Abstract
Hollow, tubular organs including oesophagus, trachea, stomach, intestine, bladder and urethra may require repair or replacement due to disease. Current treatment is considered an unmet clinical need, and tissue engineering strategies aim to overcome these by fabricating synthetic constructs as tissue replacements. Smart, functionalised synthetic materials can act as a scaffold base of an organ and multiple cell types, including stem cells can be used to repopulate these scaffolds to replace or repair the damaged or diseased organs. Epithelial cells have not yet completely shown to have efficacious cell–scaffold interactions or good functionality in artificial organs, thus limiting the success of tissue-engineered grafts. Epithelial cells play an essential part of respective organs to maintain their function. Without successful epithelialisation, hollow organs are liable to stenosis, collapse, extensive fibrosis and infection that limit patency. It is clear that the source of cells and physicochemical properties of scaffolds determine the successful epithelialisation. This article presents a review of tissue engineering studies on oesophagus, trachea, stomach, small intestine, bladder and urethral constructs conducted to actualise epithelialised grafts.
Keywords: Tubular scaffolds, epithelialisation, hollow organs, biofunctionalisation
Introduction
Hollow organs may be affected by a variety of disease processes – congenital malformation, autoimmune disease, inflammation, infection and cancer to name but a few potential problems. Current treatment generally relies on resection and/or replacement of this tissue. Resection of tissue is often only successful when relatively small segments of the organ are affected. Ultimately, having less surface area by large-scale removal of tissue leads to the organ having reduced functionality as seen in problems such as short bowel syndrome arising from loss of a component of the digestive system.1–5 Attempts to replace tissue have been made using mucosal grafts from other parts of the body; however, this can cause subsequent problems at the donor site: reconstruction of hollow organ tissue is susceptible to leakage, rejection, stricture formation, stenosis and may require continuous stenting to maintain patency.6–8 Epithelialisation is crucial to maintain patency of organs, and a lack of epithelial cell layer can lead to over-proliferation of underlying fibroblast layer leading to stricture formation, stenosis and potential graft failure9 in addition to organ-specific functions. (Table 1)
Table 1.
Types of epithelial cells present in specific hollow organs.
| Organ | Epithelium type | Function | References |
|---|---|---|---|
| Trachea | Many cell types within epithelium: Ciliated pseudostratified columnar Secretory goblet Serous cells Basal neuroendocrine cells Basal stem cells |
Moisten and protect airways Barrier to pathogens Mucociliary elevator |
Delaere and Van Raemdonck10 |
| Oesophagus | Stratified squamous | Rapid turnover Protective barrier function against the abrasive effects of food |
Ozeki et al.11, Kalabis et al.12 |
| Stomach | Stratified squamous above cardia Simple columnar with gastric pit invagination below cardia |
Mucus cells produce protective alkaline mucus to prevent digestion of stomach wall from HCl producing cells | Young et al.13 |
| Small Intestine | Simple columnar (enterocytes, goblet cells, enteroendocrine cells, M cells and Paneth immune cells) | Selectively absorb digested material from intestinal lumen Release mucus Barrier to pathogens |
Day14 |
| Urinary Bladder | Referred to as urothelium Transitional Basal layer: compact and cuboidal Intermediate: columnar Surface cells: dome cells which are imperbeable to urine |
Epithelium can contract and expand in response to volume of bladder: allows bladder to change shape according to volume of urine without damaging epithelium Protects underlying tissue from caustic effects of urine Protect blood–urine barrier |
Liao et al.15 |
| Urethra Prostatic Membranous Penile |
Referred to as urothelium Transitional Pseudostratified columnar/stratified squamous Pseudostratified columnar |
Mucus-secreting cells to protect underlying tissue from urine Protect blood–urine barrier |
Liao et al.16 |
This increasing burden of unmet clinical need is driving the search for effective procedures to develop functional epithelialised organs. Tissue engineering has already advanced sufficiently to create various organs synthetically for transplantation or reconstruction: examples include the world’s first tissue-engineered bladder.17 However, epithelialisation of these synthetic organs is a process that is proving difficult to replicate in vitro.
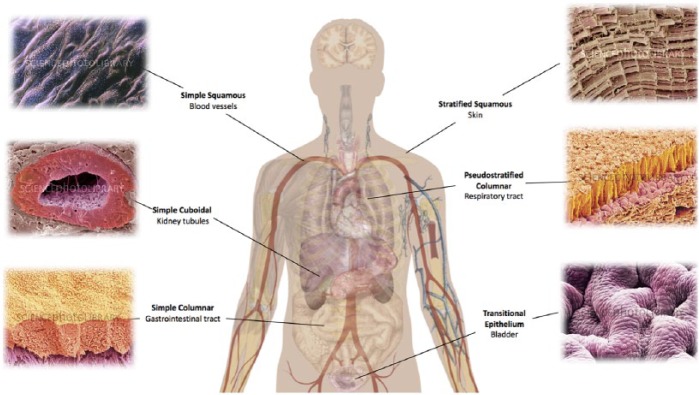
Understanding the nature of epithelial cells is an important consideration when designing epithelialised tissue-engineered structures. Epithelial cells are finely tuned to their specific organ (Table 1). Epithelial cells can be lining hollow organs as surface epithelium13 (Figure 1). At this interface, epithelial cells carry out functions such as creating a protective barrier for underlying organ; absorption of luminal contents; secreting substances into the lumen such as mucus by the goblet epithelial cells in the trachea or digestive enzymes secreted by the stomach and small intestine; controlling passage of materials across body surface by selective diffusion; and containment of luminal contents.18 Cells are usually found as a continuous sheet of cells that, in some organs, can be stacked to form layers. The cells reside upon a basement membrane, which demarcates the border between epithelial cells and underlying cells. This basement membrane is not penetrated by blood vessels, and therefore, epithelial cells rely on simple diffusion for supply of oxygen and nutrients.19
Figure 1.
Types of epithelium corresponding to distinct physiological systems.
Diagram template adapted from Wiki Commons20 and SEM pictures from Science Photo Library.21
This article focuses specifically on the epithelialisation of hollow organs such as trachea, oesophagus, stomach, small intestine, colon, urinary bladder and urethra, where their tubular forms distinguish them from other more solid, visceral organs such as the heart and liver and give a prominent role to the luminal epithelial layer, which is in contact with the external environment. This review aims to evaluate the materials and fabrication methods, which have been successful in producing scaffolds and their limitations, with a view to present these as lessons in designing more optimal scaffolds for a functional epithelium associated with tubular structures.
A host of factors determine epithelial–material interactions which include mimicking extracellular matrix (ECM). For example, scaffold pores should be large enough to allow vascular infiltration and angiogenesis, but not too large to prevent formation of cell layering and epithelial cells slipping through; hydrophilic surface to promote cell adhesion; appropriate tensile strength appropriate to the replaced organ; biodegradable and appropriate rate of degradation to allow successive replacement with native tissue; surface morphology to support cell adhesion; ability to mould into appropriate tubular structures; non-immunogenic; non-toxic; responsive to growth; and easy to produce and transplant into patient.22,23
Numerous types of scaffolds have been developed for hollow organ development. The wide range of materials available and their interaction with epithelial cells are illustrated in Table 2.
Table 2.
Epithelialisation of tissue-engineered scaffolds.
| Organ | Scaffold Type | Source of the Cell | In vitro/species | Degree of epithelialisation | Comments | References |
|---|---|---|---|---|---|---|
| Trachea | Decellularised | Autologous bone marrow–mesenchymal stem cells | Human | Patient’s endoscopy 15 months after surgery showed complete epithelialisation | Inflammation after transplant. Epithelialisation took over 1 year, and graft took 18 months to be mechanically stable but since has been operational | Elliott et al.6 |
| Trachea | Fibrin gel | Respiratory epithelial cells | In vitro | Epithelial cell profileration and differentiation was adequate. Similar results to collagen-coated microporous membranes control | Fibrin can be produced from autologous cells. Clinical application possible using injection moulding technique: research is scalable. Collagen-coated surfaces proliferated faster than fibrin | Cornelissen et al.24 |
| Trachea | Decorin + PCL + gelatin | Tracheal epithelial cells | In vitro | Electrospun meshes with decorin woven into the fibres. Cells spread over the surface of this scaffold and maintained their phenotype | The outcome is good, with decorin enhancing non-immunogenic response. In-vivo experimentation is needed | Hinderer et al.25 |
| Oesophagus | Decellularised | Oesophageal epithelial cell | Rat | Squamous stratified epithelial cell layer forms after 11 days. Once implanted, minor inflammatory response and angiogenesis in graft | There is some inflammatory response when using this scaffold, although not severe | Bhrany et al.26 |
| Oesophagus | 1. PCL 2. SF 3. PCL + SF Repeated with BM protein attachment |
Oesophageal epithelial cell | In vitro | SF–enhanced epithelial cell attachment and proliferation when combined and individually. This was improved by basement membrane (BM) protein attachment | Important result as supports the idea that basement membrane proteins are essential to epithelial regeneration | Lv et al.27 |
| Oesophagus | PCL and PCL–gelatin | Human oesophageal epithelial cells | In vitro | PCL–gelatin compound showed higher proliferation of cells to scaffolds although proliferation is seen on both | No clear stratification of oesophageal cell layers or squamous morphology formation | Kuppan et al.28 |
| Oesophagus | PLGA scaffold precoated with collagen type VI | Canine oesophageal epithelial cells | In vitro and abdominal cavity of dog | ‘Cobblestone-shaped morphology’ and presence of cytokeratins characteristics of epithelial cells: cell maintained oesophageal morphology over 4 weeks | PLGA is often deemed too expensive for wide use. Canine studies may have limited translatability to human models | Bao et al.29 |
| Oesophagus | 1. AlloDerm (decellularised skin scaffold) 2. PLLA 3. PLGA 4. PCL Repeated with collagen precoating |
Rat oesophageal epithelial cells | In vitro | AlloDerm showed comparatively better epithelialisation when compared with synthetic models. There was faster monolayer formation, stratification and keratinisation | At lower calcium concentrations, there is increased proliferation; at higher calcium concentrations, there is increased differentiation. The pore size of synthetic scaffolds limited the formation of continuous epithelial layers | Beckstead et al.30 |
| Oesophagus | 1. Chitosan 2. Chitosan + fibronectin 3. Chitosan + elastin |
Oesophageal epithelial cells | In vitro | Cells fail to adhere to chitosan only and chitosan + elastin. Chitosan + fibronectin formed strong adhesion contacts followed by de-adhesion | Long-term adhesion of cells is triggered when extracellular proteins such as fibronectin and chitosan polymer are present | Feng et al.31 |
| Stomach | PGA mesh coated with PLLA | Stomach epithelium organoid units | Rat | H&E staining showed presence of gastric epithelial cells | The use of organoid units limited full analysis of epithelialisation. There is also focus on patch formation rather than organ replacement | Maemura et al.1 |
| Bladder | Decellularised | Human bladder cells | In vitro | Urothelial cells proliferated on scaffold but were poorly attached | Basal lamina maintained may improve epithelial cell attachment. This decellularisation protocol may be restricted to thinner, less-dense scaffolds with loose collagen arrangements | Rosario et al.32 |
| Bladder | Decellularised | Canine bladder cells | Rat | Urothelium adhered and proliferated on scaffold, forming a multi-layered structure with positive cytokeratin result | Good result | Han et al.33 |
| Urethra | PLLA | Rabbit urothelial cells | In vitro | Good adhesion and proliferation of urothelial cells to scaffold, which had been modified with non-knitted filaments | In-vitro study cannot evaluate how the scaffold copes with in-vivo biophysical stresses. Exposure to urine and genitourinary compounds may affect the cell viability which cannot be deduced from this study | Fu et al.34 |
| Urethra | Decellularised bladder matrix | Mesothelial cells | Rabbit | Grafts placed in rabbit were covered with loose collagen matrix. No stricture formation and multilayer urethral architecture by 1 month | Good outcomes, but restricted to biological models | Gu et al.35 |
| Urethra | Gelatin sponge | Porcine buccal mucosal cells | Pig | Gelatin sponge was partially absorbed, complete epithelialisation of the implant was seen after 1 month. However, there was inflammation and epithelium degenerated after 2 months | The degeneration of the mucosa is not ideal. The environment of the urethral epithelium needs to be examined to determine challenges to epithelial cell survival | Li et al.36 |
PCL: polycaprolactone; SF: silk fibroin; PLGA: poly(lactic-co-glycolic) acid; PLLA: poly(lactic acid).
Types of scaffolds
Biological scaffolds
Decellularised scaffolds
Biological, decellularised scaffolds can be created from donor human or animal tissue. Decellularisation involves removing cells expressing major histocompatibility complex (MHC) class I and II antigens to stop an immunogenic response using detergent.26 Decellularisation of tissue can be done using chemical treatment of NaClO4;37 however, this method has not been widely used. Detergent-enzymatic method has been very popular and generally yields good results.11,38–42
This method removes immunogenic components of tissue while maintaining structural integrity to cope with the biological flow stresses in vivo. The scaffold is biologically active due to native ECM proteins and with pro-angiogenic, chemotactic growth factors remain intact even after the decellularisation process.42 They facilitate cells forming crucial cell–ECM interactions, culminating in organ remodelling required for transplantation.17 However, there are several limitations to this method.
Decellularised scaffold relies on donor organs; thus, it does not overcome the global issue of transplant donor organ shortage. Furthermore, decellularisation does not lead to absence of inflammatory response but it is a comparatively reduced inflammatory response in comparison with allogeneic or xenogeneic grafts. While this may be some form of progress, inflammation can still arise. Both inflammation stenosis and stricture formation have been observed in various decellularised tubular scaffolds, in the absence of cells.16,35,43 Therefore, decellularised scaffolds may require stenting to prevent graft collapse and the long-term biodegradability of decellularised scaffolds being unknown. There is also a lack of uniformity between scaffolds and unable to tailor the graft to the requirements of the recipient.44
Fibrin gel
Fibrin gel is created from fibrinogen and thrombin found in the blood to create a gel-like substance, and this can be easily extracted from autologous blood.45 Bronchial epithelial cells were shown to produce confluent layer and ciliary production when seeded on fibrin gels.24 However, in another study, the cells that grow show less structured layering, rounder cells and more immature cilia formation than original tissue, as cytokeratin patterns in experimental models do not correspond to cytokeratin patterns in native trachea.46 And the significance of epithelial tissue is reiterated by Heikal et al.,47 where fibrin constructs with cells were not implanted and it led to fibrosis and stenosis.
Advantages of fibrin gel are that it is easy to seed cells and mould the gel into appropriate structures. However, due to its relatively fragile nature, it needs to be supported by a mesh if used to replace tubular organs.48
Collagen
Abundant in the ECM, collagen is a good source to use when culturing epithelial cells. A collagen-coated polypropylene mesh has been used for airway reconstruction,49 while collagen scaffold–incorporated fibroblasts have been shown to regenerate tissue and enhance wound healing. After 14 days, epithelialisation and cartilage formation was observed throughout the scaffold, more rapidly than the control.50 This use of collagen-modified scaffolds with stem cell–epithelial cell co-culture encourages mesenchymal cell migration into the scaffold, which may produce basement membrane proteins and growth factors.51
Basic fibroblast growth factor (bFGF) was incorporated into a collagen vitrigel membrane, which then covered an artificial trachea made of Marlex® polypropene mesh and collagen sponge. There was stratified epithelium, columnar cells and ciliated cells at day 5, 7, and 14, respectively.52
OptiMaix-3D collagen-coated scaffolds are prone to epithelial cells passing through or clustering rather than seeding uniformly on the surface. Two-dimensional (2D) scaffolds showed monolayer formation and no cell migration through the scaffold but have limited applicability.53–55 As collagen coating may lead to slow or partially epithelialised surfaces, coating the collagen surface with L-C co-polymer keeps this collagen layer intact and yields more positive results.56
The useful effects of collagen may not be specifically restricted to its physical properties as a scaffold, but also after its degradation, it can improve vascular growth and lead to desmin-positive tissue formation.57
Chitosan
Chitosan is a natural polymer derived from chitin. It can be easily modified and complexed with other proteins. Unmodified chitosan leads to no cell adhesion. Modified chitosan scaffold coated with fibronectin or elastin transiently leads to the formation of strong cell adhesion contacts, but cells eventually undergo de-adhesion. There has been some link to collagen–chitosan complexes supporting oesophageal epithelial cell adhesion and proliferation.31
Gelatin
Gelatin is often complexed with other materials. It is shown to be a biodegradable and bioabsorbable natural polymer, with neovascularisation and epithelial growth seen and degeneration after 2 months. Epithelial markers such as pan-cytokeratin staining, while initially positive, become negative after 4 months. It is a good material for initial adhesion and growth of cells, but poor at maintaining differentiation of buccal mucosa.36
Gelatin has also been combined with other proteins such as decorin–gelatin electrospun complexes. There was greater adhesion of cells and increased cell layer formation in comparison with simple gelatin scaffold. To improve differentiation, there is a need for greater exposure to biophysical flow stress exposure. Also, the pore sizes created by electrospinning technique are too large for successful epithelialisation.25
Gelatin was also combined with dextran sulphate to form dextran sulphate–gelatin membrane. Full tracheal regeneration was observed, but it took 2–3 months and there is a high risk of stenosis in the organ. Furthermore, immunogenic reaction was seen towards this membrane. It is worth noting that hyaluronate-rich extracellular components allow it to have strong cell–scaffold interactions.9
Synthetic scaffolds
Synthetic polymers being increasingly investigated as natural materials prove to be mechanically weak. However, while the physical strength, biocompatibility and bioabsorbability of these polymers are promising, poor cell adhesion hinders their use. Synthetic scaffold success with regard to epithelialisation relies on four main stages epithelial cells have to progress: migration to correct site; adhesion of cells to surface; proliferation of cells to increase in number and repopulate area; and finally, differentiation to mature cell type or cell type seen in vivo models. Different factors target different stages, and thus, the challenge is to create a scaffold material that can successfully progress through all four of these stages.
Silicone stents were used as a scaffold for urothelial growth with an attempt to grow bladder epithelial cells in porcine models. Better results were in fact seen on latex scaffolds, but this may not be universally clinically applicable.4 Polyglycolic acid (PGA) mesh using poly(lactic acid) (PLLA) glue was used to seed stomach epithelial cells such as gastric patches. The results show neomucosa formation with smooth muscle proliferation and no clear discontinuity between donor and recipient mucosa.1 Previous work on polycaprolactone (PCL) has been developed in the fields of bone58,59 and oesophageal27 tissue engineering. Electrospun PCL was seeded with primary oesophageal epithelial cells. While PCL nanofibres show high tensile strength and slower degradation, there was greater cell proliferation on PCL–gelatin hybrid. A PCL–silk fibroin hybrid also promoted the epithelial cell attachment and proliferation. Mitochondrial activity increased when the material was coated with extracted basement membrane proteins.27 These findings demonstrate the importance of a combination of molecular profiles to enhance cell attachment. Poly(lactic-co-glycolic) acid (PLGA) is a biodegradable polymer, and the material shows rapid degradation and useful biocompatibility properties. It also has reduced irritation of sensitive tissues and so may be applicable to urethral stents.60 Precoating with collagen type IV has shown to increase adhesion and proliferation but differentiation is limited.29 A mesh knitted with PLGA and polypropylene for tracheal reconstruction showed good mechanical properties, which were enhanced after coating with polyurethane. However, there was patchy ciliated columnar epithelium intermittently along the graft, rather than the desired confluent layer, even after 6 weeks.61
Scaffold fabrication with three-dimensional printing
A range of biofabrication methods can be used to develop tubular scaffolds (Figure 2). Conventional methods to create scaffolds for tissue engineering such as gas foaming62 and phase separation63 are useful, and there is a need to regenerate the scaffold’s submicron internal architecture and initiate a degree of bioactivity for scaffolds to support epithelialisation. Additive manufacturing methods or three-dimensional (3D) printing can offer methods that can enable precise reproduction of the tissue’s size and shape.64 There are a variety of bioprinting methodologies that include stereolithography apparatus, thermal inkjet printing, fused deposition modelling and powder binding. Stereolithography (SLA)65 uses an excess layer of liquid photopolymer or epoxy resin. A low-power ultraviolet (UV) laser is then used to cure the excess liquid into a solid object. The excess raw materials and supporting structures must be removed and then cured in a UV chamber. A platform is positioned such that a thin layer of photopolymer/epoxy resin (0.05–0.15 mm) exists above the platform, exposing it to the UV laser.66 As the UV beam comes into contact with the liquid plastic, it instantly hardens forming a thin, solid layer at the surface of the platform.67 Each layer produced represents a cross section of the desired 3D object. The platform then moves to allow the superposition of subsequent layers until the desired thickness is reached. This system can be used with living cells and biomaterials.68 Thermal inkjet printing shows promise in regenerative medicine and has generated the foundation for future organ-printing technologies.69,70 With this method, living cells are printed in the form of droplets from a printhead onto a substrate (as opposed to printing them on scaffolds) in accordance with instructions sent digitally from a computer to the printer. The droplets are ejected using compression generated either mechanically or using thermal energy. The droplet size can be as small as 10–150 pL. This can be modified by altering the pulse frequency, temperature gradient or the viscosity of the bio-ink. Fused deposition modelling has a printhead similar to that used in a thermal inkjet printer.71 Layers of material are created by the deposition of material such as plastic as the printhead moves.70,72 The process is repeated allowing very precise control of the amount and location of each droplet of material at each layer.73 As the material is heated, it fuses as it cools to the layers below.73
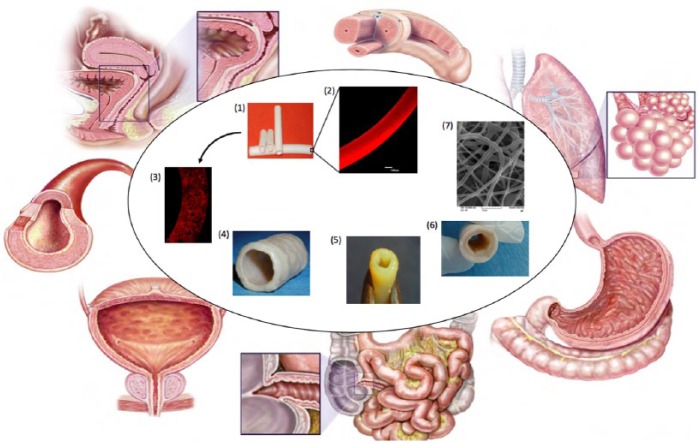
Figure 2.
Possible biofabrication methods to develop hollow, tubular scaffolds to replace/repair tubular organs.
(1) Solvent evaporation of polycaprolactone; (2) magnified cross section of (1);74 (3) magnified cross section of solvent exchanged polycaprolactone-based scaffold;74 (4) decellularised tracheal segment;75 (5) 3D-printed tubular tissue;76 (6) hybrid scaffold (polyurethane outer coat on a decellularised oesophagus); (7) electrospun tubular tissue.77 Figure of structures of natural tubular organs is adapted from Basu and Ludlow.78
There is also powder binding, by which a layer of powdered material placed on a surface and a solvent (or liquid binder) is selectively deposited onto the powdered surface by a printhead. The solvent (or liquid binder) causes the powdered material to bind together to form a fragile but solid material of a predetermined geometry. In addition to hollow, tubular scaffolds, stents are widely used to address disease and damaged tubular structures. Strut structure, high radial strength (needed to maintain tubular diameter), low recoil, high radiopacity (to ensure precise positioning)79 and conformability (lack of conformability or increased stent rigidity) leading to failure are significant features.80 Table 3 contains a summary of the advantages and disadvantages of various 3D printers.
Table 3.
Advantages and disadvantages of 3D printers.
| Type of printer | Advantages | Disadvantages |
|---|---|---|
| Stereolithography | High resolution can reach submicron scale | Expensive laser systems Laser could damage living cells Limited to UV-curable substances |
| Thermal inkjet printer | Use of small droplet volume permits high-resolution printing | Requires the use of material that has a high gelation rate which limits the materials that can be used |
| Fused deposition binding | Objects can be produced using cheap systems | During the processing stages, rough surfaces are produced. Low resolution |
| Powder binding | Low cost Fast printing speed Wide variety of powder material |
Low resolution Difficult to remove the solvent/liquid binder |
3D: three-dimensional; UV: ultraviolet.
Stainless steel can be used to make stents which has the main advantage of being highly biocompatible and sufficient mechanical strength. However, there were a number of limitations such as high strut thickness, limited flexibility and low corrosion resistance.79 Cobalt–chromium stent alloys are also used which allow for thinner struts without compromising radial strength or resistance to corrosion. The introduction of a platinum–chromium alloy stent appears to incorporate many properties such as radiopacity, thin struts, high radial strength and biocompatibility. These stents can be designed as drug-eluting stents81 and can potentially modify to ensure epithelialisation or to eliminate patency-limiting factors through the introduction of functional epithelium mimicking bio-factors.
Properties considered when fabricating scaffolds for hollow organ development
Pore size and porosity
Pore size and porosity play an important role in mimicking natural ECM and for cells to attach. Electrospinning, salt sintering and 3D printing82 are some methods by which pores are introduced. Porosity determines the mechanical strength of the polymer and the rate of biodegradability in non-biostable materials. Epithelial adhesion is shown to be optimal in scaffolds with pore sizes <10 µm.18 Nanosized porous or fibrous surfaces have also been shown to be advantageous to cell and protein adhesion within scaffold surface but larger pores created by methods such as electrospinning produce larger diameter pores do not optimally suit epithelial cell seeding.83
Hence, a laminated model seems to present a better solution with large pore sizes on the basal layer but smaller pore sizes on the luminal surface to allow for epithelial adhesion and prevent cell penetration. In the small intestine, cell sheets of varying porosity and cell size were compounded to make multi-layered scaffold.84 The bilayered concept was also explored using electrospun scaffold where smaller pores are used in luminal surface and bigger pores for basal surface which is conducive for fibroblasts.39 There is a need for scaffolds with a smaller pore size at the luminal surface for optimal epithelium attachment and proliferation. However, larger pores have shown to encourage bronchial epithelial cell aggregation, integration and vascular growth.85
Stretchability/stiffness
The role of scaffold stiffness was long recognised to influence cell–material interactions, where soft polyacrylamide gels (E = 0.1−1 kPa) would direct mesenchymal stem cell differentiation towards neuronal phenotype such as brain. Relatively harder gels (E = 8 to 17 kPa) directed mesenchymal stem cells (MSCs) to become muscle cells, while the stiffest scaffolds (E = 25 to 40 kPa) produced osteogenic cells.86 Recently, peristalsis has been shown to stimulate micromechanical processes such as rearranging lateral cell–cell adhesions and aligning cytoskeletal components.87
Surface modification
Surface modifications to scaffolds such as introducing bioactive molecules88–90 can alter the surface chemistry, thereby modulating cell attachment and proliferation. Plasma treatment can introduce hydroxyl and carbonyl groups that increase hydrophilicity of the scaffold and increase cell adhesion.91 The argon plasma ablation of polyethylene led to oxidation and increased surface roughness which had positive effect on fibroblasts cells.92
An optimal scaffold mimics the basic structure of the ECM. The ECM co-ordinates the binding of cells. Cells also respond to the ECM via integrin receptors which recognise and interact with ECM components. Subsequently, leading to signal transduction intracellularly modifies cell behaviour.
Therefore, an ideal scaffold must be more than a passive support for cells. It is a much more dynamic and influential structure: binding various signals (such as growth factors and hormones) that are tailored to the surrounding cell type is responsive to the action of cells and adjusts nutrient supply to the cell accordingly.
The concentration of calcium to which epithelial cells are exposed enhances different stages of epithelial cell growth. Cells cultured under low calcium conditions show greater proliferative capacity. When calcium concentration increases, there is raised differentiation of epithelial cells and reduced proliferation. In synthetic scaffolds, it is the crucial step of adhesion, that is, one of the great challenges of tissue engineering. The calcium concentration was initially low, and after reaching confluence, it increased.30
Such chemical and biological modifications on a scaffold can influence the surface wettability of a scaffold surface. Surface wettability refers to the hydrophilic or hydrophobic nature of the scaffold surface. Hydrophilic scaffolds tend to resist proteins, while hydrophobic scaffolds absorb proteins. Absorption of proteins might lead to distortion of the 3D conformation of the protein, making changes to the degree of cell adhesion and migration.23 Moderately, hydrophilic materials are optimum for adsorption of proteins. Neither super hydrophilic nor super hydrophobic materials have shown to be ideal.93
Cell sources for epithelialisation
Current scaffolds seem unable to rely on native cells for repopulation, as independently forming functional epithelium can be time-consuming and may impact the regeneration of underlying tissues due to reciprocal differentiation factors released from neighbouring tissue layers.30,94 The current studies on cell sources used for epithelisation are listed in Table 4.
Table 4.
Typical cell sources used for epithelialisation.
| Organ | Cell type | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Trachea | Endogenous adult stem cells: Located in basal layers of airways Near pulmonary neuroendocrine cell rests Bronchioalveolar junction Alveolar epithelial surface |
Used endogenously for lung repair and regeneration as seen on alveolar surface injuries, no immunogenic response as host cells | Ageing of lungs arises with decreasing repair capacity due to endogenous stem cell failure with age. These cells have been discussed in rodent models but not fully established and have been recently discovered in humans | Chistiakov95 |
| Trachea | ESCs | Very pluripotent and can differentiate to a variety of cell lineages | Ethical problems with procurement. Immunogenicity problem may require immune suppression to prevent host response to graft | Roomans96 |
| Trachea; small intestine | BM-MSCs | Evidence showing epithelium derived from BM-MSCs in mice models (86). Capacity to differentiate into cells outside their lineage (87) |
No direct conclusive evidence to show differentiation to epithelium. Controversial, as some believe intraepithelial lymphocytes may be interpreted as donor-derived epithelial cells rather than there being actual epithelial lineage (88). | Gomperts et al.97,98, MacPherson et al.99 |
| Trachea | Adipocyte mesenchymal stem cells | Differentiation to epithelial cells seen in rat models | Immunogenicity problem, may require immune suppression to prevent host response to graft | Suzuki et al.51 |
| Trachea | Amniotic fluid stem cells/amniotic fluid progenitor cells | Wide range of pluripotency of a variety of embryonic germ-layer origins, less tumour inducing than ESCs | Immunogenicity problem, may require immune suppression to prevent host response to graft | Chistiakov95 |
| Trachea | hiPS | Less ethical concern surrounding production and usage and ESC-like pluripotency | Not used in airway tissue engineering and may take time to programme genes to produce hiPS from somatic cell | Chistiakov95 |
| Trachea | Skin epithelial cells | Easy to access and use, show transdifferentiation to airway cells sustained for several months | Inflammation and degrees of stenosis seen post-surgery. Only done in canine models | Kim et al.100 |
| Oesophagus | Squamous epithelial cells | Epithelial cells formed layered structures mimicking native oesophagus. Cytokeratin and alpha-actin staining showed differentiation of transplanted primary epithelial cells | Cells were co-cultured with myoblast cells, so may be confounding factor and co-culturing products enhancing cell differentiation. Cell source was aborted foetus which is difficult to use clinically for multiple reasons | Cen et al.101 |
| Oesophagus | Oral mucosal tissue | Full epithelialisation occurred on the specific area epithelial sheets transplanted, as well as spreading past this area | One case had large oesophageal ulceration, stricture and dysphagia | Ohki et al.102 |
| Bladder | Oral mucosal tissue | Cell sheets contained both progenitor and proliferative cell populations | Exposure to urine affected the viability of the cells and their expression of epithelial cell markers. Lack of elasticity as seen in native bladder. Contraction due to inflammation of bladder wall occurs | Watanabe et al.103 |
| Urethra | Oral cells (seeded as composite model of epithelial oral mucosal cell sheet and muscle cells on collagen scaffold) | Reduced stricture, lumen lined by stratified epithelial cell layer | Positive result may be due to co-culture with muscle cells, growth on collagen scaffold and the support this provided for angiogenesis | Mikami et al.57 |
| Urethra | Bladder urothelial cells | Transitional epithelium as normally found in urethra is seen. Four out of six boys demonstrated urothelium for 8 years | Only four out of six boys eventually grew urothelium and the urothelium was not always present in biopsies taken. Difficult to harvest and culture in vitro | Fossum et al.5 |
| Urethra | Simple squamous mesothelium epithelium | No stricture formation when presented with intervention. Multilayered structure forms with differentiation to urothelium from mesothelium | Epithelium grown on graft was more irregular and contained fewer layers. Smooth muscle formed irregularly underneath and mainly at points of anastomosis. Takes 6 months for full epithelialisation | Gu et al.35 |
ESC: embryonic stem cell; BM-MSC: bone marrow–mesenchymal stem cell; hiPS: human-induced pluripotent stem cell.
Autologous source
It is also important to consider the immunogenic potential of grafted cells, a common concern in allogeneic or xenogeneic transplants. One way to solve this problem is relying on autologous cell transplantation. Studies have been carried out to establish culture systems for tracheal epithelial cells using tissue explant technique.104,105 While it would be intuitive to directly seed epithelial cells to allow epithelialisation, there is difficulty in epithelial cell extraction, optimal cell adhesion and sustained differentiation.41,106
There have been attempts to locate endogenous stem cells found within the site of the organ by looking at models in response to organ injury and determining where the new generation of cells to repair the organ and replace lost cells arise from, hoping to use this pool of undifferentiated cells for seeding. However, there are some cases where this proves difficult, such as in patients with reduced intestinal length due to ulcerative colitis or Crohn’s disease and may not have the capacity to provide the number of stem cells for adequate population of the graft. One solution for this may be in-vitro expansion of cells; however, intestinal epithelial cells have poor in-vitro growth, and this may reduce the clinical translatability of this method.14
Stem cells
Pluripotent stem cell is a viable option. Bone marrow–mesenchymal stem cells (BM-MSCs) hold much potential, as BM-MSCs show cytokeratin expression and migration to replace damaged epithelial cells. Several papers reiterate the idea that epithelial progenitor cells are derived from bone marrow, which circulate and then recruit to the site of injury to reconstitute the repaired epithelium to some extent.97,98 Adipocyte MSCs showed a pseudostratified columnar epithelium along with goblet cells, cilia and angiogenesis in rat models for tracheal epithelial growth.51 Human embryonic stem cells hold great potential but are wrapped in controversy. However, amniotic fluid stem cells or amniotic fluid progenitor cells display similar characteristics of bone marrow stem cells, but in rodent models have been shown to have ‘higher healing properties’, perhaps by influencing local oxygen levels. The stem cells have similar properties to the embryonic stem cells, but there are less ethical dilemmas surrounding these cells as well as less risk of being teratogenic.95 Another cell source is human-induced pluripotent stem cells, and these can show embryonic stem cell–like activity using similar signalling pathways by modification of around four key genes.107
Transdifferentiation of skin epithelial cells to tracheal epithelial cells presents a different method of obtaining epithelial cells. Results show cilia formation, and cells remain viable for several months. Despite inflammation after 1 month post-surgery and some stenosis 4 months post-surgery, this presents an interesting avenue of alternative cell sources for epithelialisation.100
Co-culture
The use of cells seeded on scaffolds is actually one of some debates. Some papers argue that research should move to focus on ensuring the scaffold has sufficient factors to stimulate cell migration, proliferation and differentiation in vivo rather than using valuable resources procuring cells and fine-tuning technique to graft onto scaffold.
The bipotential scaffold fabricated by Tada et al.108 aimed to show that native tissue infiltration is able to produce mucosal repair using native cells without the need for seeding.
Epithelialisation has shown to be optimal when co-cultured with fibroblasts or media conditioned with fibroblasts. The interaction between neighbouring mesenchymal cells and epithelial cells is crucial in differentiation of epithelium and graft development. Fibroblasts produce essential ECM, which also supports epithelial cells, secrete growth factor molecules such as bFGF, epidermal growth factor (EGF) and keratinocyte growth factor among others, each helping to develop the epithelium and surrounding mesenchyme.109
Nasal respiratory epithelial cells and fibroblasts were grown together for 1 week using a fibrin and titanium mesh in ovine models, which reconstituted the basement membrane.47 Fibroblasts continue to have this positive effect even in larger tracheal defects with ciliated, pseudostratified epithelium still seen.106 Kobayashi et al.110 also co-cultured epithelial cells with fibroblasts, leading to pseudostratified cilia goblet and basal cells formation and reciprocally, fibroblasts increasing mucin secretion by epithelial cells. However, their novel work was co-culturing fibroblasts and adipose-derived stem cells with tracheal epithelial cells. It transpires that each cell plays its own unique role in epithelial cell regeneration. The fibroblasts drive differentiation and pseudostratification of the epithelial cell layer, and adipose-derived stem cells drive proliferation, multilayering of epithelial cell sheets and accelerate neovascularisation. There are detailed synergistic effects of epithelialisation on both types of cells, as well as ion channel and basement membrane construction.53
The inclusion of adipose-derived stem cells has indicated that other cells may also be implicated in improving epithelialisation shown by promising results with BM-MSCs and chondrocytes co-culture.95 Pfenninger et al. co-cultured many human epithelial cells with various combinations of other cells including chondrocyte pellets, articular cartilage chips and collagen membrane plus chondrocytes. Epithelial cells were seeded internally and chondrocyte externally on the luminal surface of a decellularised tracheal grafts and placed in a bioreactor. Both cell types covered the matrix within 72 h and improved epithelialisation and graft survival.109
Endothelial and epithelial cells have crucial and differing roles in response to injury. The epithelial cells contain the extent of the injury and stimulate mesenchymal hyperplasia to allow proliferation of cells to replace injured cells, while endothelial cells maintain and preserve epithelial cells and perfuse the injured tissue. This in turn produces factors for fibroblast migration and remodelling and further enhances epithelium growth.111 This supports Beckstead in the oesophageal model, who has suggested that the regeneration of epithelium is crucial as it is linked to the regrowth of the underlying muscular layers in the oesophagus.30
Other techniques of improving epithelialisation
The way in which cells are seeded onto the scaffold is important in epithelialisation of the tubular structures. Conventionally, cells are usually seeded onto scaffold while in a solution with the appropriate media, with individual or clusters of cells forming attachments. This method works fairly well; however, alternative methods of cell seeding onto scaffolds have been investigated. Figure 3 summaries the various factors involved to create the ideal tubular scaffold.
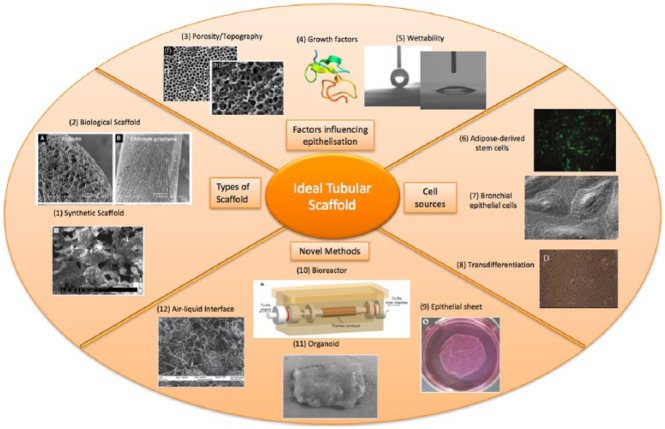
Figure 3.
Factors influencing an ideal tubular scaffold.
(1) SEM of a synthetic scaffold;30 (2) various SEM of materials and porous scaffolds;82 (3) porogen to induce homogenous honeycomb-structured pores (Everett et al.90); (4) 3D rendering of epidermal growth factor;112 (5) modulating surface wettability; measurements of water contact angle; (6) fluorescence image of adipose-derived stem cells;51 (7) SEM of bronchial epithelial cells; (8) skin epithelial cells transdifferentiation;100 (9) epithelial cell sheets;113 (10) bioreactor;114 (11) organoid (Maemura et al.1); and (12) air–liquid interface.47
Air–liquid interface
Air–liquid interface cultures are useful in airway epithelium formation.39 In submerged conditions, murine embryonic stem cells differentiated to non-ciliated secretory Clara cells, but when using air–liquid interface culturing techniques, the stem cells differentiated to all three cell types of airway epithelium ciliated, basal, and secretory.115
Epithelial cell sheets
The use of epithelial cell sheets has been shown to have regenerative potential even without scaffold support, commonly used therapeutically in oesophageal endoscopic submucosal dissection.102,113,116 The cell sheet is formed by lowering the temperature of the flask containing cells to around 20°C rather than trypsinising cells to seed them on scaffold in solution; however, mild fibrosis and substantial degree of constriction are still seen.94,117,118
In the context of synthetic scaffolds, cell sheet seeding may overcome the practical problem of epithelial cell adhesion as epithelial cells often infiltrate into the scaffold pores rather than forming a surface layer.55 A skin graft of epithelial cells seeded upon cartilage sheets wrapped with external abdominal oblique muscle flaps and a silicone stent was performed and are well-vascularised and remodelled except for thin layers that led to poor epithelialisation.119
Organoid units
An organoid is a bud of an organ which preserves the various cell layers on a smaller scale than the native organ, thereby allowing for interactions between different cell layers such as small intestines.120 In intestinal models, epithelial differentiation through goblet and/or Paneth cell formation, as well as a progenitor cell layer forming below, is consistent with gastric epithelium and expression of gastric stem cell markers.121 However, most of the cells die after implanting the construct as the complex organoid structure no longer receives adequate nutrition to the inner, more densely packed layers. In the gastric model, heterogeneity of cell types in different regions of the stomach meant that organoids may not have the full variety of cells seen in the native stomach.121
Bioreactor
Exposure of the cell–scaffold construct within in-vivo environment may enhance tissue formation. All hollow organs mediate an interface between internal and external environments, and exposure of the graft to this interface allows important additional tissue development, such as immune cell lymphoid tissue.14
A bioreactor can simulate this in-vivo environment as the graft matures. There was greater chondrocytes seeding on collagen scaffold when the scaffold rotated 5 to 20 r/min in a bioreactor.122 This mimics in-vivo physiological signals such as shear stress, compression and pressure, thereby allowing cells to respond to them in vitro.
Using in-vivo bioreactor such as implanting urethral scaffolds in peritoneal cavities of rabbits and scaffolds was well covered in fibroblasts and mesothelium. There was no stricture formation when scaffolds were transplanted into rabbits.123 Similarly, omentum was used as a bioreactor where oesophageal scaffolds were implanted. Results showed vascularisation, and its anatomical position can be used as a pedicle for subsequent transposition.124 In-vivo bioreactors should be explored further to understand its interaction with host tissue.
Angiogenesis
The delivery of nutrients and oxygen to epithelium plays a key role in epithelisation. The diffusion limit of nutrients and oxygen is approximately 200 µm, and the lack of vessels severely restrict the size of tissue-engineered scaffolds. Hence, angiogenesis is important.125 Therefore, vascular endothelial growth factor (VEGF) can be introduced to improve vessel infiltration. The administration of VEGF is, however, difficult but continuous delivery of VEGF may be possible through a bioreactor. Improved oxygen delivery may decrease lactate concentration in the graft and improve epithelial metabolism.126 This has been investigated using perfluorocarbon-based artificial oxygen carrier (Oxygent). This has benefits in maintaining a functional basal lamina and decreased lethal airway obstruction, but also may lead to decreased chondrocyte function.127
Concluding remarks and future direction
Biological scaffolds so far have presented relatively more successful results for tubular scaffold epithelialisation, originating from their ability to provide tissue-specific cues for cell–matrix interaction. Biomimicry of the natural tubular structures with synthetic scaffolds with the state-of-the-art materials and fabrication methodologies might be the way forward for effective epithelisation. Current non-biological approaches involve seeding cells on suitable scaffolds, but still lack the full range of crucial structures that mimic the ECM which are required to replicate these organ-specific cellular cues.
The best bioactive scaffolds would be those that use cell-signalling pathways to mimic the in-vivo repair and regeneration process. This is the strength of decellularised scaffolds, despite them lacking suitable mechanical strength, which could lead to graft failure. Furthermore, topography and physicochemical characteristics such as porosity, material strechability and surface wettability play a major role in epithelialisation. Embedding relevant growth factors within the scaffold may further enhance epithelial cell binding. Ultimately, to produce functional organs, it will be unlikely to rely solely on optimising cell seeding. It would be practical to improve scaffold intrinsic properties to allow autologous cells to migrate towards the scaffold of interest and transform into a functional tissue that can restore physiological homeostasis.
Footnotes
Author contribution: Authors Rhea Saksena and Chuanyu Gao contributed equally to this article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
References
- 1. Maemura T, Kinoshita M, Shin M, et al. Assessment of a tissue-engineered gastric wall patch in a rat model. Artif Organs 2012; 36: 409–417. [DOI] [PubMed] [Google Scholar]
- 2. Hu J, Chen X, Zhou Z. [Intestinal stem cells and tissue engineering technique used in treating intestinal diseases]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2007; 21: 175–179. [PubMed] [Google Scholar]
- 3. Atala A. Tissue engineering of human bladder. Br Med Bull 2011; 97: 81–104. [DOI] [PubMed] [Google Scholar]
- 4. Fossum M, Zuhaili B, Bergmann J, et al. Minced urothelium to create epithelialized subcutaneous conduits. J Urol 2010; 184: 757–761. [DOI] [PubMed] [Google Scholar]
- 5. Fossum M, Skikuniene J, Orrego A, et al. Prepubertal follow-up after hypospadias repair with autologous in vitro cultured urothelial cells. Acta Paediatr 2012; 101: 755–760. [DOI] [PubMed] [Google Scholar]
- 6. Elliott MJ, De Coppi P, Speggiorin S, et al. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet 2012; 380: 994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baiguera S, Gonfiotti A, Jaus M, et al. Development of bioengineered human larynx. Biomaterials 2011; 32: 4433–4442. [DOI] [PubMed] [Google Scholar]
- 8. Spitz L. Esophageal replacement: overcoming the need. J Pediatr Surg 2014; 49: 849–852. [DOI] [PubMed] [Google Scholar]
- 9. Klin B, Weinberg M, Vinograd I, et al. Experimental repair of tracheal defects using a new biodegradable membrane. J Laparoendosc Adv Surg Tech A 2007; 17: 342–349. [DOI] [PubMed] [Google Scholar]
- 10. Delaere P, Van Raemdonck D. Tracheal replacement. J Thorac Dis 2016; 8: S186–S196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ozeki M, Narita Y, Kagami H, et al. Evaluation of decellularized esophagus as a scaffold for cultured esophageal epithelial cells. J Biomed Mater Res A 2006; 79: 97–103. [DOI] [PubMed] [Google Scholar]
- 12. Kalabis J, Wong GS, Vega ME, et al. Isolation and characterization of mouse and human esophageal epithelial cells in 3D organotypic culture. Nat Protoc 2012; 7: 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Young BL, Stevens A, Heath J. Wheater’s functional histology: a text and colour atlas. 5th ed. London, UK: Churchill Livingstone Elsevier, 2011. [Google Scholar]
- 14. Day RM. Epithelial stem cells and tissue engineered intestine. Curr Stem Cell Res Ther 2006; 1: 113–120. [DOI] [PubMed] [Google Scholar]
- 15. Liao WB, Song C, Li YW, et al. Tissue-engineered conduit using bladder acellular matrix and bladder epithelial cells for urinary diversion in rabbits. Chin Med J (Engl) 2013; 126: 335–339. [PubMed] [Google Scholar]
- 16. Liao W, Yang S, Song C, et al. Tissue-engineered tubular graft for urinary diversion after radical cystectomy in rabbits. J Surg Res 2013; 182: 185–191. [DOI] [PubMed] [Google Scholar]
- 17. Atala A, Bauer SB, Soker S, et al. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006; 367: 1241–1246. [DOI] [PubMed] [Google Scholar]
- 18. Kevin JM, Sarah LT, Magali S-G. A novel porous scaffold fabrication technique for epithelial and endothelial tissue engineering. J Mater Sci Mater Med 2013; 24: 1659–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joshi SD, Davidson LA. Epithelial machines of morphogenesis and their potential application in organ assembly and tissue engineering. Biomech Model Mechanobiol 2012; 11: 1109–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wikimedia Commons. Human body diagrams, http://commons.wikimedia.org/w/index.php?title=Human_body_diagrams&oldid=81542289 (accessed 2 May 2015).
- 21. Science Photo Library. https://www.sciencephoto.com/ (accessed 5 November 2016).
- 22. Patel M, Fisher JP. Biomaterial scaffolds in pediatric tissue engineering. Pediatr Res 2008; 63: 497–501. [DOI] [PubMed] [Google Scholar]
- 23. Jell G, Minelli C, Stevens MM. Biomaterial-related approaches: surface structuring. In: Meyer U, Meyer T, Handschel J, et al. (eds) Fundamentals of tissue engineering and regenerative medicine. Berlin, Germany: Springer, 2009, pp. 469–484. [Google Scholar]
- 24. Cornelissen CG, Dietrich M, Krüger S, et al. Fibrin gel as alternative scaffold for respiratory tissue engineering. Ann Biomed Eng 2012; 40: 679–687. [DOI] [PubMed] [Google Scholar]
- 25. Hinderer S, Schesny M, Bayrak A, et al. Engineering of fibrillar decorin matrices for a tissue-engineered trachea. Biomaterials 2012; 33: 5259–5266. [DOI] [PubMed] [Google Scholar]
- 26. Bhrany AD, Benjamin L, Farwell DG, et al. Development of an esophagus acellular matrix tissue scaffold. Tissue Eng 2006; 12: 319–330. [DOI] [PubMed] [Google Scholar]
- 27. Lv J, Chen L, Zhu Y, et al. Promoting epithelium regeneration for esophageal tissue engineering through basement membrane reconstitution. ACS Appl Mater Interfaces 2014; 6: 4954–4964. [DOI] [PubMed] [Google Scholar]
- 28. Kuppan P, Sethuraman S, Krishnan U. PCL and PCL-gelatin nanofibers as esophageal tissue scaffolds: optimization, characterization and cell-matrix interactions. J Biomed Nanotechnol 2013; 9: 1540–1555. [DOI] [PubMed] [Google Scholar]
- 29. Bao C, Ding F, Mei J. [Experimental studies on tissue engineered esophagus reconstructed with artificial biodegradable scaffold]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2006; 20: 1235–1239. [PubMed] [Google Scholar]
- 30. Beckstead BL, Pan S, Bhrany AD, et al. Esophageal epithelial cell interaction with synthetic and natural scaffolds for tissue engineering. Biomaterials 2005; 26: 6217–6228. [DOI] [PubMed] [Google Scholar]
- 31. Feng Z, Chian KS, Ong WF, et al. Dual requirements of extracellular matrix protein and chitosan for inducing adhesion contact evolution of esophageal epithelia. J Biomed Mater Res A 2007; 82: 778–801. [DOI] [PubMed] [Google Scholar]
- 32. Rosario DJ, Reilly GC, Ali Salah E, et al. Decellularization and sterilization of porcine urinary bladder matrix for tissue engineering in the lower urinary tract. Regen Med 2008; 3: 145–156. [DOI] [PubMed] [Google Scholar]
- 33. Han P, Luo J, Zhi W, et al. [Constructing tissue-engineered urothelial structures in vitro and in vivo]. Sichuan Da Xue Xue Bao Yi Xue Ban 2008; 39: 481–484, 510. [PubMed] [Google Scholar]
- 34. Fu W, Wang Z, Li G, et al. A surface-modified biodegradable urethral scaffold seeded with urethral epithelial cells. Chin Med J (Engl) 2011; 124: 3087–3092. [PubMed] [Google Scholar]
- 35. Gu GL, Xia SJ, Zhang J, et al. Tubularized urethral replacement using tissue-engineered peritoneum-like tissue in a rabbit model. Urol Int 2012; 89: 358–364. [DOI] [PubMed] [Google Scholar]
- 36. Li P, Cai M, Li Z, et al. [Long-term observation of prefabricated urethra with buccal mucosa in expanded capsule]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2009; 23: 1487–1490. [PubMed] [Google Scholar]
- 37. Kiselevsky MV, Anisimova NY, Lebedinskaya OV, et al. Optimization of a method for preparation and repopulation of the tracheal matrix for allogenic transplantation. Bull Exp Biol Med 2011; 151: 107–113. [DOI] [PubMed] [Google Scholar]
- 38. Teti A. Regulation of cellular functions by extracellular matrix. J Am Soc Nephrol 1992; 2: S83-S87. [DOI] [PubMed] [Google Scholar]
- 39. Morris GE, Bridge JC, Brace LA, et al. A novel electrospun biphasic scaffold provides optimal three-dimensional topography for in vitro co-culture of airway epithelial and fibroblast cells. Biofabrication 2014; 6: 35014. [DOI] [PubMed] [Google Scholar]
- 40. Zang M, Zhang Q, Chang EI, et al. Decellularized tracheal matrix scaffold for tracheal tissue engineering. Plast Reconstr Surg 2013; 132: 549e–559e. [DOI] [PubMed] [Google Scholar]
- 41. Conconi MT, De Coppi P, Di Liddo R, et al. Tracheal matrices, obtained by a detergent-enzymatic method, support in vitro the adhesion of chondrocytes and tracheal epithelial cells. Transpl Int 2005; 18: 727–734. [DOI] [PubMed] [Google Scholar]
- 42. Marzaro M, Vigolo S, Oselladore B, et al. In vitro and in vivo proposal of an artificial esophagus Maurizio. J Biomed Mater Res A 2006; 77: 795–801. [DOI] [PubMed] [Google Scholar]
- 43. Poghosyan T, Gaujoux S, Vanneaux V, et al. In vitro development and characterization of a tissue-engineered conduit resembling esophageal wall using human and pig skeletal myoblast, oral epithelial cells, and biologic scaffolds. Tissue Eng Part A 2013; 19: 2242–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berthiaume F, Maguire TJ, Yarmush ML. Tissue engineering and regenerative medicine: history, progress, and challenges. Annu Rev Chem Biomol Eng 2011; 2: 403–430. [DOI] [PubMed] [Google Scholar]
- 45. Janmey PA, Winer JP, Weisel JW. Fibrin gels and their clinical and bioengineering applications. J R Soc Interface 2009; 6: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Heikal M, Aminuddin B, Jeevanan J, et al. A scanning electron microscopic study of in vivo tissue engineered respiratory epithelium in sheep. Med J Malaysia 2008; 63(Suppl. A): 34. [PubMed] [Google Scholar]
- 47. Heikal MYM, Aminuddin BS, Jeevanan J, et al. Autologous implantation of bilayered tissue-engineered respiratory epithelium for tracheal mucosal regenesis in a sheep model. Cells Tissues Organs 2010; 192: 292–302. [DOI] [PubMed] [Google Scholar]
- 48. Flanagan TC, Cornelissen C, Koch S, et al. The in vitro development of autologous fibrin-based tissue-engineered heart valves through optimised dynamic conditioning. Biomaterials 2007; 28: 3388–3397. [DOI] [PubMed] [Google Scholar]
- 49. Omori K, Nakamura T, Magrufov A, et al. Cricoid regeneration using in situ tissue engineering in canine larynx for the treatment of subglottic stenosis. Ann Otol Rhinol Laryngol 2004; 113: 623–627. [DOI] [PubMed] [Google Scholar]
- 50. Nomoto Y, Okano W, Imaizumi M, et al. Bioengineered prosthesis with allogenic heterotopic fibroblasts for cricoid regeneration. Laryngoscope 2012; 122: 805–809. [DOI] [PubMed] [Google Scholar]
- 51. Suzuki T, Kobayashi K, Tada Y, et al. Regeneration of the trachea using a bioengineered scaffold with adipose-derived stem cells. Ann Otol Rhinol Laryngol 2008; 117: 453–463. [DOI] [PubMed] [Google Scholar]
- 52. Tani A, Tada Y, Takezawa T, et al. Regenerative process of tracheal epithelium using a collagen vitrigel sponge scaffold. Laryngoscope 2013; 123: 1469–1473. [DOI] [PubMed] [Google Scholar]
- 53. Kobayashi K, Suzuki T, Nomoto Y, et al. A tissue-engineered trachea derived from a framed collagen scaffold, gingival fibroblasts and adipose-derived stem cells. Biomaterials 2010; 31: 4855–4863. [DOI] [PubMed] [Google Scholar]
- 54. Saxena AK, Ainoedhofer H, Höllwarth ME. Culture of ovine esophageal epithelial cells and in vitro esophagus tissue engineering. Tissue Eng Part C Methods 2010; 16: 109–114. [DOI] [PubMed] [Google Scholar]
- 55. Saxena AK, Ainoedhofer H, Höllwarth ME. Esophagus tissue engineering: in vitro generation of esophageal epithelial cell sheets and viability on scaffold. J Pediatr Surg 2009; 44: 896–901. [DOI] [PubMed] [Google Scholar]
- 56. Sato T, Araki M, Nakajima N, et al. Biodegradable polymer coating promotes the epithelization of tissue-engineered airway prostheses. J Thorac Cardiovasc Surg 2010; 139: 26–31. [DOI] [PubMed] [Google Scholar]
- 57. Mikami H, Kuwahara G, Nakamura N, et al. Two-layer tissue engineered urethra using oral epithelial and muscle derived cells. J Urol 2012; 187: 1882–1889. [DOI] [PubMed] [Google Scholar]
- 58. Rentsch B, Bernhardt R, Scharnweber D, et al. Embroidered and surface coated polycaprolactone-co-lactide scaffolds: a potential graft for bone tissue engineering. Biomatter 2012; 2: 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tarafder S, Bose S. Polycaprolactone-coated 3D printed tricalcium phosphate scaffolds for bone tissue engineering: in vitro alendronate release behavior and local delivery effect on in vivo osteogenesis. ACS Appl Mater Interfaces 2014; 6: 9995–9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kotsar A, Isotalo T, Mikkonen J, et al. A new biodegradable braided self-expandable PLGA prostatic stent: an experimental study in the rabbit. J Endourol 2008; 22: 1065–1069. [DOI] [PubMed] [Google Scholar]
- 61. Shi H, Xu Z, Qin X, et al. Experimental study of replacing circumferential tracheal defects with new prosthesis. Ann Thorac Surg 2005; 79: 672–676. [DOI] [PubMed] [Google Scholar]
- 62. Nam YS, Yoon JJ, Park TG. A novel fabrication method of macroporous biodegradable polymer scaffolds using gas foaming salt as a porogen additive. J Biomed Mater Res 2000; 53: 1–7. [DOI] [PubMed] [Google Scholar]
- 63. Nam YS, Park TG. Porous biodegradable polymeric scaffolds prepared by thermally induced phase separation. J Biomed Mater Res 1999; 47: 8–17. [DOI] [PubMed] [Google Scholar]
- 64. de Mel A. Three-dimensional printing and the surgeon. Br J Surg 2016; 103: 786–788. [DOI] [PubMed] [Google Scholar]
- 65. Chae MP, Rozen WM, McMenamin PG, et al. Emerging applications of bedside 3D printing in plastic surgery. Front Surg 2015; 2: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Palermo E. What is stereolithography? Live Science, http://www.livescience.com/38190-stereolithography.html (2013, accessed 3 November 2016).
- 67. Hull CW. Apparatus for production of three-dimensional objects by stereolithography, Patent US4575330 A, 1986. [Google Scholar]
- 68. Seliktar D, Dikovsky D, Napadensky E. Bioprinting and tissue engineering: recent advances and future perspectives. Isr J Chem 2013; 53: 795–804. [Google Scholar]
- 69. Ozbolat IT, Yu Y. Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans Biomed Eng 2013; 60: 691–699. [DOI] [PubMed] [Google Scholar]
- 70. Ventola CL. Medical applications for 3D printing: current and projected uses. P T 2014; 39: 704–711. [PMC free article] [PubMed] [Google Scholar]
- 71. Cui X, Breitenkamp K, Finn MG, et al. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A 2012; 18: 1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hockaday LA, Kang KH, Colangelo NW, et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication 2012; 4: 35005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mertz L. Dream it, design it, print it in 3-D: what can 3-D printing do for you? IEEE Pulse 2013; 4: 15–21. [DOI] [PubMed] [Google Scholar]
- 74. de Mel A, Yap T, Cittadella G, et al. A potential platform for developing 3D tubular scaffolds for paediatric organ development. J Mater Sci Mater Med 2015; 26: 1–8. [DOI] [PubMed] [Google Scholar]
- 75. Kojima K, Vacanti CA. Tissue engineering in the trachea. Anat Rec (Hoboken) 2014; 297: 44–50. [DOI] [PubMed] [Google Scholar]
- 76. Itoh M, Nakayama K, Noguchi R, et al. Scaffold-free tubular tissues created by a bio-3D printer undergo remodeling and endothelialization when implanted in rat aortae. PLoS ONE 2015; 10: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sohrabi A, Naderi M, Gorjipour F, et al. A new design for electrospinner collecting device facilitates the removal of small diameter tubular scaffolds and paves the way for tissue engineering of capillaries. Exp Cell Res 2016; 347: 60–64. [DOI] [PubMed] [Google Scholar]
- 78. Basu J, Ludlow JW. Platform technologies for tubular organ regeneration. Trends Biotechnol 2010; 28: 526–533. [DOI] [PubMed] [Google Scholar]
- 79. Jorge C, Dubois C. Clinical utility of platinum chromium bare-metal stents in coronary heart disease. Med Devices (Auckl) 2015; 8: 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Allocco D, Jacoski M, Huibregtse B, et al. Coronary stents platinum chromium stent series – the TAXUSTM ElementTM (IONTM), PROMUS ElementTM and OMEGATM stents. Interv Cardiol 2011; 6: 134–141. [Google Scholar]
- 81. McFadden EP, Stabile E, Regar E, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet 2004; 364: 1519–1521. [DOI] [PubMed] [Google Scholar]
- 82. Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B Rev 2013; 19: 485–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Leong MF, Chian KS, Mhaisalkar PS, et al. Effect of electrospun poly(D,L-lactide) fibrous scaffold with nanoporous surface on attachment of porcine esophageal epithelial cells and protein adsorption. J Biomed Mater Res A 2009; 89: 1040–1048. [DOI] [PubMed] [Google Scholar]
- 84. Knight T, Basu J, Rivera EA, et al. Fabrication of a multi-layer three-dimensional scaffold with controlled porous micro-architecture for application in small intestine tissue engineering. Cell Adh Migr 2013; 7: 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Crowley C, Klanrit P, Butler CR, et al. Surface modification of a POSS-nanocomposite material to enhance cellular integration of a synthetic bioscaffold. Biomaterials 2016; 83: 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell 2006; 126: 677–689. [DOI] [PubMed] [Google Scholar]
- 87. Bokka KK, Jesudason EC, Warburton D, et al. Quantifying cellular and subcellular stretches in embryonic lung epithelia under peristalsis: where to look for mechanosensing. Interface Focus 6: 20160031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Heitz J, Svorcík V, Bacáková L, et al. Cell adhesion on polytetrafluoroethylene modified by UV-irradiation in an ammonia atmosphere. J Biomed Mater Res A 2003; 67: 130–137. [DOI] [PubMed] [Google Scholar]
- 89. de Mel A, Punshon G, Ramesh B, et al. In situ endothelialisation potential of a biofunctionalised nanocomposite biomaterial-based small diameter bypass graft. Biomed Mater Eng 2009; 19: 317–331. [DOI] [PubMed] [Google Scholar]
- 90. Everett W, Scurr DJ, Rammou A, et al. A material conferring hemocompatibility. Sci Rep 2016; 6: 26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chong DST, Turner L-A, Gadegaard N, et al. Nanotopography and plasma treatment: redesigning the surface for vascular graft endothelialisation. Eur J Vasc Endovasc Surg 2015; 49: 335–343. [DOI] [PubMed] [Google Scholar]
- 92. Reznickova A, Novotna Z, Kolska Z, et al. Enhanced adherence of mouse fibroblast and vascular cells to plasma modified polyethylene. Mater Sci Eng C Mater Biol Appl 2015; 52: 259–266. [DOI] [PubMed] [Google Scholar]
- 93. Bacakova L, Filova E, Parizek M, et al. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol Adv 2011; 29: 739–767. [DOI] [PubMed] [Google Scholar]
- 94. Kanzaki M, Yamato M, Hatakeyama H, et al. Tissue engineered epithelial cell sheets for the creation of a bioartificial trachea. Tissue Eng 2006; 12: 1275–1283. [DOI] [PubMed] [Google Scholar]
- 95. Chistiakov DA. Endogenous and exogenous stem cells: a role in lung repair and use in airway tissue engineering and transplantation. J Biomed Sci 2010; 17: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Roomans GM. Tissue engineering and the use of stem/progenitor cells for airway epithelium repair. Eur Cell Mater 2010; 19: 284–299. [DOI] [PubMed] [Google Scholar]
- 97. Gomperts BN, Belperio JA, Rao PN, et al. Circulating progenitor epithelial cells traffic via CXCR4/CXCL12 in response to airway injury. J Immunol 2006; 176: 1916–1927. [DOI] [PubMed] [Google Scholar]
- 98. Gomperts BN, Belperio JA, Fishbein MC, et al. Keratinocyte growth factor improves repair in the injured tracheal epithelium. Am J Respir Cell Mol Biol 2007; 37: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. MacPherson H, Keir PA, Edwards CJ, et al. Following damage, the majority of bone marrow-derived airway cells express an epithelial marker. Respir Res 2006; 7: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kim JH, Kong WH, Kim JG, et al. Possibility of skin epithelial cell transdifferentiation in tracheal reconstruction. Artif Organs 2011; 35: 122–130. [DOI] [PubMed] [Google Scholar]
- 101. Cen S, Li W, Huang F. [Preliminary research on construction of artificial esophagus with cultured squamous epithelial cells and myoblast cells seeded on small intestinal submucosa]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2006; 20: 1040–1043. [PubMed] [Google Scholar]
- 102. Ohki T, Yamato M, Murakami D, et al. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut 2006; 55: 1704–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Watanabe E, Yamato M, Shiroyanagi Y, et al. Bladder augmentation using tissue-engineered autologous oral mucosal epithelial cell sheets grafted on demucosalized gastric flaps. Transplantation 2011; 91: 700–706. [DOI] [PubMed] [Google Scholar]
- 104. Shi HC, Lu D, Li HJ, et al. In vitro isolation and cultivation of rabbit tracheal epithelial cells using tissue explant technique. In Vitro Cell Dev Biol Anim 2013; 49: 245–249. [DOI] [PubMed] [Google Scholar]
- 105. Stewart CE, Torr EE, Mohd Jamili NH, et al. Evaluation of differentiated human bronchial epithelial cell culture systems for asthma research. J Allergy 2012; 2012: 943982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Okano W, Nomoto Y, Wada I, et al. Bioengineered trachea with fibroblasts in a rabbit model. Ann Otol Rhinol Laryngol 2009; 118: 796–804. [PubMed] [Google Scholar]
- 107. Geoghegan E, Byrnes L. Mouse induced pluripotent stem cells. Int J Dev Biol 2008; 52: 1015–1022. [DOI] [PubMed] [Google Scholar]
- 108. Tada Y, Suzuki T, Takezawa T, et al. Regeneration of tracheal epithelium utilizing a novel bipotential collagen scaffold. Ann Otol Rhinol Laryngol 2008; 117: 359–365. [DOI] [PubMed] [Google Scholar]
- 109. Pfenninger C, Leinhase I, Endres M, et al. Tracheal remodeling: Comparison of different composite cultures consisting of human respiratory epithelial cells and human chondrocytes. In Vitro Cell Dev Biol Anim 2007; 43: 28–36. [DOI] [PubMed] [Google Scholar]
- 110. Kobayashi K, Nomoto Y, Suzuki T, et al. Effect of fibroblasts on tracheal epithelial regeneration in vitro. Tissue Eng 2006; 12: 2619–2628. [DOI] [PubMed] [Google Scholar]
- 111. Zani BG, Kojima K, Vacanti CA, et al. Tissue-engineered endothelial and epithelial implants differentially and synergistically regulate airway repair. Proc Natl Acad Sci U S A 2008; 105: 7046–7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Barnham KJ, Torres AM, Alewood D, et al. Role of the 6-20 disulfide bridge in the structure and activity of epidermal growth factor. Protein Sci 2008; 1738–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Takagi R, Yamato M, Murakami D, et al. Fabrication and validation of autologous human oral mucosal epithelial cell sheets to prevent stenosis after esophageal endoscopic submucosal dissection. Pathobiology 2011; 78: 311–319. [DOI] [PubMed] [Google Scholar]
- 114. Asnaghi MA, Jungebluth P, Raimondi MT, et al. A double-chamber rotating bioreactor for the development of tissue-engineered hollow organs: from concept to clinical trial. Biomaterials 2009; 30: 5260–5269. [DOI] [PubMed] [Google Scholar]
- 115. Coraux C, Nawrocki-Raby B, Hinnrasky J, et al. Embryonic stem cells generate airway epithelial tissue. Am J Respir Cell Mol Biol 2005; 32: 87–92. [DOI] [PubMed] [Google Scholar]
- 116. Takagi R, Yamato M, Kanai N, et al. Cell sheet technology for regeneration of esophageal mucosa. World J Gastroenterol 2012; 18: 5145–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ohki T, Yamato M, Ota M, et al. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology 2012; 143: 582–588. e2. [DOI] [PubMed] [Google Scholar]
- 118. Penfield JD, Gorospe EC, Wang KK. Tissue-engineered cell sheets for stricture prevention: a new connection between endoscopy and regenerative medicine. Gastroenterology 2012; 143: 526–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Weidenbecher M, Tucker HM, Awadallah A, et al. Fabrication of a neotrachea using engineered cartilage. Laryngoscope 2008; 118: 593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sala FG, Matthews JA, Speer AL, et al. A multicellular approach forms a significant amount of tissue-engineered small intestine in the mouse. Tissue Eng Part A 2011; 17: 1841–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Speer AL, Sala FG, Matthews JA, et al. Murine tissue-engineered stomach demonstrates epithelial differentiation. J Surg Res 2011; 171: 6–14. [DOI] [PubMed] [Google Scholar]
- 122. Lin CH, Hsu SH, Huang CE, et al. A scaffold-bioreactor system for a tissue-engineered trachea. Biomaterials 2009; 30: 4117–4126. [DOI] [PubMed] [Google Scholar]
- 123. Gu GL, Zhu YJ, Xia SJ, et al. Peritoneal cavity as bioreactor to grow autologous tubular urethral grafts in a rabbit model. World J Urol 2010; 28: 227–232. [DOI] [PubMed] [Google Scholar]
- 124. Saxena AK. Esophagus tissue engineering: Designing and crafting the components for the hybrid construct approach. Eur J Pediatr Surg 2014; 24: 246–262. [DOI] [PubMed] [Google Scholar]
- 125. Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev 2011; 63: 300–311. [DOI] [PubMed] [Google Scholar]
- 126. Tan Q, Steiner R, Yang L, et al. Accelerated angiogenesis by continuous medium flow with vascular endothelial growth factor inside tissue-engineered trachea. Eur J Cardiothorac Surg 2007; 31: 806–811. [DOI] [PubMed] [Google Scholar]
- 127. Tan Q, El-Badry AM, Contaldo C, et al. The effect of perfluorocarbon-based artificial oxygen carriers on tissue-engineered trachea. Tissue Eng Part A 2009; 15: 2471–2480. [DOI] [PubMed] [Google Scholar]