Abstract
Although endovascular stenting has been used as an interventional therapy to treat cardio- and cerebro-vascular diseases, it is associated with recurrent vascular diseases following stent thrombosis and in-stent restenosis. In this study, a metallic stent was coated with dopamine-conjugated hyaluronic acid with different ratios of catechol group to improve hemocompatibility and re-endothelialization. Especially, we were interested in how much amount of catechol group is appropriate for the above-mentioned purposes. Therefore, a series of dopamine-conjugated hyaluronic acid conjugates with different ratios of catechol group were synthesized via a carbodiimide coupling reaction. Dopamine-conjugated hyaluronic acid conjugates were characterized with 1H-nuclear magnetic resonance and Fourier transform infrared spectroscopy, and the amount of catechol group in dopamine-conjugated hyaluronic acid was measured by ultraviolet spectrometer. Co-Cr substrates were polished and coated with various dopamine-conjugated hyaluronic acid conjugates under pH 8.5. Dopamine-conjugated hyaluronic acid amounts on the substrate were quantified by micro-bicinchoninic acid assay. Surface characteristics of dopamine-conjugated hyaluronic-acid-coated Co-Cr were evaluated by water contact angle, scanning electron microscopy, and atomic force microscopy. The hemocompatibility of the surface-modified substrates was assessed by protein adsorption and platelet adhesion tests. Adhesion and activation of platelets were confirmed with scanning electron microscopy and lactate dehydrogenase assay. Human umbilical vein endothelial cells were cultured on the substrates, and the viability, adhesion, and proliferation were investigated through cell counting kit-8 assay and fluorescent images. Obtained results demonstrated that optimal amounts of catechol group (100 µmol) in the dopamine-conjugated hyaluronic acid existed in terms of various properties such as hemocompatibility and cellular responses.
Keywords: Vascular stent, hyaluronic acid, dopamine, thrombosis, restenosis
Introduction
The clinical progress after intravascular metal stent treatment is often complicated by thrombosis and restenosis.1 When the metallic biomaterials contact with blood, proteins in blood plasma would be adsorbed onto the surface and the adsorbed protein layer dictates the inflammation and thrombotic responses of platelets.2 During the above procedures, the surface of material first interacts with the body environment and plays a more important role than the bulk material.3 In addition, the delivery of antimitotic or antithrombotic particulate pharmaceuticals or carriers to arterial lumens following angioplasty or endarterectomy, or chemotherapeutic agents to tumor vasculature might be practicable.4 Thus, it is an effective method to directly immobilize various biocompatible substances, such as poly(ethylene glycol) (PEG), zwitterionic materials, heparin, and hyaluronic acid (HA), onto the surface of a metallic biomaterial in order to obtain a good biocompatibility.5,6
Among the above-mentioned biocompatible substances, HA is a strongly hydrophilic anionic polysaccharide and contains a large number of hydroxyl and carboxyl groups, which provide hydrogen bond donors/acceptors and are negatively charged.7 The antifouling and antithrombotic properties of HA with respect to protein adsorption have been demonstrated well in the previous studies.8 However, the protocols involved in the modification of the substrate or HA for fabricating such antifouling surfaces are intricate.
Dopamine (3,4-dihydroxyphenylethylamine (DA)) is a highly reactive molecule that oxidizes at alkaline pH values to convert the adherent catechol/quinone forms, which can be used for facile modification of various surfaces such as polymers, metals, and ceramics.9 In the adhesion of dopamine to substrates, various interactions were involved by the surface chemistry including covalent bond with amino and thiol groups, coordination bond, and hydrogen bonding. By exploiting this fact, dopamine-conjugated materials have been employed to attach onto surfaces for compatible and simple surface modification through a dopamine-assisted strategy.10 However, many literatures for cytotoxicity of dopamine have been reported that dopamine readily undergo oxidation and generate superoxide radical (O2−), H2O2, semiquinones and quinones, especially in the presence of transition metal ions.11 The quinones and semiquinones can react with glutathione, causing glutathione depletion and the formation of glutathionyl conjugates and covalently bind to amine and thiol groups on proteins, potentially interfering with protein function. Thus, oxidation reactions appear to be involved in the toxicity of dopamine to cells in culture.12 Although the surface modification method using dopamine is very simple and effective,13 none of the papers exploring this area has considered proper concentration of dopamine in/on biomaterials with in vitro blood compatibility and cell culture studies.
Dopamine-conjugated hyaluronic acid (HA-DA) has been utilized to modify the surface of biomedical devices and to develop a cell-supporting matrix in various fields of biomedical research. However, most researchers applied a fixed concentration of dopamine without any cause and did not focus on the optimal concentration of catechol in the dopamine-based conjugates for enhancement of biological activity of biomaterials.14–16 In our previous work, we had developed the growth factor–loaded stents modified with dopamine-conjugated heparin and HA-DA conjugates (with 300 µmol of dopamine per 1 g of HA-DA) to induce rapid and tight re-endothelialization; HA-DA conjugates alone could not affect endothelial cells to improve cell attachment and proliferation onto metal stent.14 Recently, Feng Wu et al.17 and Renliang Huang et al.18 reported the effect of HA-DA conjugates on blood compatibility of metal substrates and discovered the existent of specific ratio between HA and dopamine to prevent protein adsorption and to inhibit platelet adhesion. Nevertheless, not only they could not suggest the quantitatively defined ratio of HA and dopamine but also they did not reveal any apparent reason. In this study, we synthesized HA-DA with different ratio of catechol and prepared HA-coated Co-Cr substrates via oxidation reaction of dopamine to improve blood compatibility and cytocompatibility of Co-Cr substrates. Especially, we tried to quantitatively figure out appropriate concentration of catechol in dopamine-conjugated biomaterials as a coating material with maintaining biological integrity of HA. The HA-DA as a coating material was synthesized, and their physico-chemical properties were characterized. HA-DA conjugates were immobilized to Co-Cr substrates with different concentrations of dopamine, existing the same concentration of HA. The surfaces of HA-DA-coated Co-Cr substrates were characterized by field emission–scanning electron microscopy (FE-SEM), water contact angle, attenuated total reflectance–Fourier transform infrared spectroscopy (ATR-FTIR), atomic force microscopy (AFM), and micro-bicinchoninic acid (BCA) assay. Blood compatibility was evaluated by protein adsorption and platelet adhesion. Cytocompatibility was also assessed by live/dead assay, cell counting kit-8 (CCK-8), and fluorescence staining of actin filament of cultured human endothelial cells.
Materials and methods
Materials
Cobalt–chromium (Co-Cr) alloy plates were purchased from Minitubes (Lyon, France). HA (13 kDa) was purchased from Lifecore (Chaska, USA). Dopamine hydrochloride (DA), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) hydrochloride, N-hydroxysuccinimide (NHS), 4-morpholineethanesulfonic acid (MES) buffer (1 M), tris(hydroxymethyl)-aminomethane (Tris) buffer, human plasma fibrinogen (FBG), Triton-X 100, and phalloidin-tetramethylrhodamine B isothiocyanate were purchased from Sigma–Aldrich (St. Louis, USA). Dialysis membrane (molecular weight cut-off = 3.5 kDa) was purchased from Spectrum Laboratories (Rancho Dominguez, USA). Phosphate-buffered saline (PBS) was purchased from AMRESCO (Solon, USA). Human platelet-rich plasma (PRP) and platelet-poor plasma (PPP) were supplied by Seoul Eastern Blood Center (Seoul, South Korea). Lactate dehydrogenase (LDH) activity assay kit was purchased from Takara Bio Inc. (Mountain View, USA). Human umbilical vein endothelial cells (HUVECs) were purchased from American Type Culture Collection (ATCC) (Manassas, USA). Formaldehyde solution was purchased from JUNSEI (Tokyo, Japan). Endothelial cell growth medium 2 (EGM) was manufactured by Lonza (Valais, Switzerland). Micro BCA™ Protein assay kit and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Thermo Scientific (Rockford, USA). CCK-8 and live/dead assay kit were purchased by DOJINDO (Kumamoto, Japan) and Life Technologies (Carlsbad, USA), respectively.
Synthesis of HA-DA conjugates
The HA-DA was synthesized by carbodiimide coupling chemistry (Figure 1). Briefly, HA (500 mg) was fully dissolved in MES buffer (50 mL, 0.5 M, pH 5.5), and EDC (288 mg, 1.5 mmol) and NHS (173 mg, 1.5 mmol) were gradually added into the HA solution at 15 min intervals. To prepare a series of HA-DA conjugates with catechol groups in varying ratio, HA succinimidyl succinate mixtures were reacted with different concentrations of dopamine hydrochloride: 19.14 mg (0.1 mmol), 38.28 mg (0.2 mmol), 95.7 mg (0.5 mmol) and 191.4 mg (1 mmol), respectively. The reaction mixtures were stirred at room temperature for 4 h in maintaining pH 5.5. The crude solution was purified by dialysis for 3 days against acidified deionized water (pH 5.5) to inhibit oxidation of catechol groups and 1 day against neutral water for pH adjustment. HA-DA conjugates with different catechol groups were lyophilized and obtained in white cottony mass.19,20 The chemical structure of HA-DA conjugates was characterized by 1H-nuclear magnetic resonance (NMR) (Ascend 400 MHz, Bruker, USA) and ATR-FTIR (4100; JASCO, Easton, USA). The amount of catechol groups in HA-DA conjugates was quantified by ultraviolet (UV) spectrometer (Thermo Electron Corporation, CA, USA), measuring absorbance at 280 nm with a dopamine standard curve. Following catechol concentration in HA-DA (µmol per 1 g), the products were designated by HA-DA50, HA-DA100, HA-DA250, and HA-DA400, respectively (Table 1).
Figure 1.
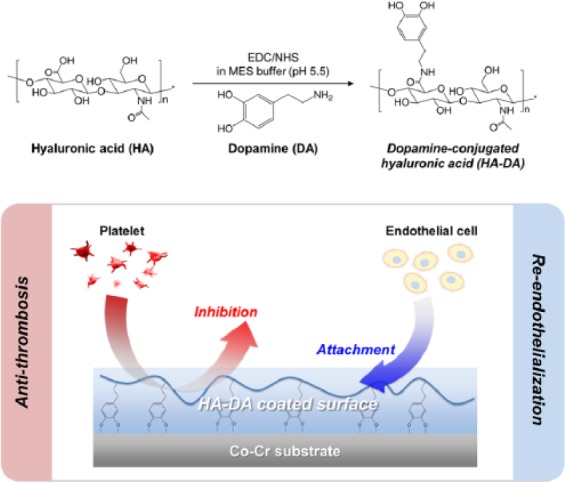
Synthetic scheme of dopamine-conjugated hyaluronic acid (HA-DA) and a hypothesis on biocompatibility of surface-modified Co-Cr substrates with HA-DA conjugates.
Table 1.
Amount and degree of substitution (DS) of dopamine in HA-DA conjugates.
| Sample | Feed amount of DA (mmol) | Dopamine of HA-DA (µmol/g) | DS of dopamine in synthesized conjugate (%) |
|---|---|---|---|
| HA-DA50 | 0.1 | 49.52 ± 3.88 | 1.98 |
| HA-DA100 | 0.2 | 96.75 ± 7.92 | 3.86 |
| HA-DA250 | 0.5 | 262.15 ± 12.04 | 10.05 |
| HA-DA400 | 1.0 | 385.69 ± 16.53 | 15.39 |
Preparation of HA-DA-coated Co-Cr substrate
To prepare the smooth and homogenous surfaces of Co-Cr alloy, Co-Cr substrates (10 × 10 mm2) were ground for 6 min with 400 and 1500 Cw sand papers and then polished for 6 min with 0.3 µm of alumina suspension using rotary equipment (GLP-S25; GLP Korea Co. Ltd., Gwangmyeong, Korea). The polished Co-Cr substrates were washed in distilled water, ethanol, and acetone with sonication and stored in vacuum oven until use.
The HA-DA-coated Co-Cr substrates were prepared with the same HA concentration, but in different concentrations of dopamine. HA-DA conjugates were dissolved in Tris buffer (10 mM, pH 8.5), and the Co-Cr substrates were statically immersed into the solution at 50°C for 4 h.21,22 The HA-DA-coated Co-Cr substrates were gently washed with distilled water at three times and dried in vacuum drying oven at low temperature. The samples were abbreviated in the equal code to HA-DA conjugates: HA-DA50, HA-DA100, HA-DA250, and HA-DA400.
Surface characterization
To confirm the HA-DA conjugates on Co-Cr substrates, the surface chemical composition of HA-DA50-, HA-DA100-, HA-DA250-, or HA-DA400-coated Co-Cr and bare Co-Cr was evaluated by ATR-FTIR in the range between 650 and 4000 cm−1. The amount of immobilized HA-DA on the Co-Cr substrates was quantified by micro-BCA protein assay kit, which can react with complexed between copper ions and amide bonds in HA-DA conjugates. For calculation, standard curves were half-diluted 10 times at 0.25 mg/mL of each of HA-DA series. Substrates were hydrated in deionized water for 1 h and transferred to new 24-well plate. Micro-BCA working solution was added with 1 mL to each well and then incubated at 37°C for 2 h. After incubation, changed color, violet, was measured at 562 nm using a microplate reader (Multiskan Spectrum; Thermo Electron Corporation).23 Contact angles were measured to compare the hydrophilicity between the bare Co-Cr surface and the HA-DA-coated surfaces. 10 µL of water was dropped on the surfaces, and the measurement was carried out using a contact angle goniometer (DigiDrop; GBX Surface Science Technology, Phoenix, USA). The surface morphologies and topographies of HA-DA-coated Co-Cr were analyzed by FE-SEM (Hitachi High-Tech S-2500C, Japan) and AFM (XE-100 Park system, Korea).
Blood compatibility
Protein adsorption test was experimented with fibrinogen and albumin. Bare and each of HA-DA-coated Co-Cr substrates were hydrated with PBS for 1 h. Substrates were incubated at 37°C with fibrinogen (0.1 mg/mL in PBS) solution for 1 h on static condition. After incubation, substrates were washed with deionized water to remove non-adsorbed proteins and incubated with micro-BCA reagent solution for 2 h at 37°C. Absorbance values of reacted solutions were observed by UV spectra at 562 nm.
Human platelets were used for the platelet adhesion test. The bare Co-Cr and HA-DA-coated Co-Cr substrates were sterilized 70% ethanol and UV radiation for 10 min. Substrates were hydrated in PBS for 1 h. The diluted PRP using PPP, which is concentrated to 5 × 104 cells/µL, were added onto each substrate and incubated for 1 h at 37 °C. After incubation, the substrates were rinsed by PBS solution at three times, immersed into 2.5% glutaldehyde solution for 1 h to fix the adhered platelets and then dehydrated by 50%, 70%, 80%, 90%, 95%, and 100% ethanol at 5 min intervals. Finally, the platelet adhesion onto the substrates was dried in vacuum drying oven and analyzed by FE-SEM.19
To quantify the amount of adherent platelets on different substrates surfaces, LDH assay was performed. As above, platelet adhesion was preceded with 1 × 105 cells/mL of platelets for 2 h at 37°C, and the rinsed substrates with PBS were moved to new 24-well plate. 1 mL of 2% Triton X-100 was added to each substrate and incubated at 37°C. After 15 min, 100 µL of the lysed solution were mixed with 100 µL of LDH assay reagent in a 96-well plate. Subsequently, the absorbance was measured by a microplate reader at 490 nm.24
In vitro cell study
To evaluate the cytocompatibility of HA-DA-coated Co-Cr substrates, HUVECs were cultured in EGM-2 supplemented with 5% inactivated fetal bovine serum (FBS) and 1% P/S in an atmosphere of 5% CO2 at 37°C. The HA-DA-coated Co-Cr substrates were sterilized by 70% ethanol and UV radiation for 10 min before cell study. The substrates was fully rinsed in PBS solution and then transferred to each wells of 24-well plate. The HUVECs suspension (1 × 104 cells/cm2) was seeded onto the substrate in well and cultured in an atmosphere of 5% CO2 at 37°C for 24 h. After each time point (1, 3, 7, and 10 days), the effect of catechol groups in HA-DA conjugates on the proliferative activity of HUVECs on the substrates was assessed by CCK-8 assay. The bare Co-Cr and HA-DA-coated Co-Cr substrates were transferred to a new 24-well plate and rinsed twice with sterilized PBS, and serum-free media containing CCK-8 solution in a ration of 1:10 was added. After incubation under 5% CO2 at 37°C for 2 h, the absorbance of the solution was measured using a microplate reader (Multiskan Spectrum; Thermo Electron Co., Vantaa, Finland) at 450 nm. The cytotoxicity of HUVECs was analyzed using staining with live/dead assay kit according to the instruction of manufactures. The live and dead cells were visualized by a fluorescence microscopy (Olympus CKX 41, Japan).25,26
To evaluate the attachment activity of cultured cells on the HA immobilized surfaces with different amount of catechol groups, HUVECs grown on the HA-DA-coated substrates were stained by DAPI and rhodamine-conjugated phalloidin on 1 day. The cells on the substrates were fixed using 4% formaldehyde for 30 min at room temperature and washing with PBS for three times each 10 min. The substrates were treated in 2% Triton X-100 solution during 10 min. After washing with PBS, the substrates were immersed in 1% BSA solution for 1 h to prevent nonspecific binding. The cells were reacted with rhodamine-conjugated phalloidin for 1 h and then washed with PBS for three times each 10 min. HUVECs nuclei were stained by DAPI for 5 min and followed washing step. Fixed with mounting solution, counterstained substrates were observed by fluorescence microscopy.27
Statistical analysis
All values were presented as mean ± standard deviation (SD). The statistically significant of the differences was analyzed by Student’s t-test. The difference was considered to be significant at p-value of less than 0.05.
Results and discussions
Chemical characteristics of HA-DA conjugates
To prepare a series of HA-DA conjugates with different ratios of catechol group, the activated carboxyl groups on HA backbone were covalently coupled with the amine groups of dopamine by the EDC/NHS coupling reaction. The chemical structure of HA-DA conjugates was characterized using 1H-NMR, as shown in Figure 2(a). The N-acetyl peak of HA backbone appeared around 2 ppm. Multiplets from 2.8 to 3.7 ppm associated with disaccharide unit and anomeric protons composed of d-glucuronic acid and N-acetyl-d-glucosamine in the HA, and the position between 6.7 and 7.2 ppm corresponds to the catechol ring of dopamine.28 Moreover, the area under this peak increased with increasing dopamine feed ratio. The degree of substitution (DS) of dopamine in the HA backbone was quantitatively estimated from the integration ratios between acetyl protons in HA and aliphatic protons in dopamine. The DS of dopamine per 100 units of disaccharides was evaluated. Integration and comparison of the peaks attributed to catechol and acetyl groups in HA-DA conjugates yielded to be about 2% dopamine incorporation for HA-DA50, 4% for HA-DA100, 10% for HA-DA250, and 16% for HA-DA400, respectively. The amount of dopamine in HA-DA conjugates was verified using UV absorbance spectra of catechol. The maximum absorbance wavelength at 280 nm extremely increased according to the increase of dopamine in HA-DA conjugates from HA-DA50 to HA-DA400 (Figure 2(b)).6 The DS of HA-DA was readily confirmed to be 1.98% (49.52 µmol of catechol group per 1 g of HA-DA50), 3.86% (96.75 µmol of catechol group per 1 g of HA-DA100), 10.05% (262.15 µmol of catechol group per 1 g of HA-DA250), and 15.39% (385.69 µmol of catechol group per 1 g of HA-DA400) (Table 1). In addition, the functional groups and chemical bonds of HA-DA conjugates were identified by FTIR (Figure 2(c)). A broad band centered at 3300 cm−1 was assigned to the vibration of the hydroxyl groups of HA. The absorption of HA-DA conjugates exhibited additional peaks consistent with the main vibration modes of dopamine such as the C–H vibration at 2850 cm−1 and the C=C ring stretching at 1520 cm−1. The absorption peaks located at 1720, 1630, and 1410 cm−1 were attributed to C=O, amid bond, and C−O stretching vibrations, respectively. The peaks of amide bond between HA and dopamine were overlapped with that of amide bond on HA backbone.28 These results correspond to the expected structure by chemical composition, suggesting that the dopamine conjugation to HA via amid bond was accomplished well.
Figure 2.
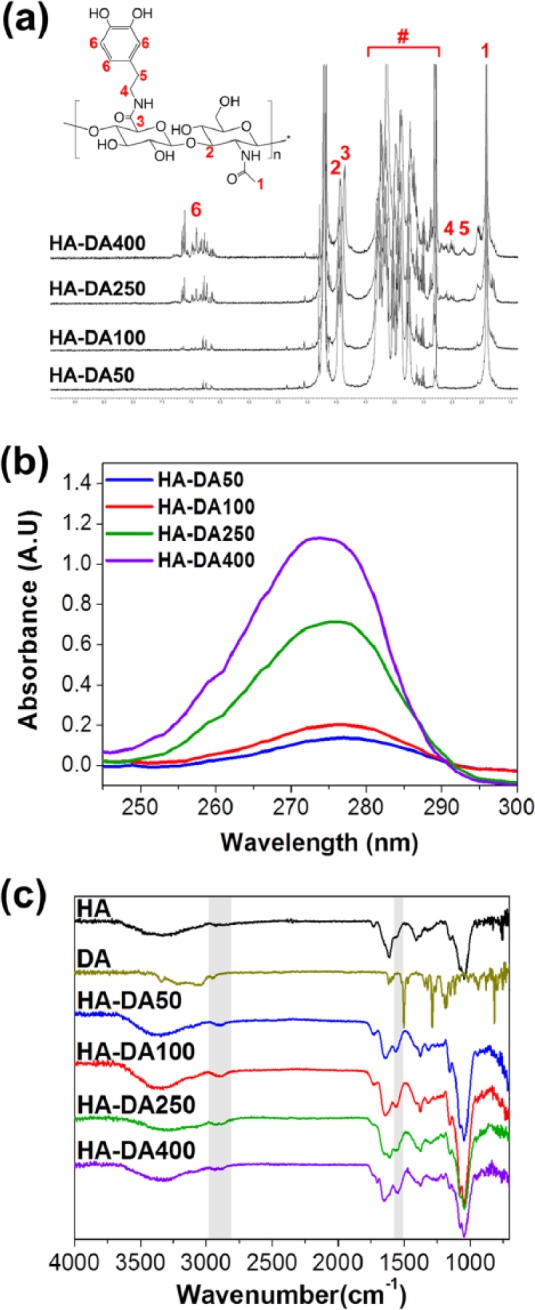
(a) 1H-NMR of synthesized HA-DA conjugates (# = disaccharide unit of HA), (b) UV absorbance at 280 nm of a series of HA-DA conjugates with different ratio of catechol, and (c) FTIR spectra of HA, dopamine, and HA-DA conjugates.
Surface characteristics of HA-DA-coated Co-Cr substrates
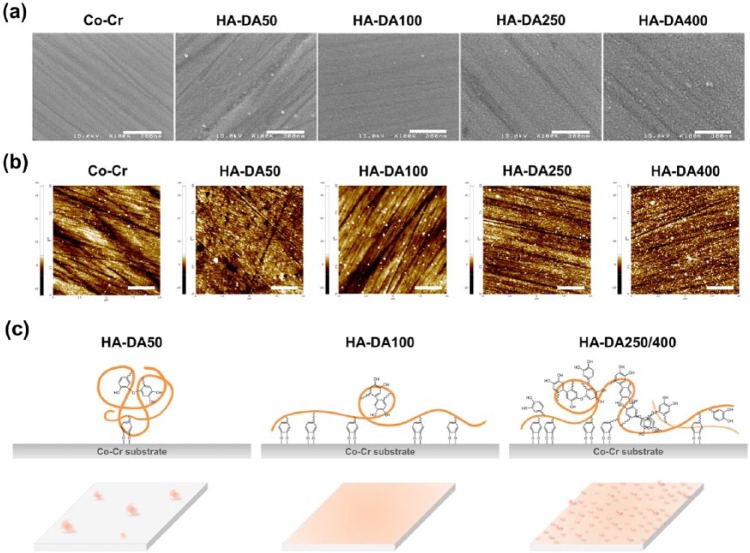
The HA-DA-coated Co-Cr substrates were prepared by forming strong coordination bonds to give catechol–metal complexes under basic condition. The surfaces morphologies of HA-DA-coated Co-Cr substrates were analyzed by FE-SEM and AFM. The SEM images of the bare Co-Cr and modified Co-Cr surfaces showed that the modified Co-Cr substrates were rougher than that of bare Co-Cr, and the roughness of HA-DA-coated Co-Cr surfaces increased as the amount of dopamine in HA-DA increased except HA-DA100 substrate (Figure 3(a)). The roughness of the substrates was exhibited and quantitatively measured by AFM in terms of root mean square (rms) roughness (Rq) (Figure 3(b)). The numerical roughness values intactly reflected the results of SEM image (Table 2). The roughness values of HA-DA50/250/400-coated Co-Cr surfaces were 1.95, 2.03, and 2.77 nm respectively, whereas that of HA-DA100-coated Co-Cr was 1.69 nm. The HA-DA100-coated substrate was displayed to be mostly smooth surface with low Rq value. It is supposed that HA-DA conjugates were self-assembled and aggregated due to relatively balanced hydrophilic (HA) and hydrophobic (DA) segments, π–π stacking of aromatic rings in catechol, and self-polymerization of dopamine.29 In the case of HA-DA50, before oxidized dopamine forms coordinate bond with metal ion of substrates, HA-DA might first aggregate and the surface was heterogeneously covered with aggregated HA-DA (Figure 3(c)). In HA-DA250 and HA-DA400 substrates, it is considered that the various reactions including hydrophilic/phobic interaction, pi-stacking, self-polymerization, and aggregation were complexly and excessively generated between surface-polymer and polymer-polymer and consequently made rough surfaces. Meanwhile, it seems that HA-DA100 with relevant ratio of HA and DA formed smaller aggregates than other HA-DA conjugates, simultaneously was coordinated onto Co-Cr substrate and covered the surface to be uniform and smooth. This is a tentative hypothesis and required further study.
Figure 3.
(a) FE-SEM micrographs of surfaces (scale bar = 300 nm), (b) topographic AFM images (scale bar = 2.5 µm), and (c) hypothetical images of the surfaces of bare Co-Cr, HA-DA50-coated Co-Cr, HA-DA100-coated Co-Cr, HA-DA250-coated Co-Cr, and HA-DA400-coated Co-Cr substrates.
Table 2.
Surface characteristics of HA-DA-coated Co-Cr substrates.
| Sample | Dopamine on surface (nmol/cm2) | Roughness (nm) | Water contact angle (degree) |
|---|---|---|---|
| Co-Cr | – | 1.67 ± 0.24 | 77.8 ± 2.2 |
| HA-DA50 | 3.04 ± 0.09 | 1.95 ± 0.26 | 57.3 ± 2.6 |
| HA-DA100 | 4.56 ± 0.12 | 1.69 ± 0.05 | 53.1 ± 2.6 |
| HA-DA250 | 13.31 ± 0.47 | 2.03 ± 0.17 | 51.4 ± 4.1 |
| HA-DA400 | 18.83 ± 0.50 | 2.77 ± 0.27 | 39.5 ± 3.1 |
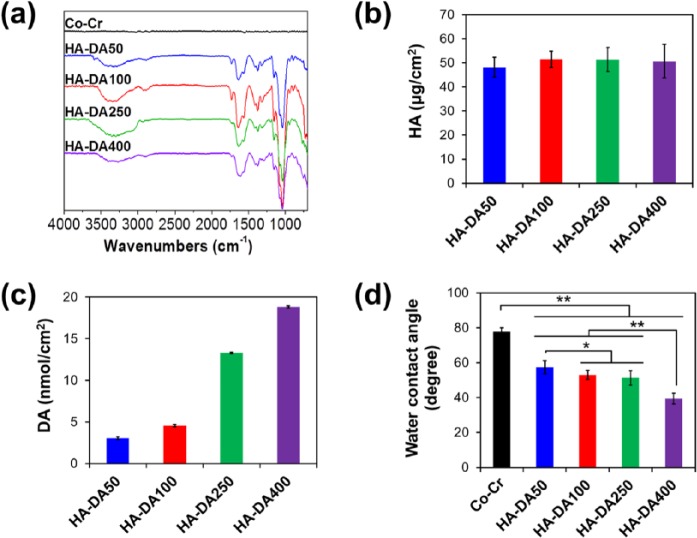
The surface chemical bonding structures of HA-DA-coated Co-Cr substrates were confirmed by ATR-FTIR, and the absorption spectra are displayed in Figure 4(a) that was similar to Figure 2(c); −OH stretching of HA at 3300 cm−1, C–H stretching of dopamine at 2850 cm−1, and C=C ring stretching of dopamine at 1520 cm−1.6 The amount of HA and catechol in HA-DA conjugates on Co-Cr surfaces was measured using a micro-BCA assay kit. In all samples, HA was equally immobilized to be about 50 µg/cm2 on the modified Co-Cr substrates (Figure 4(b)), and the concentrations of catechol on the surface gradually increased from 2.36 to 6.73 nmol/cm2, depending on the increase in dopamine in HA-DA conjugates (Figure 4(c)).
Figure 4.
(a) ATR-FTIR spectra, the amounts of (b) HA, (c) DA, and (d) water contact angle under static condition (mean ± SD, n = 10, *p < 0.01 and **p < 0.001) of the surfaces of bare Co-Cr and modified Co-Cr substrates.
Surface hydrophilicity is an important factor in blood-contacting medical devices, which influences protein adsorption and platelet adhesion leading to thrombosis formation. The hydrophilicity of the substrates was characterized by water contact angle analysis. As shown in Figure 4(d), the water contact angle on bare Co-Cr was 77.8°. The water contact angle of HA-DA-coated-Co-Cr substrates decreased after modification with HA-DA conjugates. The water contact angles of modified surfaces showed a similar reduction in HA-DA50 (57.3°), HA-DA100 (53.1°), and HA-DA250 (51.4°) substrates. In particular, the hydrophilicity of modified Co-Cr substrates with HA-DA400 conjugates dramatically decreased 39.5° due to the highly rough surfaces.30 The data exhibited that the Co-Cr substrates became more hydrophilic by immobilizing hydrophilic HA onto the substrate and increasing surface roughness, compared to bare Co-Cr control.
Blood compatibility
Protein absorption plays a significant role in hemocompatibility of biomaterials, which is the first step when biomaterial surface contacts with blood, subsequently induced platelet and cell adhesion.10 Many factors such as surface free energy, surface charge character, surface morphologies, and solution environment affect protein adsorption.31 Protein adsorption on a biomaterial surface usually occurs in a mixture of solutions containing many biomolecules and, thus, biological interactions at the surface are the overall result of complex cooperation, competition, and interference between the biomolecules and the biomaterial.32 Because handling all of the mixed biological solutions and analyzing the individual interactions are very difficult, fibrinogen was chosen as a model for the protein adsorption assay on the substrates. Fibrinogen is a typical protein in blood that adsorbs on the surface of the biomedical devices within minutes following implantation.33 It is well known that the adsorbed fibrinogen on biomaterial influences the platelets adhesion and blood compatibility.
The adsorption of fibrinogen onto the surface-modified Co-Cr is presented in Figure 5(a). HA-DA50- (87.05 µg/cm2) and HA-DA100- (78.89 µg/cm2) coated substrates exhibited considerably lower fibrinogen adsorption as compared to bare Co-Cr (117.86 µg/cm2). Meanwhile, the values of HA-DA250 and HA-DA400 substrates (166.21 and 215.46 µg/cm2, respectively) were as much as 141%−182% higher than the control Co-Cr. The results of surface-modified substrates with HA-DA50 and HA-DA100 are certainly due to the smooth and hydrophilic surface preventing proteins from approaching to these surfaces. Compared to HA-DA400-coated Co-Cr, which is relatively hydrophilic but has rough surface, much less fibrinogen adsorption occurred on HA-DA100 surfaces. It has been reported that protein adsorption onto the surface is strongly influenced by roughness rather than hydrophilicity. Surface roughness correlates with the adhesion strength of proteins on both hydrophilic and hydrophobic substrates due to the relatively high surface area for protein-surface interactions.34,35 Therefore, relatively rougher HA-DA250 and HA-DA400 substrates had much larger interaction interfaces with proteins than those of HA-DA50 and HA-DA100 substrates.
Figure 5.
(a) the amount of absorbed fibrinogen (mean ± SD, n = 12, *p < 0.001 compared with other groups), (b) SEM micrographs of adhering platelets, and (c) quantitative analysis of adhered and activated platelets on substrates (mean ± SD, n = 6, *p < 0.01 and **p < 0.001).
The adhesion and activation of platelet studies on the substrates were examined using human platelets and plasma. Their morphology and the amount of adhered platelets were determined using FE-SEM image and LDH assay, respectively. Figure 5(b) indicates that the adhered platelets on all substrates except HA-DA100 substrate were observed to be pseudopodia, which is the activated platelet, whereas most of the platelets on the HA-DA1000-coated surfaces showed proper round shape. The platelets on the modified Co-Cr substrate with HA-DA100 were observed that they maintained their original round shape, no pseudopodia.19 However, the platelets on HA-DA250- and HA-DA400-coated Co-Cr substrates were excessively activated and agglomerated between themselves. Platelet which extends their pseudopodia is known as activated form leading to thrombosis formation, which is accelerated by the released cytokines and chemokines from activated platelets.36 The round shapes and the low density of adhered platelet on HA-DA100 substrate were caused by surface properties including roughness and hydrophilicity, in common with protein adsorption. With increasing concentration of dopamine in HA-DA conjugates from 250 to 400 µmol, the surface roughness increased, and the amount of adhered protein also increased. In HA-DA50 substrate, the irregular and rough surface also led to adhere and activate platelets onto the substrate. The adhered proteins on surface are the major mediator of platelet adhesion to biomaterials pre-adsorbed with plasma. The HA-DA100-coated substrate with such low protein adsorption exhibited high platelet inhibition and poor platelet activity.
The amounts of adherent platelets were quantitatively assessed by measuring the LDH level released when the adherent platelets were lysed with a Triton X-100 buffer (Figure 5(c)).24 The adhered and activated platelets on HA-DA100-coated surface were lower than that of bare Co-Cr and other HA-DA-series-coated surfaces, supporting SEM images. The result indicated that the HA-DA100-coated Co-Cr substrates have good blood compatibility in resisting the adsorption of proteins and adhesion of platelets.
Cytocompatibility
To evaluate the cytocompatibility of the HA-DA-coated Co-Cr substrates with different concentration of catechol, HUVECs were cultured on the substrate surfaces for 10 days and evaluated by live/dead and CCK-8 assay. Figure 6(a) illustrates that most of the cells were not uniformly distributed on the surface in the case of bare Co-Cr, whereas on HA-DA-coated Co-Cr, they were better spread out than those adhering to control. The HUVECs on the HA-DA immobilized substrates were similarly attached on 1 day, viewed as stable growth on HA-DA100-coated Co-Cr substrates than other groups in process of culture time, and partially detached from all samples except HA-DA100-coated Co-Cr on 10 days. The number of cells on the substrates increased for culture period in accordance with fluorescence microscopy images of the live/dead dye (Figure 6(b)). HA is involved in a great number of biological functions, such as cell proliferation and migration. Moreover, an important biological role is related to HA oligosaccharides that stimulate cytokine secretion and endothelial cell proliferation.37 Especially, the endothelial cell functions have been implicated in CD 44, which is a cell surface glycoprotein as HA receptor.38 However, it has been reported that dopamine has cytotoxicity because the catechol can interact with amino acid containing aromatic ring structure, such as tyrosine, tryptophan, and phenylalanine, by π–π stacking, covalently bind with amine and thiol groups in proteins via Schiff base reaction and Michael-type addition,39 and ultimately denature the membrane proteins of cells. The cytotoxicity of HA-DA conjugates containing excess dopamine groups have canceled out cell compatibility of HA on modified Co-Cr substrates. Consequentially, the force of cell–cell interaction was stronger than that between cell and surfaces, and the cell sheets were separated from the surface of HA-DA250- and HA-DA400-coated substrates. In HA-DA50 groups, the bare Co-Cr was partially exposed due to the uniformly covered surface with HA, and the cells could not sturdily bind onto the surface. Figure 6(c) shows the morphology and F-actin cytoskeleton of initially adhered cell on unmodified and modified Co-Cr substrates at 1 day. The cells on HA-DA-coated substrates gently spread in comparison with that on bare Co-Cr, and the amount of cells on the modified surfaces was roughly similar. In particular, the cells on HA-DA100-coated Co-Cr substrate only showed strong F-actin staining, which is crucial for cell adhesion.40 This result was supposed that the cells strongly bound to cytocompatible surface containing HA-DA100 conjugates and a number of actin filaments were formed, compared with other groups. Accordingly, it is interesting to note that the HA-DA100 conjugates not only inhibited platelet adhesion and activation with prevention of protein adsorption but also accelerated adhesion and proliferation of endothelial cells through blood compatibility and cytocompatibility studies.
Figure 6.
(a) Fluorescence microscopy images showing HUVECs growing on different substrates for 10 days and stained with live (green) and dead (red) viability assay kit (scale bar = 500 µm), (b) cell viability (mean ± SD, n = 8, *p < 0.001 compared with other groups), and (c) phalloidin-labeled F-actin filament (red), DAPI nuclear staining (blue), and overlaid fluorescent image of immunostained cellular components for cells on the substrates at 1 day (scale bar = 50 µm).
Conclusion
A series of HA-DA conjugates with different catechol were successfully grafted on Co-Cr substrates via the dopamine oxidation reaction. The chemical structure of HA-DA conjugates was confirmed, and the surface characterizations of HA-DA-coated Co-Cr substrates were assessed by various analyses. Their surface properties improved that either too few or too many dopamine in HA-DA conjugates could not completely cover the Co-Cr substrates and make the surface rough. This difference in catechol concentration also subsequently affected the blood compatibility and cytocompatibility of the modified Co-Cr substrates. The smoothly and uniformly surface-modified Co-Cr substrates with HA-DA100 conjugates showed excellent blood compatibility as compared with bare Co-Cr and other groups. Moreover, the HA-DA100 substrates presented the proper cytocompatibility and good cell adhesion activity in vitro. Presumably, in HA-DA50-coated substrate, bare Co-Cr surface was partially exposed due to the self-aggregation of HA-DA conjugates before the immobilization onto surface, and its irregular surface led to platelet adhesion. Meanwhile, the composite with excess dopamine such as HA-DA250 and HA-DA400 conjugates tend to increase surface roughness and had possibility that residual dopamine groups react with proteins in blood and cell membrane, denature these proteins, and cause poor blood and cell compatibility. The HA-DA100 conjugates with appositely balanced ratio between HA and DA showed potential for use as a material in vascular stent, because they not only impeded protein adsorption and platelet adhesion but also enhanced the attachment and proliferation of endothelial cells. Obtained results demonstrated that HA-coated Co-Cr substrates might be a promising blood compatible material for blood-contacting biomedical applications including vascular stents.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by the Pioneer Research Center Program (2014M3C1A3056052) and Next Generation Medical Device Platform Program (2015M3A9E2028580) funded by the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (MSIP), and Strategic Core Materials Technology Development Program (10062079) funded by Ministry of Trade, Industry and Energy (MOTIE), Republic of Korea.
References
- 1. Alfonso F, Byrne R, Rivero F, et al. Current treatment of in-stent restenosis. J Am Coll Cardiol 2014; 63: 2659–2673. [DOI] [PubMed] [Google Scholar]
- 2. Schmidt DR, Waldeck H, Kao WJ. Protein adsorption to biomaterials. In: Puleo DA, Bizios R. (eds) Biological interactions on materials surfaces. New York: Springer, 2009, pp. 1–18. [Google Scholar]
- 3. Lewis CG, Belniak RM, Hopfer SM, et al. Cobalt in periprosthetic soft tissue: observations in 6 revision cases. Acta Orthop Scand 1991; 62: 447–450. [DOI] [PubMed] [Google Scholar]
- 4. Deglau TE, Maul TM, Villanueva FS, et al. In vivo PEG modification of vascular surfaces for targeted delivery. J Vasc Surg 2012; 55: 1087–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chung HJ, Lee H, Bae KH, et al. Facile synthetic route for surface-functionalized magnetic nanoparticles: cell labeling and magnetic resonance imaging studies. ACS Nano 2011; 5: 4329–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hong S, Yang K, Kang B, et al. Hyaluronic acid catechol: a biopolymer exhibiting a pH-dependent adhesive or cohesive property for human neural stem cell engineering. Adv Funct Mater 2013; 23: 1774–1780. [Google Scholar]
- 7. Suh KY, Khademhosseini A, Yang JM, et al. Soft lithographic patterning of hyaluronic acid on hydrophilic substrates using molding and printing. Adv Mater 2004; 16: 584–588. [Google Scholar]
- 8. Lih E, Chi SY, Son TI, et al. Facile surface modification of nitinol with dopamine-conjugated hyaluronic acid for improving blood compatibility. J Biomater Tissue Eng 2016; 6: 780–787. [Google Scholar]
- 9. Huang R, Liu X, Ye H, et al. Conjugation of hyaluronic acid onto surfaces via the interfacial polymerization of dopamine to prevent protein adsorption. Langmuir 2015; 31: 12061–12070. [DOI] [PubMed] [Google Scholar]
- 10. Lee H, Dellatore SM, Miller WM, et al. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007; 318: 426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weingarten P, Zhou QY. Protection of intracellular dopamine cytotoxicity by dopamine disposition and metabolism factors. J Neurochem 2001; 77: 776–785. [DOI] [PubMed] [Google Scholar]
- 12. Lai CT, Peter HY. Dopamine-and L-β-3,4-dihydroxyphenylalanine hydrochloride (L-DOPA)-induced cytotoxicity towards catecholaminergic neuroblastoma SH-SY5Y cells: effects of oxidative stress and antioxidative factors. Biochem Pharmacol 1997; 53: 363–372. [DOI] [PubMed] [Google Scholar]
- 13. Xi ZY, Xu YY, Zhu LP, et al. A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly(DOPA) and poly(dopamine). J Memb Sci 2009; 327: 244–253. [Google Scholar]
- 14. Choi DH, Kang SN, Kim SM, et al. Growth factors-loaded stents modified with hyaluronic acid and heparin for induction of rapid and tight re-endothelialization. Colloids Surf B Biointerfaces 2016; 141: 602–610. [DOI] [PubMed] [Google Scholar]
- 15. Kim SM, Park KS, Lih E, et al. Fabrication and characteristics of dual functionalized vascular stent by spatio-temporal coating. Acta Biomater 2016; 38: 143–152. [DOI] [PubMed] [Google Scholar]
- 16. Park HJ, Jin Y, Shin J, et al. Catechol-functionalized hyaluronic acid hydrogels enhance angiogenesis and osteogenesis of human adipose-derived stem cells in critical tissue defects. Biomacromolecules 2016; 17: 1939–1948. [DOI] [PubMed] [Google Scholar]
- 17. Wu F, Li J, Zhang K, et al. Multifunctional coating based on hyaluronic acid and dopamine conjugate for potential application on surface modification of cardiovascular implanted devices. ACS Appl Mater Interfaces 2016; 8: 109–121. [DOI] [PubMed] [Google Scholar]
- 18. Huang R, Liu X, Ye H, et al. Conjugation of hyaluronic acid onto surfaces via the interfacial polymerization of dopamine to prevent protein adsorption. Langmuir 2015; 31: 12061–12070. [DOI] [PubMed] [Google Scholar]
- 19. Kim K, Kim K, Ryu JH, et al. Chitosan-catechol: a polymer with long-lasting mucoadhesive properties. Biomaterials 2015; 52: 161–170. [DOI] [PubMed] [Google Scholar]
- 20. Lee MS, Lee JE, Byun E, et al. Target-specific delivery of siRNA by stabilized calcium phosphate nanoparticles using dopa–hyaluronic acid conjugate. J Control Release 2014; 192: 122–130. [DOI] [PubMed] [Google Scholar]
- 21. Lee H, Lee Y, Statz AR, et al. Substrate-independent layer-by-layer assembly by using mussel-adhesive-inspired polymers. Adv Mater 2008; 20: 1619–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yimsiri P, Mackley MR. Spin and dip coating of light-emitting polymer solutions: matching experiment with modelling. Chem Eng Sci 2006; 11: 3496–3505. [Google Scholar]
- 23. Milleret V, Buzzi S, Gehrig P, et al. Protein adsorption steers blood contact activation on engineered cobalt chromium alloy oxide layers. Acta Biomater 2015; 24: 343–351. [DOI] [PubMed] [Google Scholar]
- 24. Yang Z, Wang J, Luo R, et al. The covalent immobilization of heparin to pulsed-plasma polymeric allylamine films on 316L stainless steel and the resulting effects on hemocompatibility. Biomaterials 2010; 31: 2072–2083. [DOI] [PubMed] [Google Scholar]
- 25. Neto AI, Cibrão AC, Correia CR, et al. Nanostructured polymeric coatings based on chitosan and dopamine-modified hyaluronic acid for biomedical applications. Small 2014; 10: 2459–2469. [DOI] [PubMed] [Google Scholar]
- 26. Kim TG, Lee Y, Park TG. Controlled gene-eluting metal stent fabricated by bio-inspired surface modification with hyaluronic acid and deposition of DNA/PEI polyplexes. Int J Pharm 2010; 384: 181–188. [DOI] [PubMed] [Google Scholar]
- 27. Joo H, Byun E, Lee M, et al. Biofunctionalization via flow shear stress resistant adhesive polysaccharide, hyaluronic acid-catechol, for enhanced in vitro endothelialization. J Ind Eng Chem 2016; 34: 14–20. [Google Scholar]
- 28. Mueller DD, Morgan TD, Wassenberg JD, et al. Proton and carbon-13 NMR of 3-O and 4-O conjugates of dopamine and other catecholamines. Bioconjug Chem 1993; 4: 47–53. [DOI] [PubMed] [Google Scholar]
- 29. Ye H, Xia Y, Liu Z, et al. Dopamine-assisted deposition and zwitteration of hyaluronic acid for the nanoscale fabrication of low-fouling surfaces. J Mater Chem B 2016; 4: 4084–4091. [DOI] [PubMed] [Google Scholar]
- 30. Wenzel RN. Resistance of solid surfaces to wetting by water. Ind Eng Chem 1936; 28: 988–989. [Google Scholar]
- 31. Roach P, Farrar D, Perry CC. Interpretation of protein adsorption: surface-induced conformational changes. J Am Chem Soc 2005; 127: 8168–8173. [DOI] [PubMed] [Google Scholar]
- 32. Wagner M, Gutermann A, Podlech J, et al. Major histocompatibility complex class I allele-specific cooperative and competitive interactions between immune evasion proteins of cytomegalovirus. J Exp Med 2002; 196: 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stamatin L, Cristescu R, Socol G, et al. Laser deposition of fibrinogen blood proteins thin films by matrix assisted pulsed laser evaporation. Appl Surf Sci 2005; 248: 422–427. [Google Scholar]
- 34. Akkas T, Citak C, Sirkecioglu A, et al. Which is more effective for protein adsorption: surface roughness, surface wettability or swelling? case study of polyurethane films prepared from castor oil and poly(ethylene glycol). Polym Int 2013; 62: 1202–1209. [Google Scholar]
- 35. Conti M, Donati G, Cianciolo G, et al. Force spectroscopy study of the adhesion of plasmaproteins to the surface of a dialysis membrane: role of thenanoscale surface hydrophobicity and topography. J Biomed Mater Res A 2002; 61: 370–379. [DOI] [PubMed] [Google Scholar]
- 36. Huang Q, Yang Y, Hu R, et al. Reduced platelet adhesion and improved corrosion resistance of superhydrophobic TiO2-nanotube-coated 316L stainless steel. Colloids Surf B Biointerfaces 2015; 125: 134–141. [DOI] [PubMed] [Google Scholar]
- 37. Genasetti A, Vigetti D, Viola M, et al. Hyaluronan and human endothelial cell behavior. Connect Tissue Res 2008; 49: 120–123. [DOI] [PubMed] [Google Scholar]
- 38. Culty M, Nguyen HA, Underhill CB. The hyaluronan receptor (CD44) participates in the uptake and degradation of hyaluronan. J Cell Biol 1992; 116: 1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Waldmeier PC, Buchle AM, Steulet AF. Inhibition of catechol-O-methyltransferase (COMT) as well as tyrosine and tryptophan hydroxylase by the orally active iron chelator, 1, 2-dimethyl-3-hydroxypyridin-4-one (L1, CP20), in rat brain in vivo. Biochem Pharmacol 1993; 45: 2417–2424. [DOI] [PubMed] [Google Scholar]
- 40. Tang T, Rosenkranz A, Assmann KJM, et al. A role for Mac-1 (CDIIb/CD18) in immune complex–stimulated neutrophil function in vivo: mac-1 deficiency abrogates sustained Fcγ receptor–dependent neutrophil adhesion and complement-dependent proteinuria in acute glomerulonephritis. J Exp Med 1997; 186: 1853–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]