Abstract
The aim of this study was to investigate in vitro and model the effect of temperature (T) and water activity (aw) conditions on growth and toxin production by some toxigenic fungi signaled in cheese. Aspergillus versicolor, Penicillium camemberti, P. citrinum, P. crustosum, P. nalgiovense, P. nordicum, P. roqueforti, P. verrucosum were considered they were grown under different T (0–40 °C) and aw (0.78–0.99) regimes. The highest relative growth occurred around 25 °C; all the fungi were very susceptible to aw and 0.99 was optimal for almost all species (except for A. versicolor, awopt = 0.96). The highest toxin production occurred between 15 and 25 °C and 0.96–0.99 aw. Therefore, during grana cheese ripening, managed between 15 and 22 °C, ochratoxin A (OTA), penitrem A (PA), roquefortine-C (ROQ-C) and mycophenolic acid (MPA) are apparently at the highest production risk. Bete and logistic function described fungal growth under different T and aw regimes well, respectively. Bete function described also STC, PA, ROQ-C and OTA production as well as function of T. These models would be very useful as starting point to develop a mechanistic model to predict fungal growth and toxin production during cheese ripening and to help advising the most proper setting of environmental factors to minimize the contamination risk.
Keywords: Aspergillus, Penicillium, mycotoxin, ochratoxin, sterigmatocystin, roquefortine
1. Introduction
Several microorganisms contribute to the features of the final product during cheese-making [1]. The starter microbiota, usually artificially inoculated, is mainly composed of lactic acid bacteria such as Lactococcus lactis [2], which starts the cheese-making process by producing lactic acid and allowing the syneresis of the curd. Moreover, the starter culture degrades the proteins and may produce CO2 in some processes [3]. The secondary microbiota, mainly coming from the environment, or added as for blue cheese [4], becomes dominant after changes in the substrate, i.e., loss of water from the curd, increased salt and pH [5]. Several types of organisms, like salt-tolerant bacteria, yeasts and filamentous fungi [6,7,8] contribute to create the sensorial and nutritional characteristics of the final product with their proteolytic and lipolytic activities [4,7]. Some fungi, like Penicillium camemberti and P. roqueforti, are well known as ripening agents in appreciated cheeses [9,10,11]. Unfortunately, other fungi, e.g., P. nordicum, can act as spoiling agents and/or mycotoxin producers on products of animal origin [12,13,14], cheese included (Table 1).
Table 1.
Spoiling agents reported in cheese, mycotoxin produced and their toxic effect.
| Fungi | Mycotoxin | Toxic Effect | Reported By |
|---|---|---|---|
| Aspergillus spp. | Sterigmatocystin (STC) | Carcinogenic, mutagenic [28] | [29,30,31,32] |
| P. brevicompactum | Mycophenolic acid (MPA) | Mutagenic, possible acute toxicity [33] | [34] |
| P. camemberti | Cyclopiazonic acid | Neurotoxic, possible acute toxicity [28] | [19,35] |
| P. citrinum | Citrinin (CIT) | Nephrotoxic, teratogenic [36,37] | [18,38] |
| P. crustosum | Penitrem A (PA) | Neurotoxic [28] | [21] |
| P. expansum | Patulin | Carcinogenic, mutagenic, teratogenic, harmful to liver, possible acute toxicity [28] | [39,40] |
| P. roqueforti, P. crustosum | Roquefortine C (ROQ-C) | Neurotoxic [28] | [40,41] |
| P. roqueforti | PR Toxin | Mutagenic, Carcinogenic [42] | [39,43] |
| P. verrucosum, P. nordicum | Ochratoxin A (OTA) | Nephrotoxic, carcinogenic, hepatotoxic [28] | [44] |
Fungal activity is modulated by abiotic and biotic factors [15]; however, knowledge of the ecological needs of fungal mycoflora associated with cheese is poor [16]. Some studies have considered the role of temperature (T) on mycotoxin production by cheese-related molds, but generally only a few temperatures and few mycotoxins were considered [17]. Some data are available, regarding citrinin (CIT; [18]), cyclopiazonic acid (CPA; [19,20]), Penitrem A (PA; [21,22]), PR-Toxin [17,23,24,25] and roquefortine C (ROQ-C; [20,23,24]) under defined T regimes. The role of water activity (aw) and pH has been poorly studied [16]; ochratoxin A (OTA) production by P. verrucosum on YES medium under different pH and aw regimes, reported by Schmidt-Heydt, et al. [26], is the only research available.
Therefore, the aim of this study was to investigate and model the ecological needs of some fungi frequently reported as cheese contaminants [27] or commonly used as cheese ripening agents, focusing both on growth and mycotoxin production under different T and aw regimes. Penicillium camemberti, P. citrinum, P. crustosum, P. nalgiovense, P. nordicum, P. roqueforti, P. verrucosum and Aspergillus versicolor were considered. In a preliminary study, aimed at describing the fungal population associated with cheese during ripening, most of these species were found on the rind of grana type cheeses, sampled during the long aging period.
This is a preliminary study and will contribute to the development of a mechanistic model for the prediction of mycotoxin contamination in ripening cheese.
2. Results
2.1. Role of Temperature, Water Activity and Incubation Time on Fungal Growth
2.1.1. Temperature
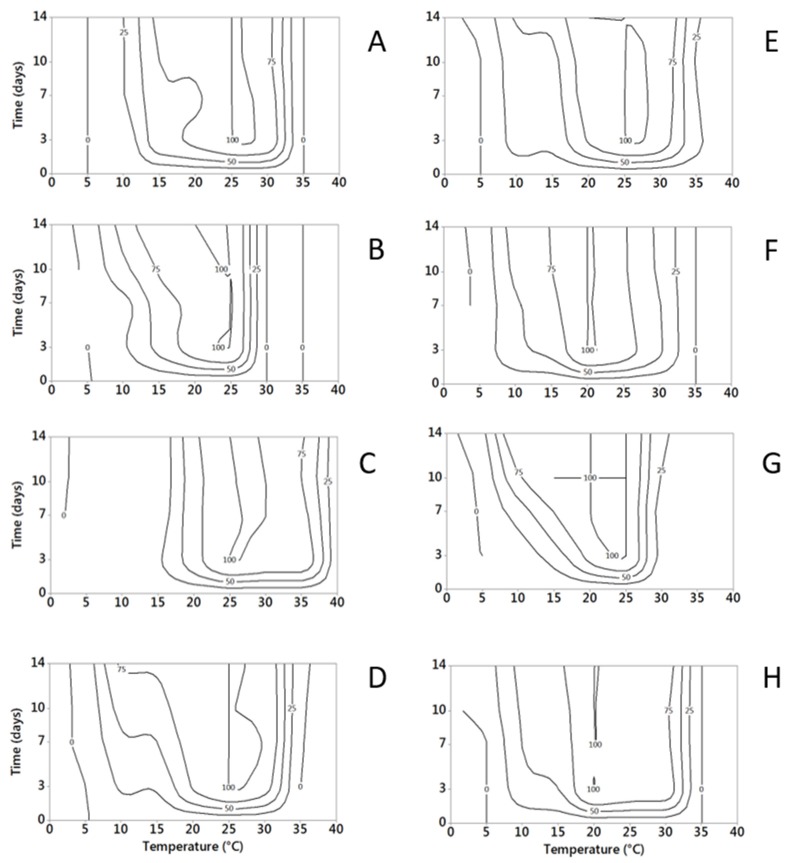
The effect of T on fungal growth, at different incubation times (0–14 days), is shown in Figure 1 using surface response curves of relative growth; maximum diameter of fungal colony after 1 day incubation is also reported in Table 2.
Figure 1.
Surface response curves of fungal relative growths (expressed as percentage on the maximum growth, numbers on the isoplethes) at different incubation times (3, 7, 10, 14 days) under different T regimes (0–40 °C, step 5 °C; aw = 0.99). (A) A. versicolor; (B) P. camemberti; (C) P. citrinum; (D) P. crustosum; (E) P. nalgiovense; (F) P. nordicum; (G) P. roqueforti; (H) P. verrucosum.
Table 2.
Fungal maximum colony diameter (cm) reached after 10 days of incubation at the reported temperature or water activity (aw), in Experiment 1 and 2, respectively.
| Species | Experiment 1. Temperature * | Experiment 2. Water Activity ** | ||
|---|---|---|---|---|
| Diameter Max (cm) | Temperature (°C) | Diameter Max (cm) | aw | |
| A. versicolor | 3.1 | 25 | 3.1 | 0.96 |
| P. camemberti | 4.3 | 25 | 4.0 | 0.99 |
| P. citrinum | 4.2 | 30 | 3.1 | 0.99 |
| P. crustosum | 5.1 | 25 | 5.0 | 0.99 |
| P. nalgiovense | 4.8 | 25 | 4.1 | 0.99 |
| P. nordicum | 3.7 | 20 | 3.3 | 0.99 |
| P. roqueforti | 5.5 | 25 | 5.5 | 0.99 |
| P. verrucosum | 4.0 | 20 | 4.0 | 0.99 |
* In experiment 1.Temperature ranged between 0 °C and 40 °C, 5 ± 1 °C step and media aw = 0.99; ** In experiment 2. Water activity ranged between 0.87 and 0.99, step 0.03 aw and T = 20 ± 1 °C
Fungal growth occurred from 5 to 30 °C for P. nordicum and P. verrucosum, from 5 to 35 °C for P. citrinum, P. crustosum, P. nalgiovense and P. roqueforti, from 10 to 30 °C and from 5 to 25 °C, for A. versicolor and P. camemberti, respectively. The highest relative growth occurred with T = 25 °C for the majority of fungi, with the exceptions of P. citrinum (T = 30 °C), P. verrucosum and P. nordicum (T = 20 °C). In Table 2, maximum colony growth values after 10-day of incubation are reported for all the selected fungi. This incubation time was considered because one of the fungi (P. roqueforti) reached the maximum possible diameter of 5.5 cm.
2.1.2. Water Activity
Fungal growth increased with the rise of aw, within the range considered (0.87–0.99 aw), as a general trend for all the species (Figure 2); A. versicolor and P. roqueforti grew from 0.87 aw, while aw = 0.90 was the minimum for all the others except P. camemberti and P. crustosum. Maximum growth was observed at 0.99 aw for all the species except A. versicolor, with 0.96 aw as optimal. At the optimal aw, all the fungal colonies reached maximum diameter between 7 and 14 days; the maximum colony diameters after 10 days of incubation is reported in Table 2.
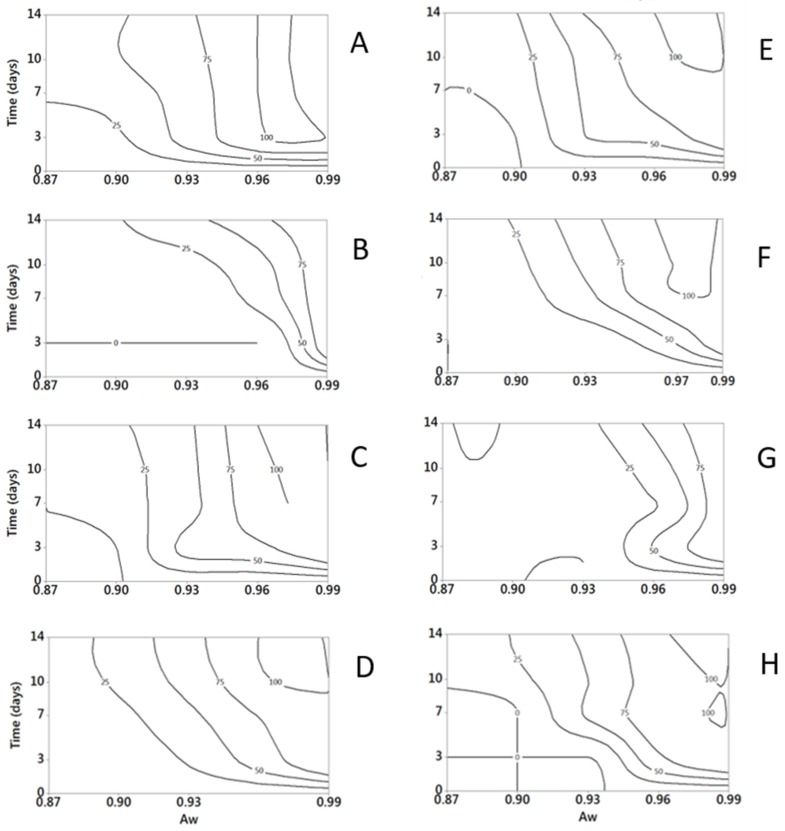
Figure 2.
Surface response curves of fungal relative growth (expressed as percentage on the maximum growth, numbers on the isoplethes) at different incubation times (3, 7, 10, 14 days) under different aw regimes (0.87–0.99; step 0.03; T = 20 °C). (A) A. versicolor; (B) P. camemberti; (C) P. citrinum; (D) P. crustosum; (E) P. nalgiovense; (F) P. nordicum; (G) P. roqueforti; (H) P. verrucosum.
Incubation times of up to 56 day, applied for aw = 0.87 and aw = 0.90, showed P. camemberti, P. crustosum and P. nordicum growth only at aw = 0.90, while a relevant growth was observed for all the other species at both aw considered.
2.2. Modeling the Influence of Temperature and Water Activity on Mycelial Growth
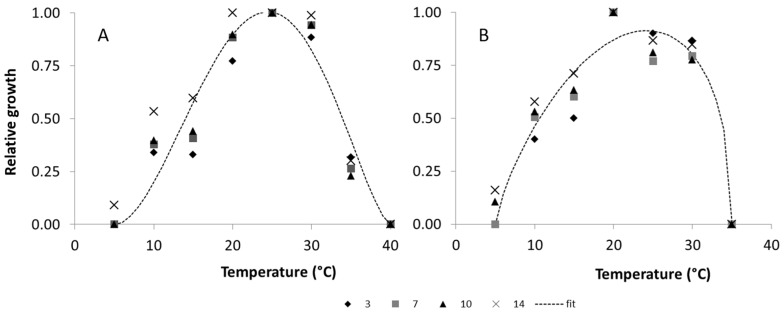
Fungal relative growth for each fungus, in different T regimes, showed a comparable trend at each incubation time (examples in Figure 3). Therefore, 36 mean relative growth values were used for T model fitting (9 T regimes and 4 incubation times, mean of 3 replicates). Regarding aw, fungal growth decreased under sub-optimal values and 3 day incubation time was not included in data analysis. Instead of 32 mean relative growth values for aw (8 aw regimes and 4 incubation time, mean of 3 replicates), were therefore used 15 mean values (5 aw regimes and 3 incubation time, mean of 3 replicates) for aw model fitting.
Figure 3.
Dynamic of relative growth of (A) P. nalgiovense and (B) P. verrucosum, after 3, 7, 10 and 14 days of incubation, at different temperature regimes (0–40 °C). Data were fitted (dotted line) by a Bete function (see Table 3 for equation parameters).
2.2.1. Temperature
The best fitting of fungal growth data as function of T was obtained, for all fungi considered, by the Bete equation [45] in the form:
| (1) |
where y is the relative growth of the colonies, a, b and c are the equations parameters and Teq is the equivalent T calculated as follows:
| (2) |
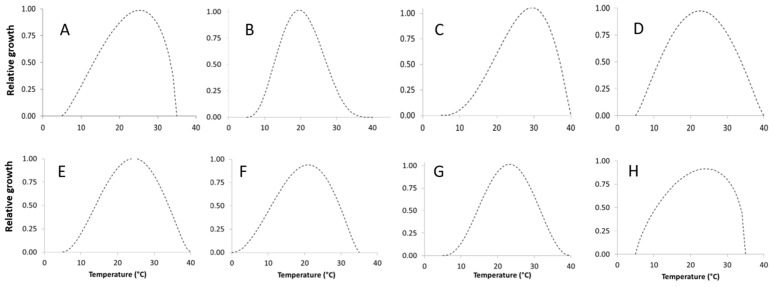
where T is the temperature regime and Tmin and Tmax are minimum and maximum temperature, respectively, at which the fungus is able to grow. Parameters, Tmin/Tmax and R2 values are reported in Table 3. The goodness of fit, measured with R2, was always good, ranging from 0.798 to 0.96. Fungal growth increased from 5 °C (0 °C for P. nordicum) to 20 °C (P. camemberti, and P. verrucosum) or 25 °C (A. versicolor, P. crustosum, P. citrinum, P. nalgiovense, P. roqueforti), and then quickly decreased (Figure 4). P. camemberti and P. nordicum showed a very similar behavior, with a fast relative growth up to 25 °C, followed by a rapid decrease (Figure 4).
Table 3.
Parameters of the equations developed to calculate relative growth and mycotoxin production for the selected fungi. The Bete and the logistic equations were used to describe fungal growth respectively as function of temperature (T) and water activity (aw). The Bete equation was also used to describe relative mycotoxin production as function of T.
| Fungi | Variable | Tmin/Tmax | Estimated Parameters (Standard Error) | |||
|---|---|---|---|---|---|---|
| a | b | c | R2 | |||
| Relative Growth | ||||||
| A. versicolor | T | 5/35 | 6.85 (0.353) * | 2.09 (0.122) | 0.63 (0.122) | 0.964 |
| aw | −32.719 (3.478) | −36.351 (3.958) | 0.953 (0.039) | 0.956 | ||
| P. camemberti | T | 5/40 | 3.22 (0.150) | 0.72 (0.049) | 4.10 (0.779) | 0.823 |
| aw | 29.12 (156.829) | −24.07 (11.615) | 198.77 (32,649.12) | 0.925 | ||
| P. citinum | T | 5/40 | 8.03 (0.387) | 2.33 (0.114) | 1.12 (0.132) | 0.961 |
| aw | 55.72 (8.565) | −59.74 (9.294) | 1.04 (0.053) | 0.966 | ||
| P. crustosum | T | 5/40 | 3.95 (0.379) | 1.01 (0.115) | 1.24 (0.274) | 0.798 |
| aw | 32.69 (8.161) | −34.57 (9.079) | 1.22 (0.233) | 0.927 | ||
| P. nalgiovense | T | 5/40 | 4.70 (0.245) | 1.25 (0.074) | 1.56 (0.209) | 0.899 |
| aw | 59.29 (9.254) | −64.24 (10.1) | 1.01 (0.044) | 0.970 | ||
| P. nordicum | T | 0/35 | 5.15 (0.200) | 1.50 (0.063) | 1.41 (0.14) | 0.900 |
| aw | 32.72 (3.478) | −36.35 (3.958) | 0.94 (0.039) | 0.956 | ||
| P. roqueforti | T | 5/40 | 4.26 (0.262) | 1.08 (0.083) | 2.45 (0.498) | 0.902 |
| aw | 73.08 (44.606) | −75.11 (47.168) | 1.28 (0.601) | 0.967 | ||
| P. verrucosum | T | 5/35 | 5.01 (0.573) | 1.80 (0.250) | 0.44 (0.043) | 0.936 |
| aw | 58.61 (8.441) | −63.15 (9.176) | 1.03 (0.043) | 0.979 | ||
| Relative Mycotoxin Production | ||||||
| A. versicolor STC | T | 5/35 | 6.31 (1.842) | 1.95 (0.659) | 0.57 (0.386) | 0.727 |
| P. crustosum PA | T | 5/35 | 4.78 (0.457) | 1.31 (0.145) | 2.19 (0.607) | 0.942 |
| P. crustosum ROQ-C | T | 5/35 | 5.14 (0.330) | 1.43 (0.103) | 1.53 (0.258) | 0.981 |
| P. nordicum OTA | T | 5/35 | 3.65 (0.138) | 0.87 (0.042) | 1.79 (0.185) | 0.991 |
| P. roqueforti ROQ-C | T | 5/35 | 5.19 (0.440) | 1.48 (0.139) | 0.79 (0.161) | 0.986 |
| P. verrucosum OTA | T | 5/35 | 6.30 (0.224) | 1.72 (0.066) | 0.87 (0.074) | 0.998 |
* Standard error of parameters were reported in parenthesis.
Figure 4.
Dynamic of relative growth of the studied fungi, at different temperature regimes (0–40 °C). Data were fitted by a Bete function (see Table 3 for equation parameters). (A) A. versicolor; (B) P. camemberti; (C) P. citrinum; (D) P. crustosum; (E) P. nalgiovense; (F) P. nordicum; (G) P. roqueforti; (H) P. verrucosum.
2.2.2. Water Activity
The influence of different aw regimes on fungal growth was well described using a Logistic equation, in the following form:
| (3) |
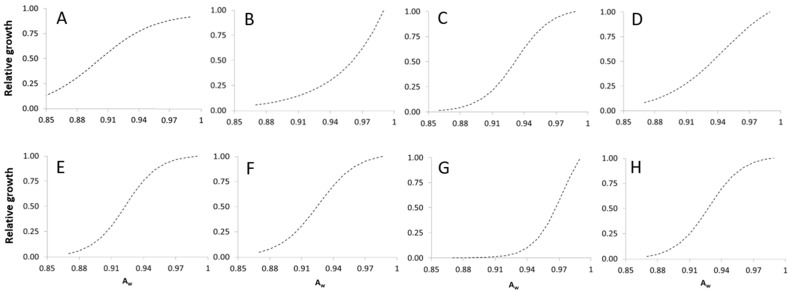
in which y is the fungal relative growth, a, b, and c are equation parameters, reported in Table 3. All fungi showed an S-shaped growth, except P. camemberti and P. roqueforti, with a J-shaped trend [46], without the upper plateau (Figure 5). The functions developed showed a very good fitting to growth data with R2 ranging between 0.925 and 0.979.
Figure 5.
Logistic equations (lines, refer to Table 3 for equation parameters) defining the dynamics of fungal growth at different aw regimes (0.78–0.99). (A) P. versicolor; (B) P. camemberti; (C) P. citrinum; (D) P. crustosum; (E) P. nalgiovense; (F) P. nordicum; (G) P. roqueforti; (H) P. verrucosum. (for Figure 5A suitable aw start from 0.78 but the same range of other fungi was used).
2.3. Modeling the Combined Effect of Temperature and Water Activity on Mycelial Growth
The combined effect of T and aw was also considered, merging the functions previously developed, as follows:
| (4) |
in which y is the relative growth, computed referring to the maximum growth observed.
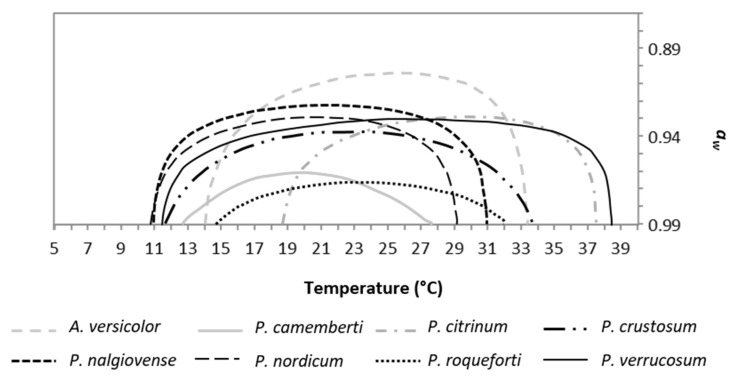
The output was plotted in a single chart (Figure 6), where the curves represent the combination of conditions that allow to reach 50% relative growth, for each fungus, as function of T and aw.
Figure 6.
Boundaries, derived from Equation (4), summarizing the combination of T and aw conditions to reach relative growth =0.5 for each fungus considered in the study.
Focusing attention on T values, the number of fungal species able to grow increased from 10 °C up to 20 °C, then decreased starting from 27 °C. P. nordicum started growth from 10 °C, immediately followed by P. nalgiovense, P. verrucosum, P. crustosum, P. camemberti, A. versicolor, P. roqueforti (from 14 °C), and P. citrinum (from 19 °C). All fungi were able to grow between 20 and 27 °C. Then, moving to higher temperatures, P. camemberti was the first to reduce the relative growth below 50% (T limit around 27 °C), followed by P. nordicum (29 °C), P. nalgiovense (31 °C), P. roqueforti (32 °C), A. versicolor and P. crustosum (34 °C), P. citrinum and P. verrucosum (37–39 °C).
Considering aw, the number of species with growth >50% gradually decreased as aw values moved from 0.99 to 0.87. P. roqueforti showed a relative growth below 50% at 0.97 aw, followed by P. camemberti and P. crustosum (0.96 and 0.94 aw respectively), P. citrinum, P. nalgiovense, P. nordicum, and P. verrucosum (around 0.93 aw) and A. versicolor (0.90 aw).
2.4. Influence of Temperature and Water Activity on Mycotoxin Production
All the mycotoxins investigated were detected (µg/L > LOD) and reported in ng/mm2 (Table 4), except CPA, PR and CIT in P. citrinum, P. roqueforti and P. verrucosum, respectively. Significant differences in the produced amount of toxins, depending on the fungus and ecological conditions tested, were noticed, except for CIT produced by P. camemberti. In particular, the optimum temperature for mycotoxin production was commonly between 20 and 25 °C, and the optimum aw was 0.99, except for MPA optimally produced by P. roqueforti at the combination 20 °C and 0.96 aw. No mycotoxin was detected in fungal colonies grown with aw < 0.93 after the 14-day incubation.
Table 4.
Mycotoxin production under different regimes of temperature (T) and water activity (aw) after 14 days of incubation. Data are reported as mean ng of toxin produced per mm2 of fungal colony area (ng/mm2; three replicates).
| A. versicolor | P. camemberti | P. citrinum | P. crustosum | P. nordicum | P. roqueforti | P. verrucosum | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | aw | STC | CIT | CPA | CIT | PA | ROQ | OTA | MPA | ROQ | PR | CIT | OTA | |||||||||
| Temperature | ||||||||||||||||||||||
| 5 | 0.99 | <LOD # | a † | 1.1 | <LOD | a | 0.9 | a | 7.4 | b | 6.7 | a | <LOD | a | <LOD | a | <LOD | a | <LOD | <LOD | <LOD | a |
| 10 | 0.99 | 7.4 | bc | 1.0 | 0.5 | a | 66.8 | b | 31.7 | c | 237.4 | b | 0.2 | a | 0.9 | c | 112.3 | b | <LOD | <LOD | <LOD | a |
| 15 | 0.99 | 16.9 | c | 0.7 | 0.4 | a | 63.8 | b | 150.9 | e | 630.8 | bc | 4.0 | b | 1.7 | d | 246.9 | c | <LOD | <LOD | 0.8 | b |
| 20 * | 0.99 | 81 | d | 3.8 | 7.6 | b | 1187.8 | c | 152.7 | e | 679.5 | c | 9.0 | b | 0.4 | b | 324.9 | d | <LOD | <LOD | 4.6 | d |
| 25 | 0.99 | 91.4 | d | 0.5 | 20.3 | c | 1147.5 | c | 197.2 | e | 824.3 | c | 3.7 | b | <LOD | a | 282.0 | d | <LOD | <LOD | 12.6 | e |
| 30 | 0.99 | 284 | e | <LOD | <LOD | a | 1728.9 | c | 81.8 | d | 615.6 | bc | 0.1 | a | <LOD | a | 248.0 | c | <LOD | <LOD | 2.5 | c |
| 35 | 0.99 | 6.6 | b | <LOD | <LOD | a | 2918.1 | c | <LOD | a | <LOD | a | <LOD | a | <LOD | a | 0.1 | a | <LOD | <LOD | <LOD | a |
| Water activity | ||||||||||||||||||||||
| 20 | 0.93 | <LOD | <LOD | <LOD | 733 | 25. 6 | 149.0 | <LOD | <LOD | 68.2 | <LOD | <LOD | <LOD | |||||||||
| 20 | 0.96 | 4.4 | 1.0 | 0.3 | 100.2 | 59.6 | 644.9 | 0.8 | 21.1 | 335.5 | <LOD | <LOD | <LOD | |||||||||
* data collected at 20 °C and 0.99 aw are common for the trial at different T and different aw regimes. # LOD is specific for each mycotoxin. See materials and methods for details. † Different letters define significant difference according to Tukey test (p ≤ 0.01).
2.5. Modeling the Effect of Temperature and Water Activity on Toxin Production
2.5.1. Temperature
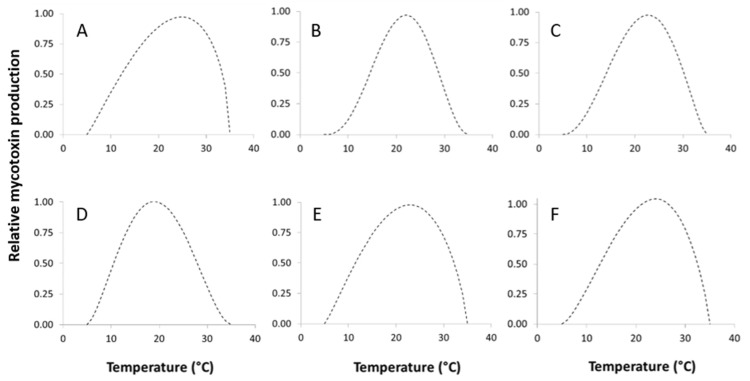
The best fitting of toxin production data as function of T was obtained by the Bete equation (Equation (1); [45]). Good results are reported for STC produced by A. versicolor, OTA produced by P. nordicum and P. verrucosum, PA produced by P. crustosum and ROQ-C produced by P. crustosum and P. roqueforti (Table 3, Figure 7), with R2 ≥ 0.94 for all fungi except A. versicolor (R2 = 0.727).
Figure 7.
Dynamic of mycotoxins production rate for: (A) STC—A. versicolor; (B) PA—P. crustosum; (C) ROQ-C—P. crustosum; (D) OTA—P. nordicum; (E) ROQ-C—P. roqueforti; (F) OTA—P. verrucosum, at different temperature regimes (5–35 °C). Data were fitted by a Beta function (see Table 3 for details).
Regarding the other fungi/toxins, toxin production was possible only for a few temperature regimes (i.e., ROQ-C and MPA produced by P. roqueforti). Therefore, no data modeling was performed.
2.5.2. Water Activity
The range of aw that allowed toxin production was limited to 0.93–0.99, with only 3 points available. Therefore, no modeling was applied to this dataset.
3. Discussion
The ripening of hard cheese is carried out commonly in a temperature range from 10 to 20 °C, as defined in the guidelines of many products Protected for Denomination of Origin, e.g., Fontina, Fiore Sardo and Emmentaler. For Italian grana type cheeses, the environmental conditions during aging are between 15 °C and 22 °C, as reported in the “Parmigiano Reggiano” and “Grana Padano” cheese production guidelines (www.politicheagricole.it). Grana cheeses are long—ripened high quality products; according to preliminary unpublished data, starting from 9 month storage, the aw varies between 0.92 and 0.85 in the crust and between 0.94 and 0.87 if a wider layer is considered, while the relative humidity of storehouses ranges between 72% and 88%.
Most of the fungi studied grew optimally around 25 °C, but for P. citrinum the relative growth was higher at 30 °C, and for P. verrucosum and P. nordicum at 20 °C. The susceptibility to aw regimes was considerable; aw = 0.99 was optimal for all species except the more xerophilic A. versicolor, which grew best with aw = 0.96. A. versicolor, P citrinum, P. nalgiovense, P. roqueforti and P. verrucosum grew down to 0.87 aw; aw = 0.90 was the limit for P. nordicum and P. camemberti and aw = 0.94 for P. crustosum, even at the longest incubation times considered in this study (56 days).
Therefore, all fungi studied can grow in the range of T common for the storage of hard, long maturing grana type cheeses. Recently Marin, et al. [47] reported Penicillium spp. as the dominant species on hard cheese; Penicillium and Aspergillus spp. were highly tolerant to water restriction, making them more competitive with other fungi during cheese ripening. This is confirmed in this study, where the lower aw limit for growth was 0.87 for several Penicillium species and for A. versicolor.
Fungal growth in artificial media with modified aw, as managed in this study, possibly suffered from the high amount of salt supplemented in the lowest aw regimes. It is well know that compounds added to modify aw (glycerol or NaCl), interfere with fungal metabolism, the former enhancing fungal growth, being a carbon source, and the latter becoming toxic for fungi at high dosages [47,48,49]. Toxicity more than aw could have limited the fungal activity of some Penicillium species; therefore, further trials on cheese will be required to define cardinal aw regimes.
No toxin production is reported in literature for P. nalgiovense; this fungus was not considered for mycotoxin analysis, but only to check its potential competition with other fungi. It was noticed that it was more xerophilic than other Penicillia studied and showed more than 50% relative growth up to aw = 0.93 after 14 days of incubation. Penicillium nalgiovense and P. crustosum were the fastest growing fungi at 10 °C, P. citrinum and P. nordicum at 5 °C. Growth speed is important because it favors fungi competiveness. Therefore, these data suggest that P. nalgiovense could effectively compete with other fungi present in cheese, depending on the abiotic conditions of exposure.
As well-known from literature, the range of abiotic conditions that allows toxin production is commonly narrow than that allowing growth and this is confirmed in the present study. CPA and MPA production started at 10 °C, while P. camemberti and P. roqueforti grew from 5 °C. OTA production started at 10 and 15 °C, respectively for P. nordicum and P. verrucosum, while growth was observed from 5 °C. MPA was detected up to 20 °C, while growth continued up to 30 °C.
Water activity resulted as the most limiting factor for toxin production; in fact, growth was observed down to 0.87–0.90 aw, depending on the fungal species, while toxin production stopped at 0.93 aw for CIT, PR and ROQ-C, 0.99 aw for OTA by P. verrucosum and 0.96 aw for all the other fungi.
Even though toxic metabolites have been reported in P. camemberti and P. roqueforti, they are used as ripening agents, and CIT, CPA, MPA and ROQ-C were effectively detected in fungal cultures in the range of conditions considered in this study. Therefore, strains included in starter inocula should be preliminarily checked for toxigenicity. This can be inferred also from Dall’Asta, de Dea Lindner, Galaverna, Dossena, Neviani and Marchelli [44], who found OTA contamination increased duiring storage of blue cheeses.
CIT is the toxin detected in the highest amount in this study, around 3000 ng/mm2 of fungal colony, in P. citrinum grown at 35 °C and 0.99 aw. CIT was produced at all the temperature conditions considered, in agreement with Bailly, Querin, Le Bars-Bailly, Benard and Guerre [18], while it rapidly decreased from 1200 to 100 ng/mm2 when aw moved from 0.99 to 0.96 (T = 20 °C).
CIT production by P. camemberti is a matter of concern because it was detected at 5 °C, T typically applied for home storage by consumers. Fortunately, according to Manabe [50], few P. camemberti strains were able to produce CIT. No CIT was produced in the present study by P. verrucosum, even if previously reported [27,51]. This could be due to its susceptibility to salt concentration. According to Schmidt-Heydt, et al. [26], when salt concentration is equal to or above 20 g/L, P. verrucosum shifts from producing CIT to OTA. As the amount of NaCl used to modify the aw in our study was about 70 g/L minimum, lacking of CIT production at different aw regimes is not surprising.
PA, typically produced by P. crustosum, was detected in all the considered conditions except at 35 °C, with the optimum at 25 °C, in agreement with Larsen, et al. [52] and Kokkonen, et al. [53].
ROQ-C and MPA are typically P. roqueforti extrolites [23,24,27], but ROQ-C is also produced by P. crustosum [54,55]. ROQ-C was produced by P. roqueforti at all the considered conditions. Several authors agree on the very high incidence of toxigenic strains in P. roqueforti populations [23,24,56,57]. Significant reduction in ROQ-C was observed with T lower than 12 °C, NaCl concentrations 8% and modified atmosphere (1%–5% O2 and 20%–40% CO2; [48]). A substantial support in describing the role of abiotic factors will come from the genome of P. roqueforti recently published [58].
STC, a very stable compound [59], has so far only beendetected on the rind of hard cheeses [31]. In this study, STC was produced by A. versicolor over the T range 10–35 °C and with aw ≥ 0.96, but STC production with lower aw cannot be excluded in cheese.
CPA was supposed to be produced by P. citrinum and by P. camemberti, but it was only detected in the latter colonies, incubated between 10 and 25 °C and with aw ≥ 0.96. The amount of CPA measured increased with temperature and aw increase, in agreement with Le Bars [19] who also confirmed the high incidence of toxigenic strains in P. camemberti populations.
Penicillium nordicum and P. verrucosum are OTA producers and in this study, as expected, P. nordicum was more efficient at slightly lower T and higher aw, 0.96–0.99, compared to P. verrucosum. The latter resulted toxigenic also at very low aw, 0.87–0.93, with a long incubation time, in agreement with Schmidt-Heydt et al. [60] and Schmidt-Heydt, et al. [26]. They also underlined the efficacy of salt addition in limiting OTA production by P. nordicum. Since the ambient T and the cheese rind aw during cheese ripening are favorable, possible contaminations by P. nordicum and P. verrucosum must not be underrated, even if OTA is sometimes undetectable [56].
Growth and toxin production rates by the studied fungi was well described by Bete function and by logistic regression in different T and aw regimes, respectively. The Bete equation is in agreement with good modelling results obtained by Rossi, et al. [61] for fungi involved in Fusarium head blight complex and deoxynivalenol and zearalenon production, by Nazari, et al. [62] for Fusarium langsethiae/F. sporotrichioides and T-2/HT-2 toxins production, by Battilani, et al. [63] to model A. flavus growth on maize and recently for A. carbonarius growth on grapes [64]. Other modeling approaches are described in literature to predict fungal growth as T function [65,66], but the use of Bete equation is more advisable when functions are developed to be used for mechanistic model development [67]. Furthermore, Bete equation was used to model other key steps of fungal infection cycle as function of T, like A. flavus sporulation [68] and A. carbonarius germination. However, further tests of growth on cheese will be necessary to develop a good model.
4. Conclusions
The highest risk of toxin production in cheese should occur between 15 and 25 °C, where 4 out of 8 of the species considered in this study had their optimal toxigenic activity. In particular, between 15 and 22 °C, 3 fungi, P. crustosum, P. nordicum and P. roqueforti, are expected to cause major problems, with OTA, PA, ROQ-C and MPA as expected toxins. STC and CIT should also be monitored, even if their production is optimized with higher T regimes. Regarding aw, if the cheese rind has an aw below 0.93, mycotoxin production should not be at very high risk, at least within the first 2 weeks of development of a spoiling mold. Nevertheless, some contamination data reported in literature after long ripening periods are in contrast with this statement. This study underlines two important factors regarding mycotoxin-producting fungi in cheese: (i) they can grow in conditions comparable with those used for cheese ripening and (ii) a multi-mycotoxin contamination of cheese is possible. Therefore, the models developed in this study should be validated/adjusted with data obtained on cheese. This will be a good starting point to develop a model to predict contamination by different mycotoxins. In the meantime, monitoring ripened cheese for toxin contamination is strongly suggested, especially when their presence will be predicted on the basis of abiotic and biotic data.
5. Materials and Methods
5.1. Fungal Strains
The fungi included in this study are all toxigenic, except for P. nalgiovense, which is however considered because of its prevalence in ripened pork meat and signaled in cheese, and as a possible competitor of toxigenic species. Characterized strains were purchased from CBS-NAW fungal collection (http://www.cbs.knaw.nl/; Table 5).
Table 5.
Fungal strains and related mycotoxins considered in this study.
| Species | Code | Mycotoxin (Abbreviation) |
|---|---|---|
| A. versicolor | CBS 108959 | Sterigmatocystin (STC) |
| P. camemberti | CBS 122399 | Citrinin (CIT), Cyclopyazonic Acid (CPA) |
| P. citrinum | CBS 122396 | CIT, CPA |
| P. crustosum | CBS 115503 | Penitrem A (PA), Roquefortine C (ROQ-C) |
| P. nalgiovense | CBS 109609 | # |
| P. nordicum | CBS 112573 | Ochratoxin A (OTA) |
| P. roqueforti | CBS 221.30 | Mycophenolic Acid (MPA), ROQ-C, PR Toxin (PR-TOXIN) |
| P. verrucosum | CBS 325.92 | CIT, OTA |
# No toxins are reported for this fungus.
5.2. Culture Media
Ingredients for media were purchased from Himedia Laboratories (Mumbai, India).
Czapek Yeast Agar (CYA, [69]), supplemented with sodium chloride (NaCl) (Carlo Erba, Milan, Italy) to modify the original aw = 0.99, was used to perform the ecological trials (Table 6).
Table 6.
Amounts of sodium chloride (NaCl) added to Ckzapek Yeast Agar (CYA) to modify medium water activity (aw; [70]).
| NaCl (g/100 mL) | aw |
|---|---|
| 7.01 | 0.96 |
| 11.98 | 0.93 |
| 16.56 | 0.90 |
| 19.40 | 0.87 |
| 23.55 | 0.84 |
| 30.10 | 0.81 |
| 39.90 | 0.78 |
5.3. Inoculum Preparation, Inoculation and Incubation
A conidia suspension was prepared using 7-day old colonies grown on Malt Extract Agar, (MEA, [71]). The spores were collected using 20 mL of sterile bi-distilled water added to each Petri dish, gently agitating the culture to remove conidia. The suspension was adjusted to a concentration of 106 spores/mL using an haemocytometer, in agreement with fungal CFU/g detected in naturally contaminated cheese. Then, 10 μL of conidial suspension of each strain was centrally inoculated in 60 mm Ø Petri plates and incubated in the proper conditions; the trial was managed in triplicate.
Two experiments were performed to assess the role of: (i) T and (ii) aw on fungal growth and mycotoxin production.
Regarding T, CYA inoculated plates, all prepared without any aw modification, were incubated at temperatures ranging between 0 °C and 40 °C, 5 ± 1 °C step, for 14 days.
Regarding aw, the CYA medium was adjusted to aw values from 0.87 to 0.99 step 0.03 aw, and incubated at 20 ± 1 °C for 14 days. Longer incubation times, up to 56 days, were considered for the aw regimes ≤0.90.
5.4. Fungal Growth Measurement
The inoculated Petri dishes were observed after 3, 7, 10 and 14 days, and two perpendicular colony diameters were measured; a weekly schedule was applied for longer incubation times (from 14 to 56 days). After 14 days of incubation (56 days for aw ≤ 0.90), the plates were sealed in plastic bags and stored at −20 °C before mycotoxin analysis.
5.5. Mycotoxin Analysis
Ochratoxin A, CPA, PR-toxin, MPA, ROQ-C, PA and CIT were considered as produced by the proper fungi (reported in Table 5); they were measured at the end of the incubation time (14 day old cultures).
5.5.1. Reagents and Standards
The chemicals and solvents used for the extraction and clean-up solutions were ACS grade or equivalent (Carlo Erba, Milan, Italy). All the water used was de-ionized and, for HPLC, purified through a Milli-Q treatment system (Millipore, London, UK). For HPLC analysis, methanol and acetonitrile were HPLC grade (Merck, Darmstadt, Germany). Mycotoxin standards were obtained from Sigma-Aldrich (St. Louis, MO, USA) and Biopure (Tulln, Austria). Working standard solutions were prepared by dilution with acetonitrile and kept at −20 °C.
5.5.2. Toxin Extraction
Toxin extraction was performed by putting the fungal colony and agar media in a flask containing 40 mL of acetonitrile. Then, the mix was vigorously shaken using a rotary-shaking stirrer for 1 h in order to smash the agar medium into little pieces, filtered (folded filter paper 595 ½, Whatman, Sigma-Aldrich, St. Louis, MO, USA) and diluted using the HPLC mobile phase before being analyzed.
5.5.3. HPLC-MS/MS Analysis
The mycotoxins (STC, CPA, CIT, ROQ, MPA, OTA, PR toxin, PA), were analyzed using an HPLC-MS/MS system, consisting of a LC 1.4 Surveyor pump, a Quantum Discovery Max triple-quadrupole mass spectrometer (Thermo-Fisher Scientific, San Jose, CA, USA) and a PAL 1.3.1 sampling system (CTC Analitycs AG, Zwingen, Switzerland); the system was controlled by Xcalibur 1.4 software (Thermo-Fisher). The mycotoxins were separated on a Betasil RP-18 column (5 µm particle size, 150 × 2.1 mm, Thermo-Fisher); except for PA, a mobile-phase gradient water-acetonitrile (both acidified with 0.2% formic acid) from 65:35 to 25:75 in 6 min, then isocratic for 5 min was used; for PA, the mobile-phase gradient water-acetonitrile (both acidified with 0.2% formic acid) was from 40:60 to 10:90 in 5 min, then isocratic for 3 min. The flow rate was always 0.2 mL/min and the injection volume 20 µL. The ionization was carried out with an ESI interface (Thermo-Fisher) in positive mode as follows: spray capillary voltage 4200 kV, sheath and auxiliary gas 35 and 10 psi, respectively, temperature of the heated capillary 270 °C. The selected fragment ions and the parent ion [M]+ were: 310, 281 and 253 m/z for STC ([M]+ 325 m/z); 196, 182 and 140 m/z for CPA ([M]+ 337 m/z); 233, 205 and 191 m/z for CIT ([M]+ 251 m/z); 334, 322 and 193 m/z for ROQ-C ([M]+ 390 m/z); 303, 275 and 207 m/z for MPA ([M]+ 321 m/z); 358, 341 and 239 m/z for OTA ([M]+ 404 m/z); 279, 173, 161 and 145 m/z for PR toxin ([M]+ 321 m/z); 616, 558 and 332 m/z for PA ([M]+ 634). The collision energy was different for each mycotoxin (ranging from 15 to 33 V) and the argon collision pressure was 1.5 mTorr. Quantitative determination was performed using LC-Quan 2.0 software (Thermo-Fisher Scientific); LODs were 30 µg/L for MPA, CPA, PR-toxin, PA and STC, 20 µg/L for ROQ-C, 10 µg/L for CIT and OTA.
5.6. Data Analyses
Statistical analyses were performed using SPSS v.23 (SPSS Inc., Armonk, NY, USA, 2012). Mycotoxin production data were statistically compared by using a OneWay-ANOVA Test transforming all values by y = ln before analysis to homogenize the variance. Tukey test was applied to highlight significant differences between means.
Data on fungal growth, intended as the fungal culture diameter, at different T or aw regimes, were considered separately for each incubation time. They were standardized (rated on the maximum value observed), to obtain relative growth in a 0–1 scale, with 0 = no growth, and 1 = maximum growth. Relative growth of each fungus, at all incubation times, were jointly analyzed. Thirty six mean values were used for T (9 T regimes and 4 incubation times, mean of 3 replicates) and 20 values for aw (5 aw regimes and 4 incubation times, mean of 3 replicates). The same approach was applied to obtain relative mycotoxin production. Different nonlinear regression models were fitted to the rate data in order to describe fungal growth and mycotoxin production as function of T and aw; the equation parameters were estimated applying the non-linear regression procedure of the statistical package PASW SPSS statistics v.23 (SPSS Inc., Armonk, NY, USA, 2012) which minimizes the residual sum squares using the Levenberg-Marquardt algorithm. The best model was chosen based on the adjusted R2 and on the number of iterations required by the algorithm to converge on parameter estimates, as indicators of goodness of fit.
Minitab 17 (Minitab Inc., State College, PA, USA) was used to develop the surface response contour plots of data, in relation to the combinations T × time of incubation and aw × time of incubation, for each considered fungus. For each combination T × time or aw × time, relative growth, computed as previously described, was used as input for data plotting (relative growth values were transformed from 0–1 scale to 0–100 scale to satisfy the type of data input requested by Minitab). Two-dimension surface response contour plots were drawn, with five quoted lines (contour levels): 0%, 25%, 50%, 75% and 100% of the relative growth. This kind of data presentation is considered useful when many data are collected in the study and it is not easy to report all data and to compare and comment results.
In order to represent the combined effect of T and aw, the equations developed to describe fungal growth as function of these two variables were combined and the 50% relative growth values obtained for each fungus were plotted.
Acknowledgments
The present work was supported by the Consorzio per la tutela del Formaggio Grana Padano and the Consorzio del Formaggio Parmigiano Reggiano. Simone Decontardi carried out this work within the PhD School “Agrisystem” of the Università Cattolica del Sacro Cuore (Italy).
Author Contributions
P.B., M.C.L., S.D. and A.P. conceived and designed the experiments; S.D. performed the experiments; M.C.L. and P.B. analyzed the data; T.B. and S.D. contributed reagents/materials/analysis tools; S.D., M.C.L. and P.B. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Banjara N., Suhr M.J., Hallen-Adams H.E. Diversity of yeast and mold species from a variety of cheese types. Curr. Microbiol. 2015;70:792–800. doi: 10.1007/s00284-015-0790-1. [DOI] [PubMed] [Google Scholar]
- 2.Gkatzionis K., Yunita D., Linforth R.S.T., Dickinson M., Dodd C.E.R. Diversity and activities of yeasts from different parts of a Stilton cheese. Int. J. Food Microbiol. 2014;107:109–116. doi: 10.1016/j.ijfoodmicro.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Cogan T.M., Hill C. Cheese starter cultures. In: Fox P.F., editor. Cheese: Chemistry, Physics and Microbiology. Volume 1. Springer; New York, NY, USA: 1993. pp. 193–255. [Google Scholar]
- 4.De Santi M., Sisti M., Barbieri E., Piccoli G., Brandi G., Stocchi V. A combined morphologic and molecular approach for characterizing fungal microflora from a traditional Italian cheese (Fossa cheese) Int. Dairy J. 2010;20:465–471. doi: 10.1016/j.idairyj.2010.02.004. [DOI] [Google Scholar]
- 5.Malacarne M., Summer A., Panari G., Pecorari M. Caratterizzazione chimico-fisica della maturazione del Parmigiano-Reggiano. Sci. Tec. Latt. Casearia. 2006;57:215–228. [Google Scholar]
- 6.Cakmakci S., Gundogdu E., Hayaloglu A.A., Dagdemir E., Gurses M., Cetin B., Tahmas-Kahyaoglu D. Chemical and microbiological status and volatile profiles of mouldy Civil cheese, a Turkish mould-ripened variety. Int. J. Food Sci. Technol. 2012;47:2405–2412. doi: 10.1111/j.1365-2621.2012.03116.x. [DOI] [Google Scholar]
- 7.Milani E., Shahidi F., Mortazavi S.A., Reza Vakili S.A., Ghoddusi H.B. Microbiological, biochemical and rheological changes throughout ripening of kurdish cheese. J. Food Saf. 2014;34:168–175. [Google Scholar]
- 8.Schiavano G.F., Barbieri E., Sisti M., Gioacchini A.M., de Santi M., Vallorani L., Casadei L., Piccoli G., Guescini M., Stocchi V., et al. Characterization of microflora and volatile organic compounds of Fossa (pit) cheese. Ind. Aliment. 2012;51:19–32. [Google Scholar]
- 9.Gaborit P., Menard A., Morgan F. Impact of ripening strains on the typical flavour of goat cheeses. Int. Dairy J. 2001;11:315–325. [Google Scholar]
- 10.Gripon J.C. Mould-ripened cheese. In: Fox P.F., editor. Cheese Chemistry, Physics and Microbiology, Major Cheese Groups. Volume 2 Elsevier Science Publisher Ltd.; Cambridge, UK: 1993. [Google Scholar]
- 11.Panelli S., Buffoni J.S., Bonacina C., Feligini M. Identification of moulds from the Taleggio cheese environment by the use of DNA barcodes. Food Control. 2012;28:385–391. doi: 10.1016/j.foodcont.2012.05.022. [DOI] [Google Scholar]
- 12.Battilani P., Pietri A., Giorni P., Formenti S., Bertuzzi T., Toscani T., Virgili R., Kozakiewicz Z. Penicillium population in dry-cured ham manufacturing plants. J. Food Prot. 2007;70:975–980. doi: 10.4315/0362-028X-70.4.975. [DOI] [PubMed] [Google Scholar]
- 13.Comi G., Chiesa L., Panseri S., Orlic S., Iacumin L. Evaluation of different methods to prevent Penicillium nordicum growth on and ochratoxin A production in country-style sausages. World Mycotoxin J. 2013;6:411–418. doi: 10.3920/WMJ2013.1548. [DOI] [Google Scholar]
- 14.Rodríguez A., Rodríguez M., Martín A., Núñez F., Córdoba J.J. Evaluation of hazard of aflatoxin B1, ochratoxin A and patulin production in dry-cured ham and early detection of producing moulds by qPCR. Food Control. 2012;27:118–126. doi: 10.1016/j.foodcont.2012.03.009. [DOI] [Google Scholar]
- 15.Filtenborg O., Frisvad J.C., Thrane U. Moulds in food spoilage. Int. J. Food Microbiol. 1996;33:85–102. doi: 10.1016/0168-1605(96)01153-1. [DOI] [PubMed] [Google Scholar]
- 16.Hymery N., Vasseur V., Coton M., Mounier J., Jany J.L., Barbier G., Coton E. Filamentous fungi and mycotoxins in cheese: A review. Compr. Rev. Food Sci. Food Saf. 2014;13:437–456. doi: 10.1111/1541-4337.12069. [DOI] [PubMed] [Google Scholar]
- 17.Chang S.C., Wei Y.H., Wei D.L., Chen Y.Y., Jong S.C. Factors affecting the production of eremofortin C and PR toxin in Penicillium roqueforti. Appl. Environ. Microbiol. 1991;57:2581–2585. doi: 10.1128/aem.57.9.2581-2585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailly J.D., Querin A., Le Bars-Bailly S., Benard G., Guerre P. Citrinin production and stability in cheese. J. Food Prot. 2002;65:1317–1321. doi: 10.4315/0362-028X-65.8.1317. [DOI] [PubMed] [Google Scholar]
- 19.Le Bars J. Cyclopiazonic acid production by Penicillium camemberti thom and natural cccurrence of this mycotoxin in cheese. Appl. Environ. Microbiol. 1979;6:1052–1055. doi: 10.1128/aem.38.6.1052-1055.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taniwaki M.H., Hocking A.D., Pitt J.I., Fleet G.H. Growth and mycotoxin production by fungi in atmospheres containing 80% carbon dioxide and 20% oxygen. Int. J. Food Microbiol. 2010;143:218–225. doi: 10.1016/j.ijfoodmicro.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 21.Lund F., Filtenborg O., Frisvad J.C. Associated mycoflora of cheese. Food Microbiol. 1995;12:173–180. doi: 10.1016/S0740-0020(95)80094-8. [DOI] [Google Scholar]
- 22.Wagener R.E., Davis N.D., Diener U.L. Penitrem A and roquefortine production by Penicillium commune. Appl. Environ. Microbiol. 1980;39:882–887. doi: 10.1128/aem.39.4.882-887.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erdogan A., Sert S. Mycotoxin-forming ability of two Penicillium roqueforti strains in blue moldy tulum cheese ripened at various temperatures. J. Food Prot. 2004;67:533–535. doi: 10.4315/0362-028X-67.3.533. [DOI] [PubMed] [Google Scholar]
- 24.Finoli C., Vecchio A., Galli A., Dragoni I. Roquefortine C occurrence in blue cheese. J. Food Prot. 2001;64:246–251. doi: 10.4315/0362-028X-64.2.246. [DOI] [PubMed] [Google Scholar]
- 25.Taniwaki M.H., van Dender A.G.F. Growth of fungi and mycotoxin production on cheese under modified atmospheres. Int. J. Food Microbiol. 2001;68:125–133. doi: 10.1016/S0168-1605(01)00487-1. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt-Heydt M., Graf E., Stoll D., Rolf G. The biosynthesis of ochratoxin A by Penicillium as one mechanism of adaptation to NaCl rich foods. Food Microbiol. 2012;29:233–241. doi: 10.1016/j.fm.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Samson R.A., Frisvad J.C. Penicillium subgenus Penicillium: New taxonomy schemes and mycotoxins and other extrolites. Stud. Mycol. 2004;49:1–266. [Google Scholar]
- 28.Altug T. Introduction to Toxicology and Food. CRC Press; Boca Raton, FL, USA: 2003. pp. 1–168. [Google Scholar]
- 29.Abd Alla E.A., Metwally M.M., Mehriz A.M., Abu Sree Y.H. Sterigmatocystin—incidence, fate and production by A. versicolor in Ras cheese. Mycotoxin Res. 1997;13:61–66. doi: 10.1002/food.19960400604. [DOI] [PubMed] [Google Scholar]
- 30.Metwally M., El-Sayed A.A., Mehriz A., Abu S.Y. Toxigenic fungi isolated from Roquefort cheese. Mycopathologia. 1979;66:187–190. doi: 10.1007/BF00683970. [DOI] [PubMed] [Google Scholar]
- 31.Northolt M.D., Egmond H.P.V., Soentoro P., Deijll E. Fungal growth and the presence of sterigmatocystin in hard cheese. J. Assoc. Off. Anal. Chem. 1980;63:115–119. [PubMed] [Google Scholar]
- 32.Rank C., Nielsen K.F., Larsen T.O., Varga J., Samson R.A., Frisvad J.C. Distribution of sterigmatocystin in filamentous fungi. Fungal Biol. 2011;115:406–420. doi: 10.1016/j.funbio.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Engel G., von Milczewski K.E., Prokopek D., Teuber M. Strain-specific synthesis of Mycophenolic acid by Penicillium roqueforti in blue-veined cheese. Appl. Environ. Microbiol. 1982;43:1034–1040. doi: 10.1128/aem.43.5.1034-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lafont P., Siriwardana M.G., Lafont J. Contamination of cheeses by mycotoxins. Med. Nutr. 1979;15:257–262. [Google Scholar]
- 35.Zambonin C.G., Monaci L., Aresta A. Determination of cyclopiazonic acid in cheese samples using solid-phase microextraction and high performance liquid chromatography. Food Chem. 2011;75:249–254. doi: 10.1016/S0308-8146(01)00218-7. [DOI] [Google Scholar]
- 36.Ammar H., Michailis G., Lisovsky T. A screen of yeast respiratory mutants for sensitivity against the mycotoxin citrinin identifies the vascular ATPase as an essential factor for the toxicity mechanism. Curr. Genet. 2000;37:277–284. doi: 10.1007/s002940070001. [DOI] [PubMed] [Google Scholar]
- 37.Singh N.D., Sharma A.K., Dwivedi P., Patil R.D., Kumar M. Citrinin and endosulfan induced maternal toxicity in pregnant wistar rats: Pathomorphological study. J. Appl. Toxicol. 2007;27:589–601. doi: 10.1002/jat.1242. [DOI] [PubMed] [Google Scholar]
- 38.Ostry V., Malir F., Ruprich J. Producers and important dietary sources of ochratoxin A and citrinin. Toxins. 2013;5:1574–1586. doi: 10.3390/toxins5091574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erdogan A., Gurses M., Sert S. Isolation of moulds capable of producing mycotoxins from blue mouldy Tulum cheeses produced in Turkey. Int. J. Food Microbiol. 2003;85:83–85. doi: 10.1016/S0168-1605(02)00485-3. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez-Bodega M.A., Mauriz E., Gomez A., Martin J.F. Proteolytic activity, mycotoxins and andrastin A in Penicillium roqueforti strains isolated from Cabrales, Valdeon and Bejes-Tresviso local varieties of blue-veined cheeses. Int. J. Food Microbiol. 2009;136:18–25. doi: 10.1016/j.ijfoodmicro.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 41.Cakmakci S., Gurses M., Hayaloglu A.A., Cetin B., Sekerci P., Dagdemir E. Mycotoxin production capability of Penicillium roqueforti in strains isolated from mould-ripened traditional Turkish civil cheese. Food Addit. Contam. Part A. 2015;32:245–249. doi: 10.1080/19440049.2014.997808. [DOI] [PubMed] [Google Scholar]
- 42.Siemens K., Zawistowski J. Occurrence of PR imine, a metabolite of Penicillium roqueforti, in Blue cheese. J. Food Prot. 1993;56:317–319. doi: 10.4315/0362-028X-56.4.317. [DOI] [PubMed] [Google Scholar]
- 43.Minervini F., Monaci L., Montagna M.T., Dragoni I. Assessment of mycotoxin production by Aspergillus and Penicillium fungi isolated from dairy products. Ind. Aliment. 2002;41:1336–1340. [Google Scholar]
- 44.Dall’Asta C., De Dea Lindner J., Galaverna G., Dossena A., Neviani E., Marchelli R. The occurrence of ochratoxin A in blue cheese. Food Chem. 2008;106:729–734. doi: 10.1016/j.foodchem.2007.06.049. [DOI] [Google Scholar]
- 45.Analytis S. Über die relation zwischen biologischer entwicklung und temperatur bei phytopathogenen pilzen. J. Phytopathol. 1977;90:64–76. doi: 10.1111/j.1439-0434.1977.tb02886.x. [DOI] [Google Scholar]
- 46.Grimm K., Ram N. Nonlinear growth curves in developmental research. Child Dev. 2011;82:1357–1371. doi: 10.1111/j.1467-8624.2011.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marin P., Palmero D., Jurado M. Effect of solute and matric potential on growth rate of fungal species isolated from cheese. Int. Dairy J. 2014;36:89–94. doi: 10.1016/j.idairyj.2014.01.012. [DOI] [Google Scholar]
- 48.Fontaine K., Hymery N., Lacroix M.Z., Puel S., Puel O., Rigalma K., Gaydou V., Coton E., Mounier J. Influence of intraspecific variability and abiotic factors on mycotoxin production in Penicillium roqueforti. Int. J. Food Microbiol. 2015;215:187–193. doi: 10.1016/j.ijfoodmicro.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 49.Giorni P., Magan N., Pietri A., Battilani P. Growth and aflatoxin production of an Italian strain of Aspergillus flavus: Influence of ecological factors and nutritional substrates. World Mycotoxin J. 2011;4:425–432. doi: 10.3920/WMJ2011.1300. [DOI] [Google Scholar]
- 50.Manabe M. Fermented foods and mycotoxins. Mycotoxins. 2001;51:25–28. doi: 10.2520/myco.51.25. [DOI] [Google Scholar]
- 51.Samson R.A., Houbraken J., Thrane U., Frisvad J.C., Andersen B. Food and Indoor Fungi. CBS KNAW Biodiversity Center; Utrecht, The Netherlands: 2010. p. 390. [Google Scholar]
- 52.Larsen T.O., Gareis M., Frisvad J.C. Cell cytotoxicity and mycotoxin and secondary metabolite production by common penicillia on cheese agar. J. Agric. Food Chem. 2002;50:6148–6152. doi: 10.1021/jf020453i. [DOI] [PubMed] [Google Scholar]
- 53.Kokkonen M., Jestoi M., Rizzo A. The effect of substrate on mycotoxin production of selected Penicillium strains. Int. J. Food Microbiol. 2005;99:207–214. doi: 10.1016/j.ijfoodmicro.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 54.Eriksen G.S., Moldes-Anaya A., Faeste C.K. Penitrem A and analogues: Toxicokinetics, toxicodynamics including mechanism of action and clinical significance. World Mycotoxin J. 2013;6:263–272. doi: 10.3920/WMJ2013.1574. [DOI] [Google Scholar]
- 55.Tancinova D., Mokry M., Barborakova Z., Maskova Z. Mycobiota of spices and aromatic herbs. Potravin. Sci. J. Food Ind. 2014;8:172–177. [Google Scholar]
- 56.Kokkonen M., Jestoi M., Rizzo A. Determination of selected mycotoxins in mould cheeses with liquid chromatography coupled to tandem with mass spectrometry. Food Addit.Contam. 2005;22:449–456. doi: 10.1080/02652030500089861. [DOI] [PubMed] [Google Scholar]
- 57.Polonsky J., Merrien M.A., Scott P.M. Roquefortine and isofumigaclavine A, alkaloids from Penicillium roqueforti. Ann. Nutr. Aliment. 1977;31:963–968. [PubMed] [Google Scholar]
- 58.Cheeseman K., Ropars J., Renault P., Dupont J., Gouzy J., Branca A., Abraham A.L., Ceppi M., Conseiller E., Debuchy R., et al. Multiple recent horizontal transfers of a large genomic region in cheese making fungi. Nat. Commun. 2014;5:2876. doi: 10.1038/ncomms3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Egmond H.P. Mycotoxins in dairy products. Food Chem. 1983;11:289–307. doi: 10.1016/0308-8146(83)90076-6. [DOI] [Google Scholar]
- 60.Schmidt-Heydt M., Magan N., Geisen R. Stress induction of mycotoxin biosynthesis genes by abiotic factors. FEMS Microbiol. Lett. 2008;284:142–149. doi: 10.1111/j.1574-6968.2008.01182.x. [DOI] [PubMed] [Google Scholar]
- 61.Rossi V., Pattori E., Ravanetti A., Giosuè S. Effect of constant and fluctuating temperature regimes on sporulation of four fungi causing head blight of wheat. J. Plant Pathol. 2002;84:95–105. [Google Scholar]
- 62.Nazari L., Manstretta V., Rossi V. A non-linear model for temperature-dependent sporulation and T-2 and HT-2 production of Fusarium langsethiae and Fusarium sporotrichioides. Fungal Biol. 2016;120:562–571. doi: 10.1016/j.funbio.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 63.Battilani P., Camardo Leggieri M., Rossi V., Giorni P. AFLA-maize, a mechanistic model for Aspergillus flavus infection and aflatoxin B1 contamination in maize. Comput. Electron. Agric. 2013;94:38–46. doi: 10.1016/j.compag.2013.03.005. [DOI] [Google Scholar]
- 64.Battilani P., Camardo Leggieri M. OTA-grapes, a mechanistic model to predict ochratoxin A risk in grapes, a step beyond the systems approach. Toxins. 2015;7:3012–3029. doi: 10.3390/toxins7083012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dantigny P., Guilmart A., Bensoussan M. Basis of predictive mycology. Int. J. Food Microbiol. 2005;100:187–196. doi: 10.1016/j.ijfoodmicro.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 66.Garcia D., Ramos A.J., Sanchis V., Marín S. Modelling mould growth under suboptimal environmental conditions and inoculum size. Food Microbiol. 2010;27:909–917. doi: 10.1016/j.fm.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 67.Rossi V., Giosuè S., Caffi T. Modelling plant diseases for decision making in crop protection. In: Oerke E.C., Gerhards R., Menz G., Sikora R.A., editors. Precision Crop Protection—The Challenge and Use of Heterogeneity. Springer; Dordrecht, The Netherlands: 2010. pp. 241–258. [Google Scholar]
- 68.Giorni P., Camardo Leggieri M., Magan N., Battilani P. Comparison of ecological needs for sporulation of Aspergillus flavus sclerotia on natural and artificial substrates. Fungal Biol. 2012;116:637–742. doi: 10.1016/j.funbio.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 69.Frisvad J.C., Smedsgaard J., Larsen T.O., Samson R.A. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud. Mycol. 2004;49:201–241. [Google Scholar]
- 70.Dallyn H., Fox A. Spoilage of material of reduced water activity by xerophilic fungi. In: Gould G.H., Corry J.E.L., editors. Microbial Growth and Survival in Extremes of Environment. Volume 15. Academic Press; London, UK: 1980. pp. 129–139. [Google Scholar]
- 71.Pitt J.I. The Genus Penicillium and Its Teleomorphic States Eupenicillium and Talaromyces. Academic Press Inc.; London, UK: 1979. [Google Scholar]