: HS= hemorrhagic shock; TXA= tranexamic acid; ADAM-17= A disintegrin and metalloproteinase;
For TNFα, there was a baseline amount in sham-vehicle animals (32.36±4.03 ng/mg protein) that was reduced in the sham-TXA group (19.83±1.66 ng/mg protein). The marked rise in TNFα after hemorrhagic shock (87.48±4.80 ng/mg protein) was significantly reduced by TXA (47.76±3.90 ng/mg protein).
As in the small bowel, pulmonary syndecan-1 immunostaining (Fig. 6) revealed that hemorrhagic shock (484,698±53,715 RFU) resulted in a significant loss of cell-surface syndecan-1 compared to sham vehicle (925,290±47,593 RFU) that was restored by TXA treatment (857,961±45,433 RFU). Additionally, TXA had no effect on shams (992,006±62,749 RFU) compared to sham vehicles (p=0.41).
Figure 6. Pulmonary syndecan-1 is maintained by intraluminal tranexamic acid following hemorrhagic shock.
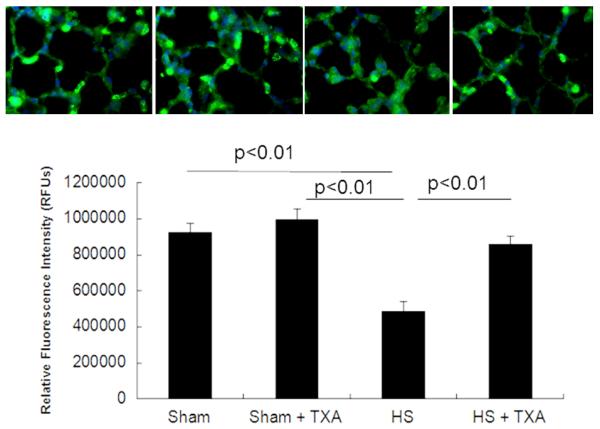
C57BL/6J mice underwent 90 minutes of hemorrhagic shock followed by the intraluminal injection of tranexamic acid into the small intestine. Three hours later, lungs were analyzed for pulmonary syndecan-1. Shown are immunostained images (100x magnification) and the corresponding quantitation. . Results are expressed as mean ± SE; n=8/group. Abbreviations: HS= hemorrhagic shock; TXA= tranexamic acid; RFU= relative fluorescent units.
Intraluminal TXA lessens systemic ADAM-17 and TNFα and reduces systemic syndecan-1 shedding
As expected, systemic levels of ADAM-17 and TNFα reflected those of both the gut and lung (Table 1). Both ADAM-17 and TNFα were significantly increased after hemorrhagic shock compared to sham vehicle and sham TXA groups. Administration of TXA following shock resulted in a reduction of systemic levels of both. Similarly, protein levels of both were lessened by TXA in shams compared to vehicle treatment. Lastly, systemic syndecan-1 mirrored changes in the organs (Table 1). Higher systemic syndecan-1 was detected in plasma after hemorrhagic shock, reflecting lower levels in the lung and gut. Similarly, there was less shed syndecan-1 systemically after TXA treatment.
Table 1.
Systemic parameters
| Sham-vehicle | Sham-TXA | HS | HS-TXA | |
|---|---|---|---|---|
| ADAM17 (pg/ml) | 1.97±0.10 | 1.40±0.16# | 4.75±0.48#* | 2.59±0.25#*^ |
| ADAM17 (%) | 100% | 49±5# | 446±22#* | 237±13#*^ |
| TNFα (pg/ml) | 161±17 | 121±5# | 489±44#* | 321±48#*^ |
|
Syndecan-1
(pg/ml) |
204±13 | 211±12 | 321±29#* | 244±17^ |
p<0.05 vs sham-vehicle
p<0.05 vs sham-TXA
p<0.05 vs HS; % indicates ADAM-17 activity as a % of sham-vehicles. Results are presented as mean±SEM.
DISCUSSION
The important role of digestive enzymes in shock-induced gut dysfunction with subsequent systemic organ failure has been established by other investigators.(23-25). These studies led to concept of intestinal autodigestion from leakage of enzymes from the lumen into the wall of the intestine which then “autodigest” the intestinal wall (26). Using a model of pancreatic duct exteriorization, Fishman et al. recently determined that trauma/hemorrhagic shock alone is not sufficient to cause significant gut injury and dysfunction in the absence of intraluminal pancreatic proteases (27), highlighting the important role of these proteases in the systemic pro-inflammatory state following severe trauma.
In the current study we have shown that inhibition of intestinal proteases by the administration of intraluminal tranexamic acid following hemorrhagic shock reduces intestinal and distant organ (lung) histopathologic injury and inflammation, verifying and expanding on the work by DeLano et al.(8) Using an in vitro model of intestinal epithelial cells, Diebel et al recently demonstrated that TXA was protective to the mucus barrier function, suggesting an additional gut protective property of TXA.(28) We have additionally demonstrated for the first time that TXA’s protection is mediated in part by intestinal sheddase inhibition of ADAM-17 and by the intestinal shedding agonist, TNFα, both which preserved intestinal syndecan-1 and limited destruction of the intestine.
Ectodomain shedding of syndecan-1 is regulated by multiple signaling pathways converging on a diverse group of metalloproteinases, referred to as sheddases. The ADAM family comprises the largest group of these sheddases with metalloproteinases also playing a role (29). We sought to specifically investigate the role of intestinal sheddase inhibition as syndecan-1 has been showed to play a pathologic role after hemorrhagic shock and our recent work suggests that resuscitation strategies can differentially modulate syndecan-1 shedding (14,20,30,31). Whether intraluminal TXA represents an additional potential therapeutic to mitigate hemorrhagic shock –induced syndecan-1 shedding in patients is currently not known.
Although most studies on syndecan-1 refer to it as a marker of endothelial injury, it is actually found on both on endothelial and epithelial cells (32). ADAM17, also known as TNFα converting enzyme (TACE), as well as TNFα, are known modulators of syndecan-1 shedding. ADAM-17 is co-expressed with syndecan-1 on epithelial cells and TNFα is secreted by epithelial cells (11). TXA inhibited both ADAM-17 and TNFα, but not syndecan-1, in sham treated animals suggesting that it acts to inhibit sheddase activity rather than at the level of syndecan-1.
The use of oral TXA as a gut protease inhibitor and an antifibrinolytic that lessens bleeding makes it an attractive agent to study for use in trauma. Studies to date have used intravenous TXA in patients in hemorrhagic shock.(33) However, the oral form can easily be translated to the battlefield as an early intervention for service members when timely evacuation is not possible or in civilian settings when transport times are prolonged in patients at risk for bleeding or in conjunction with the intravenous form in patients with active hemorrhage. Like intravenous TXA, oral TXA is also effective in controlling bleeding (34,35). Both the oral and IV forms are inexpensive, light weight, easy to administer, have minimal storage requirements and low volume requirements, and have good safety profiles. For oral therapy to be efficacious , adequate absorption from the gastrointestinal tract would need to occur and there is no data that the authors are aware of on the absorption of TXA in the setting of shock gut. However, we have shown in a model of severe gut ischemia/reperfusion that gut absorption can still occur, albeit at a slower rate (36). There are other limitations of the current model that could impair clinical translation. Studies to date have injected TXA directly into the small intestine and throughout its entire length and mixed in polyethylene glycol. (8,37) Fishman et al, when studying the effects of a mucus surrogate, injected polyethylene glycol into just the duodenum and demonstrated both gut (distal ileum) and lung protection (27), suggesting that direct contact of a protease inhibitor may not be required for protection. More proximal administration of TXA has not been tested and importantly, it is not known if oral (ie, gastric) administration would be effective in protecting the small intestine, particularly the ileum. Lastly the dose of enteral TXA used in the current study and the published literature (8) is higher than the FDA approved dose of oral TXA.
In summary, in a rodent model of hemorrhagic shock, intraluminal administration of TXA lessened gut and lung injury and inflammation. TXA inhibited gut activity of ADAM-17 and TNFα and lessened intestinal syndecan-1 shedding. Pulmonary and systemic inhibition followed the same pattern as the intestine. This study represents an early attempt to investigate the potential efficacy of oral TXA as a therapeutic adjunct to lessen gut and subsequent distant organ injury after hemorrhagic shock. Its use may be particularly relevant in patients or soldiers who may not have access to timely medical care.
Acknowledgements
Experiments were performed at the University of Texas, Houston, and the University of Maryland, Baltimore.
This work was supported by the National Institutes of Health RO1GM107482 (RAK) and the 2014 American Medical Association Seed Grant (AL).
Footnotes
Author contributions: Conception and study design RAK; Conduct of study ZP, KB, AB; Analysis of data ZP, KB, AB, RAK; Preparation of figures ZP, KB, RAK; Drafted manuscript RAK; Edited and revised manuscript ZP, KB, AL, RAK
The authors have no conflicts of interest to report
This work was presented in part as a poster at the annual meeting of the Shock Society, Denver, CO, in June 2014.
References
- 1.Rappold JF, Pusateri AE. Tranexamic acid in remote damage control resuscitation. Transfusion. 53:96S–99S. doi: 10.1111/trf.12042. [DOI] [PubMed] [Google Scholar]
- 2.Shakur H, Roberts R, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichaishvili T, Gupta S, Herrera J, Hunt B, Iribhogbe P, Izurieta M, Khamis H, Komolafe E, Marrero MA, Mejia-Mantilla J, Miranda J, Morales C, Olaomi O, Olldashi F, Perel P, Peto R, Ramana PV, Yutthakasemsunt S, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised,placebo-controlled trial. Lancet. 2010;376:23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 3.Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military application of tranexamic acid in Trauma Emergency Resuscitation (MATTERs) study. Arch Surg. 2012;147:113–9. doi: 10.1001/archsurg.2011.287. [DOI] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists ACOG committee opinion no. 557: Management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013 Apr;121(4):891–6. doi: 10.1097/01.AOG.0000428646.67925.9a. [DOI] [PubMed] [Google Scholar]
- 5.Irwin A, Khan SK, Jameson SS, Tate RC, Copeland C, Reed MR. Oral versus intravenous tranexamic acid in enhanced-recovery primary total hip and knee replacement: results of 3000 procedures. Bone Joint J. 2013 Nov;95-B(11):1556–61. doi: 10.1302/0301-620X.95B11.31055. [DOI] [PubMed] [Google Scholar]
- 6.Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;57:1005–1032. doi: 10.2165/00003495-199957060-00017. [DOI] [PubMed] [Google Scholar]
- 7.Deitch Edwin. Role of the Gut Lymphatic System in Multiple Organ Failure. Curr Opin Crit Care. 2011;7:92–98. doi: 10.1097/00075198-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 8.DeLano Frank A., Hoyt David B., Schmid-Schönbein Geert W. Pancreatic digestive enzyme blockade in the intestine increases survival after experimental shock. Science Translational Medicine. 2013;5.169:169ra11–169ra11. doi: 10.1126/scitranslmed.3005046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitsuoka H, Kistler EB, Schmid-Schönbein GW. Protease inhibition in the intestinallumen: Attenuation of systemic inflammation and early indicators of multiple organ failure in shock. Shock. 2002;17:205–209. doi: 10.1097/00024382-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Rosario HS, Penn AH, Schmid-Schönbein GW. Pancreatic digestive enzymes are potent generators of mediators for leukocyte activation and mortality. Shock. 2003;20:138–143. doi: 10.1097/01.shk.0000073866.47824.ae. [DOI] [PubMed] [Google Scholar]
- 11.Kistler EB, Alsaigh T, Chang M, Schmid-Schönbein GW. Impaired small-bowel barrier integrity in the presence of lumenal pancreatic digestive enzymes leads to circulatory shock. Shock. 2012;38:262–267. doi: 10.1097/SHK.0b013e31825b1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang M, Kistler EB, Schmid-Schönbein GW. Disruption of the mucosal barrier during gut ischemia allows entry of digestive enymes into the intestinal wall. Shock. 2012;37:297–305. doi: 10.1097/SHK.0b013e318240b59b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altshuler AE, Kistler EB, Schmid-Schönbein GW. Autodigestion: Proteolytic Degradation and Multiple Organ Failure in Shock. Shock. 2015 Nov 25; doi: 10.1097/SHK.0000000000000544. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng Z, Pati S, Potter D, Brown R, Holcomb JB, Grill R, Wataha K, Park PW, Xue H, Kozar RA. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan-1. Shock. 2013;40(3):195–202. doi: 10.1097/SHK.0b013e31829f91fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ban K, Peng Z, Pati S, Witkov RB, Park PW, Kozar RA. Plasma-mediated gut protection after hemorrhagic shock is lessened in syndecan-1−/− mice. Shock. 2015 Aug 10; doi: 10.1097/SHK.0000000000000452. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pruessmeyer J, Martin C, Hess FM, Schwartz N, Schmidt S, Kogel T, Hoettecke N, Schmidt B, Sechi A, Uhlig S, Ludwig A. A disintegrin and metalloproteinase 17 (ADAM17) mediates inflammation-induced shedding of syndecan-1 and -4 by lung epithelial cells. J Biol Chem. 2009;(1):555–64. doi: 10.1074/jbc.M109.059394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cesaro A, Abakar-Mohamat A, Brest P, Lassalle S, Selva E, Filippi J, Hebuterne X, Hugot JP, Doglio A, Galland F, et al. Differential expression and regulation of ADAM17 and TIMP3 in acute inflamed intestinal epithelia. Am J Physiol Gastrointest Liver Physiol. 2009;296(6):G1332–43. doi: 10.1152/ajpgi.90641.2008. [DOI] [PubMed] [Google Scholar]
- 18.Jastrow K, Gonzalez EA, McGuire MF, Suliburk JW, Kozar RA, Iyengar S, Motschall DA, McKinley BA, Moore FA, Mercer DW. Early cytokine production risk stratifies trauma patients for multiple organ failure. J Am Coll Surg. 2009;209:320–331. doi: 10.1016/j.jamcollsurg.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Henry-Stanley MJ, Zhang B, Erlandsen SL, Wells CL. Synergistic effect of tumor necrosis factor-alpha and interferon-gamma on enterocyte shedding of syndecan-1 and associated decreases in internalization of Listeria monocytogenes and Staphylococcus aureus. Cytokine. 2006 Jun;34(5-6):252–9. doi: 10.1016/j.cyto.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Kozar RA, Peng Z, Zhang R, Holcomb JB, Pati S, Park P, Ko TC, Paredes A. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112:1289–1295. doi: 10.1213/ANE.0b013e318210385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiino DC, Palange D, Feketeova E, Bonitz RP, Xu da Z, Lu Q, Sheth SU, Peña G, Ulloa L, De Maio A, Feinman R, Deitch EA. Activation of toll-like receptor 4 is necessary for trauma hemorrhagic shock-induced gut injury and polymorphonuclear neutrophil priming. Shock. 2012;38(1):107–14. doi: 10.1097/SHK.0b013e318257123a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart ML, Ceonzo KA, Shaffer LA, Takahashi K, Rother RP, Reenstra WR, Buras JA, Stahl GL. Gastrointestinal ischemiareperfusion injury is lectin complement pathway dependent without involving C1q1. J Immunol. 2005;174:6373–80. doi: 10.4049/jimmunol.174.10.6373. [DOI] [PubMed] [Google Scholar]
- 23.Schmid-Schonbein GW, Hugli TE. A new hypothesis for microvascular inflammation in shock and multiorgan failure: self-digestion by pancreatic enzymes. Microcirculation. 2005;12:71–82. doi: 10.1080/10739680590896009. [DOI] [PubMed] [Google Scholar]
- 24.Deitch EA, Shi HP, Lu Q, et al. Serine proteases are involved in the pathogenesis of trauma-hemorrhagic shock-induced gut and lung injury. Shock. 2003;19:452–456. doi: 10.1097/01.shk.0000048899.46342.f6. [DOI] [PubMed] [Google Scholar]
- 25.Caputo FJ, Rupani B, Watkins AC, et al. Pancreatic duct ligation abrogates the trauma hemorrhage-induced gut barrier failure and the subsequent production of biologically active intestinal lymph. Shock. 2007;28:441–446. doi: 10.1097/shk.0b013e31804858f2. [DOI] [PubMed] [Google Scholar]
- 26.Schmid-Schonbein GW. Inflammation and the autodigestion hypothesis. Microcirculation. 2009;16:289–306. doi: 10.1080/10739680902801949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fishman JE, Sheth SU, Levy G, Alli V, Lu Q, Xu D, Qin Y, Qin X, Deitch EA. Intraluminal nonbacterial intestinal components control gut and lung injury after trauma hemorrhagic shock. Ann Surg. 2014 Dec;260(6):1112. doi: 10.1097/SLA.0000000000000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diebel ME, Diebel LN, Manke CW, Liberati DM, Whittaker JR. Early tranexamic acid administration: A protective effect on gut barrier function following ischemia/reperfusion injury. J Trauma Acute Care Surg. 2015;79:1015–1022. doi: 10.1097/TA.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 29.Biancheri P, Di Sabatino A, Corazza RG, MacDonald TT. Proteases and the gut barrier. Cell Tissue Res. 2013;351:269–280. doi: 10.1007/s00441-012-1390-z. [DOI] [PubMed] [Google Scholar]
- 30.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, Protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011;254(2):194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 31.Haywood-Watson RJ, Holcomb JB, Gonzalez EA, Peng Z, Pati S, Park PW, Wang WW, Zaske AM, Menge T, Kozar RA. Modulation of Syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS ONE. 2011;6(8):e23530. doi: 10.1371/journal.pone.0023530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chappell D, Jacob M, Hofmann-Kiefer K, Rehm M, Welsch U, Conzen P, Becker BF. Antithrombin reduces shedding of the endothelial glycocalyx following ischaemia/reperfusion. Cardiovasc Res. 2009;83(2):388–96. doi: 10.1093/cvr/cvp097. [DOI] [PubMed] [Google Scholar]
- 33.Cole E, Davenport R, Willett K, Brohi K. Tranexamic acid use in severely injured civilian patients and the effects on outcomes: a prospective cohort study. Ann Surg. 2015 Feb;261(2):390–4. doi: 10.1097/SLA.0000000000000717. [DOI] [PubMed] [Google Scholar]
- 34.Eftekharian HR, Rajabzadeh Z. The efficacy of preoperative oral tranexamic acid on intraoperative bleeding during rhinoplasty. Craniofac Surg. 2015 Dec 10; doi: 10.1097/SCS.0000000000002273. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Alipour M, Tabari M, Keramati M, Zarmehri AM, Makhmalbaf H. Effectiveness of oral Tranexamic acid administration on blood loss after knee artroplasty: a randomized clinical trial. Transfus Apher Sci. 2013 Dec;49(3):574–7. doi: 10.1016/j.transci.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Kozar RA, Schultz SG, Hassoun HT, DeSoignie R, Weisbrodt NW, Haber MH, Moore FA. The type of sodium-coupled solute modulates small bowel mucosal injury, transport function and ATP after Ischemia/reperfusion injury in rats. Gastroenterology. 2002 Sep;123(3):810–6. doi: 10.1053/gast.2002.35389. [DOI] [PubMed] [Google Scholar]
- 37.Delano FA, Schmid-Schȍnbein GW. Pancreatic digestive enzyme blockage in the small intestine prevents insulin resistance in hemorrhagic shock. Shock. 2014;41(1):55–61. doi: 10.1097/SHK.0000000000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]


