Abstract
A planar energetic molecule with high density, 5,5′-dinitramino-3,3′-azo-1,2,4-oxadiazole (4), was obtained by the nitration of 5,5′-diamino-3,3′-azo-1,2,4-oxadiazole using 100% nitric acid. In addition, selected nitrogen-rich salts were prepared. Among them, the neutral compound 4 and its hydroxylammonium salt, 6, were further confirmed by single-crystal X-ray diffraction. Physicochemical and energetic properties including density, thermal stability and sensitivity were investigated. The energetic performance from the calculated heats of formation and experimental densities indicates that many of them have potential applications as energetic materials.
Keywords: oxadiazole, planar structure, nitramino group, azo bridge, energetic materials
Graphical abstract
A dense compound with a planar structure, 5,5′-dinitramino-3,3′-azo- 1,2,4-oxadiazole, has been synthesized, as well as its energetic salts. Some of them were found to have potential applications as energetic compounds.
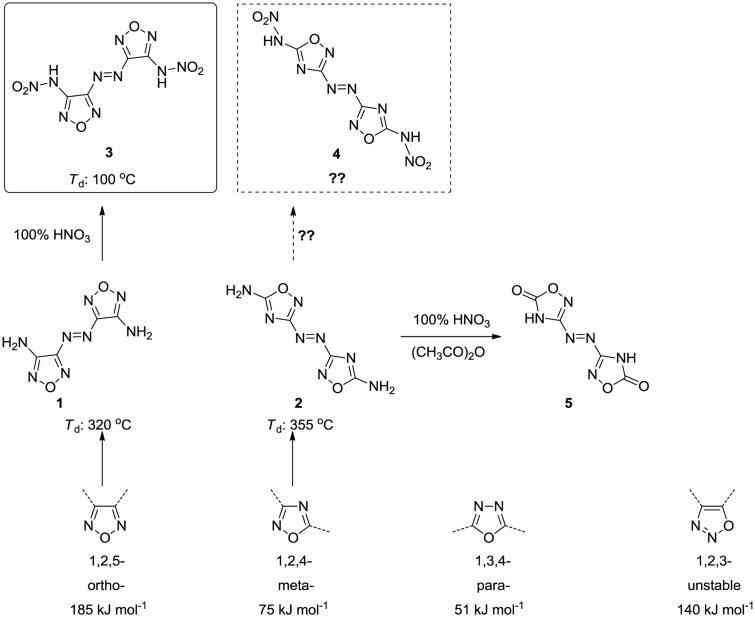
One of the most attractive approaches to the syntheses of dense energetic compounds is through molecules with planar structures.[1] Such a structure could lead to high density, good detonation performance and low sensitivity.[2] Furthermore, a single nitrogen-rich heterocyclic ring (such as tetrazole[3], triazole[4], pyrazole[5] and oxadiazole[6]) with diverse energetic functional groups also is aromatic and has attracted considerable interest. Most studies show that when these heterocyclic azoles are connected by azo bridges, not only is the planar structure maintained, but also the heat of formation is enhanced.[7]
The presence of the oxadiazole ring allows good oxygen balance to be achieved readily. There are four isomers of oxadiazole: 1,2,5-oxadiazole (furazan), 1,2,4-oxadiazole, 1,3,4-oxadiazole and 1,2,3-oxadiazole. Since molecular structure determines properties, insight into the oxadiazole structures helps to understand the key factors that determine their thermal stability and detonation properties. Very often energetic compounds based on oxadiazole are disubstituted. Apart from the unstable 1,2,3-oxadiazole, the other three isomers can form ortho-, meta-, and para-linked compounds (Scheme 1); such a combination can influence both the electron delocalization and the degree of conjugation. Additionally, the properties of the oxadiazole derivatives depend significantly on both the position of the substituents with respect to the oxadiazole and on different energetic moieties, such as 5,5′-diamino-3,3′-azo-1,2,5- oxadiazole (1)[8] and 5,5′-diamino-3,3′-azo-1,2,4-oxadiazole (2)[9]. Although both of the isomers have a planar structure, they have different thermal stabilities (Scheme 1).
Scheme 1.
Four different regioisomeric forms of oxadiazole and planar disubstituted ortho/meta oxadiazole derivatives.
Further nitration of 5,5′-diamino-3,3′-azo-1,2,5-oxadiazole with 100% nitric acid led to the formation of the dinitramino substituted product, 5,5′-dinitramino-3,3′-azo-1,2,5-oxadiazole (3).[10] Although 3 is very sensitive very sensitive toward impact and friction, but it has a high density and excellent detonation properties. Based on these attractive properties, we were encouraged to synthesize 5,5′-dinitramino-3,3-azo'-1,2,4- oxadiazole (4), which has a nitramino substituent and an azo bridge; thus, expected to be planar and to exhibit good detonation performance.
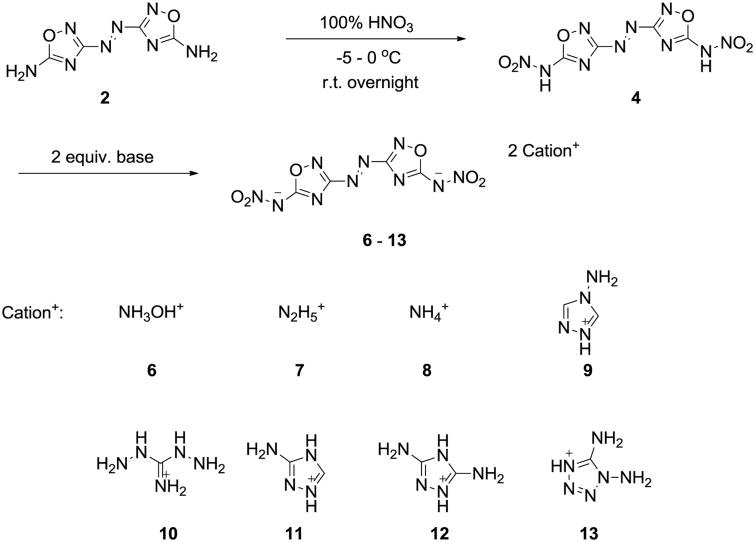
Many attempts to nitrate 5,5′-diamino-3,3-azo-1,2,4-oxadiazole (2) using a variety of nitrating reagents failed to yield the desired compound, 4.[9] When a mixture of 100% nitric acid and acetic anhydride was used, the carbonyl substituted product, 5, rather than 4, was formed. However, by using 100% nitric acid, the synthesis of 4 was successful when it precipitated from the acid solution at room temperature. The pure product was obtained in good yield by filtration followed by washing with trifluoroacetic acid. A series of energetic salts 6–13 were easily prepared by reacting 4 with two equivalents of energetic bases (Scheme 2).
Scheme 2.
Synthesis of 5,5′-dinitramino-3,3′-azo-1,2,4-oxadiazole (4) and its energetic salts.
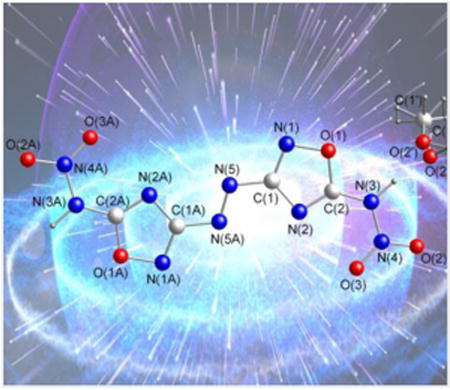
Compound 4 crystallizes in the orthorhombic space group Pbca with a density of 1.634 g cm-3 at 100 K (Figure 1a). The presence of solvated methanol gives rise to the low calculated density. The bond length of the oxadiazole ring are in accordance with those of other oxadiazoles.[9,11] The bond length of N5-N5A is 1.258 Å, slightly shorter than that in 3 at 1.261 Å.[13] The entire molecule is essentially planar as shown by the torsion angles of C2-N3-N4-O3 (-1.7 °) and N5A-N5-C1-N2 (-1.3 °). The packing structure of 4 along the c axis can be seen in Figure 1b. Extensive hydrogen bonding interactions between oxygen atom O2 from the nitro group and N2 [N2···O4 2.875 Å], as well as the oxygen atom O2′(O2”) and N3 are observed (Figure 1b).
Figure 1.
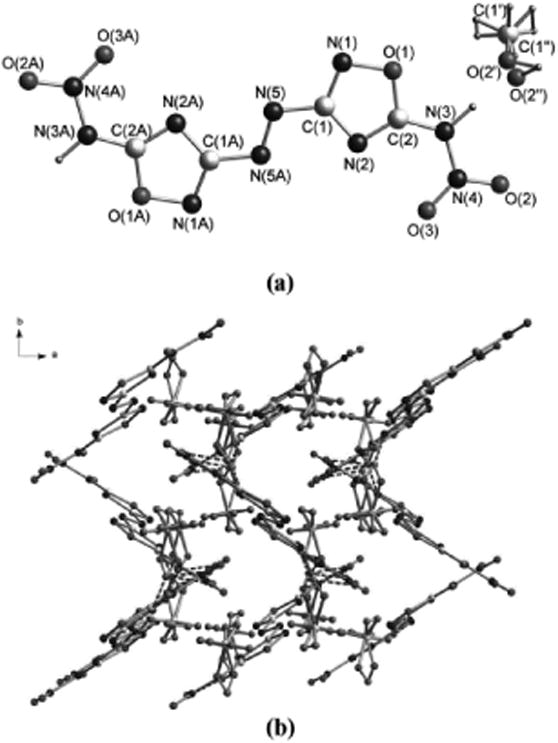
(a) Single-crystal X-ray structure of 4 with thermal ellipsoids shown at 50% probability. (b) A view of the packing down the c-axis of the unit cell for 4.
Compound 6 crystallizes as a dihydrate in the triclinic space group P-1 with a density of 1.750 g cm-3 at 296 K. The oxadiazole rings have bond lengths and angles comparable to 4. The azo double bond distance is 1.248 Å, slightly shorter than that in the parent compound 4. The anion has a planar structure, and considerable hydrogen bonding interactions between the cation and anion are seen in Figure 2b. The details can be found in the Supporting Information (SI).
Figure 2.
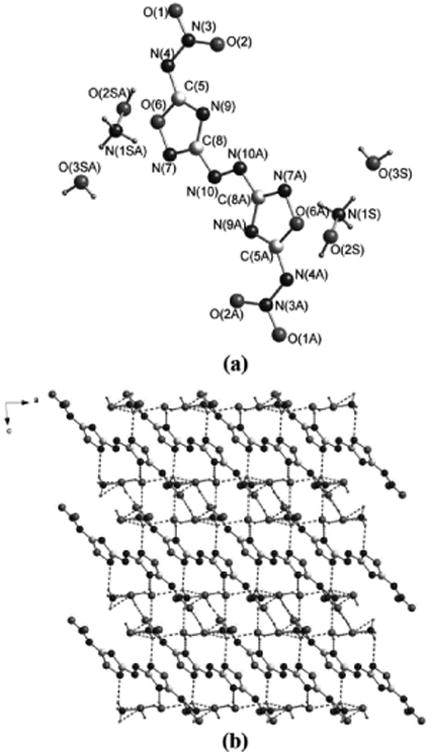
(a) Single-crystal X-ray structure of 6 with thermal ellipsoids shown at 50% probability. (b) A view of the packing down the b-axis of the unit cell for 6.
The values for the decomposition temperature, density, heat of formation, calculated detonation properties as well as sensitivity are given in Table 1. Compounds 4, 6 and 7 have higher experimental densities than that of RDX. Compound 4 with a density of 1.902 g cm-3 (25 °C) is the densest compound in this work and is slightly higher than that of 3 (1.890 g cm-3). The measured densities for 6 and 7 are 1.864 and 1.811 g cm-3, respectively. All of the compounds exhibit positive heats of formation. Among them, because of the presence of two 1,5-diaminotetrazolate cations, 13 has the highest value at 3.47 kJ g-1.
Table 1. Physicochemical and energetic properties of compounds 3, 4, 6–13 compared with RDX.
| ρ[a] (g·cm-3) | Dv[b] (m s-1) | P[c] (GPa) | ΔH[d]f(kJ mol-1)/(kJ g-1) | Tdec[e] (°C) | IS[f] (J) | FS[g] (N) | |
|---|---|---|---|---|---|---|---|
| 3[h] | 1.890 | 9517 | 41.1 | 820.2/2.86 | 100 | 2 | 10 |
| 4 | 1.902 | 9190 | 37.5 | 487.2(1.70) | 140 | 2 | 10 |
| 6 | 1.864 | 9243 | 39.2 | 514.2(1.46) | 169 | 10 | 160 |
| 7 | 1.811 | 9240 | 35.8 | 726.5(2.07) | 175 | 8 | 120 |
| 8 | 1.752 | 8670 | 31.1 | 411.8(1.29) | 261 | 12 | 240 |
| 9 | 1.737 | 8557 | 29.2 | 1220.0(2.69) | 213 | 20 | 240 |
| 10 | 1.705 | 8570 | 27.6 | 711.5(1.53) | 160 | 17 | 160 |
| 11 | 1.741 | 8381 | 27.5 | 938.6(2.07) | 241 | 18 | 160 |
| 12 | 1.755 | 8466 | 27.7 | 904.8(1.87) | 183 | 16 | 160 |
| 13 | 1.738 | 8955 | 32.8 | 1685.6(3.47) | 188 | 12 | 120 |
| RDX | 1.80 | 8795 | 34.9 | 70.3/(0.32) | 204 | 7.5 | 120 |
Density measured by a gas pycnometer at 25 °C;
Calculated detonation velocity;
Calculated detonation pressure;
Calculated molar enthalpy of formation in solid state;
Temperature of decomposition (onset);
Impact sensitivity;
Friction sensitivity;
ref. 10.
The precursor dinitramino compound 4 exhibits a low decomposition temperature at 140 °C. However, it is still considerably higher than the 1,2,5-dinitramino derivative 3 (Tdec = 100 °C). In addition, the ammonium salt, 8, is the most thermally stable at 261 °C. The decomposition temperatures of 9 and 11 also exceed 200 °C, which is higher than RDX. Both 7 and 8 have higher decomposition temperatures than the ortho substituted (5,5′-dinitramino-3,3′-azo-1,2,5-oxadiazole, analogues. The others show relatively high decomposition temperatures, which vary from 160 °C (10) to 188 °C (13).
The relatively high thermal stability of the dinitramino compound 4 tweaked our interest. The nucleus independent chemical shift (NICS) has been demonstrated to be an important parameter employed in the evaluation of thermal stability based on the anisotropic effects of π conjugation.[12] By using Multiwfn v3.3.6,[13] the shielding maps and anisotropic effects of 3 and 4 visualized as isochemical shielding surfaces (ICSS)[14] were calculated and are given in the SI (Figures S1 and S2). It can be seen that in the 1,2,4-oxadiazole ring (4) the shielding surfaces are larger than in the 1,2,5-oxadiazole ring (3). There is still deshielding associated with the azo bridge in 4 which indicates that the π delocalization in 4 is more uniform than that in 3. In addition, the π conjugation in 4 is much more prevalent than in 3, since the C-N bond takes part in the delocalized π-electron system, but does not in 3. Thus, these results support the observation that 4 (Tdec = 140 °C) has a higher thermal stability than 3 (Tdec = 100 °C), which is mainly due to the adequately increased π conjugation.
The sensitivities towards impact and friction were determined using BAM methods.[15] Compound 4 is very sensitive with an impact sensitivity of 2 J and a friction sensitivity of 10 N. In contrast, its energetic salts (6–13) show moderate impact and friction sensitivities. By using the calculated heats of formation and the experimental densities (gas pycnometer) of the new energetic compounds, the detonation pressures (P) and detonation velocities (Dv) were calculated by using the EXPLO5 v6.01 program.[16] As can be seen in Table 1, the neutral compound, 4, the hydroxylammonium salt, 6, and the hydrazinium salt, 7, show excellent detonation properties which are much higher than those of RDX. Compound 6 has a detonation velocity of 9243 m s-1 and detonation pressure of 39.2 Gpa. These properties coupled with the rather high thermal stability and hydrolytic stabilities make 6 a very attractive candidate for energetic applications. For 8–13, the calculated detonation velocities fall in the range between 8381 m s-1 (11) and 8955 m s-1 (13), and detonation pressures from 27.5 GPa (11) to 32.8 GPa (13).
In conclusion, 5,5′-dinitramino-3,3′-azo-1,2,4-oxadiazole (4) with a planar structure and its nitrogen-rich ionic derivatives (6–13) were synthesized and fully characterized. The neutral compound 4 is very sensitive, but its salts possess good detonation properties and relatively low sensitivities to impact and friction. The hydroxylammonium salt, 6, exhibits the best detonation performance of these compounds with a detonation velocity of 9243 m s-1 and detonation pressure of 39.2 GPa, which are much higher than those of RDX. These superior detonation properties together with the moderate thermal stability (Td: 169 °C) and sensitivity (IS: 10 J; FS: 160 N) make it a very attractive energetic material.
Supplementary Material
Acknowledgments
We are grateful to the Office of Naval Research (N00014-12-1-0536) and the Defense Threat Reduction Agency (HDTRA 1-11-1-0034). The authors acknowledge Dr. Orion Berryman (NSF—CHE1337908 and CoBRE NIGMS P20GM103546) for considerable assistance with crystal structuring.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- 1.Ma Y, Zhang A, Zhang C, Jiang D, Zhu Y, Zhang C. Crys Growth Des. 2014;14:4703–4713. [Google Scholar]
- 2.a) Gao H, Shreeve JM. Chem Rev. 2011;111:7377–7436. doi: 10.1021/cr200039c. [DOI] [PubMed] [Google Scholar]; b) Zhang C, Wang X, Huang H. J Am Chem Soc. 2008;130:8359–8365. doi: 10.1021/ja800712e. [DOI] [PubMed] [Google Scholar]
- 3.a) Fischer D, Klapötke TM, Stierstorfer J. Angew Chem Int Ed. 2015;54:10299–10302. doi: 10.1002/anie.201502919. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2015;127:10438–10441. [Google Scholar]; b) Göbel M, Karaghiosoff K, Klapötke TM, Piercey DG, Stierstorfer J. J Am Chem Soc. 2010;132:17216–17226. doi: 10.1021/ja106892a. [DOI] [PubMed] [Google Scholar]
- 4.Haiges R, Bélanger-Chabot G, Kaplan SM, Christe KO. Dalton Trans. 2015;44:2978–2988. doi: 10.1039/c4dt03583f. [DOI] [PubMed] [Google Scholar]
- 5.Hervé G, Roussel C, Graindorge H. Angew Chem Int Ed. 2010;49:3177–3181. doi: 10.1002/anie.201000764. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2010;122:3245–3249. [Google Scholar]
- 6.a) He C, Shreeve JM. Angew Chem Int Ed. 2016;55:772–775. doi: 10.1002/anie.201509209. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2016;128:782–785. [Google Scholar]; b) Tang Y, Gao H, Mitchell LA, Parrish DA, Shreeve JM. Angew Chem Int Ed. 2016 doi: 10.1002/anie.201509985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Klapötke TM, Piercey DG. Inorg Chem. 2011;50:2732–2734. doi: 10.1021/ic200071q. [DOI] [PubMed] [Google Scholar]; b) Tang Y, Yang H, Shen J, Wu B, Ju X, Lu C, Cheng G. New J Chem. 2012;36:2447–2450. [Google Scholar]; c) Li Y, Qi C, Li S, Zhang H, Sun C, Yu Y, Pang S. J Am Chem Soc. 2010;132:12172–12173. doi: 10.1021/ja103525v. [DOI] [PubMed] [Google Scholar]; d) Yin P, Parrish DA, Shreeve JM. Chem Eur J. 2014;20:6707–6712. doi: 10.1002/chem.201402762. [DOI] [PubMed] [Google Scholar]
- 8.a) Chavez D, Hill L, Hiskey M, Kinkead S. J Energ Mater. 2000;18:219–236. [Google Scholar]; b) Zhou Q, Wang B, Zhang Y, Huo H, Li H, Ma L. Chinese J Explos Propellants. 2013;36:16–19. [Google Scholar]
- 9.Thottempudi V, Zhang J, He C, Shreeve JM. RSC Adv. 2014;4:50361–50364. [Google Scholar]
- 10.Zhang J, Shreeve JM. J Phys Chem C. 2015;119:12887–12895. [Google Scholar]
- 11.a) Kettner MA, Karaghiosoff K, Klapötke TM, Sućeska M, Wunder S. Chem Eur J. 2014;20:7622–7631. doi: 10.1002/chem.201402291. [DOI] [PubMed] [Google Scholar]; b) Klapötke TM, Mayr N, Stierstorfer J, Weyrauther M. Chem Eur J. 2014;20:1410–1417. doi: 10.1002/chem.201303825. [DOI] [PubMed] [Google Scholar]; c) Fu Z, Su R, Wang Y, Wang Y, Zeng W, Xiao N, Wu Y, Zhou Z, Chen J, Chen F. Chem Eur J. 2012;18:1886–1889. doi: 10.1002/chem.201103159. [DOI] [PubMed] [Google Scholar]; d) Fu Z, Wang Y, Yang L, Su R, Chen J, Nie F, Huang J, Chen F. RSC Adv. 2014;4:11859–11861. [Google Scholar]
- 12.a) Ghule VD, Radhakrishnan S, Jadhav PM, Tewari SP. J Energetic Mat. 2013;31:35–48. [Google Scholar]; b) Ghule V, Sarangapani R, Jadhav P, Tewari S. J Mol Model. 2011;17:1507–1515. doi: 10.1007/s00894-010-0848-8. [DOI] [PubMed] [Google Scholar]
- 13.Lu T, Chen F. J Comput Chem. 2012;33:580–592. doi: 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- 14.a) Klod S, Kleinpeter E. J Chem Soc Perkin Trans. 2001;2:1893–1898. [Google Scholar]; b) Fallah-Bagher-Shaidaei H, Wannere CS, Corminboeuf C, Puchta R, Schleyer PvR. Org Lett. 2006;8:863–866. doi: 10.1021/ol0529546. [DOI] [PubMed] [Google Scholar]; c) Chen Z, Wannere CS, Corminboeuf C, Puchta R, Schleyer PvR. Chem Rev. 2005;105:3842–3888. doi: 10.1021/cr030088+. [DOI] [PubMed] [Google Scholar]; d) Schleyer PvR, Maerker C, Dransfeld A, Jiao H, Hommes NJRvE. J Am Chem Soc. 1996;118:6317–6318. doi: 10.1021/ja960582d. [DOI] [PubMed] [Google Scholar]
- 15.a) Tests were conducted according to the UN Recommendations on the Transport of Dangerous Goods, Manual of Tests and Criteria. 5th rev. United Nations Publication; New York: 2009. [Google Scholar]; b) 13.4.2 Test 3 (a)(ii) BAM Fallhammer. :75–82. [Google Scholar]; c) 13.5.1 Test 3 (b)(i): BAM friction apparatus. :104–107. [Google Scholar]
- 16.Sućeska M. EXPLO5 6.01. Brodarski Institute; Zagreb, Croatia: 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.