Abstract
Recent observations that disease risk can be transmitted across generations, without the need for a direct exposure of the child to the index environmental insult, has sparked interest in transgenerational inheritance. Epigenetic describes processes that modify gene expression without a change in the nucleotide sequence. Epigenetic processes can be induced in response to environmental exposures and can influence disease risk, and may explain these multigenerational effects. In experimental models, a number of epigenetic mechanisms have been identified that could mediate vertical transmission of epigenetic inheritance. However, relevance of these findings to human disease is not yet clear. An alternative model is where transgenerational inheritance of disease risk requires the presence of exposure-related diseases in the mother during pregnancy (termed ‘induced epigenetic transmission model’).
A number of cross-sectional studies have investigated multigenerational effects in allergy and asthma. However, given early life origins of asthma and allergy, birth cohort studies are ideal to investigate the impact of genetic predisposition, epigenetics, and environmental exposures avoiding pitfalls such as recall bias and confounding by ongoing exposures, disease and treatment. The well characterized, three generations of the Isle of Wight cohort includes two consecutive birth cohorts, providing longitudinal data that can be studied for epigenetic transfer of information, e.g., the effect of grand parental smoking or exposure to other toxic compounds. Further large multigenerational birth cohorts are needed to establish the clinical relevance of this phenomenon and differentiate between vertical and induced transmission models, which may influence future preventive strategies.
Keywords: Cohort, multigenerational, epigenetics, Asthma, Allergy, DNA methylation
Background
Atopy, defined as the genetic susceptibility to produce IgE in response to exposure to allergens, underlies allergic diseases including asthma, eczema, rhinitis and food allergy. The majority of the US population is affected by atopy.1 Both shared genetics and environment explain the risk of allergic diseases transferred from one generation to the next. However, disentangling genetic from environmental effects is a major challenge for most common chronic conditions. Considerable evidence supports the early life origins of allergic diseases, as early as the gestational period, a concept also known as fetal or developmental programming. Hence, cohorts starting during pregnancy can assess early developmental origins of health without confounding by environment or treatment in later life. More intriguing is the observations that not only maternal exposures, e.g., smoking, but also grand maternal and paternal line environmental exposures are associated with the development of wheeze and asthma. These multigenerational effects may be explained by epigenetic mechanisms, which are thought to mediate developmental programming across generations. Without proper knowledge of epigenetic transfer across generations, we cannot decipher effects of pregnancy-related exposures from the existing parental effects. Multigenerational cohorts are key to investigating these mechanisms.
Multigenerational Cohorts
In addition to parental genetic effects, environmental exposures of parents and grandparents have been increasingly recognised as determining individual health, even later in life. Maternal exposures, especially during the prenatal period, can have a direct effect through placental transfer of nutrients, metabolites and environmental pollutants. However, a number of studies have shown how paternal and grandparental characteristics and environmental exposures affect their children’s and grandchildren’s growth and development.2–5 Thus, developmental programming can be transmitted across generations, where the index child was not exposed to the environment that triggered the change. Hence, the risk to individual’s health has to be investigated with the knowledge of the cross-generational influences. The effects on grandchildren of grand parental characteristics or environmental exposures can either be intergenerational or transgenerational in nature (Table 1). In intergenerational effects, maternal exposure (F0) has direct effects on the developing fetus, and potentially on the germ line of the fetus leading to altered phenotype of the child (F1) and grandchild (F2). Evidence for transgenerational inheritance is rarer but has been observed both in animal models 6–9 and in humans.5,10–12
Table 1.
Definitions commonly used when describing transfer of information across generations
| Epigenetic multigenerational inheritance | transmission of epigenetic information via the germline across generations in the absence of any direct environmental exposure or genetic manipulation |
| Intergenerational inheritance | maternal exposure (F0) has direct effects on the developing fetus, and potentially on the germ line of the fetus leading to altered phenotype of the child (F1) and grandchild (F2) |
| Transgenerational inheritance | the effect on subsequent generation persists in the absence of the possibility of direct environmental exposure, such as effects persisting to the great-grandchild (F3) on the maternal line or effects of pre-pubertal paternal or grand paternal exposures in the male line |
Cross-generational studies can reveal intriguing findings. For example, the Hispanic health paradox (Hispanic immigrants tend to have better health outcomes than their US-born and white counterparts) with regards to asthma and allergy was studied using a cross-sectional design encompassing four generations. This showed that asthma and allergies were low in the immigrant generation but increased with each subsequent generation who were born in the USA, with the healthy immigrant advantage lasting for two to three generations for allergies and asthma.13 The RHINESSA study (www.rhinessa.net) is recruiting the children and parents of the participants of large cross sectional studies (RHINE17 and ECRHS18) in 7 countries. These studies have investigated lung health, allergies and associated diseases over the last 20 years. However, in epidemiological studies, a prospective cohort design is preferable as it avoids selection and recall biases. One of the largest longitudinal multigenerational studies is French E3N and E4N studies. The E3N study enrolled 100,000 women between 40 to 65 years of age since 1990 focusing on a range of diseases such as cancer, diabetes, obesity and asthma.14 Their children and grandchildren have recently been recruited in a multi-generational cohort study (E4N) to investigate exposures to environmental factors associated with disease risk in an inter- and trans-generational approach.
Birth cohort studies are ideal to investigate the impact of genetic predisposition, epigenetics, and exposures during early life on the occurrence and progression of asthma and allergies. The advantage is that data and samples are available before disease development, which could help ascertain causation and help in identifying predictive biomarkers. The Lifeways cross-generation cohort study recruited ~1000 children, their parents as well as information on health status, dietary intakes and adult chronic disease in the grandparents of both lineages. Child health outcomes include wheeze and asthma as well as obesity.15 The ACROSSOLAR study is recruiting children born to ~2000 participants of the International Study of Asthma and Allergies in Childhood (ISAAC) study in Germany.16 Information is collected regarding pregnancy conditions, and asthma and allergic health status of the child up to primary school age. Thus, information is available for 3 generations (ISAAC study participants, their parents and their children). In addition, DNA for epigenetic analysis is collected. Likewise, the ALSPAC birth cohort19 has recently started enrolling both the grandparents and offspring of the original birth cohort. Table 2 provides a summary of multigenerational cohort studies with a focus on asthma and allergy.
Table 2.
Multigenerational cohorts in the field of atopic diseases
| Author/Study/Reference | Population studied | Variables | Main outcomes |
|---|---|---|---|
| The French E3N/E4N Study14 | France; N=98 995 (started 1990)* | BMI, Education, Exercise, smoking | Cancer, Diabetes, bone health, Asthma |
| Lifeways cross-generation cohort study15 | Ireland; N=1128 (families) (started 2001) 3rd Generation n=1,094 (2001–2013) |
Diet, sex smoking, age, breastfeeding, birthweight | BMI, Wheeze, asthma, |
| ACROSSOLAR study16 | Germany; N= 2051; (started in 1995)* | Maternal stress and smoking, birthweight, breast feeding, vaccinations, nutrition | Wheezing, atopic rhinitis, and eczema |
| Avon Longitudinal Study of Parents and Children; Children of the Children of the 90s http://www.bristol.ac.uk/alspac/about/ | UK (Bristol); N=14,000 (started in 1991)* | Diet, Sex, Smoking, birthweight | Asthma, Eczema, Allergies |
| The Isle of Wight birth cohort and 3rd Generation study21 | UK (Isle of Wight) N=1456 (Started 1989) 3rd Generation; n=406 (Started 2010) |
Sex, smoking, birthweight, BMI, methof of feeding, vaccination, nutrition | Asthma, Eczema, Rhinitis, Food Allergy, Lung function |
| GINIplus birth cohort (https://www.ncbi.nlm.nih.gov/pubmed/20082618) | Germany (Munich and Wesel), N=5991 (started in 1995)* | Parental and grandparental hypertension, diabetes, allergies | atopic diseases |
| LISAplus birth cohort (https://www.ncbi.nlm.nih.gov/pubmed/12358337) | Germany (Munich, Leipzig, Bad Honnef and Wesel), N=3097 (started in 1997)* | Parental and grandparental hypertension, diabetes, allergies | atopic diseases |
Note:
3rd Generation cohort is planned
Isle of Wight 3rd Generation cohort
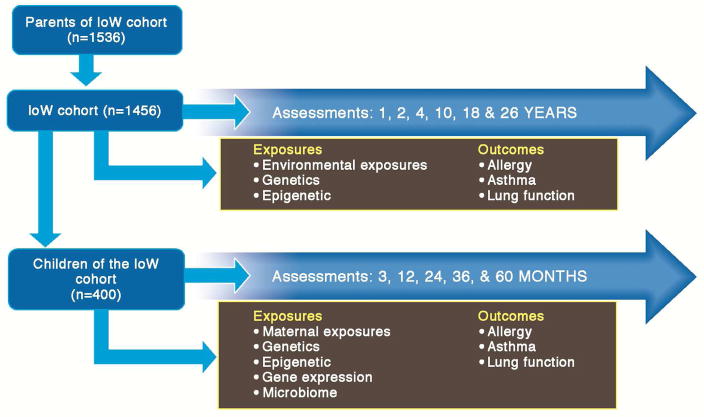
The Isle of Wight (IoW) cohort includes three generations: 1st generation (F0, grandparents) n=1536, the 2nd generation (F1, n=1456), the original birth cohort, and the 3rd generation (F2, n=403), the children of the original birth cohort participants (Figure 1). The Isle of Wight is an island just off the south coast of England with a resident population of 138,000. The IoW cohort is largely of Caucasian descent (98%). The 1st generation (grandparents) was enrolled at the time of childbirth and data and samples were collected to assess asthma and allergic status. The 2nd generation (the IoW cohort) was recruited at birth and assessed extensively and repeatedly for asthma and allergies up to the age of 27 years. Since 2010, 3rd generation children (born to at least one 2nd generation parent, mother or father) have been enrolled. The overall objective is to gain better understanding of the natural history of asthma, eczema, allergic diseases and obesity during the life course over three generations. Hence, allergic disease and exposure were extensively characterized at different ages from infancy to 27 years of age. Further, the study aims to identify environmental, genetic, and epigenetic risk factors and their transition (behavioural, genetic, epigenetic, skin and gut microbiome) over generations. An important question addresses the role of in utero exposure vs. genetic inheritance. Hence, multiple samples have been collected during pregnancy and in subsequent years from the F0, F1, and F2-generations. Importantly, there has been very low attrition with participation of 93% of the original cohort at 10 years and 90% at 18 years. This unique population-based study integrates two consecutive birth- cohorts providing data to construct intergenerational trajectories of wheezing, asthma, eczema, rhinitis, allergic sensitization and lung function with an opportunity to study the role of in utero conditions, genetics, and epigenetics in transferring health risk over generations.20 For instance, the IoW multigenerational study recently showed that using “no smoking by either mother or grandmother” as control, the odds of wheeze in early childhood was higher (2.6, CI: 0.9–7.1) when both mother and grandmother smoked during pregnancy compared to when only mother (0.9, CI: 0.4–2.3) or grandmother (0.9, CI: 0.3–2.4) smoked.21 Whether these effects across generations are transmitted via epigenetic mechanisms is being investigated.
Figure 1. Three generations of the Isle of Wight birth cohort.
The birth cohort participants were recruited in 1989–90 and have been assessed at 1, 2, 4, 10, 18 and 27 years. Data and samples were collected from the parents of the cohort in 1989–90. The third generation (children of the original birth cohort) have been recruited since 2010 and to date assessed at 3, 12, 24, 36 and 60 months.
In a mouse model, the transgenerational transmission of the asthma phenotype to F3 offspring (4th generation) following perinatal nicotine exposure of F0 has been demonstrated and presumed to be due to transgenerational transfer of epigenetic information across germline.22 However, multigenerational cohort studies are essential to confirm these effects in humans. The Isle of Wight study conducting a genome-wide assessment indicated that in this unselected population, DNA methylation (DNA-M) was transmitted from parents to offspring only at a small portion (~1%) of all the Cytosine– Guanine (CpG) sites. Among these 1% CpG sites, most (~98%) DNA-M transmission is either dominated by the mother or transmitted evenly from mother and father.23
Challenges in recruitment of multigenerational cohorts
A major problem in long-term cohort studies is the attrition of the cohort over time. Loss to follow-up is almost inevitable in studies that aim to recruit several generations sequentially. This can introduce bias, as subsequent generations are self-selected and generally over represented by those with higher education achievements and socio-economic status. The other important aspect is generalisability of findings (or lack thereof). The cohorts are usually based in one geographical location with an ethnic, economic and exposure profile that may not be shared in other geo-economic situations. Collaboration between cohorts with formation of new consortia or using existing birth cohort alliances provides an opportunity to address these challenges. A number of potential confounders that may need to be adjusted and some limitations of multigenerational trials, when investigating epigenetic and environmental affects across generations, are summarised in table 3.
Table 3.
Potential confounders and limitations of multi-generation studies
| Generation | Confounders* | opportunities | Limitations |
|---|---|---|---|
| 1st Generation | Sex, maternal smoking during pregnancy, income, education, smoking, diet, BMI | Study genetic, environmental and geneenvironmental interactions for disease development | The assessment remains limited without parental genotype and phenotype information |
| 2nd Generation | Sex, age, parental smoking, income, education, personal smoking, birthweight, gestational age, breast feeding, family size, vaccinations, physical activity, diet, BMI | Parental influences (both genetic and inutero environmental) can be assessed | Transgenerational effects (across multiple generation) cannot be assessed and inadequate for mechanistic understanding, especially epigenetic mechanisms |
| 3rd generations | Sex, age, household smoking, personal smoking, income, education, birthweight, gestational age, breast feeding, family size, vaccinations, physical activity, nutrition, diet, BMI | Grandparental (multigenerational) effects can be assessed in this generation | To confidently evaluate epigenetic transfer of information, a 4th generation is required (see text) |
Note:
with respect to outcomes such as asthma, eczema, rhinitis and lung function.
What mediates cross-generational effects? (Genetics, shared environment and epigenetics)
There are at least three possible mechanisms for the cross-generational transmission of disease risks. The first is that of shared familial environment. Environmental exposures such as tobacco smoking, dietary patterns, occupational, microbiome and geographical exposures such as air pollution and farm environments are more likely to be shared amongst family members across generations and need to be accounted for in any studies. To differentiate intrafamilial homo- and heterogeneity from intergenerational homo- and heterogeneity, it is necessary to investigate multiple family members or whole families. Typically, a birth cohort study includes only one F1-generation child. In this case, birth order itself, or an interaction with the environment of a family, provides some information on intrafamilial heterogeneity. However, to capture this process, it is necessary to recruit and study family members in successive generations with prospective ascertainment of exposures.
Secondly, genetic inheritance through generations can explain familial resemblance in phenotypes. However, this cannot account for the increased risk of disease as a result of environmental exposures of prior generations in the absence of continued exposure. The third possibility is that of epigenetic effects. Here a distinction needs to be made between intergenerational and transgenerational inheritance. As outlined above, in intergenerational inheritance, the environment of the parent can directly affect germ cells of the offspring. A true transgenerational effect can only be defined if transmitted to the F2 (on the paternal line) of F3 (on the maternal line) and possibly future generations, in the absence of further environmental exposure and with the exclusion of the possibility of germline mutations.24,25
In experimental models, a number of epigenetic mechanisms have been identified that have been shown to mediate epigenetic inheritance. For example, in yeast both prion-like- proteins, and transient expression of non-prion proteins, have been shown to convey inheritance of traits independent of DNA.26,27 In multicellular organisms, such as the nematode Caenorhabditis elegans, the RNA interference (RNAi) pathway results in non–DNA sequence–based heritable changes and environmental exposures can result in heritable phenotypic changes.28 In mammalian models such as mice, a number of different types of non-genetic intergenerational or transgenerational inheritance have been observed.26,27 The most well-known examples being heritable non-genetic changes in expression of specific loci, such as at the Agouti viable yellow (Avy) locus affecting coat color and metabolic outcome, caused by methylation silencing of an insertional mutation that results in ectopic agouti expression,29 which can be modified by maternal diet.30 In another example, Manikkam et al. demonstrated transgenerational inheritance of adult onset disease from exposure of gestating mice to an endocrine disruptor - the pesticide Methoxychlor.31 The mechanism of this non-genetic form of inheritance involved the transgenerational transmission of adult-onset disease susceptibility through epigenetic changes in the germline as epigenetic alterations in the sperm DNA of the F3 generation (great-grand offspring) could be observed after methoxychlor exposure of the F0 generation gestating female ancestors. This apparent transmission of epimutations has now been seen to be induced by a number of different environmental toxicants. However, an unknown factor is the transfer of persistent organic pollutants via the placenta from grandmothers (F0) to mothers (F1) as reported in multiple studies.32–34 In humans, it has been demonstrated that between 23–43% of the concentration of persistent organic pollutants in mothers were explained by grand-maternal concentrations.35 This leads to an exposure of F1 and thus a direct exposure of F3 oocytes when F1 mothers are pregnant with F2. Hence, transfer of persistent toxins via the placenta means that for exposures such as Methoxychlor, intergenerational inheritance cannot be excluded as effects in the F3 generation may result from direct effects of the exposure.
As discussed by Miska and Furgeson-Smith,24 transgenerational inheritance of epimutations such as observed by Manikkam and others, requires a substrate that transmits the information from one generation to the next and a mechanism for the transmitted information to be “read” or interpreted in the offspring to alter the phenotype. Evidence exists for a number of potential substrates including incomplete germline DNA-M erasure during gametogenesis,36 histone modifications,37 or non-coding RNA species in gametes.8 Robust evidence of such mechanisms operating in humans is lacking. However observations such as miRNA expression in human spermatozoa from smokers, compared with nonsmokers, suggests that similar mechanisms may operate.38
Cross-generational effects
In the F1 generation of the IOW cohort we have shown that methylation of specific CpG sites is strongly associated with eczema, asthma, smoking, and obesity in F1 adults.39–45 In addition, maternal conditions during pregnancy, such as smoking, was associated with cord blood DNA-M, for instance, AHRR CpG cg05575921, which has become a well-established marker for past smoking.46–48
Epigenetic multigenerational inheritance49,50
It is assumed that transmitted DNA-M escapes epigenetic reprogramming after fertilization, similar to an imprinted gene.51 However, this is not clearly established as it is reported that most DNA-M is erased, with only a few elements escaping erasure, 36 and as described above, other mechanisms for conveying information between generations may exist. For conceptual reasons, as the maternal line F1 and F2 are already exposed in F0, proof of maternal intergenerational inheritance focuses on exposure in the F0 generation and epigenetic changes in the F3 generation. However, as there is no suggestion that an epigenetic leap occurs from F0 to F3, we would expect to find epigenetic associations between F1 and F2. In some previous studies these steps have not been investigated sequentially and an association of exposure in F0 and phenotype outcome in F3 is taken as evidence of epigenetic transmission. In the Isle of Wight cohort study, epigenetic associations between DNA-M in F1 and F2 were not commonly observed at a genome-wide scale (only ~1% CpGs showed epigenetic associations after adjusting for multiple testing by controlling false discovery rate of 0.05). In addition, for most CpG sites that showed associations with the respective parental CpG sites, the associations were eliminated once the offspring genotype was taken into consideration. Methylation quantitative loci (single nucleotide polymorphisms) are a potential explanation of this phenomenon,52–54 indicating epigenetic associations were likely to be a epigenetic manifestation of genetic inheritance.
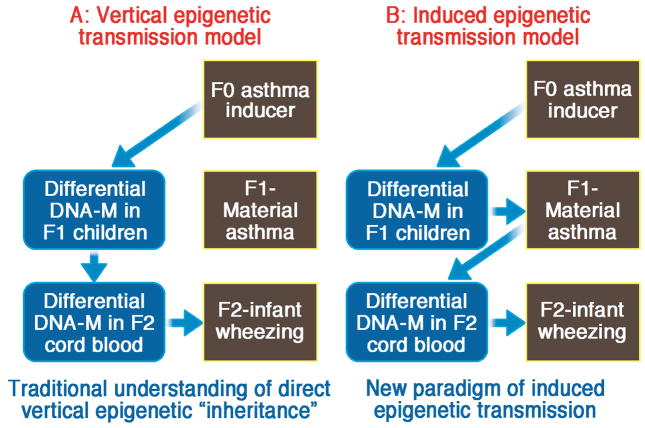
In contrast, animal studies addressing epigenetic inheritance either focused on F0-F1-F2 and not F3 55–57 or did not investigate F1 and F2 after initial exposure of F0. These experiments often reported phenotypic changes in F1 and F2 but ignored processes in F1 and F2 as explanation of reproduced DNA-M.31,58 Based on our findings in our birth cohort we believe it is critical to assess all generations from F0 to F3. It seems that it is the pathological/immunological condition arising from exposures/diseases in F0 that is likely to lead to an induced epigenetic transmission of a differential methylation of CpG sites from F1 to F3 in the maternal line. Hence, we propose that the epigenetic dogma for transgenerational inheritance that ‘exposures in great-grandmothers during pregnancy may influence disease in their great-grandchildren, even in the absence of any exposure’,59 needs to be modified by the clause ‘but only in the presence of exposure-related diseases in F1 and F2’ (Figure 2). We therefore suggest that observations purported to be transgenerational may actually arise from transmitted disease. As Figure 2 shows, we assume that DNA-M may result in disease (asthma) or exposure (smoking) during pregnancy. Then the exposure of the fetus to these diseases/exposures may initiate specific differential DNA-M in the offspring, which in turn may result in offspring experiencing diseases (e.g. asthma) or behaviour (e.g. initiating smoking). We call this the ‘induced epigenetic transmission model’.
Figure 2. Vertical and induced epigenetic transmission models.
Vertical epigenetic transmission model proposes direct transfer of epigenetic information from parents to child in successive generations (A). The alternative model, induced epigenetic transmission, proposes that maternal disease is an essential element in the chain of epigenetic transmission of information (B). DNA-M: DNA methylation
A limitation of this ‘induced epigenetic transmission model’ is that it does not explain the paternal role or a sex-specific inheritance as seen in the Isle of Wight birth cohort from F0 to F1.20 Nevertheless, it is possible that the paternal role or sex-specific inheritance is related to methylation quantitative loci or genetic inheritance that results in differential methylation in offspring.
Future Directions
The question of the existence and importance of transgenerational inheritance in humans remains unresolved. The data in animal models is stronger for environmental exposure present in F0 generation to have an effect in the F3 generation supporting true transgenerational inheritance. However, the effect of disease in the mother has not been fully explored, which may partly explain these generational effects. Robust data in human is lacking regarding the effect of environmental exposures in grandparents having an effect on disease development in grandchildren and whether this risk is mediated by epigenetic transfer of information across generations. The differentiation between intergenerational and transgenerational effects is important as there are many reasons (such as direct transfer of environmental pollutants across placenta) that needs to be excluded before true transgenerational effects can be accepted. Further studies are required in human, specifically multigenerational, well characterized cohorts with longitudinal information to avoid confounding by disease and treatment effects and recall bias. Family-based transgenerational studies will have the power to assess within family factors such as birth order and environmental exposures shared within family, as well as between family factors. Larger studies with adequate sample size and/or collaboration between studies for replication and meta-analysis will provide much needed strength to these observations. It is imperative to test the ‘induced epigenetic transmission model’ in future studies, which proposes that maternal disease/exposure is critical for changes in methylation in the successive generation. If this model is correct, then the key to prevention of asthma and allergies in the next generation is to prevent asthma and allergies during pregnancy. Epigenetic effects might also depend on sex of the child and hence sex specific analysis may reveal a differential effect. Family/sibling and sex-specific sibling effects can be studied by investigating several members of the family in multigenerational cohorts. Lastly, future studies should also investigate the functional consequences of these epigenetic signatures transferred across generations to establish the clinical relevance of this phenomenon.
Acknowledgments
Funding sources: The Isle of Wight birth cohort assessments have been supported by the National Institutes of Health USA (Grant no. R01 HL082925, R01 AI091905, R01 AI121226, and R01 HL132321) and Asthma UK (Grant no. 364).
We would like to acknowledge the help of all the staff at The David Hide Asthma and Allergy Research Centre in undertaking the assessments of 3 generations of the Isle of Wight birth cohort. We would also like to acknowledge the help of the participants and their families who have helped us over the last two and a half decades. SHA, WK, HZ, and JWH and ongoing studies in the IOW cohorts are currently supported by the National Institutes of Health (R01AI121226, and R01AI091905, and 1R01HL132321). JWH acknowledges support from the Ageing Lungs in European Cohorts (ALEC) Study (www.alecstudy.org), which has been funded by the European Union’s Horizon 2020 Research and Innovation programme under grant agreement No. 633212.
Abbreviations
- DNA-M
DNA methylation
- CpG
Cytosine–Guanine
- miRNA
micro RNA
Footnotes
Conflict of interest statement
None of the authors have any conflicts of interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. The Journal of allergy and clinical immunology. 2005;116:377–83. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Manor O, Koupil I. Birth weight of infants and mortality in their parents and grandparents: the Uppsala Birth Cohort Study. International journal of epidemiology. 2010;39:1264–76. doi: 10.1093/ije/dyq046. [DOI] [PubMed] [Google Scholar]
- 3.McCarron P, Davey Smith G, Hattersley AT. Type 2 diabetes in grandparents and birth weight in offspring and grandchildren in the ALSPAC study. Journal of epidemiology and community health. 2004;58:517–22. doi: 10.1136/jech.2003.007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith GC, Wood AM, White IR, Pell JP, Hattie J. Birth weight and the risk of cardiovascular disease in the maternal grandparents. American journal of epidemiology. 2010;171:736–44. doi: 10.1093/aje/kwp448. [DOI] [PubMed] [Google Scholar]
- 5.Kaati G, Bygren LO, Pembrey M, Sjostrom M. Transgenerational response to nutrition, early life circumstances and longevity. Eur J Hum Genet. 2007;15:784–90. doi: 10.1038/sj.ejhg.5201832. [DOI] [PubMed] [Google Scholar]
- 6.Rehan VK, Liu J, Naeem E, et al. Perinatal nicotine exposure induces asthma in second generation offspring. BMC Med. 2012;10:129. doi: 10.1186/1741-7015-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padmanabhan N, Jia D, Geary-Joo C, et al. Mutation in folate metabolism causes epigenetic instability and transgenerational effects on development. Cell. 2013;155:81–93. doi: 10.1016/j.cell.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gapp K, Jawaid A, Sarkies P, et al. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci. 2014;17:667–9. doi: 10.1038/nn.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Q, Yan M, Cao Z, et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016;351:397–400. doi: 10.1126/science.aad7977. [DOI] [PubMed] [Google Scholar]
- 10.Golding J, Northstone K, Gregory S, Miller LL, Pembrey M. The anthropometry of children and adolescents may be influenced by the prenatal smoking habits of their grandmothers: a longitudinal cohort study. Am J Hum Biol. 2014;26:731–9. doi: 10.1002/ajhb.22594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Northstone K, Golding J, Davey Smith G, Miller LL, Pembrey M. Prepubertal start of father’s smoking and increased body fat in his sons: further characterisation of paternal transgenerational responses. Eur J Hum Genet. 2014;22:1382–6. doi: 10.1038/ejhg.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svanes C, Koplin J, Skulstad SM, et al. Father’s environment before conception and asthma risk in his children: a multi-generation analysis of the Respiratory Health In Northern Europe study. International journal of epidemiology. 2016 doi: 10.1093/ije/dyw151. [DOI] [PubMed] [Google Scholar]
- 13.Balcazar AJ, Grineski SE, Collins TW. The Hispanic health paradox across generations: the relationship of child generational status and citizenship with health outcomes. Public health. 2015;129:691–7. doi: 10.1016/j.puhe.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clavel-Chapelon F. Cohort Profile: The French E3N Cohort Study. International journal of epidemiology. 2015;44:801–9. doi: 10.1093/ije/dyu184. [DOI] [PubMed] [Google Scholar]
- 15.Kelleher CC, Viljoen K, Khalil H, et al. Longitudinal follow-up of the relationship between dietary intake and growth and development in the Lifeways cross-generation cohort study 2001–2013. The Proceedings of the Nutrition Society. 2014;73:118–31. doi: 10.1017/S002966511300373X. [DOI] [PubMed] [Google Scholar]
- 16.Weinmann T, Gerlich J, Heinrich S, et al. Establishing a birth cohort to investigate the course and aetiology of asthma and allergies across three generations - rationale, design, and methods of the ACROSSOLAR study. BMC public health. 2015;15:1210. doi: 10.1186/s12889-015-2555-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toren K, Gislason T, Omenaas E, et al. A prospective study of asthma incidence and its predictors: the RHINE study. Eur Respir J. 2004;24:942–6. doi: 10.1183/09031936.04.00044804. [DOI] [PubMed] [Google Scholar]
- 18.Burney PG, Luczynska C, Chinn S, Jarvis D. The European Community Respiratory Health Survey. Eur Respir J. 1994;7:954–60. doi: 10.1183/09031936.94.07050954. [DOI] [PubMed] [Google Scholar]
- 19.Boyd A, Golding J, Macleod J, et al. Cohort Profile: the ‘children of the 90s’--the index offspring of the Avon Longitudinal Study of Parents and Children. International journal of epidemiology. 2013;42:111–27. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arshad SH, Karmaus W, Raza A, et al. The effect of parental allergy on childhood allergic diseases depends on the sex of the child. The Journal of allergy and clinical immunology. 2012;130:427–34. e6. doi: 10.1016/j.jaci.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veerish VKHJ, Karmaus W, Zhang H, Mitchell F, Ewart S, Arshad SH. Third generation study: maternal and grand-maternal smoking in pregnancy and wheeze in children. Allergy. 2014:69. [Google Scholar]
- 22.Rehan VK, Liu J, Sakurai R, Torday JS. Perinatal nicotine-induced transgenerational asthma. Am J Physiol Lung Cell Mol Physiol. 2013;305:L501–7. doi: 10.1152/ajplung.00078.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han S, Zhang H, Lockett GA, Mukherjee N, Holloway JW, Karmaus W. Identifying heterogeneous transgenerational DNA methylation sites via clustering in beta regression. The Annals of Applied Statistics. 2015;9:2052–72. [Google Scholar]
- 24.Miska EA, Ferguson-Smith AC. Transgenerational inheritance: Models and mechanisms of non–DNA sequence–based inheritance. Science. 2016;354:59–63. doi: 10.1126/science.aaf4945. [DOI] [PubMed] [Google Scholar]
- 25.Heard E, Martienssen RA. Transgenerational epigenetic inheritance: myths and mechanisms. Cell. 2014;157:95–109. doi: 10.1016/j.cell.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halfmann R, Jarosz DF, Jones SK, Chang A, Lancaster AK, Lindquist S. Prions are a common mechanism for phenotypic inheritance in wild yeasts. Nature. 2012;482:363–8. doi: 10.1038/nature10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakrabortee S, Byers JS, Jones S, et al. Intrinsically Disordered Proteins Drive Emergence and Inheritance of Biological Traits. Cell. 2016;167:369–81. e12. doi: 10.1016/j.cell.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rechavi O, Houri-Ze’evi L, Anava S, et al. Starvation-induced transgenerational inheritance of small RNAs in C. elegans Cell. 2014;158:277–87. doi: 10.1016/j.cell.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan HD, Sutherland HGE, Martin DIK, Whitelaw E. Epigenetic inheritance at the agouti locus in the mouse. Nature Genetics. 1999;23:314–8. doi: 10.1038/15490. [DOI] [PubMed] [Google Scholar]
- 30.Waterland RA, Travisano M, Tahiliani KG, Rached MT, Mirza S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int J Obes (Lond) 2008;32:1373–9. doi: 10.1038/ijo.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manikkam M, Haque MM, Guerrero-Bosagna C, Nilsson EE, Skinner MK. Pesticide methoxychlor promotes the epigenetic transgenerational inheritance of adult-onset disease through the female germline. PLoS One. 2014;9:e102091. doi: 10.1371/journal.pone.0102091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vizcaino E, Grimalt JO, Fernandez-Somoano A, Tardon A. Transport of persistent organic pollutants across the human placenta. Environ Int. 2014;65:107–15. doi: 10.1016/j.envint.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Li LX, Chen L, Meng XZ, et al. Exposure levels of environmental endocrine disruptors in mother-newborn pairs in China and their placental transfer characteristics. PLoS One. 2013;8:e62526. doi: 10.1371/journal.pone.0062526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porpora MG, Lucchini R, Abballe A, et al. Placental transfer of persistent organic pollutants: a preliminary study on mother-newborn pairs. International journal of environmental research and public health. 2013;10:699–711. doi: 10.3390/ijerph10020699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu WW, Osuch JR, Todem D, et al. DDE and PCB serum concentration in maternal blood and their adult female offspring. Environ Res. 2014;132:384–90. doi: 10.1016/j.envres.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Hackett JA, Sengupta R, Zylicz JJ, et al. Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine. Science. 2013;339:448–52. doi: 10.1126/science.1229277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ost A, Lempradl A, Casas E, et al. Paternal diet defines offspring chromatin state and intergenerational obesity. Cell. 2014;159:1352–64. doi: 10.1016/j.cell.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Marczylo EL, Amoako AA, Konje JC, Gant TW, Marczylo TH. Smoking induces differential miRNA expression in human spermatozoa: a potential transgenerational epigenetic concern? Epigenetics. 2012;7:432–9. doi: 10.4161/epi.19794. [DOI] [PubMed] [Google Scholar]
- 39.Patil VK, Holloway JW, Zhang H, et al. Interaction of prenatal maternal smoking, interleukin 13 genetic variants and DNA methylation influencing airflow and airway reactivity. Clin Epigenetics. 2013;5:22. doi: 10.1186/1868-7083-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ziyab AH, Karmaus W, Holloway JW, Zhang H, Ewart S, Arshad SH. DNA methylation of the filaggrin gene adds to the risk of eczema associated with loss-of-function variants. J Eur Acad Dermatol Venereol. 2013;27:e420–3. doi: 10.1111/jdv.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Everson TM, Lyons G, Soto-Ramírez N, et al. DNA methylation loci associated with atopy and high serum IgE: a genome-wide application of recursive Random Forest feature selection. Genome Medicine. 2014 doi: 10.1186/s13073-015-0213-8. (in revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guthikonda K, Zhang H, Nolan VG, et al. Oral contraceptives modify the effect of GATA3 polymorphisms on the risk of asthma at the age of 18 years via DNA methylation. Clinical epigenetics. 2014;6:17. doi: 10.1186/1868-7083-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H, Tong X, Holloway JW, et al. The interplay of DNA methylation over time with Th2 pathway genetic variants on asthma risk and temporal asthma transition. Clinical epigenetics. 2014;6:8. doi: 10.1186/1868-7083-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soto-Ramirez N, Arshad SH, Holloway J, et al. The interaction of genetic variants and DNA methylation of the interleukin-4 receptor gene increase the risk of asthma at age 18 years. Clinical Epigenetics. 2013;5:1. doi: 10.1186/1868-7083-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yousefi M, Karmaus W, Zhang H, Ewart S, Arshad H, Holloway JW. The methylation of the LEPR/LEPROT genotype at the promoter and body regions influence concentrations of leptin in girls and BMI at age 18 years if their mother smoked during pregnancy. Int J Mol Epidemiol Genet. 2013;4:86–100. [PMC free article] [PubMed] [Google Scholar]
- 46.Philibert RA, Sears RA, Powers LS, et al. Coordinated DNA methylation and gene expression changes in smoker alveolar macrophages: specific effects on VEGF receptor 1 expression. J Leukoc Biol. 2012 doi: 10.1189/jlb.1211632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monick MM, Beach SR, Plume J, et al. Coordinated changes in AHRR methylation in lymphoblasts and pulmonary macrophages from smokers. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:141–51. doi: 10.1002/ajmg.b.32021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shenker NS, Polidoro S, van Veldhoven K, et al. Epigenome-wide association study in the European Prospective Investigation into Cancer and Nutrition (EPIC-Turin) identifies novel genetic loci associated with smoking. Hum Mol Genet. 2013;22:843–51. doi: 10.1093/hmg/dds488. [DOI] [PubMed] [Google Scholar]
- 49.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–9. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanda J, Chiou LW, Szabolcs P, et al. Immune recovery in adult patients after myeloablative dual umbilical cord blood, matched sibling, and matched unrelated donor hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18:1664–76. e1. doi: 10.1016/j.bbmt.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osada T, Berglund P, Morse MA, et al. Co-delivery of antigen and IL-12 by Venezuelan equine encephalitis virus replicon particles enhances antigen-specific immune responses and antitumor effects. Cancer immunology, immunotherapy : CII. 2012;61:1941–51. doi: 10.1007/s00262-012-1248-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bock C, Paulsen M, Tierling S, Mikeska T, Lengauer T, Walter J. CpG island methylation in human lymphocytes is highly correlated with DNA sequence, repeats, and predicted DNA structure. PLoS Genet. 2006;2:e26. doi: 10.1371/journal.pgen.0020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gertz J, Varley KE, Reddy TE, et al. Analysis of DNA methylation in a three-generation family reveals widespread genetic influence on epigenetic regulation. PLoS Genet. 2011;7:e1002228. doi: 10.1371/journal.pgen.1002228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tycko B. Allele-specific DNA methylation: beyond imprinting. Hum Mol Genet. 2010;19:R210–20. doi: 10.1093/hmg/ddq376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Relton CL, Davey Smith G. Is epidemiology ready for epigenetics? Int J Epidemiol. 2012;41:5–9. doi: 10.1093/ije/dys006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chinnery PF, Elliott HR, Hudson G, Samuels DC, Relton CL. Epigenetics, epidemiology and mitochondrial DNA diseases. Int J Epidemiol. 2012;41:177–87. doi: 10.1093/ije/dyr232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niedzwiecki M, Zhu H, Corson L, et al. Prenatal exposure to allergen, DNA methylation, and allergy in grandoffspring mice. Allergy. 2012;67:904–10. doi: 10.1111/j.1398-9995.2012.02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang H, Maity A, Arshad H, Holloway J, Karmaus W. Variable selection in semi-parametric models. Stat Methods Med Res. 2013 doi: 10.1177/0962280213499679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skinner MK. Endocrine disruptor induction of epigenetic transgenerational inheritance of disease. Mol Cell Endocrinol. 2014 doi: 10.1016/j.mce.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]