Abstract
Study design:
To review prospective and randomized trials studying anticholinergic therapy for neurogenic bladder in SCI to identify whether trials included standardized clinical evaluation tools and reporting measures now recognized to enhance clinical trial data.
Methods:
A systematic search via EMBASE, MEDLINE, CENTRAL, CINAHL (Cumulative Index to Nursing and Allied Health Literature), HTA (Health Technology Assessment), CMR (Comprehensive Microbial Resource), HAPI (Health and Psychosocial Instruments) and PsycINFO using the key term spinal cord injury crossed with oxybutynin, tolterodine, darifenacin, solifenacin, fesoterodine, trospium chloride, propiverine, propantheline and anticholinergic(s) for 1946–2015 inclusive. We then collated whether standardized clinical tools, measures and descriptors were used within each study identified: American Spine Injury Association (ASIA) impairment scale; symptom scores validated in SCI; technical methodology for urodynamics/video urodynamics; urinary diaries; and standardized urologic terminology.
Results:
A total of 1225 entries with 610 unique articles were identified, 14 randomized and 16 prospective studies. In 6/30 the population comprised SCI patients with neurogenic bladder alone; the remainder included mixed neurogenic etiologies. Classification using the ASIA impairment scale was used in <10% of studies; none used symptom scores validated in SCI; <50% reported urodynamic test methodology fully, incorporated urinary diaries or used International Continence Society Standardization Subcommittee urinary tract terminology.
Conclusion:
Integrative review of trials from 1946 to 2015 identified infrequent use of standardized clinical evaluation tools and reporting measures. Data from future trials evaluating therapies for neurogenic bladder would likely be more applicable to specific SCI patients if current standardized classification and descriptors now available were used consistently: for example, the ASIA scale, symptom scores validated in SCI, standardized urodynamic methodology, urinary diaries and urinary tract terminology. Studies recruiting SCI patients exclusively would also provide additional benefit.
Introduction
An increasing number of people are living with spinal cord injury (SCI),1, 2, 3 and management of the bladder has been identified as a priority to prolong life,4 hence the importance of studies that define the efficacy of the treatment modalities available. Depending on the level and extent of SCI, bladder involvement includes loss of autonomic function and/or urinary sphincter mechanisms, leading to the symptom complex of neurogenic lower urinary tract dysfunction, and associated complications that include urinary incontinence, reflux, urinary tract infection, urosepsis, renal dysfunction and death.3, 4
Anticholinergic drugs remain central for lowering bladder pressure and preventing urinary incontinence via relaxation of the detrusor muscle.5 Although an increasing number of anticholinergic agents have become available to treat overactive bladder in the non-neurologically impaired population, their efficacy and safety in the SCI population are most often extrapolated from data obtained in heterogeneous populations many of which do not include patients with SCI. This is obviously problematic as the idiopathic overactive bladder and neurogenic non-SCI populations differ in important ways from the SCI population, with the latter recognized to be at particular risk for vesicoureteric reflux, renal dysfunction, and comorbidities such as autonomic dysreflexia (AD). A recent meta-analysis of randomized controlled trials on the effectiveness of anticholinergic agents reported that 'compared with placebo, anticholinergic treatment in patients with NDO is associated with better patient-reported cure improvement', but 'there is still uncertainty about which anticholinergic drugs are most effective, at which dose, and by which route of administration', and 'a subgroup analysis based on different neurologic pathology was not possible'.5
To address this further, we have examined the methodologies in all studies that included SCI patients within a neurogenic bladder cohort to identify how external validity in the SCI population could be improved in future clinical trials. Using an integrative review of prospective and randomised controlled trials reporting the effectiveness of oral and intravesical anticholinergics that include data on patients with SCI, we have quantified the use of standardized clinical evaluation tools including the American Spine Injury Association (ASIA) scale,6, 7 SCI-specific validated symptom scores8, use of a urinary diary,9 and recognition and utilization of methodology and standardized terminology for the urinary tract.10, 11, 12 These are all elements that when reported provide a more comprehensive description of study subjects and hence allow clinical trial data to be more comprehensively matched to the needs of an individual SCI patient.
Materials and methods
Integrative review search strategy
A literature search was conducted from the time period 1946 to the end of September 2015 inclusive of using the following databases: EMBASE, MEDLINE, CENTRAL, CINAHL (Cumulative Index to Nursing and Allied Health Literature), HTA (Health Technology Assessment), CMR (Comprehensive Microbial Resource), HAPI (Health and Psychosocial Instruments) and PsycINFO. The keyword search term 'spinal cord injury' was crossed with a title search and a keyword search for the following terms: oxybutynin, tolterodine, darifenacin, solifenacin, fesoterodine, trospium chloride, propiverine, anticholinergics and anticholinergic. A review of references within each article identified was conducted to locate additional articles.
Inclusion criteria
To be included the studies had to have been published, be available in English and contain at least one SCI adult subject in the study population. In addition, a full-text version of the paper was required for inclusion to allow review of tools used in the description of subjects and outcome measures.
Analytic process
The analytic summary process is provided in Supplementary Appendix A. In Table 1 of Supplementary Appendix A, SCI and the anticholinergic agent terms were crossed in a keyword search. The number of entries is listed below each database for the anticholinergic agent. The total number of entries is tallied below each column. A similar approach was used in the Table 2 of Supplementary Appendix A, with SCI and the anticholinergic agent terms crossed in a title search. The total number of entries has been tallied below each column. In Table 3 of Supplementary Appendix A, the terms SCI and anticholinergic were crossed in a keyword search. This was listed in a separate table because it was deemed an alternative search term. In total, the combined entries from all databases in keyword and title searches comprised 1225 articles.
Table 1. Randomized trials involving the use of anticholinergic drugs in spinal cord injury.
| Author, year | Active drug | Placebo | Active overall n | Control overall n | Active SCI n | Control SCI n | ASIA | UDS methdology | Video UDS | Standardized terminology | Bladder diary | Symptom score | Design |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nardulli, 2012 | Group A: Oxybutynin 3x5.0 mg per day and Trospium chloride 4x20 mg per day Group B: Oxybutynin 3x5.0 mg per day and solifenacin 1x10 mg per day | Xa | 12 | X | 6 | 6 | No | Not detailed | Not specified | Yes | Yes | No | Single center |
| Stöhrer, et al.15b | Propiverine 15 mg Oxybutynin 3x5.0 mg | X | 131 total 70 (pro) 61 (oxy) | X | 122 | X | No | Not detailed | Not specified | Yes | No | No | Multicenter |
| Menarini et al.17 | Trospium chloride standard dose versus adjustable dose | X | 80 | X | 80 | No | Detailed | No | Yes | No | No | Multicenter | |
| Horstmann et al.16 b | Tolterodine, 4 mg inc. to 8 mg OR trospium chloride, 45 mg inc. to 90 mg | X | 21 total 11 (tolt) 10 (TCl) | X | 17 total (10 tetra, 7 para) | X | No | Detailed | Yes | No | Yes | No | Single center |
| Ethans et al.33 | +Tolterodine (SSDb; 4–12 mg per day) Oxybutynin (SSDb; 10–15 mg per day) Placebo | placebo | 10 | X | 10 | X | No | N/A | N/A | No | Yes | No | Crossover |
| Halaska et al.18 | Trospium chloride 2x20 mg per day Oxybutynin 2x5.0 mg per day | X | 368 total 267 (TCl) 90 (oxy) | X | X | X | No | Not detailed | Not specified | Yes | Yes | No | Multicenter |
| Lehtoranta et al.19 | Intravesical oxybutynin | Placebo | 9 | 9 | 1 | 1 (same patient crossover) | No | Not detailed | No | Yes | Yes | No | Double-blind crossover |
| Di Stasi et al.20 | Oxybutynin 5 mg oral Intravesical passive diffusion oxybutynin 15 mg Intravesical electromotive administration Oxybutynin 15 mg | Placebo | 12 | X | All N=12 SCI | Yes | Not detailed | No | No | No | No | Single center | |
| Birns et al.21 | Oxybutynin CR 1x10 mg Oxybutynin 2x5.0 mg | X | 63 (62 final) | 67 (66 final) | X | X | No | N/A | N/A | No | Yes | No | Multicenter |
| Stöhrer, et al.10 | Propiverine 15 mg | Placebo | 60 | 53 | 60 | 53 | No | Not detailed | Not specified | Yes | No | 3-point rating scale | Multicenter |
| van Kerrebroeck et al.28 b | Tolterodine 2x0.5, 1.0, 2.0 and 4.0 mg per day | Placebo | 71 total 0.5 (20), 1.0 (16), 2.0 (18), 4.0 (17). | 19 | 95 total (90 final) Of 52 group: Paraplegia − 20; Quadriplegia −11; hemiplegia − 7; unspecified SCI −8 | No | Detailed | Yes/partial | Yes | Yes | No | Multicenter | |
| Madersbacher et al.24 | Trospium chloride 2x20 mg per day Oxybutynin 3x5.0 mg per day | X | 52 (TCl) 43 (oxy) | X | X | X | No | Not detailed | No | Yes | No | Non-validated well-being questionnaire | Multicenter |
| Stöhrer, et al.25 | Trospium chloride 3x20 mg per day | Placebo | 29 | 32 | 29 | 32 | No | Detailed | Yes/partial | Yes | No | No | Multicenter |
| Throff, 1991 | Oxybutynin 3x5 mg per day Propantheline 3x15 mg per day | Placebo 3x per day | 63 (oxy) 54 (pro) | 52 (plac) | X | X | No (predates) | Some detail | no | No | No | Scored urologic history for female incontinence evaluation | Multicenter |
Abbreviations: ASIA, American Spinal Injury Association Impairment Scale; N/A, not applicable; oxy, oxybutynin; plac, placebo; pro, propiverine; SCI, spinal cord injury; SSD, self-selected dose; TCl, trospium chloride; tolt, tolterodine; UDS, urodynamic studies.
X=no information provided or could be obtained.
Unable to distinguish between active SCI and control SCI groups.
Table 2. Prospective trials of anticholinergic drugs in the management of neurogenic bladder related to SCI.
| Author, year | Active drugs compared | Active | Active SCI | ASIA | UDS methdology | Video UDS | Standardized terminology | Bladder diary | Symptom score | Multicenter |
|---|---|---|---|---|---|---|---|---|---|---|
| Kennelly et al.27 | Transdermal oxybutynin initially 3.9 and 7.8 mg per day to oxybutynin final 7.8, 9.1 and 11.7 mg per day | 24 Total 18 final | Xa | Yes | Not detailed | No | Yes | Yes | No | Yes |
| Amend et al.28 | Group A: Tolterodine and oxybutynin 15 or 30 mg Group B: Trospium chloride 90 mg and tolterodine 4–8 mg Group C: Oxybutynin 30 mg and trospium chloride 45 or 90 mg | 27 | 21 | No | Detailed | Yes | Yes | Yes | No | No |
| Zahariou et al.30 | Oxybutynin 5 mg 1x3 daily DDAVP (intranasal)+oxybutynin 5 mg 1x3 daily | 11 | 11 | No | N/A | N/A | No | No | No | No |
| George et al.31 | Oxybutynin 5 mg Propantheline 15 mg Capsaicin 1 mm/1 in 30% ethanol in saline | 18 | X | Yes | Not detailed | No | No | Yes | No | No |
| Bennett et al.32 | Oxybutynin XL 10 mg, increasing to 15, 20, 25 and 30 mg | 39 | 10 | No | N/A | N/A | No | No | No | No |
| O'Leary et al.34 | Oxbutynin XL 10 mg, increasing to 30 mg (n=5) | 10 | 10 | Yes | Detailed | Yes | No | Yes | No | No |
| Pannek et al.35 | Oxybutynin (oral) 4x5 mg per day Oxybutynin (oral) 4x5 mg per day+oxybutynin (intravesical) 3x15 mg per day dissolved in 15 ml solution | 25 | 25 | No | Detailed | No | No | Yes | No | No |
| Haferkamp et al.36 | Oxybutynin (intravesical) 0.3 mg/kg increasing to 0.9 mg/kg | 32 | 17 | No | Detailed | Yes | Yes | No | No | No |
| Vaidyananthan et al.37 | Oxybutynin 1–3x5.0 mg per day | 7 | 7 | No | N/A | N/A | No | No | No | No |
| Szollar and Lee38 | Ditropan 1–3x5.0 mg per day diluted in 30 ml saline solution | 13 | 13 | No | Detailed | Yes | No | No | No | No |
| Singh and Thomas39 | Oxybutynin (intravesical) 1x10.0 mg per day diluted in 30 ml solution | 6 | 6 | No | Detailed | Yes | No | No | No | No |
| O'Flynn and Thomas40 | Oxybutynin (intravesical) 1x5.0 mg diluted in distilled water | 15 | 12 | No | Detailed | No | No | No | No | No |
| Prasad and Vaidyananthan41 | Oxybutynin 3x5.0 mg per day dissolved in 10 ml boiled and cooled tap water | 12 | 8 | No | Detailed | No | No | No | No | No |
| Madersbacher et al.42 | Intravesical oxybutynin 5 mg tab crushed into 30 cm3 water | 13 | 13 | Predates | No | No | Predates | No | Predates | No |
| Diokno and Lapides44b | Part 1: Oxybutynin 1x5.0 mg to Probanthine (oral) 1x15 mg OR probanthine (intravenous) 1x60 mg | 8 | 5 | Predates | No | No | No | No | No | No |
| Part 2: Oxybutynin 2–3x5.0 mg per day to no medication | 8 | 8 | Predates | No | No | Predates | No | Predates | No | |
| Bodner et al.43 | 'Verapamil (alone) 240SL Oxybutynin (alone) 5 mg Verapamil+oxybutyninb | 14 | 14 | Predates | No | Yes | Predates | No | Predates | No |
Abbreviations: ASIA, American Spinal Injury Association Impairment Scale; N/A, not applicable; SCI, spinal cord injury; TCl, trospium chloride; tolt, tolterodine; UDS, urodynamic studies.
X=No information provided or could be obtained.
Only parts 1 and 2 included patients with neurogenic bladder.
Each paper identified was reviewed by the authors to extract the active drug, the control agent (if applicable), the number of subjects in the study and control group, the number of subjects included who had SCI, and hospitalizations and deaths if these data were reported.
We also identified whether standardized measures were or were not used and reported within each study, with a yes/no response for the following: (1) the ASIA impairment scale, (2) a symptom score validated in the SCI population, (3) technical methodology for urodynamics/video urodynamics, (4) urinary diaries and (5) standardized urologic terminology.
Pediatric studies, colonic studies, review articles, abstracts and posters were not included.
Results
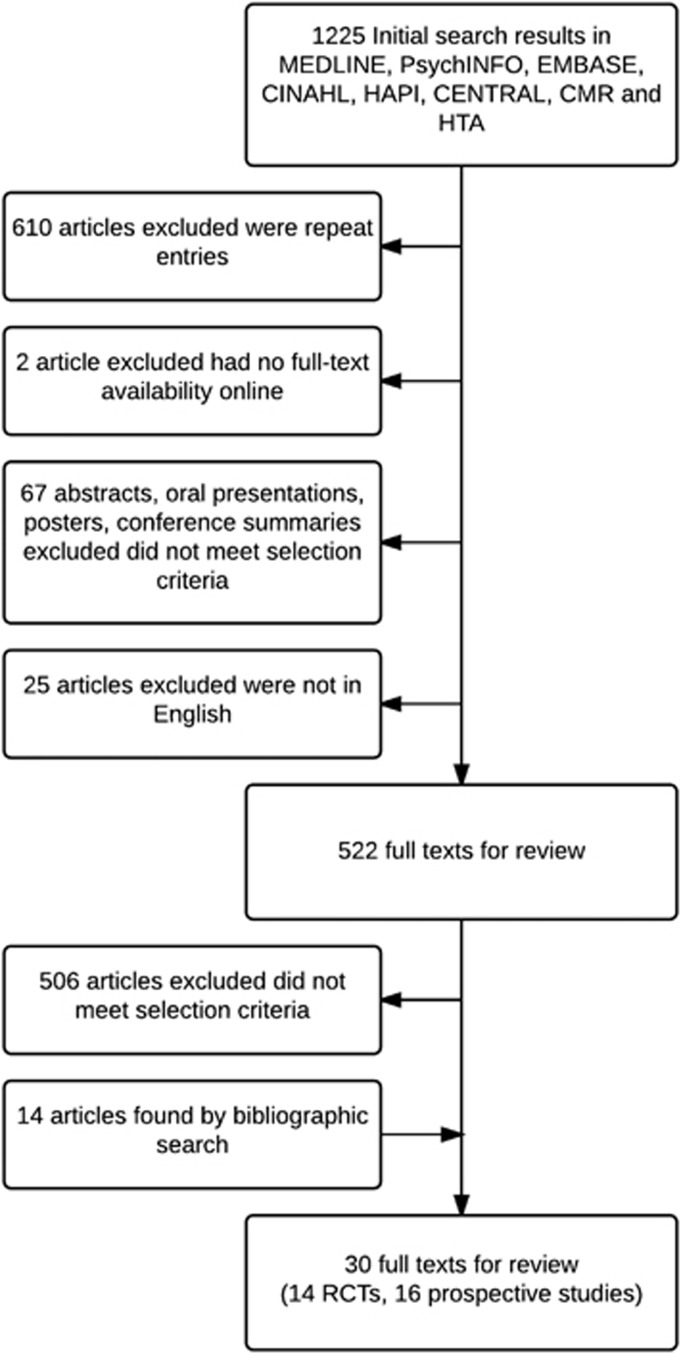
The integrative review process identified the articles shown in Figure 1. The search identified 30 citations, which were reviewed in detail.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 These studies constitute research published between the years 1972 and 2012, and 14/30 were randomized controlled trials (RCTs), the first of which was published in 1991. Table 1 summarizes the 14 randomized controlled trials reviewed. Within the RCTs, 6/14 recruited a SCI population exclusively. Seven studies included a placebo. The randomization strategy was reported in one study to include the use of permuted blocks. Three were single center, nine multicenter and two were crossover in design.
Figure 1.
Search flow strategy CINAHL, Cumulative Index to Nursing and Allied Health Literature; CMR, Comprehensive Microbial Resource; HAPI, Health and Psychosocial Instruments.
Review for the use of standardized measures identified one study that classified patients using the ASIA scale20 and one study26 that predated the development of the ASIA scale. No studies reported using a validated symptoms score; four provided conventional detailed urodynamic studies (UDS) methodology and three indicated at least some portion of the population had video urodynamics;16, 23, 25 seven reported using a urinary diary; and nine referenced standardized terminology descriptors for the lower urinary tract. Table 2 summarizes the 16 prospective studies reviewed.
Within the prospective studies, 1/16 was a multicenter study. The ASIA scale was reported in three, whereas three predated the development of this scale; none reported the use of validated symptom scores; urodynamics methods were detailed in 8 of the 13 studies where UDS was part of the design, and 6 indicated that at least some of the population had video urodynamics; 5 used a urinary diary; and 3 made reference to standardized urinary tract terminology, with 3 predating their availability.
Both prospective and randomized trials commonly included patients with multiple forms of neurogenic bladder, such as those combining multiple sclerosis and SCI. Men and women were detailed as eligible in six RCTs, but in only three were the final number of men and women enrolled clearly defined.
Discussion
This integrative review identified 30 prospective or randomized trials of anticholinergic agent use where at least one subject with SCI was included. Only six studies recruited SCI subjects exclusively, and in only three studies with a heterogeneous cohort was it possible to clearly identify the treatment arm to which the SCI subjects were assigned. This inability was due to the inclusion of subjects with neurogenic bladder because of multiple etiologies and failure of the methodology to allow distinction between them in the study results. In only four studies were the specifics of the SCI classified using the ASIA scale, and no studies used a symptom score validated in a SCI population. A urinary diary was absent in 63% (19 of 30); sufficient detail to repeat the methods used in urodynamic was lacking in the majority of studies, and video urodynamics was only used approximately in half of studies.
Considering the importance of anticholinergic medications in the management of neurogenic lower urinary tract dysfunction in patients with SCI and that there is now a choice of agents available, this represents a lack of information that compromises the ability of a clinician to match the needs of an individual SCI patient to a specific agent. This finding corroborates the conclusion of a 2012 meta-analysis of randomized trials in mixed populations with neurogenic bladder that trial data available do not allow differentiation between results in different neurogenic populations.5 From our review, we suggest that future clinical trials would more readily allow individualized matching of patients with SCI to specific therapeutic modalities if the following components were considered in their design:
Recruitment of the SCI population
Ideally, trials would be specifically tailored to the SCI population. Practically speaking, this is a challenge because this population is small compared with other neurogenic conditions, and hence multicenter studies are likely required. An advantage of a large multicenter study recruiting SCI patients alone would be the potential to stratify therapy based on the level and completeness of injury. Randomized crossover studies can be used in persisting disease states where the study drug modifies symptoms in a reversible way allowing for the subject to act as their own control, exposes the subject to both treatments and reduces the sample size needed. Examples of crossover design are the studies by Lehtoranta et al.19 and Ethans et al.33 Alternatively, N of 1 designs defined as ‘where an individual is exposed to a random sequence of control or treatment many times' may be possible in conditions where the event of interest occurs frequently in an individual. In the context of reduction in neurogenic bladder symptoms in SCI, this may be hampered because of the small effect size of drugs used, including anticholinergics.
Conduct of mixed cohort trials
When recruitment of a SCI population alone is not possible, mixed neurogenic population cohorts can be used. We found this to be common in neurogenic bladder-related studies. However, data related to individual neurologic conditions can be difficult or impossible to identify from such reports. Use of stratified randomization in neurogenic bladder whether based on disease etiology, level of function (paraplegia versus quadriplegia) or urodynamic parameters was virtually absent in the studies examined. This limited the ability to tract SCI patient's from within the overall cohort. Provision of a randomization diagram within mixed cohort studies aids readers in the interpretation of results related to SCI patients.
Incorporation of validated tools and standardized terminology
Standardized and/or validated tools exist that should be used; many of these have been validated for use in patients with SCI. These include descriptors that are specific to SCI and to urologic conditions. It is currently possible to accurately describe patients with SCI by using (1) a scale of physical impairment (ASIA scale), (2) validated history/symptom scores for neurogenic bladder or those that are specific to SCI and bladder function, (3) urinary diaries, (4) standardized urodynamic testing methodology and (5) the use of standardized lower urinary tract terminology.
(1) The ASIA impairment scale
The ASIA Impairment scale is an internationally accepted clinical tool to standardize reporting of the level, extent and degree of completeness of SCI.7 The benefits of incorporating an ASIA assessment into clinical trials have been outlined by the International Campaign for Cures of SCI Paralysis Clinical Guidelines Panel, including the ability to compare sub-populations of SCI between centers.6
Even though the majority of RCTs were reported after the current ASIA scale was developed in our review, only Di Stasi et al.20 included ASIA classification of subjects. The level of cord lesion was described in some studies as quadriplegia or paraplegia. Pannek et al.35 distinguished between subjects who had a cervical spinal cord lesion and those in whom the injury was at a thoracic level. In future, this limitation can be avoided by incorporation of ASIA classification, which is inexpensive and does not require specialized equipment to complete.6
(2) Validated symptom scores
Validated questionnaires to document history related to urinary tract function in SCI patients now exist.8 None were used in any of the 30 studies we reviewed; this may be due to the timing of development of these scores in relation to the conduct of the studies reported, or to slow adoption. However, other validated instruments that quantify quality of life in the neurogenic population in general or use validated questionnaires to document their bladder symptoms were not identified.
An example of a questionnaire recently validated in the SCI population is the Rick Hansen SCI Registry questionnaire.45 Scores validated for use in both the SCI and MS populations include the SF-Qualiveen (Short Form Qualiveen),46 the Qualiveen47 and the IQOL questionnaire.48, 49 The Qualiveen includes questions regarding urinary incontinence and storage symptoms such as urgency. The SF-Qualiveen contains only eight questions. A more broadly used single question tool is the Patient Perception of Bladder Condition Questionnaire.50 The IQOL is longer, validated in neurogenic bladder (not disease specific to SCI) and contains 28 items measured on a Likert scale for ease of subject use. Visual analog scales can also be used to assess treatment satisfaction. Where possible instruments developed and validated by expert panels should be used. The International Consultation of Incontinence Modular Questionnaire is an example and has been used in a recent study, which included subjects with neurogenic bladder.51, 52
(3) Urodynamic methodology
Trials examining the effectiveness of treatment related to the bladder include the evaluation of physiologic measures related to bladder function; the gold standard evaluation is urodynamics (UDS),9 which is fundamental in the decision regarding the need for anticholinergic therapy and assessing response to treatment. UDS measures a number of variables; UDS results can be influenced by elements of the methodology including the size of catheters used, the type of fluid infused and the use of EMG and hardware parameters including the bladder filling rate. Specifically reporting these variables allows other researchers to reproduce the methodology and facilitates translation of the findings to patients with similar physiologic function. Reports of UDS measurements should itemize methodological elements listed above or cite a methodology reference. It is important to highlight the fact that lower urinary tract function can vary from individual to individual with the same level of SCI, and hence the importance of using objective UDS data combined with the ASIA score to provide a comprehensive description of the subject.
International guidelines on the management of neurogenic bladder such as those from the European Urologic Association include the relevance of video urodynamic studies,9 but we found that they were only used occasionally. This limits the ability to speak to the effectiveness of reducing or eliminating reflux in the setting of a high-pressure neurogenic bladder.
A further consideration related to the SCI population is the potential onset of AD during UDS. Also, AD is relevant in patients with high-pressure neurogenic bladder where treatment given is intended to lower bladder pressure and the potential for AD therefore exists, but we did not find the topic of AD management discussed within any of the 30 extant articles.
(4) Urinary diaries
A patient-completed standardized urinary diary is commonly used to quantify symptoms, as evidenced in RCTs evaluating idiopathic overactive bladder. These diaries should be used as they document times of micturition and voided volumes, incontinence episodes, pad usage and other relevant information.9
(5) Standardized urologic terminology
The International Continence Society Standardization of Terminology group has provided a set of definitions that relate to patient symptoms, signs and urodynamic findings.10, 11, 12 These definitions have evolved over time, as the understanding of lower urinary tract dysfunction has increased and the technical methods for measuring bladder function have improved.
We found that more than half of studies made reference to the current terminology of the lower urinary tract definitions of the International Continence Society.
Adverse events reporting
Standardized definitions are available for the reporting of adverse events, as well as the criteria for their use. The presence or absence of certain events such as AD occurring in SCI should be reported in mixed cohort trials even where patients with other neurologic conditions are not at risk. Adverse events related to pharmacotherapy with anticholinergics such as cognitive impairment have particular relevance in SCI as coexisting traumatic brain injury may be present. However, in the context of AEs reported in the trials reviewed for this study, it was only reported that these were similar in type and frequency to those seen in the idiopathic overactive bladder population and included dry mouth, constipation, vision abnormality, headache and urinary retention.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 However, a meta-analysis concluded that side effects were more common in the neurogenic population.5 This underscores the need to document the frequency and severity of AEs in future trials with SCI subjects to examine for differences that may be apparent based on the ASIA scale.
Female/male-specific considerations
Reporting the number of males and females enrolled within a SCI cohort is relevant for a number of reasons. Bladder management options vary between men and women such as the ability to us condom drainage in men. Also, there are implications of bladder management during pregnancy; some anticholinergic drugs are contraindicated and others have not been studied sufficiently for their use to be recommended. This is important as the number of women with SCI becoming pregnant is increasing.53
We recognize limitations in this study; article omission may have occurred because of the inherent methodological risks of an integrated review. Every effort was made to find articles through bibliographic search and the use of a librarian. Our search strategy was also limited to articles published in English; some articles of relevance may have been published in other languages. Our review is limited to prospective and randomized trials, due to the increased levels of evidence that can be obtained in the hierarchy of their study design. Also, as these studies are designed before data collection, researchers have the ability to include measurements and outcomes such as the ones we have recommended in advance.
Conclusions
Despite the importance of management of neurogenic bladder in the SCI population, this integrative review of 30 trials evaluating the effects of anticholinergic drug therapy in the management of neurogenic bladder indicates that the information provided is too limited for clinicians to be able to match trial data to the needs of individual patients with SCI. This is principally because the cohorts enrolled were predominantly heterogeneous, and the number of SCI patients studied was small. However, the classification of those patients who were included was also very limited. Most studies lacked inclusion of standardized clinical evaluation tools—in particular, the ASIA scale and validated symptom scores. Many also did not incorporate a urinary diary, although most did use current standardized terminology for the urinary tract. In future, trials evaluating drug efficacy will ideally include more that are SCI patient specific, but all can use these standardized clinical evaluation tools to provide a more comprehensive classification. This will add to the applicability of trial data and the ability of clinicians to understand the acute and chronic effects of treatment modalities required by a patient population that is growing annually and where many are dependent on therapy for their lifetime.
Data archiving
There were no data to deposit.
Acknowledgments
We acknowledge the International Collaboration on Repair Discoveries (ICORD) for providing student support.
Footnotes
Supplementary Information accompanies this paper on the Spinal Cord website (http://www.nature.com/sc)
The authors declare no conflict of interest.
Supplementary Material
References
- Noonan V, Fingas M, Farry A, Baxter D, Singh A, Fehlings MG et al. Incidence and prevalence of spinal cord injury in Canada: a National Perspective. Neuroepidemiology 2012; 38: 219–226. [DOI] [PubMed] [Google Scholar]
- Dryden DM, Saunders LD, Rowe BH, May LA, Yiannakoulias N, Svenson LW et al. Utilization of health services following spinal cord injury: a 6-year follow-up study. Spinal Cord 2004; 42: 513–525. [DOI] [PubMed] [Google Scholar]
- World Health Organization International Perspectives on Spinal Cord Injury. WHO Press: Malta. 2013. [Google Scholar]
- Soden RJ, Walsh J, Middleton JW, Craven ML, Rutkowski SB, Yeo JD. Causes of death after spinal cord injury. Spinal Cord 2000; 38: 604–610. [DOI] [PubMed] [Google Scholar]
- Madhuvrata P, Singh M, Hasafa Z, Abdel-Fattah M. Anticholinergic drugs for adult neurogenic detrusor overactivity: a systematic review and meta-analysis. Eur Urol 2012; 62: 816–830. [DOI] [PubMed] [Google Scholar]
- Steeves JD, Lammertse D, Curt A, Fawcett JW, Tuszynski MH, Ditunno JF et al. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord 2007; 45: 206–221. [DOI] [PubMed] [Google Scholar]
- Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A et al. International standards for neurological classification of spinal cord injury (Revised 2011). J Spinal Cord Med 2011; 34: 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang B, Stothers L, Macnab AJ, Lazare D, Nigro M. A systematic review of questionnaires used in the management of neurogenic bladder including multiple sclerosis and spinal cord injury. Neurourol Urodyn 2015; 35: 354–364. [DOI] [PubMed] [Google Scholar]
- Pannek J, Blok B, Castro-Diaz D, Del Popolo G, Kramer G, Radziszewski P et al. Guidelines on neurogenic lower urinary tract dysfunction. Eur Assoc Urol 2013; 64: 118–140. [DOI] [PubMed] [Google Scholar]
- Stöhrer M, Goepel M, Kondo A, Kramer G, Madersbacher H, Millard R et al. The standardization of terminology in neurogenic lower urinary tract dysfunction. With suggestions for diagnostic procedures. Neurourol Urodyn 1999; 18: 139–158. [DOI] [PubMed] [Google Scholar]
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U et al. The Standardisation of Terminology of Lower Urinary Tract Function: Report from the Standardization Sub-committee of the International Continence Society. Neurourol Urodyn 2002; 21: 167–178. [DOI] [PubMed] [Google Scholar]
- Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010; 29: 4–20. [DOI] [PubMed] [Google Scholar]
- Amarenco G, Sutory M, Fagertun H, Wright M, Compion G, De Ridder D. Solifenacin is effective and well tolerated in patients with neurogenic detrusor overactivity: preliminary results from the SONIC urodynamic study. Eur Urol Suppl 2012; 11: E457–U20. [DOI] [PubMed] [Google Scholar]
- Nardulli R, Losavio L, Ranieri M, Fiore P, Megna G, Bellomo RG et al. Combined antimurscarinics for treatment of neurogenic overactive bladder. Int J Immunolopathol Pharmacol 2012; 25: 35–41. [DOI] [PubMed] [Google Scholar]
- Stöhrer M, Mürtz G, Kramer G, Warmack W, Primus G, Jinga V et al. Propiverine compared to oxybtynin in neurogenic detrusor overactivity—results of a randomized, double-blind, multicentre clinical study. Eur Urol 2007; 51: 235–242. [DOI] [PubMed] [Google Scholar]
- Horstmann M, Schaefer T, Aguilar Y, Stenzi A, Sievert KD. Neurogenic bladder treatment by doubling the recommended antimuscarinic dosage. Neurourol Urodyn 2006; 25: 441–445. [DOI] [PubMed] [Google Scholar]
- Menarini M, Del Popolo G, De Benedetto P, Haselmann J, Bödeker RH, Schwantes U et al. TcP128-Study Group. Trospium chloride in patients with neurogenic detrusor overactivity: is dose titration of benefit to the patients? Int J Clin Pharmacol Ther 2006; 44: 523–532. [DOI] [PubMed] [Google Scholar]
- Halaska M, Ralph G, Wiedemann A, Primus G, Ballering-Bruhl B, Hofner K et al. Controlled, double-blind, multicentre clinical trial to investigate long-term tolerability and efficacy of trospium chloride in patients with detrusor instability. World J Urol 2003; 20: 392–399. [DOI] [PubMed] [Google Scholar]
- Lehtoranta K, Tainia H, Lukkari-Lax E, Hakonen T, Tammela TL. Pharmacokinetics, efficacy and safety of intravesical formulation of oxybutynin in patients with detrusor overactivity. Scand J Urol Nephrol 2002; 36: 18–24. [DOI] [PubMed] [Google Scholar]
- Di Stasi SM, Giannantoni A, Navarra P, Capelli G, Storti L, Porena M et al. Intravesical oxybutynin: mode of action assessed by passive diffusion and electromotive administration with pharmacokinetics of oxybutynin and n-desethyl oxybutynin. J Urol 2001; 166: 2232–2236. [PubMed] [Google Scholar]
- Birns J, Lukkari E, Malone-Lee JG. A randomized controlled trial comparing the efficacy of controlled-release oxybutynin tables (10mg once daily) with conventional oxybutynin tables (5mg twice daily) in patients whose symptoms were stabilized on 5mg twice daily of oxybutynin. BJU Int 2000; 85: 793–798. [DOI] [PubMed] [Google Scholar]
- Stöhrer M, Madersbacher H, Richter R, Wehnert J, Dreikorn K. Efficacy and safety of propiverine in SCI-patients suffering from detrusor hyperreflexia—a double-blind, placebo-controlled clinical trial. Spinal Cord 1999; 37: 196–200. [DOI] [PubMed] [Google Scholar]
- Van Kerrebroeck PEVA, Amarenco G, Thüroff JW, Madersbacker HG, Lock MT, Messelink EJ et al. Dose-ranging study of tolterodine in patients with detrusor hyperreflexia. Neurourol Urodyn 1998; 17: 499–512. [DOI] [PubMed] [Google Scholar]
- Madersbacher H, Stöhrer M, Richter R. Trospium chloride versus oxybutynin: a randomized, double-blind, multicentre trial in the treatment of detrusor hyperreflexia. BJU Int 1995; 75: 452–456. [DOI] [PubMed] [Google Scholar]
- Stöhrer M, Bauer P, Giannetti BM, Richter R, Burgdorfer J, Murtz G. Effect of trospium chloride on urodynamic parameters in patients with detrusor hyperreflexia due to spinal cord injuries. Urol Int 1991; 47: 138–143. [DOI] [PubMed] [Google Scholar]
- Throff JW, Bunke B, Ebner A, Faber P, DeGeeler P, Hannappel J et al. Randomized, double-blind, multicentre trial on treatment of frequency, urgency and incontinence related to detrusor hyperactivity: oxybutynin versus propantheline versus placebo. J Urol 1991; 145: 813–817. [DOI] [PubMed] [Google Scholar]
- Kennelly M, Lemack G, Foote J, Trop CS. Efficacy and Safety of oxybutynin transdermal system in spinal cord injury patients with neurogenic detrusor overactivity and incontinence: an open-label, dose-titration study. Urology 2009; 74: 741–745. [DOI] [PubMed] [Google Scholar]
- Amend B, Hennenlotter J, Schaffer T, Horstmann M, Stenzl A, Sievert K. Effective treatment of neurogenic detrusor dysfunction by combined high-dosed antimuscarinics without increased side-effects. Eur Urol 532008, 1021–1028. [DOI] [PubMed] [Google Scholar]
- Madersbacher H, Murtz G, Allousi S, Domurath B, Henne T, Komer I et al. Propiverine vs oxybutynin for treating neurogenic detrusor overactivity in children and adolescents: results of a multicentre observational cohort study. BJU Int 2008; 103: 776–781. [DOI] [PubMed] [Google Scholar]
- Zahariou A, Karagiannis G, Papaioannou P, Stathi K, Michail X. The use of desmopressin in the management of nocturnal enuresis in patients with spinal cord injury. Eur Medicophys 2007; 43: 333–338. [PubMed] [Google Scholar]
- George J, Tharion G, Richard J, Macaden AS, Thomas R, Bhattacharji S. The effectiveness of intravesical oxybutynin, propantheline, and capsaicin in the management of neuropathic bladder following spinal cord injury. Scientific World J 2007; 7: 1683–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett N, O'Leary M, Patel AS, Xavier M, Erickson JR, Chancellor MB. Can higher doses of oxybutynin improve efficacy in neurogenic bladder? J Urol 2004; 171: 749–751. [DOI] [PubMed] [Google Scholar]
- Ethans KD, Nance PW, Bilrd RJ, Casey AR, Schryvers OI. Efficacy and safety of tolterodine in people with neurogenic detrusor overactivity. J Spinal Cord Med 2004; 27: 214–218. [DOI] [PubMed] [Google Scholar]
- O'Leary M, Erickson J, Smith C, McDermott C, Horton J, Chancellor MB. Effect of controlled-release oxybutynin on neurogenic bladder function in spinal cord injury. J Spinal Cord Med 2003; 26: 159–162. [DOI] [PubMed] [Google Scholar]
- Pannek J, Sommerfeld H, Bötel U, Senge T. Combined intravesical and oral oxybutynin chloride in adult patients with spinal cord injury. Urology 2000; 55: 358–362. [DOI] [PubMed] [Google Scholar]
- Haferkamp A, Staehler G, Gerner HJ, Dorsam J. Dosage escalation of intravesical oxybutynin in the treatment of neurogenic bladder patients. Spinal Cord 2000; 38: 250–254. [DOI] [PubMed] [Google Scholar]
- Vaidyananthan S, Soni BM, Brown E, Seti P, Krishnan KR, Bingley J et al. Effect of intermittent urethral catheterization and oxybutynin bladder instillation on urinary continence status and quality of life in a selected group of spinal cord injury patients with neuropathic bladder dysfunction. Spinal Cord 1998; 36: 409–414. [DOI] [PubMed] [Google Scholar]
- Szollar S, Lee SM. Intravesical oxybutynin for spinal cord injury patients. Spinal Cord 1996; 34: 284–287. [DOI] [PubMed] [Google Scholar]
- Singh G, Thomas DG. lntravesical oxybutynin in patients with posterior rhizotomies and sacral anterior root stimulators. Neurourol Urodyn 1995; 14: 65–71. [DOI] [PubMed] [Google Scholar]
- O'Flynn KJ, Thomas DG. lntravesical instillation of oxybutynin hydrochloride for detrusor hyper-reflexia. BJU Int 1993; 12: 566–570. [DOI] [PubMed] [Google Scholar]
- Prasad KVR, Vaidyanathan S. Intravesical oxybutynin chloride and clean intermittent catheterisation in patients with neurogenic vesical dysfunction and decreased bladder capacity. BJU Int 1993; 72: 719–722. [DOI] [PubMed] [Google Scholar]
- Madersbacher H, Jilg G. Control of detrusor hyperreflexia by the intravesical instillation of oxybutynine hydrochloride. Paraplegia 1991; 29: 84–90. [DOI] [PubMed] [Google Scholar]
- Bodner DR, Lindan R, Leffler E, Resnick MI. The effect of verapamil on the treatment of detrusor hyperreflexia in the spinal cord injured population. Paraplegia 1989; 27: 364–369. [DOI] [PubMed] [Google Scholar]
- Diokno A, Lapides J. Oxybutynin: a new drug with analgesic and anticholinergic properties. J Urol 1972; 108: 307–309. [DOI] [PubMed] [Google Scholar]
- Noureau L, Cobb J, Bélanger LM, Dvorak MF, Leblond J, Noonan VK et al. Development and assessment of a community follow-up questionnaire for the Rick Hansen Spinal Cord Injury Registry. Arch Phys Med Rehabil 2013; 94: 1753–1765. [DOI] [PubMed] [Google Scholar]
- Véronique B, Bryant D, Parratte B, Guyatt G. Development and Validation of the Short Form of a Urinary Quality of Life Questionnaire: SF-Qualiveen. J Urol 2008; 180: 2592–2598. [DOI] [PubMed] [Google Scholar]
- Véronique B, Parratte B, Amarenco G, Jackowski D, Didier JP, Guyatt G et al. Measuring quality of life in multiple sclerosis patients with urinary disorders using the Qualiveen Questionnaire. Arch Phys Med Rehabil 2004; 85: 1317–1323. [DOI] [PubMed] [Google Scholar]
- Wagner TH, Patrick DL, Bavendam TG, Martin ML, Buesching DP. Quality of life of persons with urinary incontinence: development of a new measure. Urology 1996; 47: 67–71. [DOI] [PubMed] [Google Scholar]
- Schurch B, Denys P, Kozma C, Reese PR, Slaton T, Barron R et al. Reliability and validity of the Incontinence Quality of Life Questionnaire in patients with neurogenic urinary incontinence. Arch Phys Med Rehabil 2007; 88: 646–652. [DOI] [PubMed] [Google Scholar]
- Coyne KS, Matza LS, Kopp Z, Abrams P. The Validation of the Patient Perception of Bladder Condition (PPBC): a single-item global measure for patients with overactive bladder. Eur Urol 2006; 49: 1079–1086. [DOI] [PubMed] [Google Scholar]
- Abrams P, Avery K, Gardener N, Donovan J, ICIQ Advisory Board. The International Consultation on Incontinence Modular Questionnaire: www.iciq.net. J Urol 2006; 175: 1063–1066. [DOI] [PubMed] [Google Scholar]
- Vasquez NN. The interaction of cortico-spinal pathways and sacral sphincter reflexes in subjects with incomplete spinal cord injury: a pilot study. Neurourol Urodyn 2015; 34: 349–355. [DOI] [PubMed] [Google Scholar]
- Pannek J, Bertschy S. Mission impossible? Urological management of patients with spinal cord injury during pregnancy: a systematic review. Spinal Cord 2011; 49: 1028–1032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.