Abstract
Background
Polybrominated diphenyl ether (PBDE) flame retardants are endocrine disrupting chemicals that exhibit estrogenic and androgenic properties and may affect pubertal timing.
Methods
Study subjects were participants between 1999 and 2013 in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS), a longitudinal cohort study of predominantly Mexican origin families in Northern California. We measured serum concentrations of four PBDEs (BDE-47, -99, -100, -153) in blood collected from mothers during pregnancy (N=263) and their children at age 9 years (N=522). We determined timing of pubertal onset in 309 boys and 314 girls using clinical Tanner staging every 9 months between 9 and 13 years of age and timing of menarche by self-report. We used Poisson regression for relative risk (RR) of earlier puberty and parametric survival analysis for time ratios (TR) of pubertal milestones.
Results
Prenatal concentrations of all 4 congeners and ΣPBDEs were associated with later menarche in girls (RRearlier menarche = 0.5, 95% Confidence Interval (CI): 0.3, 0.8 for ΣPBDEs) but earlier pubic hair development in boys (RRearlier pubarche = 2.1, 95% CI: 1.3, 3.3 for ΣPBDEs). No associations were seen between prenatal exposure and girls’ breast or pubic hair development or boys’ genital development. Childhood PBDE exposure was not associated with any measure of pubertal timing, except for an association of BDE-153 with later menarche.
Conclusions
We found that prenatal PBDE exposure was associated with later menarche in girls but earlier pubarche in boys, suggesting opposite pubertal effects in girls and boys.
Keywords: Puberty, PBDEs, flame retardants, endocrine disruption
1. Introduction
Age at onset of puberty among girls, defined as first breast development (thelarche), has been decreasing over recent decades (Biro et al. 2013; Euling et al. 2008; Herman-Giddens et al. 1997), potentially placing girls at increased risk of reproductive cancers (Kelsey et al. 1993; Riman et al. 1998), psychiatric disorders (Graber et al. 2004; Hayward et al. 1997), and behavior problems (Flannery et al. 1993; Lanza and Collins 2002; Phinney et al. 1990; Udry and Cliquet 1982). Recent evidence suggests that age of onset of puberty in boys, defined by testicular enlargement (gonadarche) and the appearance of pubic hair (pubarche), may also be decreasing (Herman-Giddens 2006; Herman-Giddens et al. 2001). The reasons for the downward shifts in timing of puberty are unclear, but one hypothesis is that exposure to endocrine disruptors – chemicals that mimic, block, or interfere with the body’s natural hormones – may impact pubertal timing in children (Chiabotto et al. 2006; Massart et al. 2006).
Polybrominated diphenyl ethers (PBDEs) are a class of brominated chemicals used for many years as flame retardants in consumer products such as furniture, textiles, and electronics (U.S. DHHS 2004). The pentaBDE mixture, which includes the congeners BDE-47, -99, -100, and 153, was widely used in furniture and other household products containing polyurethane foam until its use was discontinued in 2004. Exposure to the pentaBDE mixture is widespread, with more than 93% of Americans having detectable levels of BDE-47, -100, and 153 in their blood (Sjödin et al. 2008b). Although use of PBDE flame retardants has stopped, exposure continues because they are present in existing furniture, electronics, and other large items in the home, are not chemically bound and can leach out into house dust (Sjödin et al. 2008a), and are persistent in the environment and the body (Geyer et al. 2004; Hale et al. 2003; Sjodin et al. 2003).
Several studies have shown that PBDEs (Hamers et al. 2006; Harju et al. 2007; Stoker et al. 2005) and their hydroxylated PBDE (OH-PBDE) metabolites (Hamers et al. 2008; Meerts et al. 2000) have endocrine disrupting properties, which may differ by congener. In estrogen receptor binding assays, lower brominated PBDEs and OH-PBDEs (e.g. BDE-28, -47 and -100) exhibit estrogenic activity (Dang et al. 2007; Meerts et al. 2001), while higher brominated congeners (e.g. BDE-153 and -190) display anti-estrogenic properties (Meerts et al. 2001). The pentaBDE mixture also exhibits anti-androgenic activity in androgen receptor binding assays (Harju et al. 2007; Stoker et al. 2005).
A small number of animal studies suggest that PBDE exposure may impact timing of puberty. In female rats, gestational (Lilienthal et al. 2006); Kodavanti et al. 2010) and peripubertal (Stoker et al. 2004) exposure to pentaBDE and BDE-99 have been associated with significant delays in puberty as measured by age at vaginal opening or mammary gland development. In two studies of male rats, gestational (Kodavanti et al. 2010) and peripubertal (Stoker et al. 2004) exposure to the pentaBDE mixture were associated with later puberty, assessed by age at preputial separation, although gestational exposure to BDE-99 was non-significantly associated with earlier preputial separation in another study (Lilienthal et al. 2006).
In human studies, prenatal PBDE exposure has been associated with reduced fertility (Harley et al. 2010) altered thyroid hormone and sex hormone levels (Chevrier et al. 2011; Chevrier 2008; Eskenazi et al. 2016; Herbstman et al. 2008; Lin et al. 2011; Stapleton et al. 2011), lower infant birth weight (Harley et al. 2011) and impaired childhood neurodevelopment and behavior (Chevrier et al. 2013; Eskenazi et al. 2013; Herbstman et al. 2010; Sagiv et al. 2015) but only two studies have examined timing of puberty. Among 271 adolescent girls participating in the National Health and Examination Survey (NHANES), higher serum PBDE concentrations between ages 12 and 19 years was associated with early menarche (<12 years of age) (Chen et al. 2011). However, among 645 girls participating in the Breast Cancer and the Environment Research Program, higher exposure to several individual PBDE congeners between ages 6 and 8 years was associated with older age at onset of breast and pubic hair development (Windham et al. 2015). No epidemiologic studies have examined the association of PBDEs and puberty in boys or have examined the association of in utero exposure and puberty in boys or girls.
In the present prospective study, we examined the association of prenatal and childhood exposure to four components of the pentaBDE flame retardant mixture on timing of puberty in girls and boys.
2. Methods
2.1. Study population
Participants were children in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS), a longitudinal cohort study examining the effects of environmental exposures on children’s growth and development in a largely Latino region of California. Participants were enrolled in the study in two waves: in the first wave (CHAM1), 601 pregnant mothers were recruited from prenatal care clinics in 1999–2000 and 529 mothers stayed in the study through the birth of a live born infant. The CHAM1 children were assessed at multiple time points throughout childhood, with 326 children (54.2%) continuing to participate in the study at age 9 years. In the second wave of enrollment in 2009–2011, 305 additional mothers and their 9-year-old children were recruited through community outreach (CHAM2). Eligibility requirements were the same for both waves: the children were born between 2000 and 2002 in the Salinas Valley to Spanish- or English-speaking mothers who, at the time of pregnancy, were at least 18 years of age and were eligible for low income health insurance (Medicaid). Pubertal timing was assessed in 641 children between 9 and 13 years of age. We excluded 18 participants who did not provide serum samples for PBDE measurements, for a total of 623 children (309 boys and 314 girls). This study was approved by the Institutional Review Board at the University of California, Berkeley. Informed consent was obtained from mothers and assent was obtained from children for all activities.
2.2. Physical exam and pubertal assessment
At 7 years of age, we used Tanner stage diagrams (Tanner 1986) to obtain mothers’ report of their daughters’ pubertal stages. Between the ages 9 and 13 years, five trained research assistants assessed timing of puberty using clinical Tanner staging conducted at 9 month intervals.. We assessed girls’ stages of breast development (B1–B5) using palpation and pubic hair development (PH1-PH5) using visual inspection. Menarche status was asked at each visit and age at menarche was ascertained at the first post-menarchal visit. We visually assessed boys’ stages of genital (G1–G5) and pubic hair development (PH1-PH5) and measured testicular volume (TV) by comparison with orchidometer beads. A boy was not considered to be in stage G2 unless his TV was >3 cm3. Research assistants were trained and supervised by two pediatric endocrinologists (R.L. and L.G.). Kappas for inter-rater reliability were 0.70 for breast and 0.79 for pubic hair development in girls and 0.75 for genital and 0.86 for pubic hair development in boys. The examiners’ assessments agreed with those of the pediatric endocrinologists 90%, 92%, 92%, and 100% of the time for girls’ breast and pubic hair stage and boys’ genital and pubic hair stage, respectively, with regard to whether the child was in stage 1 versus stage 2+.
At each visit, we measured weight (Tanita TBF 300A bioimpedence scale) and height (Seca 222 stadiometer). Body mass index (BMI) was calculated as weight/height2 (kg/m2) and classified as underweight, normal weight, overweight, or obese according to CDC age- and sex-specific percentiles.(National Center for Health Statistics 2005) Child’s birth weight was obtained from medical records. Maternal pre-pregnancy BMI was based on measured height and either self-reported (CHAM1) or medical record (CHAM2) pre-pregnancy weight.
2.3. PBDE exposure assessment
PDBE concentrations were measured in serum collected from 263 CHAM1 mothers during pregnancy (N=203; mean: 25.7 weeks gestation) or at delivery (N=60) and 522 CHAM1 and CHAM2 children at age 9. Serum was stored at −80°C until shipment to the Centers for Disease Control and Prevention in Atlanta, GA, where specimens were analyzed for 10 PBDE congeners (BDE-17, -28, -47, -66, -85, -99, -100, -153, -154, and -183) by gas-chromatography isotope-dilution high-resolution mass spectrometry.(Sjödin et al. 2004) Each analytical run included laboratory blanks and spikes.
PBDE concentrations are expressed on a serum lipid basis (ng/g lipid).(Phillips et al. 1989) Limits of detection ranged from 0.8–2.6 ng/g for BDE-47 and 0.2–0.7 ng/g for other congeners. For concentrations below the limit of detection (LOD), the machine-read value was used if available or a value <LOD was imputed based on a log-normal probability distribution if not.(Lubin et al. 2004) Of the 10 congeners analyzed, 6 were detected in less than 55% of samples and were not included in this analysis. The four components of the pentaBDE commercial mixture (BDE-47, -99, -100, and -153) were detected in 97% of samples. These four congeners were examined individually and summed to generate a ΣPBDE variable.
2.4 Covariates
Information on potential confounders was collected using structured interviews conducted in the mother’s language of choice (English or Spanish) during pregnancy and/or at the 9-year visit. Potential confounders were selected a priori using directed acyclic graphs (Supplemental Figures S1 & S2). All models controlled for mother’s education and years of residence in the United States at time of pregnancy, child’s birth order, duration of breastfeeding, family income at age 9, and cohort (CHAM1 vs. CHAM2). Models of girls’ pubertal onset also included father’s presence in the home at age 9 and maternal age at menarche. Models of prenatal exposure included maternal pre-pregnancy BMI (continuous variable) while models of childhood exposure included child’s birth weight and BMI (categorical variable for normal, overweight, or obese) at age 9.
2.5. Statistical analysis
We examined the associations of PBDE concentrations with timing of puberty in two ways. First, we examined the relative risk (RR) of earlier onset of puberty (defined as thelarche ≤9 years and pubarche ≤10.5 years in girls; gonadarche ≤10.5 years and pubarche ≤12 years in boys) and earlier menarche (<12 years) using Poisson regression with robust variance estimates. The age cut-offs for earlier thelarche, pubarche, and gonadarche were chosen because they were the study visit closest to the median ages of onset in this sample. The cut-off for earlier menarche was chosen because it was the nearest whole year to the median age at menarche for the study (11.7 years). Second, we modeled time to onset of Tanner 2 and menarche using parametric accelerated failure time (AFT) models assuming a 2-parameter Weibull distribution. For these models, we used the Stata intcens module, which allows for interval-censored data (i.e., pubertal onset occurring at an unknown time between two observations), in addition to the more typical right-censoring (i.e., pubertal onset after the end of observation). The AFT models allow the estimation of a time ratio (TR), which measures the association with the exposure in terms of time (age at puberty), rather than a hazard ratio, which estimates instantaneous risk. For example, if the average age of the onset of telarche in the sample was 10 years of age, a TR=0.9 would imply a 10% reduction in the age of telarche, or an onset at 9 years.
A number of children were left censored because they had already reached one of the pubertal milestones at the start of the observation period (age 9). Among girls, 43.0% had reached thelarche and 20.4% had reached pubarche at the 9-year visit. For these girls, we set the parameters of the AFT models to assume thelarche or pubarche had occurred in either the 7–9 year-old or 5–7 year-old interval based on the mother’s report of her daughter’s Tanner stages at age 7. Among boys, 8% had reached gonadarche and 2% had reached pubarche at age 9. For these boys, the models were set to assume that pubertal onset occurred between 7 and 9 years of age. ) Only two girls had already begun menstruating at age 9, when the PBDE measurement was taken.
PBDE exposure was examined continuously as a log10-transformed variable because generalized additive models suggested that this was an appropriate dose-response relationship and because this reduced the influence of individuals with very high exposure. We also examined PBDE exposure categorically by quartiles of exposure. For the main analyses, we conducted separate models for prenatal and childhood PBDE exposure, but we included prenatal and childhood concentrations in the same model in sensitivity analyses. Because of the strong interrelationship between PBDE concentrations, BMI, and timing of puberty, we also examined interaction by BMI using interaction terms for log10 PBDE concentrations*overweight/obese. We used lipid-standardized PBDE concentrations in our main models but conducted sensitivity analyses examining wet weight PBDE concentrations with serum lipid levels as a covariate (Schisterman et al. 2005).
All analyses were performed using Stata 13.1 (College Station, TX).
3. Results
The study participants were almost entirely Latino with most mothers being recent immigrants from Mexico (Table 1). Approximately three quarters of mothers had not completed high school and the majority of families were living below the federal poverty threshold. There was a high prevalence of overweight and obesity among both mothers and children.
Table 1.
Characteristics of the study population (N=314 girls, N=309 boys), CHAMACOS Study, Salinas Valley, CA.
| Girls N (%) |
Boys N (%) |
|
|---|---|---|
| Maternal ethnicity | ||
| Latino | 301 (96.8) | 298 (96.8) |
| Non-Latino | 10 (3.2) | 10 (3.2) |
| Maternal residence in the US at delivery (years) | ||
| ≤1 | 53 (17.0) | 74 (24.0) |
| 2–5 | 95 (30.5) | 74 (24.0) |
| 6–10 | 76 (24.4) | 75 (24.3) |
| ≥11 | 60 (19.2) | 55 (17.8) |
| Entire life | 28 (9.0) | 31 (10.0) |
| Maternal education | ||
| ≤6th grade | 138 (44.4) | 126 (40.9) |
| 7–12th grade | 99 (31.8) | 105 (34.1) |
| ≥High school graduate | 74 (23.8) | 77 (25.0) |
| Maternal pre-pregnancy BMI | ||
| Underweight | 1 (0.4) | 4 (1.4) |
| Normal weight | 95 (33.7) | 107 (38.4) |
| Overweight | 110 (39.0) | 101 (36.2) |
| Obese | 76 (27.0) | 67 (24.0) |
| Mother’s age at menarche | ||
| < 12 years | 73 (23.5) | 48 (15.5) |
| 12–13 years | 139 (44.7) | 153 (49.5) |
| > 13 years | 99 (31.8) | 108 (35.0) |
| Child’s birthweight | ||
| ≥2500g | 293 (95.1) | 287 (93.5) |
| < 2500g | 15 (4.9) | 20 (6.5) |
| Child’s birth order | ||
| 1 | 107 (34.4) | 105 (34.0) |
| 2 | 94 (30.2) | 94 (30.4) |
| 3+ | 110 (35.4) | 110 (35.6) |
| Months of breastfeeding | ||
| Never | 27 (8.5) | 24 (7.5) |
| < 1 month | 27 (8.5) | 43 (12.5) |
| 1–6 months | 100 (31.4) | 103 (32.3) |
| 6–12 months | 79 (24.8) | 70 (21.9) |
| > 12 months | 86 (27.0) | 79 (24.8) |
| Household income at 9 years | ||
| At or below poverty line | 227 (72.3) | 231 (75.0) |
| Above poverty line | 87 (27.7) | 77 (25.0) |
| Child lives with biological father at 9 years? | ||
| No | 83 (27.0) | 75 (24.8) |
| Yes | 224 (73.0) | 228 (75.3) |
| Child BMI at 9-year visit | ||
| Underweight | 0 (0.0) | 0 (0.0) |
| Normal weight | 148 (47.4) | 127 (41.6) |
| Overweight | 47 (15.1) | 52 (17.1) |
| Obese | 117 (37.5) | 126 (41.3) |
| Cohort | ||
| CHAM1 | 172 (54.8) | 151 (48.9) |
| CHAM2 | 142 (45.2) | 158 (51.1) |
The distribution of serum PBDE concentrations is shown in Supplemental Table S1. More than 97% of mothers and 99% of 9-year-old children had detectable concentrations of the four main congeners of the pentaBDE mixture in their blood. BDE-47 was the dominant congener with median concentrations that were 3–7 times higher than BDE-99, -100, and -153. Maternal prenatal PBDE concentrations in this study population tended to be lower than among pregnant women participating in NHANES (Castorina et al. 2011) while concentrations in children at age 9 were slightly higher than children age 12–19 in NHANES (Centers for Disease Control and Prevention 2015).
In girls, the median age at thelarche was 9.3 years, pubarche was 10.4 years, and menarche was 11.7 years (Table 2). Age at thelarche in this cohort was comparable to Latino girls in the Breast Cancer and the Environment Research Program (BCERP) (median: 9.3 years) who were assessed between 2004 and 2011 (Biro et al. 2013). In boys, the median age at gonadarche was 10.7 years and at pubarche was 12.1 years, which was older than among Latino boys participating in the Pediatric Research Office Settings (PROS) study between 2005–2010 (gonadarche median: 10.0, pubarche median: 11.4 years)(Herman-Giddens et al. 2012).
Table 2.
Timing of pubertal landmarks in boys and girls, CHAMACOS Study, Salinas Valley, CA
| Outcome | n | Age in Years (Median) | “Earlier” puberty1 N (%) |
|---|---|---|---|
| Girls | |||
| Thelarche (B2) | 314 | 9.3 | 42.3% |
| Pubarche (PH2) | 314 | 10.4 | 55.3% |
| Menarche | 314 | 11.7 | 54.5% |
| Boys | |||
| Gonadarche (G2) | 309 | 10.7 | 48.6% |
| Pubarche (PH2) | 309 | 12.1 | 52.0% |
Defined as thelarche ≤9.0 years, pubarche ≤10.5 years, and menarche <12.0 years in girls; gonadarche ≤10.5 years and pubarche ≤12.0 years in boys.
The association of PBDE exposure and timing of puberty in girls is shown in Table 3. When we examined relative risk of earlier thelarche (≤ age 9), pubarche (≤ age 10.5), and menarche (< age 12), prenatal PBDE concentrations were associated with decreased risk of earlier menarche. Each 10-fold increase in prenatal ΣPBDEs was associated with a 50% reduction in risk of earlier menarche (RR=0.5, 95% confidence interval (CI): 0.3, 0.9) and similar results were seen for the individual PBDE congeners. PBDE concentrations in childhood were also associated with reduced risk of earlier menarche, but did not reach statistical significance.
Table 3.
Adjusted Relative Risks (RR) and Time Ratios (TR) of Maternal and Child Lipid-Adjusted Serum PBDE Concentrations (log10) with Pubertal Development in Girls.
| Early Development | Age at Onset | |||||
|---|---|---|---|---|---|---|
| Thelarche1 | Pubarche2 | Menarche3 | Thelarche | Pubarche | Menarche | |
|
|
|
|
|
|
|
|
| RR (95% CI) | RR (95% CI) | RR (95% CI) | TR (95% CI) | TR (95% CI) | TR (95% CI) | |
| Prenatal PBDE Concentrations (N = 140)4 | ||||||
| BDE-47 | 0.9 (0.6, 1.5) | 0.9 (0.6, 1.3) | 0.6 (0.4, 0.9)* | 1.02 (0.97, 1.08) | 1.04 (0.99, 1.09) | 1.03 (0.99, 1.06) |
| BDE-99 | 1.0 (0.6, 1.5) | 1.0 (0.7, 1.4) | 0.6 (0.4, 0.9)* | 1.02 (0.97, 1.07) | 1.02 (0.98, 1.07) | 1.02 (0.99, 1.06) |
| BDE-100 | 0.9 (0.5, 1.4) | 1.0 (0.7, 1.4) | 0.6 (0.4, 0.9)* | 1.01 (0.96, 1.07) | 1.03 (0.98, 1.07) | 1.02 (0.98, 1.05) |
| BDE-153 | 0.6 (0.3, 1.2) | 0.9 (0.6, 1.4) | 0.6 (0.3, 0.9)* | 1.01 (0.95, 1.07) | 1.02 (0.97, 1.07) | 1.01 (0.98, 1.05) |
| ΣPBDEs | 0.9 (0.5, 1.5) | 0.9 (0.6, 1.4) | 0.5 (0.3, 0.9)* | 1.02 (0.97, 1.08) | 1.03 (0.98, 1.08) | 1.03 (0.99, 1.06) |
| Age 9 PBDE Concentrations (N = 266)5 | ||||||
| BDE-47 | 0.8 (0.5, 1.3) | 1.0 (0.7, 1.4) | 0.8 (0.6, 1.1) | 1.02 (0.98, 1.06) | 1.01 (0.97, 1.05) | 1.02 (0.99, 1.05) |
| BDE-99 | 0.7 (0.5, 1.1) | 1.0 (0.7, 1.3) | 0.8 (0.6, 1.0) | 1.03 (1.00, 1.08) | 1.01 (0.98, 1.05) | 1.02 (1.00, 1.05) |
| BDE-100 | 0.7 (0.4, 1.2) | 0.9 (0.6, 1.3) | 0.7 (0.5, 1.0) | 1.02 (0.97, 1.07) | 1.02 (0.98, 1.06) | 1.03 (1.00, 1.06) |
| BDE-153 | 0.6 (0.4, 1.0) | 0.8 (0.5, 1.2) | 0.7 (0.5, 1.0) | 1.04 (0.99, 1.09) | 1.03 (0.99, 1.08) | 1.05 (1.01, 1.08)** |
| ΣPBDEs | 0.7 (0.4, 1.2) | 1.0 (0.7, 1.4) | 0.7 (0.5, 1.0) | 1.03 (0.98, 1.08) | 1.01 (0.97, 1.06) | 1.03 (1.00, 1.06) |
Defined as stage 2+ for breast development at 9 year visit
Defined as stage 2+ for pubic hair development at 10.5 year visit
Defined as menarche before 12 years of age
Models adjusted for maternal education, maternal years in US, birth order, duration of breastfeeding, family income at 9 years, cohort, father’s presence in home, maternal age at menarche, and maternal BMI.
Models adjusted for maternal education, maternal years in US, birth order, duration of breastfeeding, family income at 9 years, cohort, father’s presence in home, maternal age at menarche, child birth weight, and child BMI at 9 years of age.
p<0.05;
p<0.01
Similar results were found examining time ratios for PBDE exposure when thelarche, pubarche, and menarche were examined longitudinally (Table 3). We observed consistently increased TRs – indicating later onset – in the associations of prenatal and childhood PBDE concentrations with breast development, pubic hair development, and menarche in girls, although only the association between childhood BDE-153 and menarche was statistically significant. Each 10-fold increase in 9-year old BDE-153 was associated with a 5% (or 7.2 month) delay in menarche (TR = 1.05, 95% CI: 1.01, 1.08). We found no statistically significant associations of prenatal or childhood PBDE concentrations with breast or pubic hair development in girls.
The association of PBDE exposure and timing of puberty in boys is shown in Table 4. We observed increased risk of earlier pubarche (≤12 years) with each 10-fold increase in all of the prenatal PBDE congeners and ΣPBDEs (RR ΣPBDEs =2.0, 95% CI: 1.3, 3.3). Prenatal concentrations of BDE-100, BDE-153, and ΣPBDEs were also associated with significantly earlier pubic hair development when we examined onset of pubertal development longitudinally, with a 10-fold increase in ΣPBDEs associated with a 5% (7.2 month) decrease in age at onset of pubarche (TR: 0.95, 95% CI: 0.90, 1.00). We found no associations of childhood PBDE concentrations with timing of pubic hair development or of prenatal or childhood concentrations with timing of gonadarche in boys.
Table 4.
Adjusted Relative Risks and Time Ratios of Maternal (Prenatal) and Child (Age 9) Lipid-Adjusted Serum PBDE Concentrations (log10) with Pubertal Development in Boys.
| Early Development | Age at Onset | |||
|---|---|---|---|---|
| Gonadarche1 | Pubarche2 | Gonadarche | Pubarche | |
|
|
|
|
|
|
| RR (95% CI) | RR (95% CI) | TR (95% CI) | TR (95% CI) | |
| Prenatal PBDE Concentrations (N = 117)3 | ||||
| BDE-47 | 1.0 (0.6, 1.7) | 1.9 (1.1, 3.1)* | 0.95 (0.89, 1.01) | 0.96 (0.91, 1.01) |
| BDE-99 | 0.9 (0.5, 1.4) | 1.7 (1.2, 2.6)** | 0.96 (0.90, 1.02) | 0.95 (0.91, 1.00) |
| BDE-100 | 1.0 (0.6, 1.8) | 2.1 (1.3, 3.4)** | 0.93 (0.87, 1.00) | 0.94 (0.89, 1.00)* |
| BDE-153 | 1.1 (0.7, 1.9) | 2.4 (1.4, 4.4)** | 0.90 (0.83, 0.97)** | 0.92 (0.87, 0.98)* |
| ΣPBDEs | 1.0 (0.6, 1.7) | 2.0 (1.3, 3.3)** | 0.94 (0.88, 1.01) | 0.95 (0.90, 1.00)* |
| Peripubertal (age 9) PBDE Concentrations (N = 266)4 | ||||
| BDE-47 | 1.0 (0.7, 1.5) | 0.9 (0.6, 1.3) | 0.99 (0.96, 1.03) | 1.01 (0.98, 1.04) |
| BDE-99 | 1.0 (0.7, 1.4) | 0.8 (0.6, 1.1) | 1.00 (0.97, 1.03) | 1.02 (0.99, 1.05) |
| BDE-100 | 1.1 (0.8, 1.6) | 0.9 (0.6, 1.3) | 0.99 (0.95, 1.03) | 1.01 (0.98, 1.05) |
| BDE-153 | 1.3 (0.9, 2.0) | 1.0 (0.6, 1.5) | 0.98 (0.94, 1.03) | 1.01 (0.97, 1.06) |
| ΣPBDEs | 1.1 (0.7, 1.5) | 0.9 (0.6, 1.3) | 0.99 (0.95, 1.03) | 1.02 (0.98, 1.05) |
Defined as G2+ at 10.5 year visit
Defined as PH2+ at 12 year visit
Models adjusted for maternal education, maternal years in US, birth order, duration of breastfeeding, family income at 9 years, cohort, and maternal BMI.
Models adjusted for maternal education, maternal years in US, birth order, duration of breastfeeding, family income at 9 years, cohort, child birth weight, and child BMI at 9 years of age.
p<0.05;
p<0.01
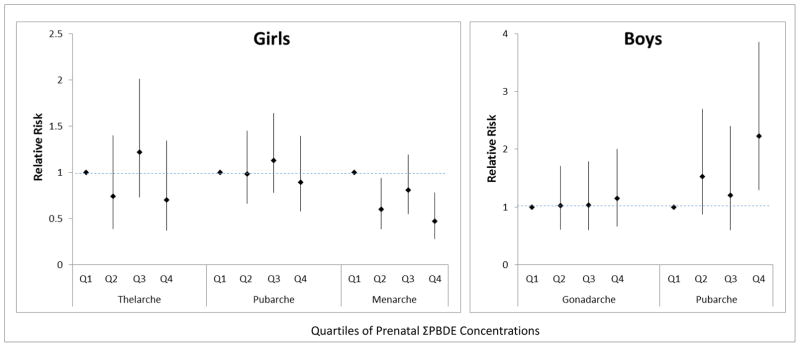
When PBDE exposure was modelled categorically by quartiles of exposure rather than continuously, results were similar. Figure 1 shows that having prenatal ΣPBDE concentrations in the highest quartile was associated with decreased risk of earlier menarche in girls and increased risk of earlier pubarche in boys relative to those in the lowest quartile.
Figure 1.
Associations of Quartiles of Prenatal PBDE Concentrations with Earlier Puberty in Girls and Boys.
When we included prenatal and childhood concentrations of PBDEs in the same models (Supplemental Tables S2 and S3), the sample size was reduced but the inferences remained largely the same (i.e. we observed associations in the direction of later menarche in girls and earlier pubarche in boys and the associations tended to be with prenatal rather than childhood PBDE concentrations). In sensitivity analyses, we controlled for blood lipids by including wet weight PBDEs and total lipids in the models, but found that it made little difference to the findings (results not shown).
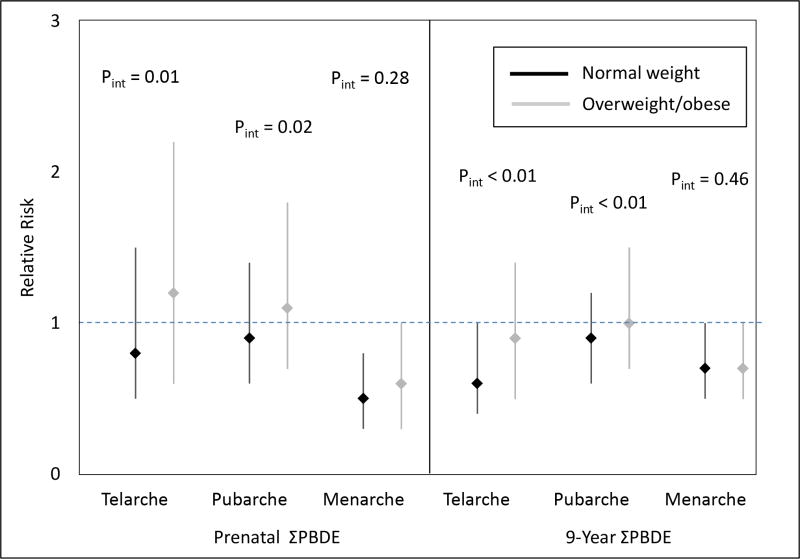
When we examined interaction by BMI, we found evidence of interaction for thelarche and pubarche (interaction p-values < 0.05) among girls, but little evidence of interaction among boys. Among girls, we observed that some prenatal and childhood PBDE concentrations were associated with later thelarche and pubarche in normal weight girls but not in overweight/obese girls (Figure 2 and Supplemental Table S4). For example, each 10-fold increase in ΣPBDEs at 9 years of age was associated with decreased risk of earlier thelarche in normal weight girls (RR=0.6, 95% CI: 0.4, 1.0) but not overweight/obese girls (RR=0.9, 95% CI: 0.5, 1.4). We observed little interaction for menarche; prenatal and childhood PBDEs were associated with later menarche in both normal weight and overweight/obese girls, although the associations were stronger and only statistically significant in normal weight girls. Among boys (Supplemental Table S5), the associations of prenatal PBDE concentrations with early pubarche persisted, results were similar in both normal weight and overweight/obese children, and all interaction p-values were > 0.05.
Figure 2.
Associations of Log2 Prenatal PBDE Concentrations with Earlier Puberty in Normal Weight and Overweight/Obese Girls.
4. Discussion
We found that prenatal exposure to PBDE congeners found in the pentaBDE flame retardant mixture was associated with later menarche in girls, but earlier pubic hair development in boys, in a low-income, predominantly Latino population. Associations for girls were stronger in normal weight rather than overweight/obese girls. Associations were predominantly for prenatal PBDE concentrations rather than for concentrations measured during childhood.
This finding is consistent with research in female rats that found developmental exposure to components of the pentaBDE mixture to be associated with later onset of puberty (Lilienthal et al. 2006; Stoker et al. 2004). The findings are less consistent with animal studies in males; although one study found non-significant associations of prenatal pentaBDE exposure with earlier onset of puberty in males (Lilienthal et al. 2006), two other studies of found prenatal (Kodavanti et al. 2010) and peripubertal (Stoker et al. 2004) exposure to be associated with later puberty. Our findings are also consistent with the only other longitudinal study of PBDEs and puberty in humans, which found early childhood exposure to six PBDE congeners, including the four examined in this study, to be associated with later onset of breast and pubic hair development in girls (Windham et al. 2015). We observed associations in the direction of later onset of breast and pubic hair development, but these associations were only statistically significant in normal weight girls. We also found associations of delayed timing of menarche (which is consistent with later puberty) with all four PBDE congeners examined, although it should be noted that menarche occurs near the mid-point of the pubertal transition and, unlike thelarche, does not mark the onset of puberty. The Windham et al. study did not examine timing of menarche.
Our findings are in contrast to Chen et al., who found PBDEs to be associated with earlier menarche in a cross-sectional study of 12–19 year old girls in NHANES.(Chen et al. 2011) However, the PBDE concentrations measured in the NHANES study were collected after the girls had begun puberty and, in most cases, after they had begun menarche. Although PBDEs have a fairly long half-life in the body, on the order of 1 – 6 years,(Geyer et al. 2004) puberty is a time of rapid growth which may cause a dilution of PBDE body burden, so that the PBDE concentrations measured between age 12 and 19 years in that study may not accurately reflect exposure before menarche.
We know of no other studies of PBDE exposure and timing of puberty in boys. However, we recently reported that prenatal concentrations of BDE-100 and BDE-153 were positively associated with hormone levels, including testosterone, luteinizing hormone, and follicle stimulating hormone, in 12 year-old boys in the CHAMACOS cohort (Eskenazi et al, 2016), which is consistent with our finding of earlier puberty in boys.
We examined PBDE concentrations prenatally and peri-pubertally (9 years of age) but our associations of later menarche in girls and earlier pubarche in boys were strongest with prenatal exposure, suggesting that this may be the more susceptible window of exposure. Very few epidemiologic studies of endocrine disruptors and pubertal timing have been able to include prenatal measures of exposure and have tended to examine chemical exposure during childhood(Windham et al. 2015) or to estimate prenatal levels from blood concentrations collected later in life.(Chen et al. 2011)
One important consideration is the role of BMI in the association of lipophilic compounds like PBDEs with timing of puberty, particularly in girls. Higher BMI in childhood is associated with earlier puberty in girls (Davison et al. 2003). We have previously reported associations of prenatal PBDE exposure with decreased BMI in girls (but increased BMI in boys) at 7 years of age (Erkin-Cakmak et al. 2015); thus, it is possible that lower BMI in childhood is on the causal pathway between prenatal PBDE exposure and later onset of puberty in girls. However, BMI may also be a confounder in this relationship. Because PBDEs are stored in adipose tissue, PDBE concentrations may be diluted by rapid growth or increased body fat, resulting in lower serum PBDE concentrations in overweight children. Thus, being overweight could induce a spurious association between lower PBDE concentrations and earlier puberty in girls. We addressed this concern by controlling for BMI of the person providing the PBDE measurement (i.e. maternal BMI for the prenatal exposure models, child BMI for the 9-year-old exposure models) in our models, but uncontrolled confounding is still a possibility. Finally, assessment of breast development is more difficult in overweight girls because of the difficulty differentiating breast tissue from adipose tissue, suggesting that BMI may also be related to misclassification of timing of thelarche. In this study, we found associations of prenatal and 9-year-old PBDE concentrations with later thelarche in normal weight girls only. While this may suggest that PBDEs have different effects on pubertal timing in normal weight versus overweight girls, it may also be because that misclassification and/or confounding prevented the observation of associations in overweight girls. The associations with age at menarche, which should not be affected by BMI-related misclassification, was similar in normal weight and overweight/obese girls.
The CHAMACOS Study is a population of Latino children from a farmworker community and findings may not be generalizable to all populations. Additionally, many children were lost to follow-up in the 9 years between birth and the start of our puberty assessments, which may have introduced bias in our study sample.
Although we had maternal report of girls’ Tanner stage at age 7 years, one limitation of this study is that we did not begin clinical Tanner staging of the cohort until the children were 9 years of age, by which time 43% of girls and 9% of boys had already begun puberty. This left censoring should not affect the validity of our relative risk analyses of earlier puberty, but may impact the results of our time ratio analysis. For the time ratio models, there is more uncertainty about the exact age of pubertal onset among the early developers, particular for thelarche among girls. This may have reduced our ability to detect associations and may explain why we see stronger associations with relative risk than with time ratios in girls. For all children, because we performed Tanner staging examinations at 9-month intervals, we were not able to observe the exact age of pubertal onset and can place it only within the interval. However, our use of parametric survival analysis (which allows for left, right, and interval censoring) helps address this problem. Finally, clinical Tanner staging, particularly of breast and genital development, is difficult to conduct and we cannot assume that all misclassification will be non-differential (for example, it may be related to BMI). We expect less misclassification in timing of pubarche or menarche, which were two outcomes where we observed the strongest associations. In conclusion, we found evidence that prenatal exposure to the PBDE flame retardants found in the pentaBDE mixture widely used in home furnishings may be associated with later puberty in girls and earlier puberty in boys. Although this finding does not help explain the observed phenomenon of earlier onset of puberty in girls, it suggests that these ubiquitous exposures may none-the-less be impacting children’s pubertal development.
Supplementary Material
Highlights.
PBDE flame retardant exposure was associated with later age at menarche in girls.
PBDEs were associated with later onset of puberty in normal weight girls.
PBDE flame retardant exposure was associated with earlier onset of puberty in boys.
Acknowledgments
Sources of Financial Support: This research was supported by the following grants: RD 83171001 and RD 826709 from the U.S. Environmental Protection Agency (US EPA) and PO1 ES009605 and RO1 ES015572 from NIEHS. The contents of this publication are solely the authors’ responsibility and do not necessarily represent the official views of the NIEHS, NIH, EPA or the Centers for Disease Control and Prevention.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Biro FM, Greenspan LC, Galvez MP, Pinney SM, Teitelbaum S, Windham GC, et al. Onset of breast development in a longitudinal cohort. Pediatrics. 2013;132(6):1019–1027. doi: 10.1542/peds.2012-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castorina R, Bradman A, Sjodin A, Fenster L, Jones RS, Harley KG, et al. Determinants of serum polybrominated diphenyl ether (PBDE) levels among pregnant women in the CHAMACOS cohort. Environ Sci Technol. 2011;45(15):6553–6560. doi: 10.1021/es104295m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, February 2015. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2015. http://www.cdc.gov/exposurereport/ [Google Scholar]
- Chen A, Chung E, DeFranco EA, Pinney SM, Dietrich KN. Serum PBDEs and age at menarche in adolescent girls: analysis of the National Health and Nutrition Examination Survey 2003–2004. Environ Res. 2011;111(6):831–837. doi: 10.1016/j.envres.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier J, Gunier RB, Harley K, Bradman A, Sjodin A, Boyd-Barr D, et al. Interaction of DDT/E and PBDE in their association with cognition and behavior in 7-year old CHAMACOS children. Proceedings of the International Society of Environmental Epidemiology Annual Meeting; Basel, Switzerland. 2013. Abstract available at: Environ Health Perspect; http://dx.doi.org/10.1289/ehp.ehbasel13. [Google Scholar]
- Chevrier J, Harley KG, Bradman A, Sjodin A, Eskenazi B. Prenatal exposure to polybrominated diphenyl ether flame retardants and neonatal thyroid-stimulating hormone levels in the CHAMACOS study. Am J Epidemiol. 2011;174(10):1166–1174. doi: 10.1093/aje/kwr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier JHK, Bradman A, Sjodin A, Holland N, Fenster L, Eskenazi B. Associations between PBDE body burden and thyroid hormone levels in pregnant women participating in the CHAMACOS study. 2008 ISEE Abstract-1228. [Google Scholar]
- Chiabotto P, Costante L, de Sanctis C. Premature thelarche and environmental pollutants. Minerva Med. 2006;97(3):277–285. [PubMed] [Google Scholar]
- Dang VH, Choi KC, Jeung EB. Tetrabromodiphenyl ether (BDE 47) evokes estrogenicity and calbindin-D9k expression through an estrogen receptor-mediated pathway in the uterus of immature rats. Toxicol Sci. 2007;97(2):504–511. doi: 10.1093/toxsci/kfm051. [DOI] [PubMed] [Google Scholar]
- Davison KK, Susman EJ, Birch LL. Percent body fat at age 5 predicts earlier pubertal development among girls at age 9. Pediatrics. 2003;111(4 Pt 1):815–821. doi: 10.1542/peds.111.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkin-Cakmak A, Harley KG, Chevrier J, Bradman A, Kogut K, Huen K, et al. In utero and childhood polybrominated diphenyl ether exposures and body mass at age 7 years: the CHAMACOS study. Environ Health Perspect. 2015;123(6):636–642. doi: 10.1289/ehp.1408417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, et al. In Utero and Childhood Polybrominated Diphenyl Ether (PBDE) Exposures and Neurodevelopment in the CHAMACOS Study. Environ Health Perspect. 2013;121(2):257–262. doi: 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Rauch SA, Tenerelli R, Huen K, Holland NT, Lustig RH, et al. In utero and childhood DDT, DDE, PBDE and PCBs exposure and sex hormones in adolescent boys: The CHAMACOS study. Int J Hyg Environ Health. 2016 doi: 10.1016/j.ijheh.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, Sorensen TI, et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 2008;121(Suppl 3):S172–191. doi: 10.1542/peds.2007-1813D. [DOI] [PubMed] [Google Scholar]
- Flannery DJ, Rowe DC, Gulley BL. Impact of pubertal status, timing, and age on adolescent sexual experience and delinquency. J Adolesc Res. 1993;8(1):21–40. [Google Scholar]
- Geyer HJ, Schramm K-W, Darnerud PO, Aune M, Feicht A, Fried KW. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Compounds. 2004;66:3867–3872. [Google Scholar]
- Graber JA, Seeley JR, Brooks-Gunn J, Lewinsohn PM. Is pubertal timing associated with psychopathology in young adulthood. J Am Acad Child Adolesc Psychiatry. 2004;43(6):718–726. doi: 10.1097/01.chi.0000120022.14101.11. [DOI] [PubMed] [Google Scholar]
- Hale RC, Alaee M, Manchester-Neesvig JB, Stapleton HM, Ikonomou MG. Polybrominated diphenyl ether flame retardants in the North American environment. Environ Int. 2003;29(6):771–779. doi: 10.1016/S0160-4120(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MH, Andersson PL, et al. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci. 2006;92(1):157–173. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Visser TJ, Van Velzen MJ, et al. Biotransformation of brominated flame retardants into potentially endocrine-disrupting metabolites, with special attention to 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) Mol Nutr Food Res. 2008;52(2):284–298. doi: 10.1002/mnfr.200700104. [DOI] [PubMed] [Google Scholar]
- Harju M, Hamers T, Kamstra JH, Sonneveld E, Boon JP, Tysklind M, et al. Quantitative structure-activity relationship modeling on in vitro endocrine effects and metabolic stability involving 26 selected brominated flame retardants. Environ Toxicol Chem. 2007;26(4):816–826. doi: 10.1897/06-308r.1. [DOI] [PubMed] [Google Scholar]
- Harley KG, Chevrier J, Schall RA, Sjodin A, Bradman A, Eskenazi B. Association of prenatal exposure to polybrominated diphenyl ethers and infant birth weight. Am J Epidemiol. 2011;174(8):885–892. doi: 10.1093/aje/kwr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Marks AR, Chevrier J, Bradman A, Sjodin A, Eskenazi B. PBDE concentrations in women’s serum and fecundability. Environ Health Perspect. 2010;118(5):699–704. doi: 10.1289/ehp.0901450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward C, Killen JD, Wilson DM, Hammer LD, Litt IF, Kraemer HC, et al. Psychiatric risk associated with early puberty in adolescent girls. J Am Acad Child Adolesc Psychiatry. 1997;36(2):255–262. [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Apelberg BJ, Witter FR, Halden RU, Patterson DG, et al. Birth Delivery Mode Modifies the Associations between Prenatal Polychlorinated Biphenyl (PCB) and Polybrominated Diphenyl Ether (PBDE) and Neonatal Thyroid Hormone Levels. Environ Health Perspect. 2008;116(10):1376–1382. doi: 10.1289/ehp.11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V, et al. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118(5):712–719. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman-Giddens ME. Recent data on pubertal milestones in United States children: the secular trend toward earlier development. Int J Androl. 2006;29(1):241–246. doi: 10.1111/j.1365-2605.2005.00575.x. discussion 286–290. [DOI] [PubMed] [Google Scholar]
- Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics. 1997;99(4):505–512. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- Herman-Giddens ME, Steffes J, Harris D, Slora E, Hussey M, Dowshen SA, et al. Secondary sexual characteristics in boys: data from the Pediatric Research in Office Settings Network. Pediatrics. 2012;130(5):e1058–1068. doi: 10.1542/peds.2011-3291. [DOI] [PubMed] [Google Scholar]
- Herman-Giddens ME, Wang L, Koch G. Secondary sexual characteristics in boys: estimates from the national health and nutrition examination survey III, 1988–1994. Arch Pediatr Adolesc Med. 2001;155(9):1022–1028. doi: 10.1001/archpedi.155.9.1022. [DOI] [PubMed] [Google Scholar]
- Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15(1):36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Coburn CG, Moser VC, MacPhail RC, Fenton SE, Stoker TE, et al. Developmental exposure to a commercial PBDE mixture, DE-71: neurobehavioral, hormonal, and reproductive effects. Toxicol Sci. 2010;116(1):297–312. doi: 10.1093/toxsci/kfq105. [DOI] [PubMed] [Google Scholar]
- Lanza ST, Collins LM. Pubertal timing and the onset of substance use in females during early adolescence. Prev Sci. 2002;3(1):69–82. doi: 10.1023/a:1014675410947. [DOI] [PubMed] [Google Scholar]
- Lilienthal H, Hack A, Roth-Harer A, Grande SW, Talsness CE. Effects of developmental exposure to 2,2,4,4,5-pentabromodiphenyl ether (PBDE-99) on sex steroids, sexual development, and sexually dimorphic behavior in rats. Environ Health Perspect. 2006;114(2):194–201. doi: 10.1289/ehp.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SM, Chen FA, Huang YF, Hsing LL, Chen LL, Wu LS, et al. Negative associations between PBDE levels and thyroid hormones in cord blood. Int J Hyg Environ Health. 2011;214(2):115–120. doi: 10.1016/j.ijheh.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112(17):1691–1696. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massart F, Parrino R, Seppia P, Federico G, Saggese G. How do environmental estrogen disruptors induce precocious puberty? Minerva Pediatr. 2006;58(3):247–254. [PubMed] [Google Scholar]
- Meerts IA, Letcher RJ, Hoving S, Marsh G, Bergman A, Lemmen JG, et al. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, and polybrominated bisphenol A compounds. Environ Health Perspect. 2001;109(4):399–407. doi: 10.1289/ehp.01109399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts IA, van Zanden JJ, Luijks EA, van Leeuwen-Bol I, Marsh G, Jakobsson E, et al. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci. 2000;56(1):95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. CDC Growth Charts. United States: 2005. [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert JT, Jr, Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18(4):495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- Phinney VG, Jensen LC, Olsen JA, Cundick B. The relationship between early development and psychosexual behaviors in adolescent females. Adolescence. 1990;25(98):321–332. [PubMed] [Google Scholar]
- Riman T, Persson I, Nilsson S. Hormonal aspects of epithelial ovarian cancer: review of epidemiological evidence. Clin Endocrinol (Oxf) 1998;49(6):695–707. doi: 10.1046/j.1365-2265.1998.00577.x. [DOI] [PubMed] [Google Scholar]
- Sagiv S, Kogut K, Gaspar F, Gunier R, Harley K, Parra K, et al. Prenatal and childhood polybrominated diphenyl ether (PBDE) exposure and attention and executive function at 9–12 years of age. 2015 doi: 10.1016/j.ntt.2015.08.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Whitcomb BW, Louis GM, Louis TA. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect. 2005;113(7):853–857. doi: 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin A, Jones RS, Lapeza CR, Focant JF, McGahee EE, 3rd, Patterson DG., Jr Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Anal Chem. 2004;76(7):1921–1927. doi: 10.1021/ac030381+. [DOI] [PubMed] [Google Scholar]
- Sjödin A, Papke O, McGahee E, Focant JF, Jones RS, Pless-Mulloli T, et al. Concentration of polybrominated diphenyl ethers (PBDEs) in household dust from various countries. Chemosphere. 2008a;73(1 Suppl):S131–136. doi: 10.1016/j.chemosphere.2007.08.075. [DOI] [PubMed] [Google Scholar]
- Sjodin A, Patterson DG, Jr, Bergman A. A review on human exposure to brominated flame retardants--particularly polybrominated diphenyl ethers. Environ Int. 2003;29(6):829–839. doi: 10.1016/S0160-4120(03)00108-9. [DOI] [PubMed] [Google Scholar]
- Sjödin A, Wong L, Jones RS, Park A, Zhang Y, Hodge C, et al. Serum Concentrations of Polybrominated Diphenyl Ethers (PBDEs) and Polybrominated Biphenyl (PBB) in the United States Population: 2003–2004. Environmental science & technology. 2008b;42(4):1377–1384. doi: 10.1021/es702451p. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Eagle S, Anthopolos R, Wolkin A, Miranda ML. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environ Health Perspect. 2011;119(10):1454–1459. doi: 10.1289/ehp.1003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker TE, Cooper RL, Lambright CS, Wilson VS, Furr J, Gray LE. In vivo and in vitro anti-androgenic effects of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture. Toxicol Appl Pharmacol. 2005;207(1):78–88. doi: 10.1016/j.taap.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Laws SC, Crofton KM, Hedge JM, Ferrell JM, Cooper RL. Assessment of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture, in the EDSP male and female pubertal protocols. Toxicol Sci. 2004;78(1):144–155. doi: 10.1093/toxsci/kfh029. [DOI] [PubMed] [Google Scholar]
- Tanner JM. Normal growth and techniques of growth assessment. Clin Endocrinol Metab. 1986;15(3):411–451. doi: 10.1016/s0300-595x(86)80005-6. [DOI] [PubMed] [Google Scholar]
- U.S. DHHS. PBDE-New Investigator Grant. 2004. Toxicological profile for polybrominated biphenyls and polybrominated diphenyl ethers (PBBs and PBDEs) [Google Scholar]
- Udry JR, Cliquet RL. A cross-cultural examination of the relationship between ages at menarche, marriage, and first birth. Demography. 1982;19(1):53–63. [PubMed] [Google Scholar]
- Windham GC, Pinney SM, Voss RW, Sjodin A, Biro FM, Greenspan LC, et al. Brominated Flame Retardants and Other Persistent Organohalogenated Compounds in Relation to Timing of Puberty in a Longitudinal Study of Girls. Environ Health Perspect. 2015;123(10):1046–1052. doi: 10.1289/ehp.1408778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.