Abstract
Chronic lymphocytic leukemia, the most common adult leukemia worldwide, is considered an indolent but incurable non-Hodgkin lymphoma. Leukemia cutis is an uncommon manifestation of chronic lymphocytic leukemia. We present a case of an adult patient who presented with skin lesion of bilateral ears, which led to the diagnosis of chronic lymphocytic leukemia. We also reviewed the cases of auricular involvement in chronic lymphocytic leukemia patients reported in the literature. Local treatment is indicated in case of leukemia cutis; however, systemic treatment is recommended when there are systemic signs and symptoms. Better awareness of disease evolution and prompt diagnosis of this leukemia cutis of chronic lymphocytic leukemia will improve the effectiveness and outcome of its management.
Keywords: Chronic lymphocytic leukemia, leukemia cutis, ear involvement
Introduction
Chronic lymphocytic leukemia (CLL) is considered an indolent but incurable non-Hodgkin lymphoma (NHL). It is the most common adult leukemia worldwide. Leukemia cutis (LC) is an uncommon manifestation of CLL. It is rarely manifested as bilateral auricular involvement. We present a case of an adult patient who presented with skin lesion of bilateral ears, which leads to the diagnosis of CLL. We also review the literature of related cases.
Case presentation
A 45-year-old male, with no significant past medical history, noted skin changes involving both ears about 3 years prior to initial presentation (Figure 1). He described it as non-tender, reddish discoloration of both auricles. He did not note significant changes in appearance or progression since that time. There were no associated B symptoms initially. The patient finally decided to seek medical attention for cosmetic reason. On examination, he was found to have plum-colored swellings involving the helices of both ears. He was also found to have cervical and axillary lymphadenopathy. Computed tomography (CT) scans confirm the presence of lymphadenopathy. There was no evidence of hepatosplenomegaly. Complete blood count (CBC) showed leukocytosis 19.1/L with lymphocyte predominance 11.1/L, hemoglobin (Hb) was 15.5 g/dL and platelet count (PLT) was 122,000/µL. Biopsies (Figure 2) of these skin lesions were performed and results were suggestive of a low-grade B-cell lymphoma (initial immunohistochemical (IHC) stain positive for CD20, few positive for CD3 and negative for CD30). A lymph node biopsy was consistent with CD5-positive B-cell lymphoproliferative neoplasm, with co-expression of dim CD20 and CD23, favoring small lymphocytic lymphoma (SLL)/CLL. Rai stage was 1. Peripheral blood smear and flow cytometry confirmed circulating CLL and fluorescence in situ hybridization (FISH) demonstrated a 13q deletion, which is a favorable prognostic factor. ZAP-70 result was indeterminate; however, CD38 expression was positive.
Figure 1.
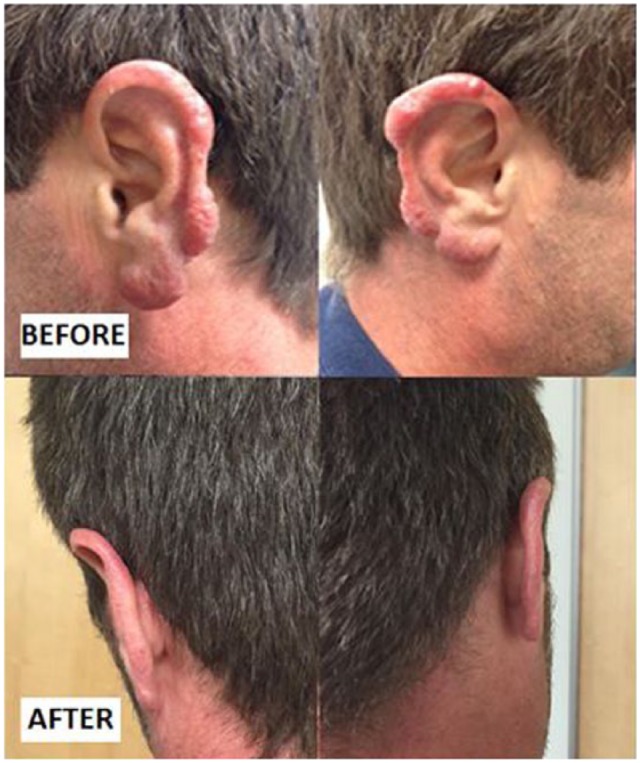
Leukemia cutis as bilateral helical lesion before and after chemotherapy.
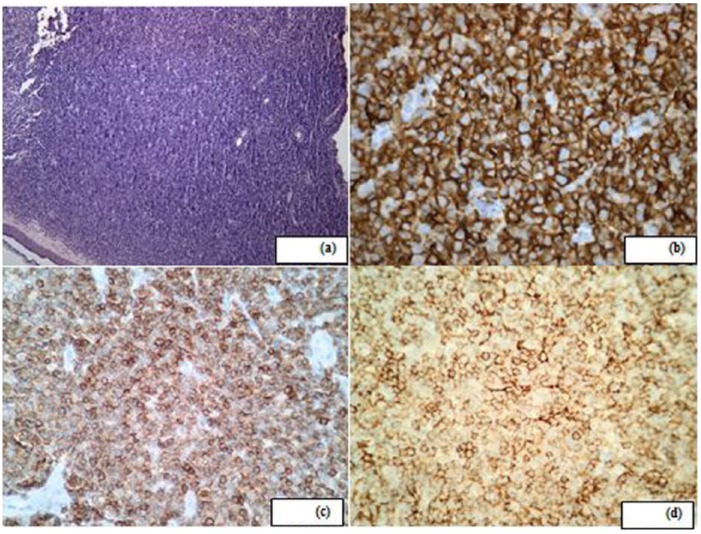
Figure 2.
(a) Histopathology demonstrating dense mononuclear cell infiltrate (hematoxylin and eosin; 10×), (b) immunohistochemical (IHC) stains strongly and diffusely positive for CD20, (c) IHC stain positive for CD5; 40× and (d) IHC stain positive for CD23; 40×.
After initial discussion regarding local radiation therapy, the patient opted for watch-and-wait approach. After 6 months, the patient developed significant fatigue and drenching night sweats for 1 month. At this time, CBC showed leukocytosis 17.1/L with lymphocyte predominance 9.7/L, Hb was 16 g/dL and PLT was 126,000/µL.
Because of systemic involvement,1 it was decided to commence treatment. We discussed the standard therapy with fludarabine, cyclophosphamide and rituximab (FCR).2,3 However, the patient was still working full-time and was planning his wedding in the near future. He, therefore, adamantly refused aggressive cytotoxic chemotherapy for fear of change in current quality of life. We, therefore, discussed less intense therapy with chlorambucil and obinutuzumab.4 This is a very active regimen and currently recommended first-line therapy for elderly or “less fit” patient. He understood that risk of earlier relapse with this regimen may be higher, but he insisted that his quality of life was paramount. He, therefore, chose to be treated with chlorambucil and obinutuzumab. He received six cycles, which he tolerated very well. His B symptoms resolved and he was fully able to perform his daily routine including work-related activities. The patient had an excellent response to therapy. The skin lesions regressed (Figure 1) and the lymphadenopathy resolved. Counts were within normal limits. Repeat peripheral blood flow cytometry did not show any immunophenotypic evidence of an abnormal population of B-lymphocytes. Upon his last follow-up, 2 years since starting chemotherapy, he still remained in complete remission.1
Discussion
CLL is classified as an indolent but incurable NHL. It is the most common adult leukemia worldwide. Among all leukemia in the United States, CLL accounts for 25%–30% of cases.5
Cutaneous eruptions in CLL have been described in 4%–20% of CLL patients and is relatively rare entity as compared to T-cell leukemia.6,7 It may be manifested primarily as LC or more commonly as secondary lesions such as purpura, pruritus, urticaria, erythroderma, pyoderma gangrenosum, cutaneous vasculitis and Sweet’s syndrome. The skin lesions, in some cases, developed at the previously affected healed sites of Borrelia burgdorferi, herpes zoster and herpes simplex.8–10 Typically, skin manifestations happen late in the natural history of the disease, often after multiple treatment and recurrence. This early presentation is far less common.
The term LC is referred to the cutaneous infiltration by malignant leukocytes, clinically manifested as cutaneous lesions.11 LC has been described in patients with acute and chronic leukemia including acute myeloid leukemia, chronic myelogenous leukemia (CML), myelodysplastic syndromes and CLL.12 LC in CLL is far less common and is seen in less than 5% of cases.
CLL can appear solely as clonal B-cell infiltrate in the skin and may be considered as part of the differential even in the absence of systemic involvement such as lymphocytosis or lymphadenopathy.13 LC, in CLL, is characterized by infiltrates comprised of monotonous population of small lymphoid cells with round nuclear contours. Cerroni et.al.14 have described three main architectural patterns in a large case series of 42 CLL patients with LC. These identifiable patterns include (1) perivascular and periadnexal (lymphoid infiltrate around vessels/adnexal structures), (2) nodular and diffuse (nodules of neoplastic cells) like in our patient and (3) relatively rare band-like (lymphoid infiltrate arranged as band-like pattern). The neoplastic B cells co-express CD20, CD43, CD23 and CD5 per IHC.
LC in CLL, as exclusive and localized auricular manifestation, was first described by Gotay15 in 1976. Auricular LC usually occurs in older patients with known history of CLL; however, it may present in relatively young patients like ours as a heralding manifestation of CLL that leads to the diagnosis of CLL. These lesions may mimic many nonmalignant conditions, such as infectious (leprosy, leishmaniasis and tuberculosis) or inflammatory; sarcoidosis, perniosis, relapsing polychondritis, auricular pseudocyst and trauma/hematoma. There are also other neoplastic conditions including primary lymphoma, (especially cutaneous T-cell lymphoma), lymphocytoma cutis, Rosai–Dorfman disease and multicentric reticulohistiocytosis that may resemble the auricular involvement of CLL. Tables 1 and 2 represent the reported cases and their outcomes in which auricular LC manifested either years3–14 after the diagnosis of CLL or presented as early manifestation of CLL followed by the identification of the underlying CLL disease, respectively. Our patient is among the youngest of the reported cases.
Table 1.
Auricular involvement in patients (years after the diagnosis of CLL).
| Case | Time period (years) | Age (years) | Gender | Clinical lesion | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Gotay15 | 14 | 65 | Male | Non-tender, violaceous bilateral symmetrical ear lobe swelling | RT | Regressed |
| Colburn et al.16 | 10 | 59 | Male | Painless, erythematous lesions on both ears | RT | Regressed |
| Jasim et al.17 | 10 | 63 | Male | Plum-colored swelling of the skin of the ears, eyebrows, tip of the nose and the scalp | Not known | Not known |
| Koletsa et al.18 | 3 | 67 | Male | Bilateral ear lobe papules | FCR | Regressed |
| Lu et al.19 | 10 | 57 | Male | Plum-colored swelling involving the prominent parts of the ears (helix, tragus and ear lobe), the eyebrows, nose and toes | None (patient declined) | NA |
CLL: chronic lymphocytic leukemia; RT: radiation therapy; FCR: fludarabine, cyclophosphamide and rituximab.
Table 2.
Auricular involvement in CLL patients (early manifestation).
| Case | Age (years) | Gender | Clinical lesion | Treatment | outcome |
|---|---|---|---|---|---|
| Porter et al.20 | 59 | Male | Painful nodules on his ears and nose | UVB phototherapy | Regressed |
| Colburn et al.16 | 61 | Male | Erythematous nodules on the helices of both ears | RT | Regressed |
| Kim and Sheth21 | 73 | Male | Cauliflower-like, non-tender plum-colored lesions involving both ears | R-CVP | Regressed |
| Vester et al.22 | 40 | Male | Painless erythematous swelling of both earlobes | RT | Regressed |
| Kindem et al.23 | 44 | Male | Erythematous nodules cover the helices and lobes of the right and left ears | Unknown | Unknown |
| Walker et al.24 | 39 | Male | Erythematous nodules and papules on the helix, tragus and lobule | RT | Regressed |
| 2015 (current case) | 45 | Male | Painless plum-colored swellings involving the helices of both ears | Chlorambucil and obinutuzumab | Regressed |
CLL: chronic lymphocytic leukemia; RT: radiation therapy; R-CVP: rituximab, cyclophosphamide, vincristine, prednisone; UVB: ultraviolet light B.
Patient survival is not affected by LC; however, occasionally it may be associated with poor prognosis in patients when LC presenting as a late presentation of B-CLL or as myeloid leukemia, blastic transformation (Richter’s syndrome) and T-cell CLL.14,16,25
Conventional therapy for CLL involves systemic chemotherapy usually combined with a monoclonal antibody2 or newer tyrosine kinase inhibitor like ibrutinib26 and idelalisib. However, LC of CLL can be treated with local therapy. Many therapeutic options have been reported that have given promising results with respect to local control of these lesions. These include radiation therapy,27 excision, intralesional steroids, ultraviolet light B20 and electro-chemotherapy28 with bleomycin. We successfully treated our patient with systemic immunochemotherapy due to the presence of systemic signs and symptoms.
Conclusion
Our case, by describing bilateral helical involvement of the ear as a characteristic finding in CLL, exemplifies that it can be a harbinger of the disease. It adds to a list of exceedingly rare and early cutaneous presentation of CLL that leads to the diagnosis. CLL should be considered in the differential diagnosis in patient when presented with bilateral auricular lesions. Further investigation, better awareness and prompt diagnosis of this LC of CLL will enhance our current understanding of disease evolution as well as effectiveness and outcome of its management.
Footnotes
Author contribution: A.R. as corresponding author contributed in the literature search, getting and preparing figures, data collection and interpretation and writing the report; all other authors contributed equally to the literature search.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article and for publication of this case report and accompanying image. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- 1. Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008; 111(12): 5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010; 376(9747): 1164–1174. [DOI] [PubMed] [Google Scholar]
- 3. Eichhorst B, Fink AM, Bahlo J, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol 2016; 17(7): 928–942. [DOI] [PubMed] [Google Scholar]
- 4. Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014; 370(12): 1101–1110. [DOI] [PubMed] [Google Scholar]
- 5. Siegel RL, Miller KD, Jemal A, et al. Cancer statistics, 2015. CA: Cancer J Clin 2015; 65(1): 5–29. [DOI] [PubMed] [Google Scholar]
- 6. Siegel RS, Gartenhaus RB, Kuzel TM. Human T-cell lymphotropic-I-associated leukemia/lymphoma. Curr Treat Option On 2001; 2(4): 291–300. [DOI] [PubMed] [Google Scholar]
- 7. Dosaka N, Tanaka T, Miyachi Y, et al. Examination of HTLV-I integration in the skin lesions of various types of adult T-cell leukemia (ATL): independence of cutaneous-type ATL confirmed by Southern blot analysis. J Invest Dermatol 1991; 96(2): 196–200. [DOI] [PubMed] [Google Scholar]
- 8. Ziemer M, Bornkessel A, Hahnfeld S, et al. “Specific” cutaneous infiltrate of B-cell chronic lymphocytic leukemia at the site of a florid herpes simplex infection. J Cutan Pathol 2005; 32(8): 581–584. [DOI] [PubMed] [Google Scholar]
- 9. Aberer W, Zonzits E, Soyer HP, et al. Post-zoster-specific skin infiltrates in chronic lymphatic leukemia. Hautarzt 1990; 41(8): 455–457. [PubMed] [Google Scholar]
- 10. Cerroni L, Hofler G, Back B, et al. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia (B-CLL) at sites typical for Borrelia burgdorferi infection. J Cutan Pathol 2002; 29(3): 142–147. [DOI] [PubMed] [Google Scholar]
- 11. Weedon D. Cutaneous infiltrates: lymphomatous and leukemic. In: Weedon D. (ed.) Skin pathology. 2nd ed. New York: Churchill Livingstone, 2002, pp. 1118–1120. [Google Scholar]
- 12. Hossfeld DK, Jaffe ES, Harris NL, et al. (eds) World Health Organization classification of tumours: pathology and genetics of tumours of haematopoietic and lymphoid tissues. Ann Oncol 2002; 13(3): 490–491. [Google Scholar]
- 13. Su WP. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol 1994; 13(3): 223–230. [PubMed] [Google Scholar]
- 14. Cerroni L, Zenahlik P, Hofler G, et al. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia: a clinicopathologic and prognostic study of 42 patients. Am J Surg Pathol 1996; 20(8): 1000–1010. [DOI] [PubMed] [Google Scholar]
- 15. Gotay V. Unusual otologic manifestation of chronic lymphocytic leukemia. Laryngoscope 1976; 86(12): 1856–1863. [DOI] [PubMed] [Google Scholar]
- 16. Colburn DE, Welch MA, Giles FJ. Skin infiltration with chronic lymphocytic leukemia is consistent with a good prognosis. Hematology 2002; 7(3): 187–188. [DOI] [PubMed] [Google Scholar]
- 17. Jasim ZF, Cooke N, Somerville JE, et al. Chronic lymphocytic leukaemia skin infiltrates affecting prominent parts of the face and the scalp. Br J Dermatol 2006; 154(5): 981–982. [DOI] [PubMed] [Google Scholar]
- 18. Koletsa T, Patsatsi A, Kostopoulos I, et al. Ear lobe involvement in chronic lymphocytic leukemia. J Dtsch Dermatol Ges 2013; 11(1): 80–82. [DOI] [PubMed] [Google Scholar]
- 19. Lu C, Li L, Qiao Q, et al. Cutaneous manifestations in a patient with chronic lymphocytic leukemia involving the head, neck and distal extremities. Exp Ther Med 2015; 9(3): 877–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Porter WM, Sidwell RU, Catovsky D, et al. Cutaneous presentation of chronic lymphatic leukaemia and response to ultraviolet B phototherapy. Br J Dermatol 2001; 144(5): 1092–1094. [DOI] [PubMed] [Google Scholar]
- 21. Kim PS, Sheth PB. Asymptomatic cauliflower ears in a 73-year-old man. Diagnosis: leukemia cutis in the setting of indolent B-cell chronic lymphocytic leukemia (B-CLL). Arch Dermatol 2011; 147(12): 1443–1448. [DOI] [PubMed] [Google Scholar]
- 22. Vester K, Treudler R, Paasch U, et al. Specific bilateral ear infiltration as an early manifestation of chronic lymphocytic leukaemia. Eur J Dermatol 2012; 22(2): 265–266. [DOI] [PubMed] [Google Scholar]
- 23. Kindem S, Traves V, Requena C, et al. Bilateral cauliflower ear as the presenting sign of B-cell chronic lymphocytic leukemia. J Cutan Pathol 2014; 41(2): 73–77. [DOI] [PubMed] [Google Scholar]
- 24. Walker JL, Cohen LM, Kroumpouzos G. Bilateral auricular involvement: a rare presenting sign of chronic lymphocytic leukemia, and successful treatment with electron beam therapy. Acta Derm Venereol 2015; 95(5): 616–617. [DOI] [PubMed] [Google Scholar]
- 25. Ratnam KV, Khor CJ, Su WP. Leukemia cutis. Dermatol Clin 1994; 12(2): 419–431. [PubMed] [Google Scholar]
- 26. Farooqui MZ, Valdez J, Martyr S, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. Lancet Oncol 2015; 16(2): 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bakst R, Yahalom J. Radiation therapy for leukemia cutis. Pract Radiat Oncol 2011; 1(3): 182–187. [DOI] [PubMed] [Google Scholar]
- 28. Sainsbury DC, Allison KP, Muir T. Electrochemotherapy treatment of a recalcitrant earlobe keloid scar with chronic lymphocytic leukaemia infiltration. J Plast Reconstr Aesthet Surg 2010; 63: e733–e736. [DOI] [PubMed] [Google Scholar]