Abstract
Introduction
This paper reports the results of an audit to assess the possible thermal hazard associated with the clinical use of ultrasound scanners in UK Hospitals for transvaginal ultrasound imaging.
Methods
An anatomically relevant phantom composed of a block of agar-based tissue mimicking material with embedded thermal sensors was developed. Seventeen hospitals around the UK were visited and a total of 64 configurations were tested. A representative typical scanning protocol was adopted, which primarily used B-mode with 30 s periods of colour-flow and pulsed Doppler modes for both gynaecology and obstetrics pre-sets.
Results
The results confirmed that the highest temperature increase is always at the surface. The greatest temperature rise measured across all the systems was 3.6℃, with an average of 2.0℃ and 2.16℃ for gynaecology and obstetrics pre-sets, respectively. For some systems, the temperature increased rapidly when selecting one of the Doppler modes, so using them for longer than 30 s will in many cases lead to greater heating. It is also shown that, in agreement with previous studies, the displayed thermal index greatly underestimates the temperature rise, particularly close to the transducer face but even to distances approaching 2 cm.
Conclusions
Overall, the results of the audit for the temperature rise during transvaginal ultrasound at clinical settings fell within the limits indicated by the national and international standards, for the pre-sets tested and following a representative typical scanning protocol. Only selected pre-sets were tested and the scanner outputs were not maximised (for example by using zoom, greater depth or narrow sector angles). Consequently, higher temperatures than those measured can certainly be achieved.
Keywords: Ultrasound safety, transvaginal ultrasound, temperature measurement, thermal index, ultrasound phantom
Introduction
Ultrasound (US) is widely used for diagnostic medical imaging; more than 8.5 million non-obstetric ultrasound examinations were performed by the NHS in England between April 2015 and March 2016. Almost 3 million obstetric scans can be estimated.1 Transvaginal ultrasound scans (TVUS) may be performed for gynaecology, fertility, obstetrics and urogenital checks. They provide clearer pictures of the uterus, ovaries and surrounding structures and can be advantageous for, e.g., early pregnancy scans. National figures for transvaginal scans are not available but in a small survey which aimed to provide a snapshot of exposure conditions during ultrasound scans performed on one day in February 2007, of the figures returned, 21 scans (9%) were classified as transvaginal, out of 231 obstetric scans.2
The foetus and other tissues imaged during transvaginal ultrasound scans are sensitive to thermal damage and may be close to the transducer during scanning. Transducer surface temperature rises of up to 6℃ are permitted for endocavitary transducers under International Standards3; a safety statement prepared by the British Medical Ultrasound Society advises that temperature rises of 4℃ or more for 5 minutes in embryonic and foetal tissue should be considered potentially hazardous, while safety guidelines issued by the same group recommend that when there is a thermal index (TI) of between 2.5 and 3.0, the scan time should be limited to less than 1 minute.4
Previously, studies have been performed to assess the likelihood that these recommended maximum temperature rises would be exceeded during scanning. A block of Tissue Mimicking Material (TMM) with embedded thermocouples has been used in the work of Calvert et al.5 Two different transducers were tested in B-mode and Colour Flow (CF) mode for 200 s. The results were compared with analytical and numerical simulations of heat transfer from the transducer (considered as a heat source). In Killingback et al.,6 a thermal phantom was developed and the output of a transducer was maximised. In this case, the measured temperature at the interface between the transducer and the phantom exceeded the IEC recommendations after 30 minutes of exposure.
In this paper, we present the results of temperature measurements on 32 scanners in 17 hospitals made using a thermal phantom maintained at 37℃. Temperature changes were measured at the interface between the transducer and a block of TMM and at clinically relevant distances within the phantom. The experimental protocol was designed based on a survey carried out amongst sonographers to identify the conditions employed for normal clinical TVUS examinations.7 Both gynaecology and obstetrics settings were tested.
Materials and methods
Phantom
A thermal phantom was designed to measure the temperature increase due to transvaginal ultrasound examinations. The general layout of the phantom is similar to thermal phantoms developed previously at the National Physical Laboratory (NPL) for transabdominal scanning8,9 and neonatal transcranial scanning.10 Five 75 µm fine wire K-type insulated thermocouples from Omega Engineering (5SRTC-TT-KI-40-1 M, Manchester, UK) were embedded into a matrix of the standard agar-based soft tissue mimicking material described in IEC 60601-2-37, Annex DD.3 The recipe, based on a 3% w/w of agar, includes glycerol to match the speed of sound of soft tissues and additives (Silicon Carbide and Aluminium Oxide) to adjust the attenuation and backscatter coefficient. The main acoustic properties of the TMM are: an amplitude attenuation coefficient which is almost linear with frequency (0.49 ± 0.05 dB cm−1 MHz−1) and a speed of sound of 1540 m s−1 ± 1%. The density of the TMM is 1070 ± 30 kg m−3 and the thermal properties are a specific heat capacity of 3770 J kg−1 K−1 ± 3%, thermal conductivity of 0.58 W m−1 K−1 and thermal diffusivity of 0.15 mm2 s−1.11 These values are comparable with those of soft tissues. In particular, the IT’IS Database of Tissue Properties12 reports values of 3676 J kg−1 K−1 and 0.53 W m−1 K−1 for the specific heat capacity and the thermal conductivity of the uterus.
The phantom housing was designed to hold a block of TMM and contain a surrounding liquid. The block was 34 mm wide by 100 mm long by 70 mm deep, sufficient to contain the field generated by TVUS transducers without allowing significant reflected energy to return to the source. The surrounding liquid, an aqueous solution of 11.9% w/w glycerol (to prevent glycerol leaching out of the TMM), was heated to close to body temperature by a 40 W rubber-coated heating pad. The fluid was continuously circulated around the housing using a micropump with an external power supply and a PID controller to maintain the temperature. The TMM was mounted inside the phantom housing and the transducer was inserted into a fluid-filled well in the phantom to mimic the endocavitary set up and to ensure that the transducer face was not surrounded by air. A foam cover was used to reduce the heat exchange with the environment. Tests performed in our laboratories showed that the fluctuations of temperature within the TMM block were less than 0.1℃ once equilibrium was reached.
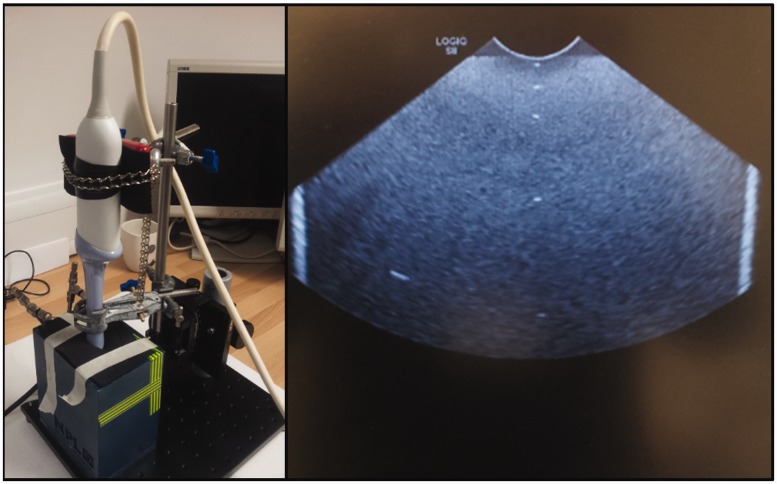
The first thermocouple was placed at the surface of the TMM block and was intended in principle to be in contact with the transducer surface. In practice, to prevent it being dragged around when the transducer was moved, this thermocouple was also embedded in the gel, with a covering layer of approximately 1 mm as measured using B-mode images. The positions of the other thermocouples were based on the work of Ramnarine et al.,13 in which the minimum, maximum and mean path length between the transducer and the embryo in transvaginal scan images was determined. Four thermocouples were placed within the TMM at half the minimum (7 mm), minimum (14 mm), mean (32 mm) and maximum (60 mm) foetal depths. The assembled device and the B-mode image of the TMM block are shown in Figure 1. The temperature data from all thermocouples were recorded using a TC08 datalogger (PicoTech, St Neots, UK) connected to a computer. The sample period was 1 s, and the time constant for the 75 µm thermocouples was approximately 100 ms. During measurements, the laptop was disconnected from the mains and run off its internal battery to reduce electrical noise and eliminate temperature offsets which were observed otherwise.
Figure 1.
Left: TVUS transducer aligned and inserted into the thermal phantom. Right: B-mode image of the phantom, the five thermocouples are clearly visible as bright spots down the centre line. The bright vertical stripes are the edges of the TMM block.
Experimental procedure
Before the experiments, the phantom was allowed to reach an equilibrium temperature. The phantom was placed on an aluminium base which was adjusted to be horizontal. It was then important to ensure that the centreline of the imaging plane of the transducer was precisely vertical so that it could be aligned through all five thermocouple junctions. The transducer was mounted on an optical post and held firmly by two clamps (Figure 1). Using a horizontal laser level for guidance, the centre of the transducer face was aligned with the axis of rotation of the optical post. This was necessary so that the height of the transducer face did not change significantly when the transducer was later rotated to align with the thermocouples. Then the transducer was gently inserted into the phantom aperture, rotated and aligned with the array of thermocouples using B-Mode imaging, aided by displaying an M-mode or Doppler line. Finally, the transducer was lowered to touch the TMM surface and a small degree of pressure was applied to compress the TMM by about 1 mm. Once the new thermal equilibrium was reached, the test was initiated.
Equipment settings and protocol
To establish the durations and the settings for a typical transvaginal scan, sonographers were consulted. Martin et al. prepared an online questionnaire and collected answers from 294 respondents.7 A group of 12 respondents were selected for more detailed follow-up in order to determine an appropriate insonation protocol. Only small differences were reported between gynaecology and obstetrics scans, so a single protocol was developed covering both. The answers showed that generally the scans last between 5 and 15 minutes, with the mean minimum time being 8.5 minutes and mean maximum time being 15.9 minutes for gynaecology, which became 7.4 minutes and 14.8 minutes for obstetrics. Most of the scanning time is spent in B-mode, while Colour Flow is used always or often by more than half of the respondents; Pulsed Wave Doppler (PW) mode is also sometimes used, with these figures only slightly varying between gynaecology and obstetrics scans. CF might be used more than once during a scan but only for 30 s for a time up to 1 or 2 minutes in total; PW is likely to be used less and for only a few seconds. Based on these answers, a well-defined protocol was developed.
The final protocol was as follows: (i) 180 s freeze (to establish a baseline temperature); (ii) 300 s B-mode; (iii) 30 s Colour Flow; (iv) 120 s B-mode; (v) 30 s Pulsed Wave Doppler; (vi) 120 s B-mode; (vii) 30 s Colour Flow; (viii) 30 s Pulsed Wave Doppler; (ix) 300 s B-mode. 3D imaging was not included in the protocol. The total duration of the test was 19 minutes, with the US on for 16 minutes. In the same survey, sonographers replied that most of the scans are performed (or at least start) using ‘pre-sets’ for either gynaecology or obstetrics. To reduce the number of variables, only these pre-sets were tested. When a sonographer was available during testing, confirmation of the selection of the correct pre-setting was obtained. As a general guideline, the ‘pre-set’ for uterus examinations was chosen for the gynaecology tests and the ‘pre-set’ for first trimester scans for the obstetrics. No other parameter was changed during the tests, but the sector for CF mode and the line for the PW mode were centred if necessary. The whole experimental procedure on a single transducer/scanner combination lasted on average 3 h.
Audit
To assess the potential thermal hazard deriving from a typical transvaginal scan, an audit was carried out at 17 hospitals around the UK, which were visited between March 2016 and August 2016. A total of 64 configurations were tested: a single configuration being defined by the combination of the individual scanner, transducer and pre-set. Some of the scanner/transducer combinations were repeated in different hospitals or departments. The scanners represented five equipment suppliers and were manufactured between 2003 and 2016. The distribution of manufacturers was consistent with the one found by Martin et al.7 A map of the locations visited is shown in Figure 2.
Figure 2.
Map of the locations visited during the audit.
Results
Results presented here show the change in temperature relative to the first 180 s, when the scanner output was frozen. A slight drift in the temperature was sometimes observed during the first 180 s; however, this was always considered negligible (average absolute value 0.03℃ over 180 s for the surface thermocouple, and lower for all the other thermocouples) and any corrections were made on the acquired data.
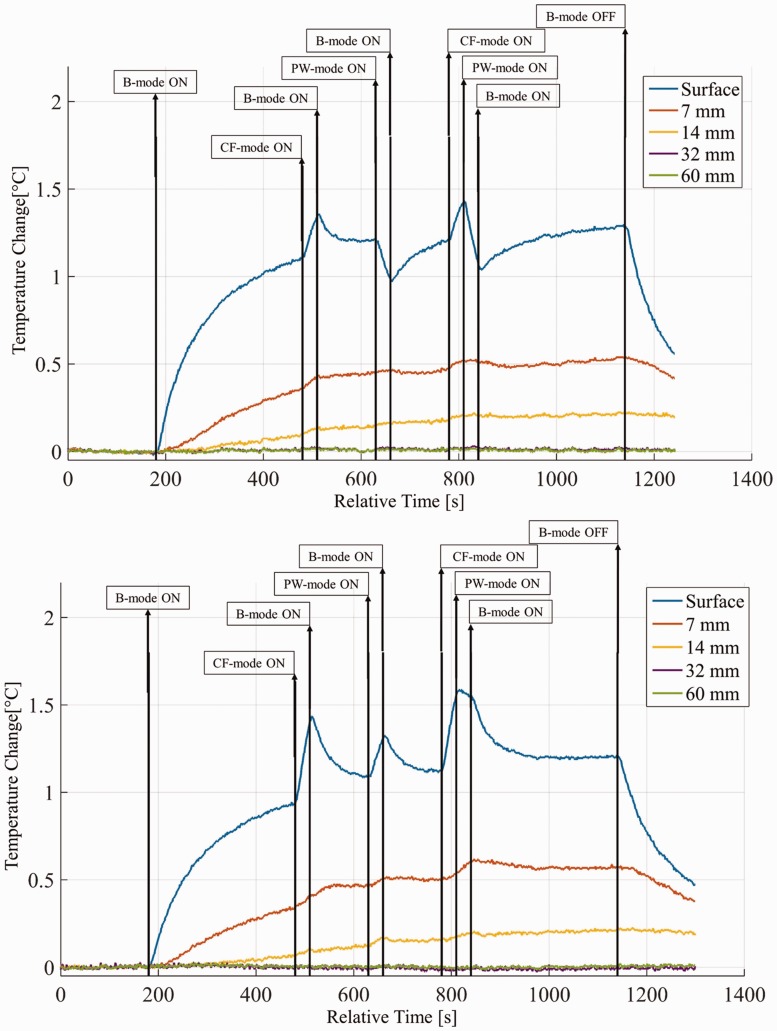
The starting temperature for the surface thermocouple varied between 33.7℃ and 36.4℃ (mean 35.6℃ ± 0.6℃). The variation was mainly due to the transducer equilibrium temperature, and reduced to 36℃ ± 0.3℃ for the second thermocouple and 36.3℃ ± 0.2℃ for the deepest one. Figure 3 shows two representative curves for the five thermocouples during a test. The times of mode change are also highlighted.
Figure 3.
Temperature profile for the gynaecology pre-settings of a GE IC5-9D transducer with a Logiq S8 scanner (top) and a Toshiba PVT-661VT with an Aplio 80 (bottom).
The results confirmed that the highest temperature increase was always at the surface. A change in mode was usually visible; however, the resulting change in temperature rise could be either positive or negative depending on the transducer and the setting. Table 1 reports a summary of representative results, in particular the maximum temperature at the surface (dTmax) and the final temperature after 16 minutes for the first three thermocouples (dTendi). Where a scanner/transducer combination is repeated, the tests were performed in different hospitals, which could imply different settings. When the tests were replicated on the same machine, by repeating the alignment procedure described in the section above, differences of less than 15% in both final and peak temperature were observed.
Table 1.
Results showing the maximum temperature rise (℃) at the surface and final temperature rise (℃) at the nearest 3 thermocouples. The two lines refer to Gynaecology and Obstetrics pre-sets respectively. If the configuration was tested more than once, values are averaged and the number of averages is in brackets. The full table is available with the online version of this article at http://journals.sagepub.com/home/ULT
| Scanner/transducer combination | dTmax | dTend1 | dTend2 | dTend3 | Scanner/transducer combination | dTmax | dTend1 | dTend2 | dTend3 |
|---|---|---|---|---|---|---|---|---|---|
| GE Logiq E9 + IC5-9D | 1.65 | 1.39 | 0.53 | 0.18 | Philips iU22 + C10-3 V | 1.29 | 0.97 | 0.50 | 0.24 |
| 2.13 | 2.12 | 0.83 | 0.36 | 1.74 | 1.54 | 0.87 | 0.40 | ||
| GE Logiq S8 + IC5-9D | 1.43 | 1.28 | 0.53 | 0.22 | Philips iU22 + C8-4 V (2) | 2.99 | 1.69 | 0.81 | 0.32 |
| 1.52 | 1.42 | 0.64 | 0.30 | 2.37 | 1.36 | 0.69 | 0.26 | ||
| GE Voluson 730 + IC5-9 | 1.97 | 1.52 | 0.82 | 0.44 | Siemens Acuson Antares + EC9-4 | 3.21 | 2.86 | 1.14 | 0.41 |
| 1.98 | 1.76 | 0.77 | 0.34 | 3.36 | 3.14 | 1.32 | 0.51 | ||
| GE Voluson 730 Expert + RIC5-9H | 1.64 | 1.16 | 0.48 | 0.15 | Siemens Acuson S2000 + MC9-4 | 2.96 | 2.65 | 1.11 | 0.41 |
| 2.23 | 2.17 | 1.01 | 0.43 | 2.80 | 2.47 | 1.17 | 0.49 | ||
| GE Voluson 730 Pro + RIC5-9 W | 1.03 | 0.99 | 0.41 | 0.17 | Toshiba Aplio 500 + 11C3 | 2.29 | 2.21 | 0.91 | 0.39 |
| 1.28 | 1.27 | 0.53 | 0.23 | 2.86 | 2.82 | 1.30 | 0.63 | ||
| GE Voluson E6 + IC5-9D | 1.14 | 1.13 | 0.46 | 0.19 | Toshiba Aplio 500 + 9C3 | 3.28 | 1.98 | 0.96 | 0.46 |
| 1.30 | 1.29 | 0.59 | 0.30 | 3.36 | 2.09 | 0.88 | 0.34 | ||
| GE Voluson E6 + RIC5-9D | 1.53 | 1.47 | 0.63 | 0.23 | Toshiba Aplio 500 + 9CV3 | 1.86 | 1.67 | 0.58 | 0.19 |
| 0.82 | 0.76 | 0.36 | 0.19 | 1.92 | 1.78 | 0.87 | 0.36 | ||
| GE Voluson E8 + RIC6-5D | 1.71 | 1.46 | 0.66 | 0.24 | Toshiba Aplio 500 + PVT-661 VT (2) | 2.64 | 2.13 | 0.87 | 0.33 |
| 1.88 | 1.62 | 0.82 | 0.36 | 2.31 | 1.92 | 0.91 | 0.34 | ||
| GE Voluson-E + IC5-9 W-RS | 1.73 | 1.72 | 0.63 | 0.22 | Toshiba Aplio 80 + PVT-661 VT | 1.58 | 1.21 | 0.57 | 0.21 |
| 1.23 | 1.21 | 0.51 | 0.22 | 1.57 | 1.03 | 0.50 | 0.21 | ||
| Hitachi Ascendus + 42410 | 1.79 | 1.59 | 0.64 | 0.22 | Toshiba Aplio MX + PVT-661 VT | 1.90 | 1.75 | 0.77 | 0.28 |
| 1.44 | 1.32 | 0.52 | 0.19 | 2.38 | 1.95 | 1.02 | 0.35 | ||
| Philips CX50 + C10-3 V | 2.68 | 1.25 | 0.66 | 0.34 | Toshiba Aplio XG + PVT-661 VT (4) | 2.08 | 1.93 | 0.80 | 0.28 |
| 2.84 | 1.61 | 0.83 | 0.40 | 2.15 | 2.12 | 1.02 | 0.41 | ||
| Philips Envisor HD + C8-4 V | 2.60 | 2.11 | 0.96 | 0.42 | Toshiba Viamo + PVT-661 VT | 1.84 | 0.95 | 0.58 | 0.25 |
| 1.75 | 1.33 | 0.62 | 0.24 | 0.27 | 0.22 | 0.18 | 0.09 | ||
| Philips iU22 + 3D9-3 V | 1.59 | 0.51 | 0.24 | 0.09 | Toshiba Xario + PVT-661 VT | 2.71 | 2.69 | 1.24 | 0.48 |
| 3.04 | 2.34 | 1.19 | 0.56 | 1.88 | 1.51 | 0.71 | 0.27 |
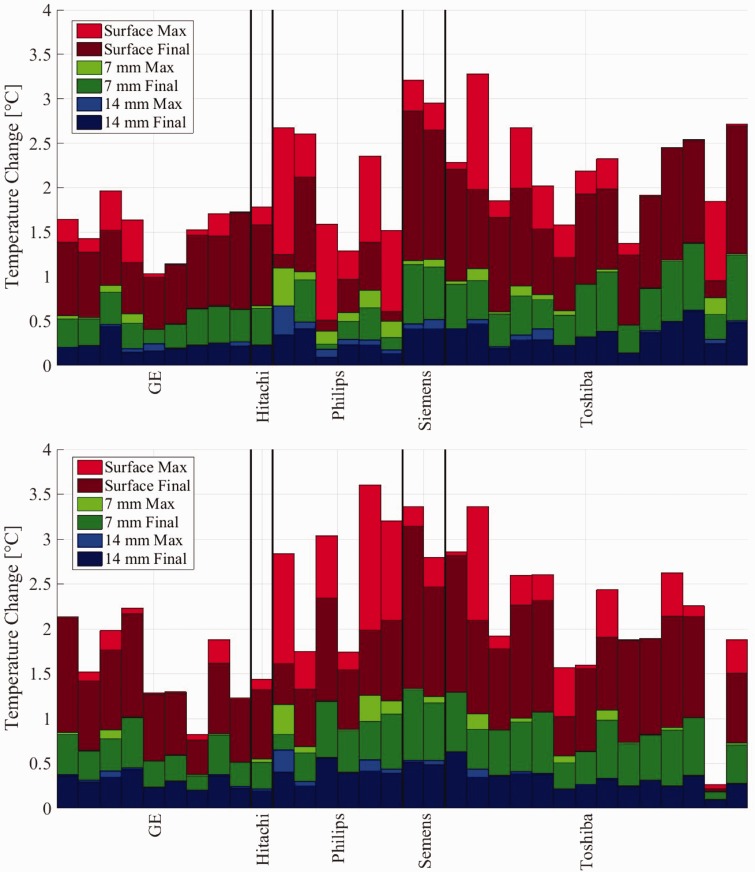
Figure 4 shows the final and maximum temperatures for gynaecology (top) and obstetrics (bottom) settings. These graphs show the results for the surface thermocouple (red) as well as the thermocouples at 7 mm (green) and 14 mm (blue). The temperature elevation at the end of the final B-mode insonation (at 19 minutes) is shown in dark red. If the peak temperature during the test was higher than the final temperature (e.g. during one of the CF or PW mode insonations), this is shown as light red. Dark and light shading is used similarly for the 7 and 14 mm thermocouples.
Figure 4.
Temperature change for the three thermocouples at the surface and 7 mm and 14 mm for gynaecology (top) and obstetrics (bottom) pre-sets. Dark colours refer to the temperature at the end of the protocol, while light colours indicate the highest temperature reached.
For gynaecology scans, at the surface, the average for the final temperature increase was 1.62 ± 0.60℃ (min. 0.51℃, max. 2.86℃) and the peak increase was 2.00 ± 0.62℃ (min. 1.03℃, max. 3.28℃). At 7 mm, the average for the final temperature was 0.71 ± 0.27℃ (min. 0.24℃, max. 1.24℃) and the peak increase was 0.78 ± 0.27℃ (min. 0.38℃, max. 1.25℃). For the thermocouple at 14 mm depth, the average for the final temperature increase was 0.28 ± 0.12℃ (min. 0.09℃, max. 0.50℃) and the peak increase was 0.33 ± 0.14℃ (min. 0.14℃, max. 0.67℃).
For obstetrics scans, at the surface the average for the final temperature increase was 1.80 ± 0.54℃ (min. 0.76℃, max. 3.14℃) and the peak increase was 2.16 ± 0.75℃ (min. 0.82℃, max. 3.61℃). At 7 mm, the average for final temperature was 0.82 ± 0.25℃ (min. 0.36℃, max. 1.32℃) and the peak increase was 0.87 ± 0.27℃ (min. 0.37℃, max. 1.33℃). Finally, for the thermocouple at 14 mm depth, the average for the final temperature increase was 0.34 ± 0.11℃ (min. 0.19℃, max. 0.63℃) and the peak increase was 0.38 ± 0.13℃ (min. 0.20℃, max. 0.65℃).
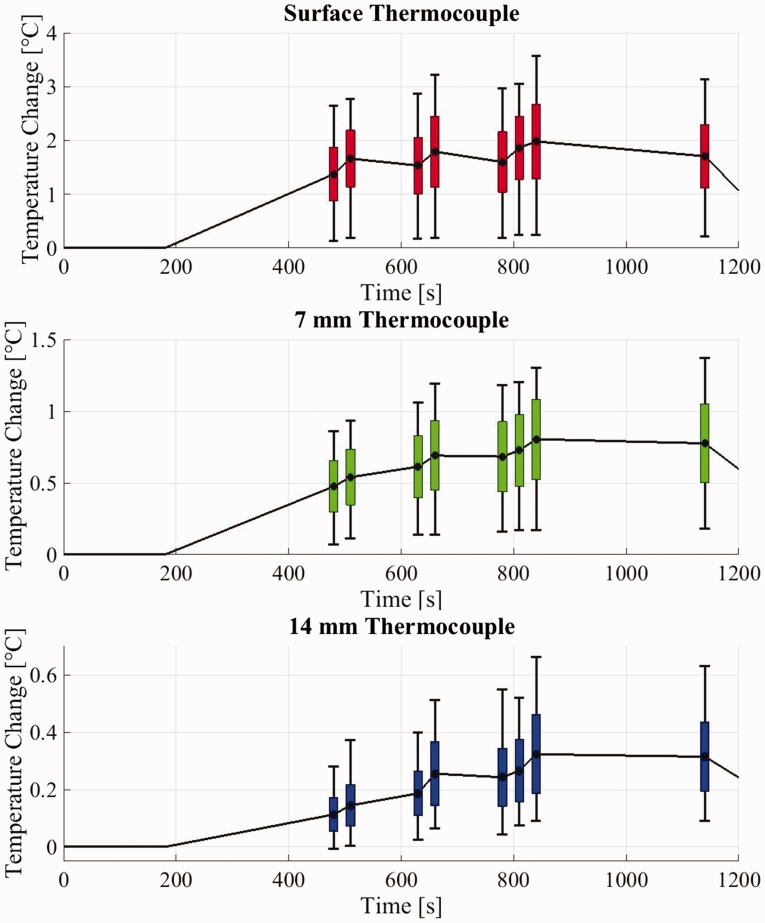
The plots in Figure 5 show the range of temperature increases for both pre-sets, for the 64 configurations tested, at specific points during the protocol, for the first three thermocouples. The dots represent the average value at the switch times (as reported in the materials and methods section), the boxes show the standard deviation, and the error bars represent the minima and maxima.
Figure 5.
Average, standard deviation and outer values for temperature changes at specific points during the protocol for the three thermocouples at the surface and 7 mm and 14 mm deep.
Although there was some variation in the results, both CF and PW modes generated on average a greater temperature rise than B-mode, both at the surface and deeper in the phantom. Looking first at the surface temperature plot, a temperature increase of up to 1.44℃ (mean 0.29 ± 0.30℃) in 30 s was registered when CF mode was activated after 5 minutes of B-mode (between 480 s and 510 s). Activation of PW mode for 30 s (between 630 s and 660 s) generated temperature increases of up to 1.28℃ (mean 0.26 ± 0.32℃), while 30 s of CF followed by 30 s of PW (between 780 s and 840 s) generated a temperature rise of up to 1.68℃ (mean 0.38 ± 0.40℃) after 10 minutes of exposure. The increases were still visible at 7 mm, where the maximum temperature variations are 0.28℃, 0.24℃ and 0.43℃, respectively. Deeper into the TMM these effects were less evident, but still measurable.
Discussion
A thermal phantom for the assessment of the temperature elevation during transvaginal ultrasound scans was developed and tested in 17 hospitals using clinical settings and a scanning protocol representative of typical clinical practice. During the audit, the number of variables and settings were reduced to a minimum in order to give an overview of the equipment around the UK at its clinical settings. For this reason, only pre-sets were used and the outputs were not maximised (for example by using zoom, or greater depth or narrow sector angles). Consequently, higher temperatures than those measured can certainly be achieved. On the other hand, in practice, other factors such as movement of the transducer, perfusion and the presence of low attenuation amniotic fluid could lead to lower temperature changes deeper into the tissue. The TMM was selected to mimic the average acoustic and thermal properties of soft tissues. However, real tissues present a big variability and some differences from TMMs (e.g. the ratio between attenuation and absorption). These considerations should be taken into account when translating these results to real clinical situations.
All of the transducers tested with this particular protocol, fell within the recommendations from National (BMUS) and International (IEC) Committees. However, activation of Doppler modes such as CF or PW can produce measurable temperature increases. Previous studies have shown that when the output is maximised, the temperature can exceed recommendations if the transducer is left on for a long period, although very long exposures are not representative of routine clinical practice.6 In our tests, an increase of more than 3℃ at the surface of the phantom was observed seven times in 64 experiments. Temperature rise of more than 1℃ was recorded at a depth of 7 mm in 22 cases, while at 14 mm only in 12 cases did the temperature rise by more than 0.5℃. At a depth of 32 mm, no variation in temperature was observed.
The results confirm that the highest temperature increase occurred at the interface between the transducer and the phantom. Since no major increase was observed when the transducer was active but not in contact with the phantom (e.g. during the alignment), and consistent with previous studies, self-heating of the transducer is expected to be the major cause of this elevation. The temperature variations registered at the surface of the phantom were in line with previous works.5 Deeper in tissue, absorption will play a more significant role in determining the overall temperature change.
It is interesting to compare the measured temperature rise with the displayed TI value. Since TI is intended to represent the ‘long time’ temperature elevation, the simplest comparison is the ratio of dTend to the B-mode TI for each of the first three thermocouples, which was manually recorded when available (here not considered if they were soft tissue or bone TI). At the surface, the average ratio was 8.0 ± 4.7 (range 1.2 to 17.2); at 7 mm the average ratio was 3.7 ± 2.0 (range 0.5 to 8.2); and at 14 mm the average ratio was 1.6 ± 0.8 (range 0.2 to 3.6). So even at depths of 14 mm, TI tends to underestimate the temperature rise. There are only a small number of Siemens and Hitachi combinations represented, and in most cases Toshiba machines did not display the TI. However, comparison of the GE and Philips systems is interesting. Looking just at this ratio at the surface, the average value for all Philips systems was 5.75; for the GE Logiq systems it was 2.62 (based only on 4 measurements), and for the GE Voluson systems it was 11.8. It appears that the difference is mostly due to the displayed TI value rather than to the surface temperature.
Conclusions
Overall, the results of the audit for the temperature rise during transvaginal ultrasound at clinical settings fell within the limits indicated by the national and international standards, for the pre-sets tested and following a representative typical scanning protocol. The greatest temperature rise measured across all the systems was 3.6℃ at the transducer surface. The protocol that was followed primarily used B-mode with 30-second periods of colour-flow and pulsed Doppler. For some systems, the temperature increased rapidly when selecting one of these other modes, so using them for longer than 30 s would have been likely to lead to greater heating in many cases, even if it is unusual during clinical scans. It is also important to recognise that only selected pre-sets have been tested and the scanner outputs have not been maximised (for example by using zoom, or greater depth or narrow sector angles). Consequently, higher temperatures could certainly be achieved than were measured, even using the same protocol.
In agreement with previous work, it has been shown14 that displayed thermal index greatly underestimates the temperature rise, particularly close to the transducer face but even to distances approaching 2 cm.
Supplementary Material
Acknowledgment
The authors wish to thank all the departments visited during the audit and the scientists and staff who greatly helped the work.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. Any mention of commercially available ultrasound equipment should not be seen as an endorsement.
Funding
The author(s) disclosed receipt of the following financial support for the research authorship and/or publication of this article: This report is an independent research commissioned and funded by the Department of Health Policy Research Programme (Transvaginal Ultrasound Scanning– Hazard and Clinical Practice, 091/0206).
Ethical approval
Not applicable
Guarantor
PM
Contributorship
EM and AS designed the hardware. All authors created the experimental protocol, PM and AS carried out the tests and analysed the data. All authors contributed to, reviewed and approved the final version of the manuscript.
Supplementary material
Supplementary material for this paper can be found at http://ult.sagepub.com/doi/suppl/10.1177/1742271X16684529
References
- 1.NHS England Analytical Services. NHS Diagnostic Imaging Dataset 2015/16 Release, https://www.england.nhs.uk/statistics/statistical-work-areas/diagnostic-imaging-dataset/diagnostic-imaging-dataset-2015-16-data/ (2016, accessed 4 October 2016).
- 2.ter Haar G. Results of a survey of exposure conditions used in ultrasound scans in the UK, February 2007. Ultrasound 2008; 16: 110–113. [Google Scholar]
- 3.International Electrotechnical Commission. IEC 60601-2-37 Medical electrical equipment — particular requirements for the safety of ultrasonic medical diagnostic and monitoring equipment, Geneva: IEC, 2005. [Google Scholar]
- 4.Safety Group of the British Medical Ultrasound Society. Guidelines for the safe use of diagnostic ultrasound equipment, http://www.bmus.org/policies-guides/BMUS-Safety-Guidelines-2009-revision-DETAILED.pdf (2009, accessed 26 August 2016).
- 5.Calvert J, Duck F, Clift S, et al. Surface heating by transvaginal transducers. Ultrasound Obstet Gynaecol 2007; 29: 427–432. [DOI] [PubMed] [Google Scholar]
- 6.Killingback AL, Newey VR, El-Brawany MA, et al. Development of a thermal test object for the measurement of ultrasound intracavity transducer self-heating. Ultrasound Med Biol 2008; 34: 2035–2042. [DOI] [PubMed] [Google Scholar]
- 7.Martin E, Shaw A, Lees C. Survey of current practice in clinical transvaginal ultrasound scanning in the UK. Ultrasound 2015; 23: 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ISUOG. ISUOG ultrasound safety phantom project, http://www.isuog.org/AboutUs/Committees/Safety+Committee/ISUOG_Safety_Phantom.htm (2012, accessed 31 August 2016).
- 9.Axell RG, Smith SF, Abramowicz J, et al. The ISUOG phantom pilot study: does the preset matter? Ultrasound Obstet Gynaecol 2013; 42: 105. [Google Scholar]
- 10.Memoli G, Gatto M, Sadhoo N, et al. Building and assessing anatomically relevant phantoms for neonatal transcranial ultrasound. J Phys Conf 2011; 279: 012007–012007. [Google Scholar]
- 11.Brewin MP, Pike LC, Rowland DE, et al. The acoustic properties, centered on 20 MHz, of an IEC agar-based tissue-mimicking material and its temperature, frequency and age dependence. Ultrasound Med Biol 2008; 34: 1292–1306. [DOI] [PubMed] [Google Scholar]
- 12.Hasgall PA, Di Gennaro F, Baumgartner C, et al. IT’IS Database for thermal and electromagnetic parameters of biological tissues. Version 3.0, 1 September 2015, www.itis.ethz.ch/database (accessed 5 October 2016).
- 13.Ramnarine KV, Nassiri DK, Pearce JM, et al. Estimation of in situ ultrasound exposure during obstetric examinations. Ultrasound Med Biol 1993; 19: 319–329. [DOI] [PubMed] [Google Scholar]
- 14.Shaw A, Pay NM and Preston RC. Assessment of the likely thermal index values for pulsed Doppler ultrasonic equipment – stages II and III: experimental assessment of scanner/transducer combinations. NPL REPORT CMAM 12, 1998.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.