ABSTRACT
Colorectal cancer (CRC) is the most commonly diagnosed cancer worldwide, and over 50% of patients will develop hepatic metastasis during the course of their disease. CXCR4 and its ligand, stromal cell-derived factor 1α (SDF-1α)/chemokine (C-X-C motif) ligand 12 (CXCL12) have been revealed as regulatory molecules involved in the spreading and progression of a variety of tumors. Here we have shown that lipopolysaccharides (LPS) promoted the migratory capacity of colon cancer cells in vivo and in vitro, which correlated with the activation of SDF-1α/CXCR4 axis and epithelial-mesenchymal transition (EMT) occurrence. Additionally, we found that LPS-induced CXCR4 expression and EMT through NF-κB signaling pathway activation. And inhibition of NF-κB pathway, which recovered the epithelial phenotype and attenuated CXCR4 expression, inhibited cell migratory capacity. Clinically, high levels of CXCR4 always correlated with metastasis and poor prognosis of CRC patients. In conclusion, LPS participate in the whole process of hepatic metastasis of CRC, not only causing liver damage resulting in the production of SDF-1α, but also enhancing the invasive potential of CRC cells by promoting CXCR4 expression and EMT occurrence, which would contribute to the enhancement of cell migration and invasion.
KEYWORDS: colorectal cancer, epithelial-mesenchymal transition, lipopolysaccharides, NF-κB, SDF-1α/CXCR4 axis
Introduction
Colorectal cancer (CRC) is the most commonly diagnosed cancer worldwide.1 Metastasis is the major cause of death in patients with CRC, and surgery is the only feasible therapy with very low mortality. The liver is the most common site of distant spread of primary CRC, and over 50% of patients will develop hepatic metastasis during the course of their disease.2,3 Therefore, it is important to uncover the biological mechanisms underlying liver metastasis of CRC and accelerate the development of new treatment strategies.
Because large amount of microbes colonize in the gut, the gut epithelium which acts as a first-barrier, is exposed consistently to the microbes and microbial products.4 It is becoming increasingly evident that the bacterial population plays an important role in colon carcinogenesis.5 For example, mutant mice that are genetically susceptible to CRC were found to develop significantly fewer tumors when maintained in germ-free conditions than in the presence of a conventional microbiota environment.6 Lipopolysaccharides (LPS) are a major constituent of the outer cell membrane of gram-negative bacteria, whose levels are common elevated in the enteric cavity and portal venous blood of the patients with CRC owing to the intestinal obstruction, increased gut permeability, bacterial overgrowth and changes in the composition of gut flora.7 And LPS can cause significant systemic inflammation. LPS-induced systemic inflammation was shown to increase hepatic recruitment of cancer cells in mice.8 In addition, recent studies have reported that LPS exert direct effects on carcinogenesis, tumor progress, invasion and metastasis.9-12 And it could significantly augment the cell adhesion and liver metastasis of human CRC cells.13,14
Recently, several studies have placed chemokines and their receptors at the center of not only physiological cell migration, but also pathological processes, such as metastasis in cancer.15 Stromal cell-derived factor 1α (SDF-1α)/CXCR4 axis is one of the few chemotactic systems that characterizes one-to-one correspondence reciprocally between the ligand and receptor.16 The chemokine receptor CXCR4 was initially described to regulate the homing of lymphocytes in inflammatory tissues.17 However, recent findings determined that the SDF-1α/CXCR4 axis plays a key role in the local progression and metastasis of various cancers including lung, pancreatic and breast cancers, as well as the CRC.18-21 Several researches have shown that strong expression of CXCR4 by CRC cells is significantly associated with liver metastasis and poor survival in patients with CRC.22-26 As the natural ligand of CXCR4, SDF-1α is highly expressed in tissues of metastatic growth and attracts cancer cells to these organs toward a concentration gradient.27
The aim of this work was to analyze whether LPS participate in the whole process of hepatic metastasis of CRC, not only causing liver damage resulting in the production of SDF-1α, but also enhancing the invasive potential of CRC cells by promoting CXCR4 expression and epithelial-mesenchymal transition (EMT) occurrence, which would contribute to the enhancement of cell migration and invasion.
Results
LPS contributed to hepatic metastasis of colon cancer cells
Mouse splenic vein metastasis assay was performed to detect the role of LPS in the hepatic metastasis of C26 cells. We subjected BALB/c mice with acute liver injury and the normal one, to combination with PBS and LPS-treated C26 cells. The acute liver injury was induced by intraperitoneal injections of LPS. Results showed that the LPS-treated BALB/c mice with splenic vein injection of LPS-challenged C26 cells formed much more and bigger liver tumors than control (Fig. 1A–D). And LPS-treated BALB/c mice with splenic vein injection of LPS-challenged C26 cells have poor survival rate (Fig. 1E). Results above indicated that LPS enhanced the invasion and migration of colon cancer cells, and LPS-induced liver inflammation also contributed to the hepatic metastasis of colon cancer cells.
Figure 1.
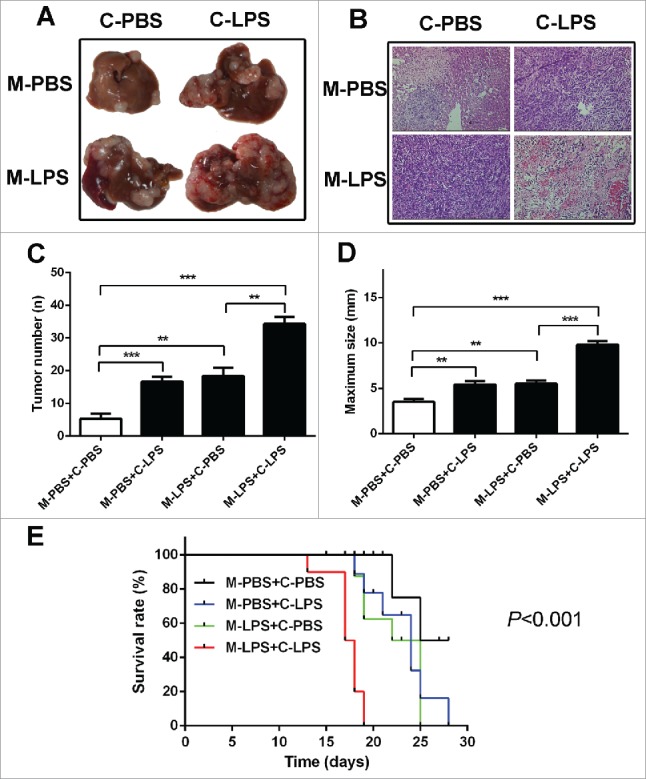
LPS contributed to hepatic metastasis of colon cancer cells. (A) Mouse splenic vein metastasis assay was performed to detect the role of LPS in the hepatic metastasis. (B) H & E staining was performed on serial sections of metastatic tumors and normal liver (× 200). (C) The numbers of nodules were quantified on mice livers (n = 10 per group). Values for individual mice are shown above the bars (**P < 0.01, ***P < 0.001). (D) The maximum size of nodules was quantified on the surface of mice liver. Values for individual mice are shown above the bars (**P < 0.01, ***P < 0.001). (E) The survival time of treated mice were monitored. C-PBS: PBS-challenged C26 cells; C-LPS: LPS-challenged C26 cells; M-PBS: PBS-challenged BALB/c mice; M-LPS: LPS-challenged BALB/c mice.
LPS induced a SDF-1α/CXCR4 dependent migration of colorectal cancer cells
SDF-1α/CXCR4 axis plays an important role in tumor cell metastasis.28 In order to detect the influence of LPS to SDF-1α/CXCR4 axis, the expression of SDF-1α and CXCR4 was determined. We found that SDF-1α expression was significantly increased in the liver of LPS-challenged BABL/c mice (Fig. 2A, B). And real time cellular analysis was used to determine the contribution of SDF-1α to the migratory capacity of C26 cells. The results showed that LPS-treated C26 cells displayed an increased migratory ability when SDF-1α was presented in the bottom chamber (Fig. 2C). To determine whether LPS contribute to migratory ability in colon cancer cells in vitro, the wound healing assay was performed. It showed that LPS-disposed C26 cells migrated at a much longer distance than control (Fig. 3A). Then we analyzed the expression level of CXCR4, the data of RT-PCR showed that LPS induced an increased levels of CXCR4 expression (Fig. 3B), which was consistent with the immunohistochemistry (IHC) and western blot results (Fig. 3C, D). Since CXCR4 was up-regulated in LPS-disposed C26 cells, we next investigated whether it was a key factor in the metastasis of C26 cells in vitro. The results of transwell assays showed that additional supplementation of CXCR4 inhibitor (AMD3100) significantly decreased the invasiveness of LPS-challenged C26 cell lines compared to control (p < 0.001) (Fig. 3E). The results above suggested that LPS induced the expression of SDF-1α and CXCR4, which promoted the invasion of CRC cells.
Figure 2.
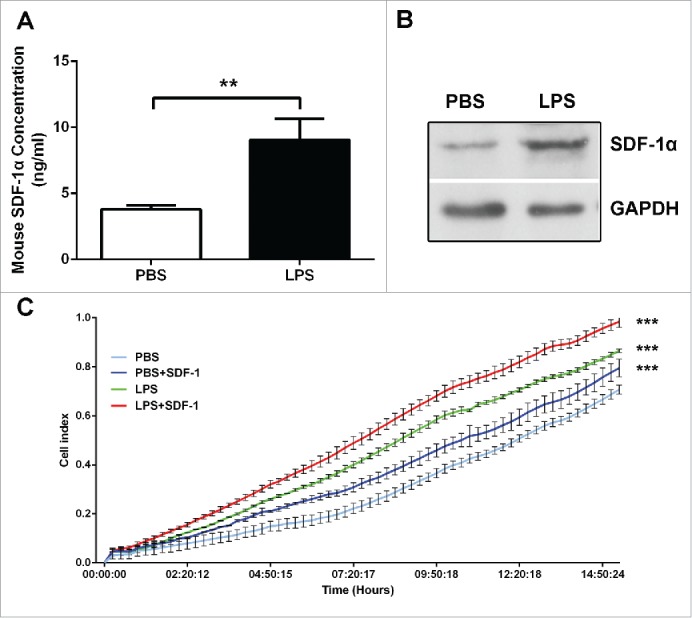
LPS induced SDF-1α expression, which was related to the hepatic metastasis of colon cancer cells. (A) The level of SDF-1α in PBS and LPS-challenged mice was evaluated by ELISA (**P < 0.01). (B) The expression of SDF-1α in mice liver was measured by protein gel blot. (C) Real-time migration assay (xCELLigence System, ACEA Biosciences Inc.) was used to detect the migratory of C26 cells (***P < 0.001).
Figure 3.
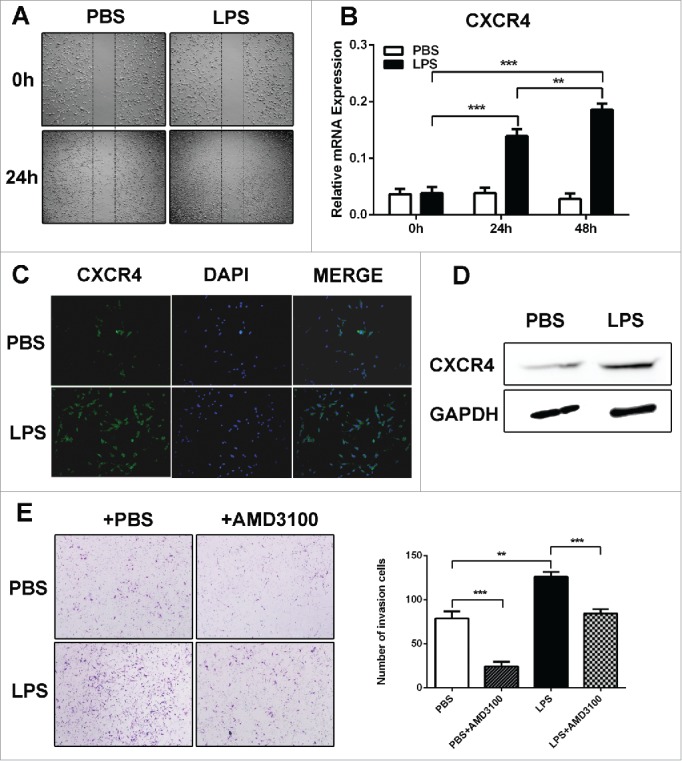
LPS promoted CRC cells migration through upregulating CXCR4 expression in vitro. (A)The wound healing assay was employed to determine the migration of PBS and LPS-disposed C26 cells. Cells were monitored 24 h to evaluate the rate of migration into the scratched area (X40). (B, C and D) RT-PCR, western blot and immunofluorescence were used to detect CXCR4 expression in PBS and LPS-disposed C26 cells, GAPDH was used as an internal reference for RT-PCR and protein gel blot. **P < 0.01, ***P < 0.001. (E) Invasiveness of cells was determined using the Transwell assay (**P < 0.01, ***P < 0.001, X200).
LPS promoted EMT phenotype in CRC cells
Additionally, in LPS-treated C26 cells, the mesenchymal markers (Vimentin and Snail) were upregulated and epithelial marker (E-cadherin) was downregulated (Fig. 4A–D). The immunofluorescence results of E-cadherin and Vimentin further support the finding that LPS induced EMT in C26 cells (Fig. 4E). It means that C26 cells with LPS treatment harbored EMT phenotype, which is responsible for the invasive capacity of C26 cells.
Figure 4.
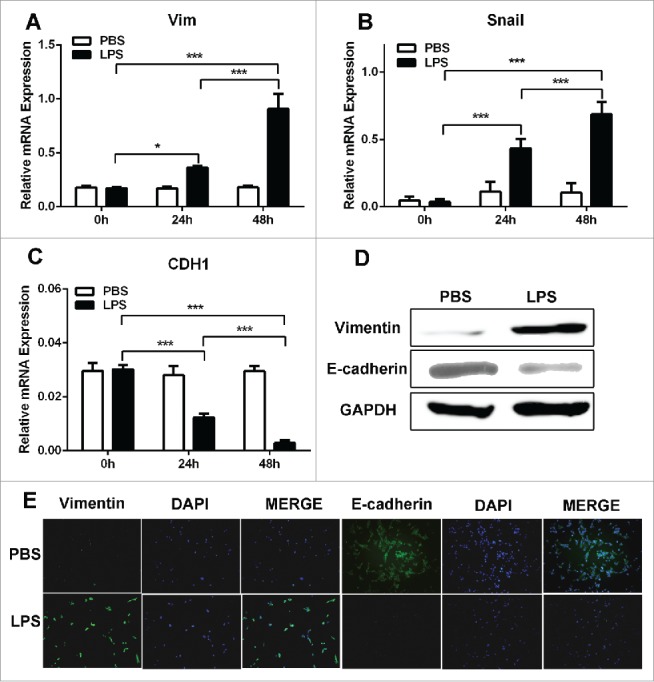
EMT contributed to the migratory capacity of LPS-disposed CRC cells. (A, B and C). RT-PCR was used to detect Vimentin, Snail and Ecadherin expression in PBS and LPS-disposed C26 cells, GAPDH was used as an internal reference. *P < 0.05, ***P < 0.001. (D) Western blot was used to detect Vimentin and Ecadherin expression in PBS and LPS-disposed C26 cells, GAPDH was used as an internal reference. (E) Immunofluorescence was used to evaluate the expression of Vimentin and Ecadherin in PBS and LPS-disposed C26 cells.
LPS induced CXCR4 expression and EMT occurrence through NF-κB pathway activation
To investigate whether nuclear factor-κB (NF-κB) pathway is required for the up-regulation of CXCR4 and EMT in response to LPS, protein gel blot was used to detect the IκB activation. The result showed that IκB was phosphorylated in C26 cells with LPS treatment (Fig. 5A). Then we used IκB inhibitor (BAY11-7028) to block signaling pathway in C26 cells, and examined the level of CXCR4 expression and EMT phenotype. We found that BAY11-7028 significantly reduced the CXCR4 and EMT markers induction in C26 cell line, compared to the control (Fig. 5B–E), suggesting that NF-κB signaling is important for the upregulation of CXCR4 and EMT markers in response to LPS stimulation.
Figure 5.
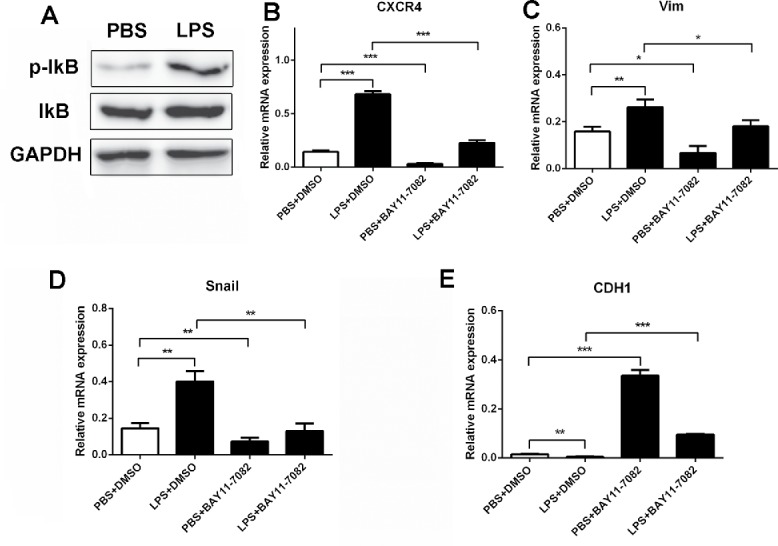
LPS induced CXCR4 expression and EMT through NF-κB pathway activation. (A)Western blot was used to detect the activation of IκB in PBS and LPS-disposed C26 cells, GAPDH was used as an internal reference. (B, C, D and E) CXCR4, Vimentin, Snail and Ecadherin expression were detected by RT-PCR. GAPDH was used as an internal reference. BAY11-7082: IκB inhibitor.
High CXCR4 expression correlated with tumor metastasis and poor prognosis
To understand the relationship between the CXCR4 expression and the characteristics of primary colorectal tumors, we compared the clinicopathologic features between the CXCR4 high expression group (n = 44) and the low expression group (n = 36) (Fig. 6A, Table 1). Table 1 shows the characteristics of patients and tumors. The levels of carbohydrate antigen 19-9 in the high expression group tended to be higher than those in the low expression group (P = 0.031). And the rate of lymph node metastasis and distant metastasis was significantly more frequent in the high expression group than that in the low expression group (P = 0.013, P = 0.024). Moreover, the overall survival rate in the high expression group was significantly poorer than that in the low expression group (Fig. 6B, P < 0.001). The data above demonstrated that CXCR4 expression was correlated with colorectal tumor metastasis and poor survival in clinical.
Figure 6.
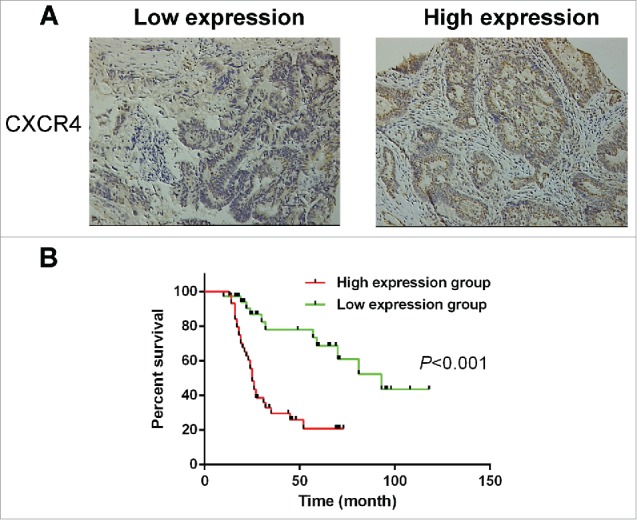
High CXCR4 expression correlated with tumor metastasis and poor prognosis. (A) The membranous expression of CXCR4 was seen to be low in 36/80 of CRC tissues (left), and high in 44/80 (right). Magnification: ×200. (B). Overall survival was analyzed in the same cohort of CRC patients and the results showed that CRC patients in the high CXCR4 expression group also have poorer overall survival than those in the low CXCR4 expression group (P < 0.001). CRC: Colorectal cancer.
Table 1.
Clinicopathologic features of the CXCR4 high expression and low expression groups for CRC patients with primary tumors.
| CXCR4 |
|||
|---|---|---|---|
| Clinicpathologic features | Low (n=36) | High (n=44) | p value |
| Age (years) | |||
| <60 | 19 | 25 | 0.718 |
| ≥60 | 17 | 19 | |
| Gender | |||
| Male | 20 | 24 | 0.928 |
| Female | 16 | 20 | |
| CEA (ng/ml) | |||
| ≤5ng/ml | 18 | 13 | 0.062 |
| >5ng/ml | 18 | 31 | |
| CA19-9 (U/ml) | |||
| ≤35U/ml | 25 | 20 | 0.031* |
| >35U/ml | 11 | 24 | |
| Location (colon/rectum) | 27/9 | 38/6 | 0.195 |
| Tumor size (cm) | |||
| <5cm | 21 | 31 | 0.258 |
| ≥5cm | 15 | 13 | |
| Lymph node metastasis (+/−) | 9/27 | 23/21 | 0.013* |
| Distance metastasis (+/−) | 13/23 | 27/17 | 0.024* |
CEA: carcinoembryonic antigen; CA 19-9: carbohydrate antigen 19-9;
*P < 0.05
Discussion
LPS are the major component of the outer membrane of gram-negative bacteria and are pivotal in increasing the metastatic potential of human CRC.29 In current work, we found that LPS promoted the migratory ability of CRC cells in vivo and in vitro, which through the activation of SDF-1α/CXCR4 axis and induction of EMT phenotype. And LPS upregulated CXCR4 expression and EMT occurrence through NF-κB signaling pathway activation. And clinically, high expression of CXCR4 was associated with the metastasis and poor prognosis of CRC patients.
Tumor metastasis is the main cause of cancer death, it is particularly urgent to understand the mechanism of organ-specific metastasis. Emerging evidences indicated that SDF-1α/CXCR4 axis plays an important role in the metastasis. In 2001, Muller et al. showed that CXCR4 was expressed more highly in breast cancer tissue than that in normal breast tissue, and SDF-1α was expressed in many organs, such as bone marrow, lymph nodes, and lungs, in which breast cancer metastases are often found.19 Cancer cells expressing high levels of CXCR4 are guided to organs expressing high SDF-1α, and this attraction between SDF-1α and CXCR4 is considered to be closely associated with organ-specific metastasis.24,30-33 In our work, we found that SDF-1α was upregulated in injured liver tissue induced by LPS, and in vitro, CXCR4 levels on the surface of C26 cells were significantly increased by LPS. In order to investigate the role of SDF-1α/CXCR4 in LPS-induced migration of C26 cells, a specific inhibitor of SDF-1α/CXCR4 axis, AMD3100, was used prior to LPS stimulation. The results showed that AMD3100 significantly diminished LPS-induced migration, which further indicated that SDF-1α/CXCR4 axis was involved in LPS-induced migration. Additionally, we analyzed the association of CXCR4 expression and clinical characteristics, we found that high CXCR4 expression was related to tumor metastasis and poor prognosis.
The occurrence of EMT is closely related to the local tumor microenvironment and tumor metastasis. EMT has been proposed as a key process in embryonic development and cancer progression, by which epithelial cells acquire mesenchymal, fibroblast-like phenotypes with reduced cell-to-cell adhesion, loss of cell polarity, with increased migration and invasiveness.34 A variety of factors in the tumor microenvironment can promote EMT, including IL-8, TGF-β and LPS.35 Previous study has shown that LPS could increase β1 integrin-mediated cell adhesion and liver metastasis, which was an important marker of EMT.13 In this study, we treated the colon cancer cells with LPS in vitro to observe the occurrence of EMT, with encouraging results. Stimulation by LPS caused E-cadherin (the epithelial marker) to disappear, Vimentin and Snail (the mesenchymal marker) to increase. Thus, C26 cells acquired a mesenchymal phenotype through EMT induced by LPS.
Previous research has indicated that the NF-κB signal transduction pathway might be involved in the process of EMT.36 The NF-κB signaling pathway has been shown to be involved in tumor cells migration and invasion.37 And, there have been studies which indicate that LPS can activate NF-κB pathway.36,38 In order to verify whether LPS affect the occurrence of EMT and CXCR4 expression via NF-κB in C26 cells, we used western blot to detect NF-κB activity. The results showed that LPS activated p-IκB. Blocking NF-κB pathway can inhibit LPS-induced EMT, and also decrease CXCR4 expression. These results indicated that NF-κB was involved in LPS-induced EMT and CXCR4 expression in C26 cells. And it suggested that CXCR4 may act as an EMT biomarker, which still need further studies.
In conclusion, our data has shown that LPS promoted the migration and invasion of colon cancer cells, which involved the activation of SDF-1/CXCR4 axis and EMT occurrence through NF-κB signaling pathway. And CXCR4 participates in malignant behaviors and may serve as a biomarker of metastasis in CRC, which will be a new therapeutic target for the metastasis of CRC.
Materials and methods
Tissue samples
Primary CRC and metastatic liver cancer tissue samples were obtained from 80 patients undergoing surgical resection of primary CRC and/or liver metastasis at the Department of Surgery, Changhai Hospital and Eastern Hepatobiliary Surgery Hospital of the Second Military Medical University from February 2007 to July 2010. After resection, patients were followed up every 3 months. Sections were reviewed by 2 experienced pathologists to verify the histologic assessment. All the specimens were adenocarcinoma. Prior informed consent was obtained and the study protocol was approved by the Ethics Committee of the Second Military Medical University.
Cell culture and treatments
The BALB/c mice colon cancer cell line, C26, was maintained in RPMI 1640 culture medium (GIBCO, Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; GIBCO, Invitrogen), 100 units/ml penicillin and 100 mg/ml streptomycin in a humidified incubator under 95% air and 5% CO2 at 37°C.
For experiments with LPS treatment, cells which were grown to 80% confluency were treated with 10 μg/ml LPS (Sigma, St. Louis, MO, USA) for 24 and 48 hours, respectively.
Animals and treatments
Male BALB/c mice, 6 to 8 weeks old, were purchased from the Shanghai Experimental Animal Center of the Chinese Academy of Sciences (Shanghai, China), which were housed under pathogen-free conditions. Mice received LPS at a 10mg/kg concentration to induce acute liver injury, and all procedures were performed in accordance with the institutional animal welfare guidelines of the Second Military Medical University.
Mouse splenic vein metastasis assay
Cells were injected into the splenic vein of BALB/c mice at 1 × 106 cells/injection site. The mice were sacrificed after 3 weeks and the numbers and size of surface liver metastases were recorded.
Immunohistochemistry analysis
The sample processing and IHC procedures were performed as described.39 Slides were independently reviewed by 2 observers to evaluate the staining pattern of the protein under the light microscope. Ten visual fields were randomly selected from each slide. The results were expressed as the percentage of tumor cells with a positive stain. Thereafter, the percentage of CXCR4-positive tumor cells was scored on a scale of 0 to 4 (0: no staining; 1: ≤10%; 2: 11% to 30%; 3: 31% to 50%; 4: ≥50%). Furthermore, the expression level of CXCR4 was divided into the following 2 groups according to score: low (0, 1, 2) and high (3, 4).
Real-time quantitative PCR
Total RNA was isolated using TRIZOL (Invitrogen, Carls-bad, CA, USA) and cDNA synthesis was performed using the PrimeScript RT reagent Kit (Takara, Kyoto, Japan) according to the manufacturer's instructions. The mRNA expression of CXCR4 and EMT markers (E-cadherin, Vimentin and Snail) were quantified by real-time quantitative PCR. Quantitative PCR was performed using SYBR Green PCR Kit (Applied BI) according to the manufacturer's instructions.
Western blotting analysis
Western blot was performed according to a previous study.40 Rabbit monoclonal anti-CXCR4, anti-Vimentin and anti-Ecadherin antibodies (1:1000; Abcam, Cambridge, UK); Rabbit monoclonal anti-SDF-1, anti-IkB, anti-pho-IkB antibody (1:1000; Cell Signaling Technology, Danvers, MA, USA), and a rabbit monoclonal anti-β-actin and anti-GAPDH antibody (1:1000; Bioworld Technology, St. Louis, MN, USA) were used. Western blots were repeated 3 times for each sample.
Migration assays
Cell motility was examined by 3 different methods: (1) Real-time migration assay41 (2) Wound-healing assay,42 and (3) Transwell assay.43 For real-time monitoring of cell migration, the xCELLigence system was used; 5 × 104 cells/well were seeded onto the top chamber of a CIM plate, which features microelectronic sensors integrated on the underside of the microporous membrane of a Boydenlike chamber. The medium with SDF-1α (100 ng/ml) was added in the bottom chamber. CIM plates were placed onto the Real-Time Cell Analyzer (RTCA) station (xCELLigence System, ACEA Biosciences Inc., San Diego, CA). Cell migration was continuously monitored by measuring changes in the electrical impedance at the electrode/cell interface, as a population of cells migrated from the top to the bottom chamber. Continuous values are represented as cell index (CI), a dimensionless parameter that reflects a relative change in measured electrical impedance. For the wound-healing assay, cells (1 × 105) were seeded in 6-well plates and incubated for 24h, monolayers were then scratched with a pipette tip. Cell migration was recorded by phase contrast microscopy at 24 hours after wound scratch. For the Transwell assay, Boyden chambers (8 μm pore size) were coated with 200 μl Matrigel at 200 μg/ml and incubated overnight. Cells (5 × 104) in medium with AMD3100 (1 μg/ml) were plated in the upper chamber, and the medium containing 5% FBS was added in the lower chamber as a chemoattractant. After 24 hours of incubation at 37°C, the cells were fixed in 4% formaldehyde and stained with crystal violet dye, and the cells that invaded through the pores to the lower surface of the filter were counted under a microscope. Three invasion chambers were used per condition. The values obtained were calculated by averaging the total number of cells from 3 filters.
Immunofluorescence staining
Cells (5 × 103) which had been pretreated by LPS (10 μg/ml) or PBS for 48 hours were seeded on a 48-well dish. After 24 hours, cells were washed with PBS, fixed in 4% paraformaldehyde for 1 hour and permeabilized with 0.1% Triton X-100 for 30 minutes at room temperature. After incubating with the blocking solution (1% bovine serum albumin in PBS), the cells were incubated with the primary antibodies at 4°C overnight and then washed with PBS and finally incubated with secondary antibodies at room temperature for 1 hour. Nuclei were counterstained with DAPI (4′6-diamidino-2-phenylindole) and the pictures were grapped by an immunofluorescence microscope with the identical exposure time.
Statistical analysis
All of the experiments were repeated at least 3 times. Analysis of variance was performed using GraphPad Prism 5.0 (GraphPad Software). Quantitative data was expressed as mean±SD for each experiment. Significance between groups was performed by a Student's test. And clinically, statistical analysis was performed using SPSS 20.0 for Windows (SPSS Inc.., Chicago, IL); differences between categorical variables were assessed by chisquare test or Fisher's exact test. Log-rank test was used to compare patients' survival between subgroups. P < 0.05 was considered statistically significant.
Abbreviations
- CRC
colorectal cancer
- FBS
fetal bovine serum
- EMT
epithelial-mesenchymal transition
- IHC
immunohistochemistry
- LPS
lipopolysaccharides
- NF-κB
nuclear factor-κB
- SDF-1α
stromal cell-derived factor 1α
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This project was supported by Key Basic Research Project of China (Grant NO. 2012CBA01303); National Natural Science Foundation of China (Grant NO. 81372312, 81372330, 81472737, 81402018, 81402020, 81401308, 81402026, 81572444, 81502417, 81502543); Special Funds for National key Sci-Tech Special Project of China (Grant NO. 2012ZX10002-016, 2012ZX10002011-011); Shanghai Science and Technology Committee (Grant NO. 14ZD1900403, 14ZR1409200, 15PJ1410600); Shanghai Municipal Education Commission (Grant NO. 14ZZ086).
References
- [1].Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69-90; PMID:21296855. [DOI] [PubMed] [Google Scholar]
- [2].Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006; 244:254-9; PMID:16858188; http://dx.doi.org/ 10.1097/01.sla.0000217629.94941.cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin 2008; 58:71-96; PMID:18287387. [DOI] [PubMed] [Google Scholar]
- [4].Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology 2009; 50:638-44; PMID:19575462; http://dx.doi.org/ 10.1002/hep.23009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rowland IR. The role of the gastrointestinal microbiota in colorectal cancer. Curr Pharm Des 2009; 15:1524-7; PMID:19442169; http://dx.doi.org/ 10.2174/138161209788168191 [DOI] [PubMed] [Google Scholar]
- [6].Proctor LM. The Human Microbiome Project in 2011 and beyond. Cell Host Micr 2011; 10:287-91; PMID:22018227; http://dx.doi.org/ 10.1016/j.chom.2011.10.001 [DOI] [PubMed] [Google Scholar]
- [7].Shah V, Kamath P. Portal hypertension and gastrointestinal bleeding In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran's gastrointestinal and liver disease, 9th ed. 2010. p. 1489-516. [Google Scholar]
- [8].McDonald B, Spicer J, Giannais B, Fallavollita L, Brodt P, Ferri LE. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Inter J Cancer 2009; 125:1298-305; PMID:19431213; http://dx.doi.org/ 10.1002/ijc.24409 [DOI] [PubMed] [Google Scholar]
- [9].Harmey JH, Bucana CD, Lu W, Byrne AM, McDonnell S, Lynch C, Bouchier-Hayes D, Dong Z. Lipopolysaccharide-induced metastatic growth is associated with increased angiogenesis, vascular permeability and tumor cell invasion. Inter J Cancer 2002; 101:415-22; PMID:12216068; http://dx.doi.org/ 10.1002/ijc.10632 [DOI] [PubMed] [Google Scholar]
- [10].Wang JH, Manning BJ, Wu QD, Blankson S, Bouchier-Hayes D, Redmond HP. Endotoxin/lipopolysaccharide activates NF-kappa B and enhances tumor cell adhesion and invasion through a beta 1 integrin-dependent mechanism. J Immun 2003; 170:795-804; http://dx.doi.org/ 10.4049/jimmunol.170.2.795 [DOI] [PubMed] [Google Scholar]
- [11].Liu X, Liang J, Li G. Lipopolysaccharide promotes adhesion and invasion of hepatoma cell lines HepG2 and HepG2.2.15. Mol Biol Rep 2010; 37:2235-9; PMID:19680784; http://dx.doi.org/ 10.1007/s11033-009-9710-4 [DOI] [PubMed] [Google Scholar]
- [12].Ikebe M, Kitaura Y, Nakamura M, Tanaka H, Yamasaki A, Nagai S, Wada J, Yanai K, Koga K, Sato N, et al.. Lipopolysaccharide (LPS) increases the invasive ability of pancreatic cancer cells through the TLR4/MyD88 signaling pathway. J Surg Oncol 2009; 100:725-31; PMID:19722233; http://dx.doi.org/ 10.1002/jso.21392 [DOI] [PubMed] [Google Scholar]
- [13].Hsu RY, Chan CH, Spicer JD, Rousseau MC, Giannias B, Rousseau S, Ferri LE. LPS-induced TLR4 signaling in human colorectal cancer cells increases beta1 integrin-mediated cell adhesion and liver metastasis. Cancer Res 2011; 71:1989-98; PMID:21363926; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-2833 [DOI] [PubMed] [Google Scholar]
- [14].Gul N, Grewal S, Bogels M, van der Bij GJ, Koppes MM, Oosterling SJ, Fluitsma DM, Hoeben KA, Beelen RH, van Egmond M. Macrophages mediate colon carcinoma cell adhesion in the rat liver after exposure to lipopolysaccharide. Oncoimmunology 2012; 1:1517-26; PMID:23264898; http://dx.doi.org/ 10.4161/onci.22303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sheu BC, Chang WC, Cheng CY, Lin HH, Chang DY, Huang SC. Cytokine regulation networks in the cancer microenvironment. Front Biosci 2008; 13:6255-68; PMID:18508658; http://dx.doi.org/ 10.2741/3152 [DOI] [PubMed] [Google Scholar]
- [16].Stetler-Stevenson W, Kleiner DJ. Molecular biology of cancer: invasion and metastasis In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Philadelphia: LWW. p. 123-36. [Google Scholar]
- [17].Murdoch C. CXCR4: chemokine receptor extraordinaire. Immun Rev 2000; 177:175-84; PMID:11138774; http://dx.doi.org/ 10.1034/j.1600-065X.2000.17715.x [DOI] [PubMed] [Google Scholar]
- [18].Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells 2005; 23:879-94; PMID:15888687; http://dx.doi.org/ 10.1634/stemcells.2004-0342 [DOI] [PubMed] [Google Scholar]
- [19].Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al.. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001; 410:50-6; PMID:11242036; http://dx.doi.org/ 10.1038/35065016 [DOI] [PubMed] [Google Scholar]
- [20].Saur D, Seidler B, Schneider G, Algul H, Beck R, Senekowitsch-Schmidtke R, Schwaiger M, Schmid RM. CXCR4 expression increases liver and lung metastasis in a mouse model of pancreatic cancer. Gastroenterology 2005; 129:1237-50; PMID:16230077; http://dx.doi.org/ 10.1053/j.gastro.2005.06.056 [DOI] [PubMed] [Google Scholar]
- [21].Belperio JA, Phillips RJ, Burdick MD, Lutz M, Keane M, Strieter R. The SDF-1/CXCL 12/CXCR4 biological axis in non-small cell lung cancer metastases. Chest 2004; 125:156S; PMID:15136483; http://dx.doi.org/ 10.1378/chest.125.5_suppl.156S [DOI] [PubMed] [Google Scholar]
- [22].Rubie C, Frick VO, Wagner M, Weber C, Kruse B, Kempf K, König J, Rau B, Schilling M. Chemokine expression in hepatocellular carcinoma versus colorectal liver metastases. World J Gastroenterol 2006; 12:6627-33; PMID:17075975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Matsusue R, Kubo H, Hisamori S, Okoshi K, Takagi H, Hida K, Nakano K, Itami A, Kawada K, Nagayama S, et al.. Hepatic stellate cells promote liver metastasis of colon cancer cells by the action of SDF-1/CXCR4 axis. Ann Surg Oncol 2009; 16:2645-53; PMID:19588204; http://dx.doi.org/ 10.1245/s10434-009-0599-x [DOI] [PubMed] [Google Scholar]
- [24].Zeelenberg IS, Ruuls-Van Stalle L, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res 2003; 63:3833-9; PMID:12839981. [PubMed] [Google Scholar]
- [25].Kim J, Takeuchi H, Lam ST, Turner RR, Wang HJ, Kuo C, Foshag L, Bilchik AJ, Hoon DS, et al.. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol 2005; 23:2744-53; PMID:15837989; http://dx.doi.org/ 10.1200/JCO.2005.07.078 [DOI] [PubMed] [Google Scholar]
- [26].Schimanski CC, Schwald S, Simiantonaki N, Jayasinghe C, Gonner U, Wilsberg V, Junginger T, Berger MR, Galle PR, Moehler M. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res 2005; 11:1743-50; PMID:15755995; http://dx.doi.org/ 10.1158/1078-0432.CCR-04-1195 [DOI] [PubMed] [Google Scholar]
- [27].Phillips RJ, Burdick MD, Lutz M, Belperio JA, Keane MP, Strieter RM. The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. Am J Respirat Critic Care Med 2003; 167:1676-86; PMID:12626353; http://dx.doi.org/ 10.1164/rccm.200301-071OC [DOI] [PubMed] [Google Scholar]
- [28].Wang B, Wang W, Niu W, Liu E, Liu X, Wang J, Peng C, Liu S, Xu L, Wang L, et al.. SDF-1/CXCR4 axis promotes directional migration of colorectal cancer cells through upregulation of integrin alphavbeta6. Carcinogenesis 2014; 35:282-91; PMID:24085800; http://dx.doi.org/ 10.1093/carcin/bgt331 [DOI] [PubMed] [Google Scholar]
- [29].Simiantonaki N, Kurzik-Dumke U, Karyofylli G, Jayasinghe C, Michel-Schmidt R, Kirkpatrick CJ. Reduced expression of TLR4 is associated with the metastatic status of human colorectal cancer. Inter J Mol Med 2007; 20:21-9; PMID:17549384. [PubMed] [Google Scholar]
- [30].Yoshitake N, Fukui H, Yamagishi H, Sekikawa A, Fujii S, Tomita S, Ichikawa K, Imura J, Hiraishi H, Fujimori T. Expression of SDF-1 alpha and nuclear CXCR4 predicts lymph node metastasis in colorectal cancer. Br J Cancer 2008; 98:1682-9; PMID:18443596; http://dx.doi.org/ 10.1038/sj.bjc.6604363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sun YX, Schneider A, Jung Y, Wang J, Dai J, Wang J, Cook K, Osman NI, Koh-Paige AJ, Shim H, et al.. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res 2005; 20:318-29; PMID:15647826; http://dx.doi.org/ 10.1359/JBMR.041109 [DOI] [PubMed] [Google Scholar]
- [32].Zhao FL, Guo W. Expression of stromal derived factor-1 (SDF-1) and chemokine receptor (CXCR4) in bone metastasis of renal carcinoma. Mol Biol Rep 2011; 38:1039-45; PMID:20563655; http://dx.doi.org/ 10.1007/s11033-010-0200-5 [DOI] [PubMed] [Google Scholar]
- [33].Iwasa S, Yanagawa T, Fan J, Katoh R. Expression of CXCR4 and its ligand SDF-1 in intestinal-type gastric cancer is associated with lymph node and liver metastasis. Anticancer Res 2009; 29:4751-8; PMID:20032431 [PubMed] [Google Scholar]
- [34].Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 2006; 7:131-42; PMID:16493418; http://dx.doi.org/ 10.1038/nrm1835 [DOI] [PubMed] [Google Scholar]
- [35].Li H, Li Y, Liu D, Liu J. LPS promotes epithelial-mesenchymal transition and activation of TLR4/JNK signaling. Tumour Biol 2014; 35:10429-35; PMID:25053598; http://dx.doi.org/ 10.1007/s13277-014-2347-5 [DOI] [PubMed] [Google Scholar]
- [36].Jing YY, Han ZP, Sun K, Zhang SS, Hou J, Liu Y, Li R, Gao L, Zhao X, Zhao QD, et al.. Toll-like receptor 4 signaling promotes epithelial-mesenchymal transition in human hepatocellular carcinoma induced by lipopolysaccharide. BMC Med 2012; 10:98; PMID:22938142; http://dx.doi.org/ 10.1186/1741-7015-10-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Oh JH, Kim JH, Ahn HJ, Yoon JH, Yoo SC, Choi DS, Lee IS, Ryu HS, Min CK. Syndecan-1 enhances the endometrial cancer invasion by modulating matrix metalloproteinase-9 expression through nuclear factor kappaB. Gynecol Oncol 2009; 114:509-15; PMID:19539355; http://dx.doi.org/ 10.1016/j.ygyno.2009.05.027 [DOI] [PubMed] [Google Scholar]
- [38].He W, Qu T, Yu Q, Wang Z, Lv H, Zhang J, Zhao X, Wang P. LPS induces IL-8 expression through TLR4, MyD88, NF-kappaB and MAPK pathways in human dental pulp stem cells. Int Endod J 2013; 46:128-36; PMID:22788664; http://dx.doi.org/ 10.1111/j.1365-2591.2012.02096.x [DOI] [PubMed] [Google Scholar]
- [39].Hu D, Du C, Xue W, Dou F, Yao Y, Gu J. The expression of chemokine receptors CCR6, CXCR2 and CXCR4 is not organ-specific for distant metastasis in colorectal cancer: a comparative study. Histopathology 2013; 63:167-73; PMID:23758411; http://dx.doi.org/ 10.1111/his.12127 [DOI] [PubMed] [Google Scholar]
- [40].Liu WT, Jing YY, Yu GF, Han ZP, Yu DD, Fan QM, Ye F, Li R, Gao L, Zhao QD, et al.. Toll like receptor 4 facilitates invasion and migration as a cancer stem cell marker in hepatocellular carcinoma. Cancer lett 2015; 358:136-43; PMID:25511737; http://dx.doi.org/ 10.1016/j.canlet.2014.12.019 [DOI] [PubMed] [Google Scholar]
- [41].Bertran E, Crosas-Molist E, Sancho P, Caja L, Lopez-Luque J, Navarro E, Egea G, Lastra R, Serrano T, Ramos E, et al.. Overactivation of the TGF-beta pathway confers a mesenchymal-like phenotype and CXCR4-dependent migratory properties to liver tumor cells. Hepatology 2013; 58:2032-44; PMID:23813475; http://dx.doi.org/ 10.1002/hep.26597 [DOI] [PubMed] [Google Scholar]
- [42].Bertran E, Caja L, Navarro E, Sancho P, Mainez J, Murillo MM, Vinyals A, Fabra A, Fabregat I. Role of CXCR4/SDF-1 alpha in the migratory phenotype of hepatoma cells that have undergone epithelial-mesenchymal transition in response to the transforming growth factor-beta. Cell Signal 2009; 21:1595-606; PMID:19586611; http://dx.doi.org/ 10.1016/j.cellsig.2009.06.006 [DOI] [PubMed] [Google Scholar]
- [43].Yang MH, Chang SY, Chiou SH, Liu CJ, Chi CW, Chen PM, Teng SC, Wu KJ. Overexpression of NBS1 induces epithelial-mesenchymal transition and co-expression of NBS1 and Snail predicts metastasis of head and neck cancer. Oncogene 2007; 26:1459-67; PMID:16936774; http://dx.doi.org/ 10.1038/sj.onc.1209929 [DOI] [PubMed] [Google Scholar]


