Abstract
CHIP28 is a 28-kDa integral membrane protein with similarities to membrane channels and is found in erythrocytes and renal tubules. A cDNA for CHIP28 was isolated from human fetal liver cDNA template by a three-step polymerase chain reaction (PCR) cloning strategy, starting with degenerate oligonucleotide primers corresponding to the N-terminal amino acid sequence determined from purified CHIP28 protein. Using the third-step PCR product as a probe, we isolated a recombinant from a human bone marrow cDNA library. The combined sequence of the PCR products and bone marrow cDNA contains 38 base pairs of 5' untranslated nucleotide sequence, an 807-bp open reading frame, and approximately 2 kilobases of 3' untranslated sequence containing a polyadenylation signal. This corresponds to the 3.1-kilobase transcript identified by RNA blot-hybridization analysis. Authenticity of the deduced amino acid sequence of the CHIP28 protein C terminus was confirmed by expression and immunoblotting. Analysis of the deduced amino acid sequence suggests that CHIP28 protein contains six bilayer-spanning domains, two exofacial potential N-glycosylation sites, and intracellular N and C termini. Search of the DNA sequence data base revealed a strong homology with the major intrinsic protein of bovine lens, which is the prototype of an ancient but recently recognized family of membrane channels. These proteins are believed to form channels permeable to water and possibly other small molecules. CHIP28 shares homology with all known members of this channel family, and it is speculated that CHIP28 has a similar function.
Full text
PDF


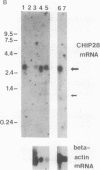
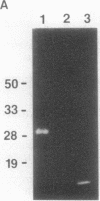
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Saboori A. M., Asimos A., Smith B. L. Purification and partial characterization of the Mr 30,000 integral membrane protein associated with the erythrocyte Rh(D) antigen. J Biol Chem. 1987 Dec 25;262(36):17497–17503. [PubMed] [Google Scholar]
- Baker M. E., Saier M. H., Jr A common ancestor for bovine lens fiber major intrinsic protein, soybean nodulin-26 protein, and E. coli glycerol facilitator. Cell. 1990 Jan 26;60(2):185–186. doi: 10.1016/0092-8674(90)90731-s. [DOI] [PubMed] [Google Scholar]
- Bennett V. The spectrin-actin junction of erythrocyte membrane skeletons. Biochim Biophys Acta. 1989 Jan 18;988(1):107–121. doi: 10.1016/0304-4157(89)90006-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Denker B. M., Smith B. L., Kuhajda F. P., Agre P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J Biol Chem. 1988 Oct 25;263(30):15634–15642. [PubMed] [Google Scholar]
- Ehring G. R., Zampighi G., Horwitz J., Bok D., Hall J. E. Properties of channels reconstituted from the major intrinsic protein of lens fiber membranes. J Gen Physiol. 1990 Sep;96(3):631–664. doi: 10.1085/jgp.96.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin M. B., Yancey S. B., Cline J., Revel J. P., Horwitz J. The major intrinsic protein (MIP) of the bovine lens fiber membrane: characterization and structure based on cDNA cloning. Cell. 1984 Nov;39(1):49–59. doi: 10.1016/0092-8674(84)90190-9. [DOI] [PubMed] [Google Scholar]
- Guerrero F. D., Jones J. T., Mullet J. E. Turgor-responsive gene transcription and RNA levels increase rapidly when pea shoots are wilted. Sequence and expression of three inducible genes. Plant Mol Biol. 1990 Jul;15(1):11–26. doi: 10.1007/BF00017720. [DOI] [PubMed] [Google Scholar]
- Harris H. W., Jr, Strange K., Zeidel M. L. Current understanding of the cellular biology and molecular structure of the antidiuretic hormone-stimulated water transport pathway. J Clin Invest. 1991 Jul;88(1):1–8. doi: 10.1172/JCI115263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann E., Rapoport T. A., Lodish H. F. Predicting the orientation of eukaryotic membrane-spanning proteins. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5786–5790. doi: 10.1073/pnas.86.15.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Voltage-sensitive ion channels. Cell. 1989 Jan 13;56(1):13–25. doi: 10.1016/0092-8674(89)90979-3. [DOI] [PubMed] [Google Scholar]
- Johnson K. D., Höfte H., Chrispeels M. J. An intrinsic tonoplast protein of protein storage vacuoles in seeds is structurally related to a bacterial solute transporter (GIpF). Plant Cell. 1990 Jun;2(6):525–532. doi: 10.1105/tpc.2.6.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landschulz K. T., Noyes A. N., Rogers O., Boyer S. H. Erythropoietin receptors on murine erythroid colony-forming units: natural history. Blood. 1989 May 1;73(6):1476–1486. [PubMed] [Google Scholar]
- Muramatsu S., Mizuno T. Nucleotide sequence of the region encompassing the glpKF operon and its upstream region containing a bent DNA sequence of Escherichia coli. Nucleic Acids Res. 1989 Jun 12;17(11):4378–4378. [PMC free article] [PubMed] [Google Scholar]
- Pao G. M., Wu L. F., Johnson K. D., Höfte H., Chrispeels M. J., Sweet G., Sandal N. N., Saier M. H., Jr Evolution of the MIP family of integral membrane transport proteins. Mol Microbiol. 1991 Jan;5(1):33–37. doi: 10.1111/j.1365-2958.1991.tb01823.x. [DOI] [PubMed] [Google Scholar]
- Rao Y., Jan L. Y., Jan Y. N. Similarity of the product of the Drosophila neurogenic gene big brain to transmembrane channel proteins. Nature. 1990 May 10;345(6271):163–167. doi: 10.1038/345163a0. [DOI] [PubMed] [Google Scholar]
- Saboori A. M., Smith B. L., Agre P. Polymorphism in the Mr 32,000 Rh protein purified from Rh(D)-positive and -negative erythrocytes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4042–4045. doi: 10.1073/pnas.85.11.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandal N. N., Marcker K. A. Soybean nodulin 26 is homologous to the major intrinsic protein of the bovine lens fiber membrane. Nucleic Acids Res. 1988 Oct 11;16(19):9347–9347. doi: 10.1093/nar/16.19.9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. L., Agre P. Erythrocyte Mr 28,000 transmembrane protein exists as a multisubunit oligomer similar to channel proteins. J Biol Chem. 1991 Apr 5;266(10):6407–6415. [PubMed] [Google Scholar]
- Sweet G., Gandor C., Voegele R., Wittekindt N., Beuerle J., Truniger V., Lin E. C., Boos W. Glycerol facilitator of Escherichia coli: cloning of glpF and identification of the glpF product. J Bacteriol. 1990 Jan;172(1):424–430. doi: 10.1128/jb.172.1.424-430.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistow G. J., Pisano M. M., Chepelinsky A. B. Tandem sequence repeats in transmembrane channel proteins. Trends Biochem Sci. 1991 May;16(5):170–171. doi: 10.1016/0968-0004(91)90065-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y. T., Cheng C. L., Conkling M. A. Root-specific genes from tobacco and Arabidopsis homologous to an evolutionarily conserved gene family of membrane channel proteins. Nucleic Acids Res. 1990 Dec 25;18(24):7449–7449. doi: 10.1093/nar/18.24.7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampighi G. A., Hall J. E., Ehring G. R., Simon S. A. The structural organization and protein composition of lens fiber junctions. J Cell Biol. 1989 Jun;108(6):2255–2275. doi: 10.1083/jcb.108.6.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R. B., Logee K. A., Verkman A. S. Expression of mRNA coding for kidney and red cell water channels in Xenopus oocytes. J Biol Chem. 1990 Sep 15;265(26):15375–15378. [PubMed] [Google Scholar]