Abstract
Recurrent bacterial infections are a significant burden worldwide, and prior history of infection is often a significant risk factor for developing new infections. For urinary tract infection (UTI), a history of two or more episodes is an independent risk factor for acute infection. However, mechanistic knowledge of UTI pathogenesis has come almost exclusively from studies in naive mice. Here we show that, in mice, an initial Escherichia coli UTI, whether chronic or self-limiting, leaves a long-lasting molecular imprint on the bladder tissue that alters the pathophysiology of subsequent infections, affecting host susceptibility and disease outcome. In bladders of previously infected versus non-infected, antibiotic-treated mice, we found (1) an altered transcriptome and defects in cell maturation, (2) a remodelled epithelium that confers resistance to intracellular bacterial colonization, and (3) changes to cyclooxygenase-2-dependent inflammation. Furthermore, in mice with a history of chronic UTI, cyclooxygenase-2-dependent inflammation allowed a variety of clinical E. coli isolates to circumvent intracellular colonization resistance and cause severe recurrent UTI, which could be prevented by cyclooxygenase-2 inhibition or vaccination. This work provides mechanistic insight into how a history of infection can impact the risk for developing recurrent infection and has implications for the development of therapeutics for recurrent UTI.
Bacterial infections, in particular recurrent bacterial infections, are a significant health, financial and quality of life burden worldwide1. Sixty percent of women will have at least one urinary tract infection (UTI) in their lifetime2 and in ~25% of women who suffer an initial UTI, a recurrent UTI (rUTI) will occur with the same or a different bacterial strain in the following six months3. Furthermore, some women will have highly recurrent UTI, with six or more episodes per year3. Two of the most important independent risk factors for UTI in sexually active premenopausal women are a history of rUTI (two or more episodes)4 and a first UTI at age ≤15 years5. However, experimental models of UTI have largely been limited to animals without prior infections6–8, so how infection history impacts the risk for and outcome of rUTI remains poorly understood. To elucidate the molecular interplay between infection history and increased susceptibility to rUTI, we investigated molecular mechanisms in a mouse model of recurrent cystitis (bladder infection) (Fig. 1a)9,10.
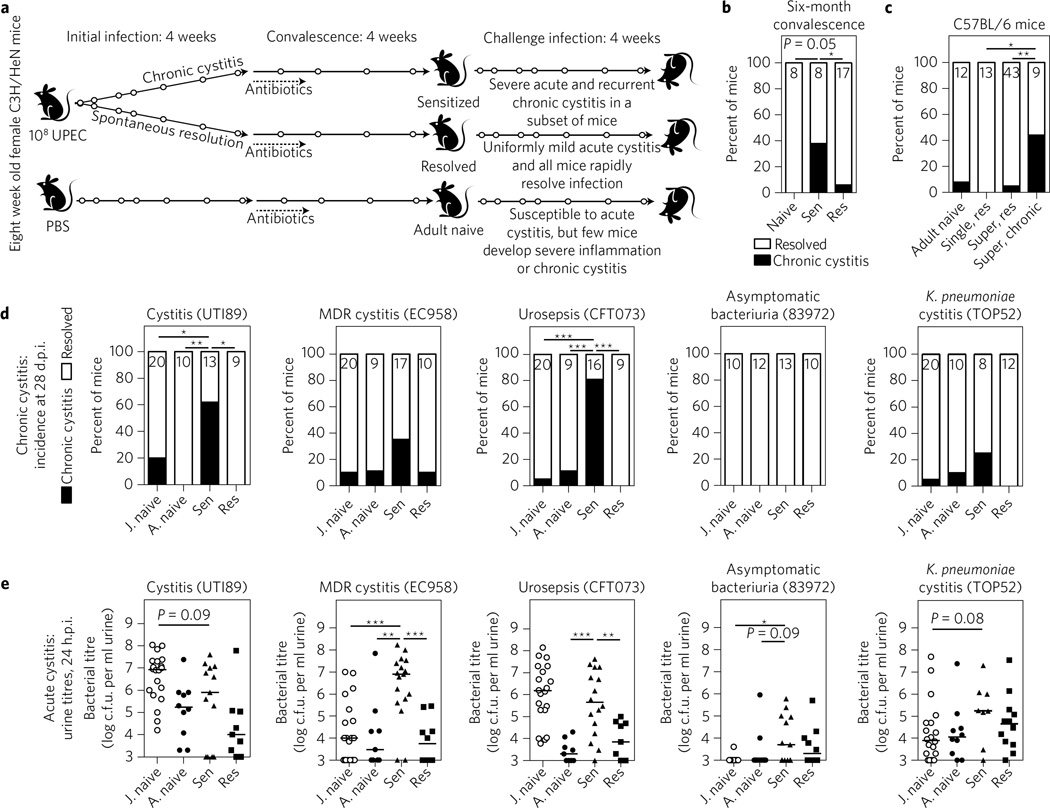
Figure 1. Mice from different genetic backgrounds with a history of chronic infection have long-lasting, enhanced susceptibility to recurrent UTI caused by clinical uropathogens.
a, Time course of sensitization experiments in C3H/HeN mice9. Open circles indicate urine collection to monitor infection status. b, For this experiment only, the convalescent period in C3H/HeN mice was extended for 6 months after the initiation of sterilizing antibiotic therapy. Shown is the incidence of chronic cystitis 28 d.p.i. after challenge with 108 c.f.u. UTI89. Sen, sensitized (initial chronic infection); Res, resolved of the initial infection. c, C57BL/6J mice were challenged with one 107 c.f.u. dose of UTI89, 4 weeks after sterilizing antibiotic therapy, and the incidence of chronic cystitis was determined. Mice were initially infected with a single dose of PBS (‘adult naive’) or 107 c.f.u. of UTI89 (‘single, res’), or were superinfected with two doses of 107 c.f.u. of UTI89 24 h apart12, which resulted in either chronic cystitis (‘super, chronic’) or resolution (‘super, res’) (Supplementary Fig. 1). d,e, Juvenile (8 weeks old; ‘J. naive’) and adult (16 weeks old, ‘A. naive’) C3H/HeN mice were challenged with 107 c.f.u. of the uropathogenic E. coli isolates UTI89, EC958, CFT073 or 83972, or the Klebsiella pneumoniae isolate TOP52. Headings indicate the type of infection from which these strains were isolated (MDR, multi-drug-resistant). All sensitized and resolved mice in this experiment were initially infected with UTI89 before antibiotic therapy. d, Incidence of chronic cystitis at 28 d.p.i. e, Urine bacterial burden at 24 h.p.i indicative of acute cystitis. Data are combined from two independent experiments except for b, which shows results for one experiment. For urine titres, data points represent actual values for each individual mouse, zeros are plotted at the limit of detection, bars indicate median values and Mann–Whitney U test was used. For the incidence of chronic cystitis, Fisher’s exact test was used and the no. of mice per group is shown at the top of each bar. *P<0.05, **P < 0.01, ***P < 0.001.
Results
In C3H/HeN mice, acute bladder infection with the prototypical uropathogenic Escherichia coli (UPEC) isolate UTI89 (ref. 11) results in two distinct outcomes: spontaneous resolution of infection within the first 7–14 days or progression to persistent bladder infection, referred to here as chronic cystitis. This bimodal outcome mimics the natural history of uncomplicated UTI in placebo-treated women: 25–75% of women spontaneously resolve symptoms and bacteriology, while the rest remain symptomatic and bacteriuric for weeks3. Antibiotic treatment of chronically infected mice sterilizes the urinary tract, allowing rapid repair of the bladder mucosal surface9. However, if infection is allowed to persist for >14 days before antibiotics, convalescent mice become predisposed to developing severe recurrent cystitis upon subsequent challenge and are thus referred to as ‘sensitized’. In contrast, mice that spontaneously resolved the initial infection are highly resistant to challenge infection and are thus called ‘resolved’ (Fig. 1a). Elevation of neutrophil chemokines and myeloid cell development factors (CXCL1, CXCL2 and G-CSF) in the serum and urine of mice 24 h post-infection (h.p.i.) is predictive of chronic infection and subsequent sensitization to rUTI (ref. 9). Similarly, in women with an acute UTI, high serum levels of neutrophil chemokines and myeloid cell development factors (CXCL1, CXCL8 and M-CSF) correlated with subsequent rUTI (ref. 10), supporting the relevance of our model.
Prior infection affects host susceptibility to recurrent UTI
To study the longevity of differential responses to subsequent infections due to prior history of infection, we challenged sensitized, resolved and age-matched mice with no history of UPEC UTI (naive) with UTI89, after six months of antibiotic-induced convalescence. We determined bacteriuria over time and assessed the incidence of chronic cystitis, which is defined as persistent high-titre (>104 c.f.u. ml−1) bacteriuria over four weeks and high-titre (>104 c.f.u.) bladder infection when humanely euthanized. Sensitized C3H/HeN mice remained significantly more susceptible to developing severe rUTI than age-matched naive mice (Fig. 1b) and resolved mice (Fig. 1b and Supplementary Fig. 1). Furthermore, we found that C57BL/6J mice with a history of chronic cystitis (induced via superinfection with two doses of UPEC 24 h apart12; Supplementary Fig. 2) were also prone to significantly increased susceptibility to rUTI (Fig. 1c). Thus, ‘sensitization’ to rUTI as a result of chronic cystitis is long-lasting and occurs in different host backgrounds.
Diverse uropathogens can cause recurrent UTI
E. coli causes up to 80% of community-acquired UTI (ref. 3). Clinical isolates recovered from women with UTI are phylogenetically, genomically and phenotypically diverse, sharing as few as 60% of their genes in pairwise strain comparisons13, and vary significantly in their ability to colonize naive mouse bladders. We thus assessed infection outcomes in sensitized, resolved and age-matched adult naive mice challenged with clinical uropathogenic strains (Supplementary Fig. 3) after an initial UTI89 infection and antibiotic therapy. Our panel of strains differed in the clinical UTI syndrome with which they were associated, and included (1) UTI89, a cystitis isolate11; (2) EC958, a multi-drug-resistant cystitis isolate14; (3) CFT073, a urosepsis isolate15; (4) 83972, an asymptomatic bacteriuria isolate16; and (5) TOP52, a cystitis isolate of Klebsiella pneumoniae, a common cause of UTI with pathogenic mechanisms similar to UPEC in juvenile naive mice17. UTI89, CFT073, EC958 and TOP52 caused chronic cystitis in 62, 81, 35 and 25% of sensitized mice, respectively (Fig. 1d), compared to 0, 11, 11 and 10% of adult naive female C3H/HeN mice, which become more resistant to chronic cystitis as they mature into adulthood18. UTI89, CFT073 and TOP52 caused no chronic cystitis in resolved mice, whereas EC958 caused chronic cystitis in one resolved mouse (Fig. 1d); this was the only incidence of chronic cystitis in a total of 124 resolved mice tested in this study, suggesting that a previous self-resolving infection results in substantial protection from subsequent infections. Strain 83972 did not cause chronic cystitis in any mice (Fig. 1d). The impact of host history was even more striking when acute cystitis was evaluated: all four UPEC strains produced higher urine titres in sensitized mice than in adult naive or resolved mice at 24 h.p.i. (Fig. 1e). TOP52 also had higher bacteriuria in sensitized mice than in naive mice, which approached statistical significance. Notably, the laboratory strain E. coli MG1655 (K-12)19 and the gut commensal E. coli strain Nissle 1917 (ref. 20) did not cause significant acute or chronic cystitis (Supplementary Fig. 4). Thus, while sensitized mice have increased susceptibility to Gram-negative uropathogens, they are not susceptible to rUTI with all E. coli. Overall, our data support different degrees of host susceptibility to rUTI that are influenced by infection history. Furthermore, a wide (but not limitless) variety of bacteria could cause UTI in susceptible hosts, which may help to explain in part the genetic diversity observed in clinical UPEC isolates13.
Convalescent bladders have an altered epithelium
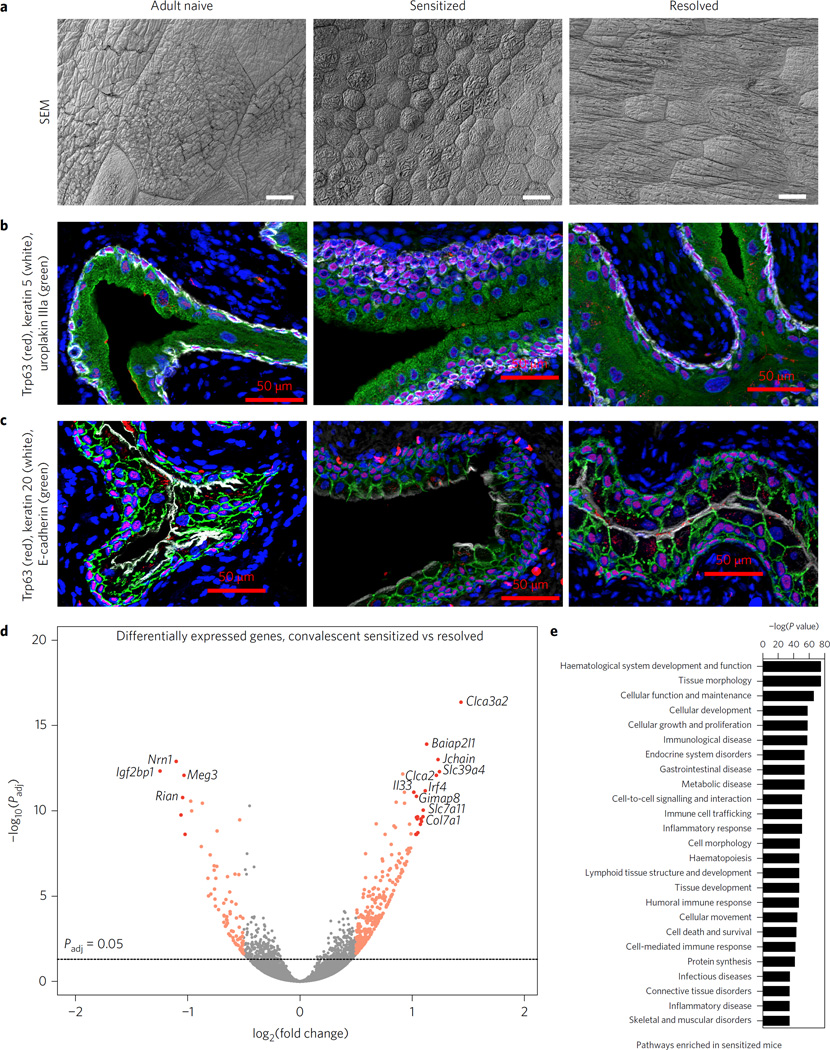
Scanning electron microscopy (SEM) of sensitized and resolved convalescent mice (four weeks after the initiation of antibiotics) and adult naive controls showed that superficial facet cells of the bladder lumen had a mean surface area 21 times smaller in sensitized mice and 6 times smaller in resolved than in adult naive mice (Fig. 2a and Supplementary Fig. 5). Immunofluorescence microscopy of bladder sections stained with markers specific for different epithelial cell types revealed striking differences in cellular morphology and differentiation in sensitized mice (Fig. 2b,c). In adult naive and resolved mice, the mature bladder epithelium (urothelium) contains layers of basal cells (keratin (Krt) 5+, Fig. 2b white; Trp63+, Fig. 2b,c red), intermediate cells (Trp63+, Fig. 2b,c red; Krt5−) and a superficial layer of large, flattened, terminally differentiated superficial cells (uroplakin IIIa+, Fig. 2b green; Krt20+, Fig. 2c white), which are bordered with a basolateral layer of E-cadherin (Fig. 2c green) and are sometimes binucleate (Fig. 2b,c blue and Supplementary Fig. 6). In contrast, the superficial cells in sensitized mice were small and rounded. They expressed uroplakin IIIa (Fig. 2b green) but had reduced expression of Krt20 (Fig. 2c white), a marker of terminal differentiation. Additionally, sensitized mice had fewer binucleate superficial cells (Supplementary Fig. 6) and exhibited amarked expansion of the intermediate and basal cell layers, suggestive of urothelial hyperplasia (Fig. 2b,c), which is consistent with proteomics data that found increased levels of keratin 14, an indicator of increased basal cell proliferation, in the sensitized urothelium10. Taken together, these observations suggest that urothelial superficial cells may fail to properly regenerate in sensitized mice, even weeks after antibiotic therapy.
Figure 2. Prior UPEC infection results in bladder epithelial remodelling that varies according to disease outcome.
a, Scanning electron microscopy was used to visualize the luminal surface of the bladder. Representative images from N = 2 replicates with a total of n = 4 mice per group are shown. Scale bars, 25 µm. b–d, Cell morphology and differentiation was assessed via immunofluorescence of paraffin-embedded bladder sections from N=3 staining experiments with bladder sections from n = 3 adult naive and resolved and n = 6 sensitized mice; representative images are shown. In b, uroplakin IIIa is in green, Trp63 in red, keratin 5 in white and nuclei in blue. In c, keratin 20 is shown in white, E-cadherin in green, Trp63 in red and nuclei in blue. Scale bars, 50 µm. d,e, RNA-seq was performed on whole bladders from n = 7 sensitized and n = 6 resolved convalescent mice. In d, 837 genes were significantly differentially expressed in sensitized mice relative to resolved mice (Padjusted < 0.05). Colours indicate each gene’s absolute log2(fold change): grey ≤0.58; orange between 0.58 and 1; and red >1. Labelled genes had absolute log2(fold change) > 1 and −log10(Padjusted) > 10. Significance was determined by a Wald test and adjusted for multiple comparisons using the Benjamini–Hochberg false-discovery rate correction. In e, pathway analysis was used to assess the biological processes enriched in the most significantly differentially expressed genes in convalescent sensitized bladders relative to resolved bladders, and significance was determined by a right-tailed Fisher’s exact test, with Padjusted < 0.05 considered significantly enriched pathways. Shown are the top 25 broad meta-pathways assembled from the specific enriched pathways by Ingenuity IPA, ordered by most significant P value.
Defects in terminal differentiation and urothelial hyperplasia have also been reported in girls and women with chronic and recurrent UTI (refs 21,22). In addition, these clinical studies reported the presence of lymphoid follicles, which are also present in sensitized mice but are not required for sensitization to rUTI (ref. 9). These findings suggest the possibility that UTI may induce lasting changes to the bladder in some individuals. This hypothesis is consistent with our proteomic evidence that chronic cystitis results in urothelial ‘remodelling’, which renders sensitized mice more susceptible to severe inflammation and mucosal wounding upon bacterial challenge, relative to resolved mice10. Notably, the observed UTI-induced bladder remodelling is distinct from the Th2-driven remodelling seen in urogenital schistosomiasis infection of the urinary bladder23 or in allergic remodelling of the lung24, as we find no evidence of increased fibrosis in bladder sections from sensitized mice (Supplementary Fig. 7).
Convalescent bladders have altered gene expression
We performed RNA-seq analysis of sensitized and resolved bladders during convalescence to expand our understanding of bladder remodelling and sensitization mechanisms. We found that sensitized and resolved bladders had distinct transcriptomic signatures (Supplementary Table 1), with 837 genes significantly differentially expressed (Fig. 2d). The whole bladder RNA-seq findings were in concordance with the previously published urothelial membrane proteome (Supplementary Fig. 8 and Supplementary Table 2). Pathway analysis of differentially expressed genes (Fig. 2e and Supplementary Tables 3 and 4) showed that immune-related pathways were significantly enriched in convalescent sensitized bladders, probably due in part to the presence of lymphoid follicles. In addition, pathways pertaining to tissue morphology, cellular development and cellular growth and proliferation were among the most highly significantly enriched pathways in sensitized compared to resolved bladders, further supporting the observation of urothelial remodelling.
UTI history impacts acute pathogenesis of recurrent infection
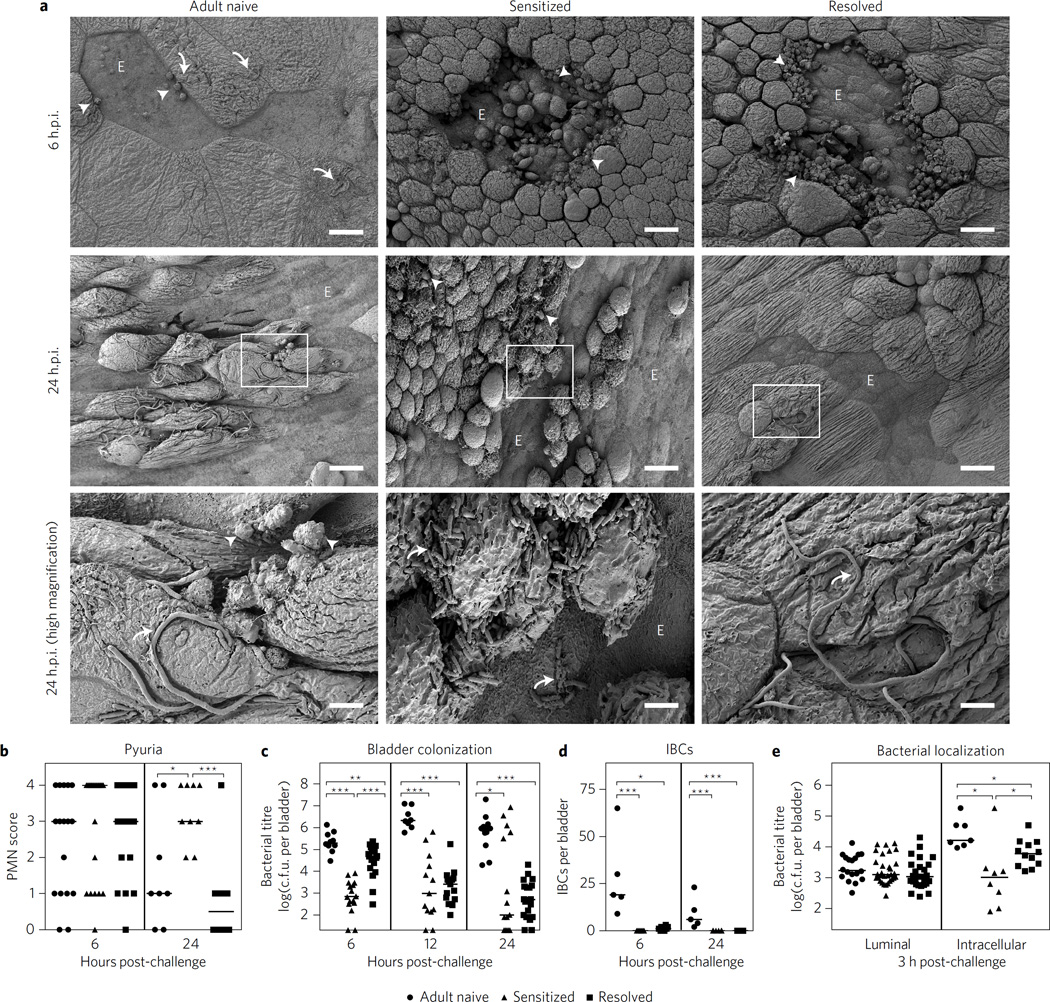
Naive mice respond to UPEC infection by exfoliating the superficial facet cells and recruiting immune cells to the bladder6,9. To assess whether infection history significantly altered these responses, we performed SEM on bladders collected at 6 and 24 h.p.i. after challenge infections of convalescent mice (Fig. 3a and Supplementary Fig. 9). Bladders of control adult naive mice had patches of exfoliation and extracellular filamentous bacteria at 6 and 24 h.p.i., with relatively few neutrophils visible by SEM. At 6 h.p.i., bladders from both sensitized and resolved mice had large patches of urothelial exfoliation bounded by transmigrating neutrophils, with few or no bacteria visible by SEM. Two of four resolved bladders at 24 h.p.i. had evidence of epithelial exfoliation, neutrophils and filamentous bacteria, but to a lesser degree than adult naive bladders (Fig. 3a), while the other two had no evidence of exfoliation, neutrophils or bacteria. In contrast, three of four imaged sensitized bladders had severe urothelial exfoliation, neutrophils and abundant extracellular rod-shaped bacteria at 24 h.p.i. (Fig. 3a). Urine sediments showed pyuria (neutrophil accumulation) in all animals at 6 h.p.i. By 24 h.p.i., pyuria had waned in adult naive and resolved mice but remained high in sensitized mice (Fig. 3b). Sensitized and resolved mice also had significantly reduced bladder colony-forming units (c.f.u.) at 6 h.p.i. compared to adult naive mice (Fig. 3c). By 24 h.p.i., bladder titres in resolved mice had uniformly decreased to 103 c.f.u., whereas sensitized mice segregated into two distinct populations having either (1) undetectable or very low titres (average of 102 c.f.u.) or (2) very high titres (average of 106 c.f.u.). In juvenile naive mice, a bimodal response to infection has been shown to be due to the presence of an acute host–pathogen checkpoint that determines infection outcomes25. Here, the distribution of bladder titres in sensitized mice was extremely polarized at 24 h.p.i. (Fig. 3c), suggesting protection from infection in some animals and severe rUTI in others.
Figure 3.
Bladder remodelling fundamentally alters acute cystitis pathogenesis upon UPEC challenge, conferring resistance to early bladder colonization. a, Representative SEM images of the luminal surface of bladders at 6 or 24 h.p.i. with 108 c.f.u. UTI89. Curved arrows show bacteria; arrowheads show neutrophils; ‘E’ denotes regions of exfoliation. The third row contains magnifications of regions denoted by white boxes in the second row. Scale bars, 25 µm for low-magnification and 5 µm for high-magnification images. N = 2 experiments with n=3–4 bladders per group. b–e, Mice were challenged with 107 c.f.u. UTI89 and acute outcomes were evaluated. Pyuria in urine sediments at 6 and 24 h.p.i. was assessed with a semi-quantitative scale (b) (PMN, polymorphonuclear neutrophil); total bladder bacterial burdens were determined at 6, 12 and 24 h.p.i. (c); intracellular bacterial communities (IBCs) were enumerated in mice at 6 and 24 h.p.i. after infection with GFP-overexpressing UTI89 (d); and bladder invasion and intracellular replication were determined at 3 h.p.i. using an ex vivo gentamicin proection assay6 (e). Data are combined from two to three independent experiments. Data points represent actual values for each individual mouse, zeros are plotted at the limit of detection, and bars indicate median values. *P<0.05, **P < 0.01, ***P<0.001, Mann–Whitney U test.
Extensive studies of UPEC pathogenesis in juvenile naive mice have demonstrated that UPEC adhere to, invade into and replicate within the superficial facet cells of the urothelium, forming biofilm-like intracellular bacterial communities (IBCs) that allow UPEC to escape elimination by urine flow and killing by professional phagocytic cells6,7. IBCs have been documented in the urine of women and children suffering from acute UTI (refs 26,27). In juvenile naive mice, mutant UPEC strains defective in IBC formation are highly attenuated28. The altered kinetics of acute bladder infection in mice with a history of infection suggested that urothelial remodelling may fundamentally change the canonical intracellular pathway defining the pathophysiology of acute host–pathogen interactions in naive mice. Indeed, we found that at 6 h.p.i. with UTI89, resolved mice had very few IBCs relative to adult naive mice, and IBCs were not detected at all in sensitized mice at 6 or 24 h.p.i. (Fig. 3d and Supplementary Figs 10 and 11). These phenotypes were probably a result of reduced epithelial invasion and/or intracellular replication inside the altered superficial urothelial cells, as at 3 h.p.i. the intracellular bacterial burden was reduced in resolved mice and even further reduced in sensitized mice, while the extracellular (luminal) bacterial burden was statistically indistinguishable among the three groups (Fig. 3e). Taken together, these experiments demonstrate that in previously infected mice, bladder remodelling confers resistance to initial colonization and IBC formation by UPEC, but that a subset of sensitized mice nevertheless succumb to severe bladder infection by 24 h.p.i.
Inhibition of cyclooxygenase-2 reduces severe recurrent UTI
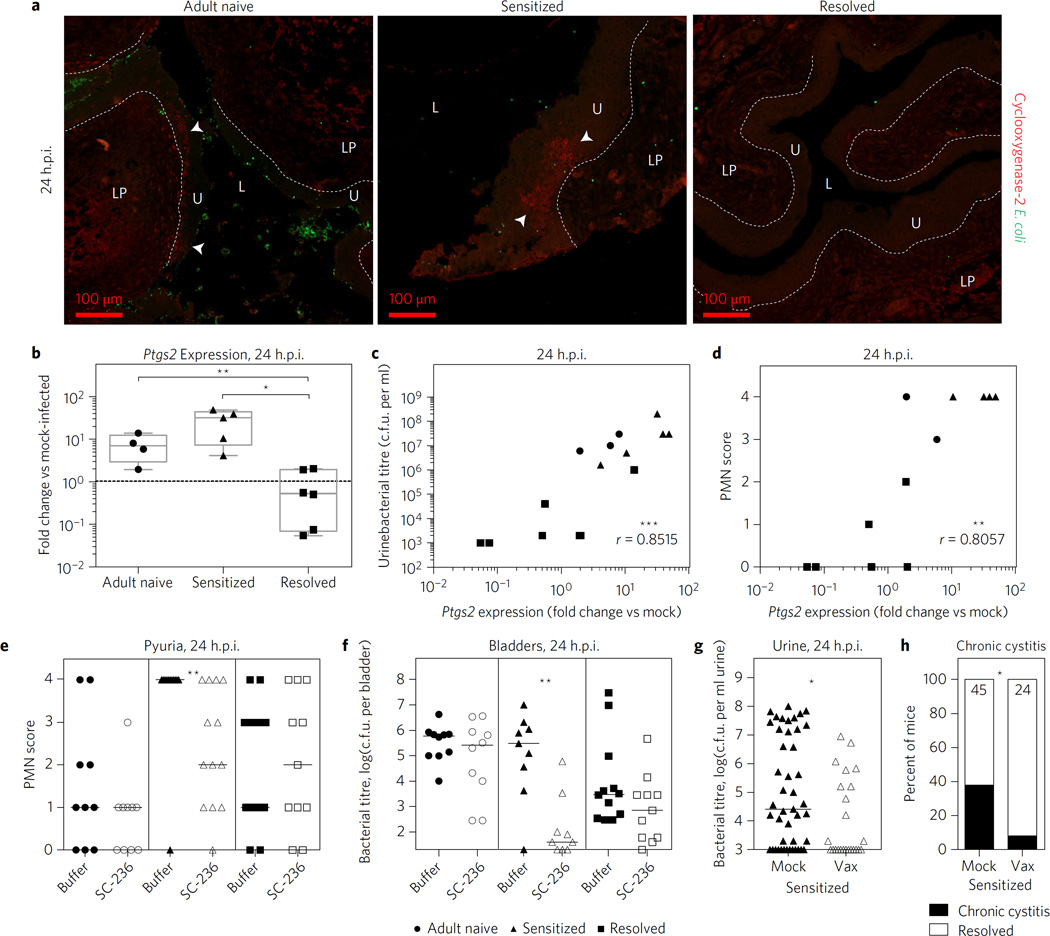
We hypothesized that the trigger for sensitized mice to develop rUTI was cyclooxygenase-2 (COX-2)-dependent inflammation. COX-2 is an enzyme expressed during inflammation that mediates the conversion of arachidonic acid to prostanoids, which act locally to modulate inflammation. Previously, we found that COX-2 inhibition in juvenile naive mice reduced the severity of acute cystitis by lowering bacterial burdens and preventing severe neutrophilic inflammation and bladder mucosal wounding10. During convalescence, UTI history did not influence expression of the COX-2 gene, Ptgs2, which was minimal in sensitized, resolved and adult naive mice (Supplementary Fig. 12). Following challenge infection, at 24 h.p.i. Ptgs2 was not expressed in resolved mice, but was induced by an average of 7.5-fold (median sevenfold) in adult naive mice (Fig. 4a,b) and in sensitized mice expression ranged from uninduced to up to ~50-fold induction (average 27-fold, median 32-fold) (Fig. 4b) with large clusters of COX-2-positive epithelial cells evident in some bladders (Fig. 4a). This bimodality of COX-2 expression in infected sensitized mice probably reflects the triggering of the previously described host–pathogen checkpoint that determines the fate of disease25. COX-2 expression at 24 h.p.i. was strongly correlated with both urine bacterial burden (Fig. 4c) and pyuria (Fig. 4d). Treatment of sensitized mice with the COX-2 inhibitor SC-236 before challenge with UTI89 prevented mucosal wounding (Supplementary Fig. 13) and resulted in a significant reduction in pyuria (Fig. 4c) and bladder bacterial burden (Fig. 4d) at 24 h.p.i. This was not the case for adult naive or resolved mice, which are resistant to severe acute and chronic cystitis. Taken together, these data indicate that even though sensitized and resolved mice demonstrate significant early urothelial resistance to colonization, COX-2-dependent bladder inflammation in sensitized mice can allow UPEC to cause severe rUTI.
Figure 4. COX-2-dependent inflammation during acute cystitis in sensitized mice allows UPEC to circumvent the early urothelial resistance to colonization.
a,b, Expression of Ptgs2 (COX-2) in bladders at 24 h.p.i. with 108 c.f.u. UTI89. In a, immunofluorescence microscopy images are shown for a mouse monoclonal antibody against COX-2. L, lumen; LP, lamina propria; U, urothelium; arrowheads indicate nests of COX-2 positive urothelial cells; dotted line indicates urothelial basement membrane. Scale bars, 100 µm, n=3–5 bladders per mouse from N = 2 experiments. COX-2 staining was not seen in any resolved bladders and was seen in about half of sensitized and adult naive bladders, of which representative images are shown. In b, qRT-PCR results are shown for bladder RNA (normalized to mock-infected adult naive bladders). Whiskers are min to max values. c,d, Ptgs2 expression at 24 h.p.i. is positively correlated with urine bacterial burden (c) and pyuria (d). e,f, Mice were pretreated with the COX-2-specific inhibitor SC-236, or mock treated, 30 min before challenge with 107 c.f.u. UTI89. In e, pyuria was determined in urine sediments collected at 24 h.p.i. PMN, polymorphonuclear neutrophil. In f, bladder bacterial burden was determined at 24 h.p.i.. g,h, After an initial 2 week chronic infection and 4–5 week convalescent period after antibiotics, sensitized mice were vaccinated (Vax) with FimH29 or mock-vaccinated (Mock), boosted or mock-boosted and then challenged with 107 c.f.u. UTI89 (Supplementary Fig. 14). Urine bacterial burden at 24 h.p.i. (g) and incidence of chronic cystitis at 28 d.p.i. (h) were determined. Data are combined from two to three independent experiments. Data points represent actual values for each individual mouse and bars indicate median values. For urine and bladder titres, zeros are plotted at the limit of detection. The Mann–Whitney U test was used for qRT-PCR and urine and bladder titres. For XY correlations a Spearman correlation was used. For incidence of chronic cystitis, Fisher’s exact test was used and the no. of mice per group is shown at the top of each bar. *P<0.05, **P < 0.01, ***P < 0.001.
Vaccination prevents recurrent UTI
The observation that sensitization to severe rUTI was long-lasting (Fig. 1b) suggests that the bladder response to UPEC is irreversibly altered in these animals. We thus tested whether vaccination could shift the balance towards protection from severe rUTI in sensitized mice. As a proof of concept, we tested systemic vaccination with the type 1 pilus adhesin FimH (Supplementary Fig. 14), which in previous studies effectively protected naive mice and cynomolgus monkeys from experimental bladder infection29,30. FimH vaccination prevented both acute (Fig. 4e) and chronic (Fig. 4f) cystitis in sensitized mice upon challenge with UTI89, compared to mock-vaccinated animals. Thus, vaccination may be a viable strategy for overcoming the enhanced susceptibility to rUTI caused by bladder remodelling as a result of chronic bladder infection. Although relatively little is known about the adaptive immune response to UTI in women and to what extent rUTI is a result of failure by the host to mount a protective adaptive response, vaccines have been shown to elicit protection from experimental UTI in naive animals31. Our findings demonstrate that targeting specific immune response pathways, for example, COX-2-mediated inflammation, can also protect against rUTI in the sensitized host.
Discussion
In a majority of women with acute uncomplicated cystitis, antibiotic treatment is effective and the disease does not recur3. However, many women experience frequent recurrences for reasons that are not well understood. Here we provide mechanistic insight into the pathophysiology of rUTI by demonstrating that bladder mucosal remodelling that occurs as a consequence of prior infection by the relatively virulent cystitis isolate UTI89 fundamentally alters the pathogenesis of acute cystitis upon subsequent UPEC challenge. Notably, the IBC cycle is considered essential for UPEC pathogenesis in naive animals. Few IBCs were detected in resolved mice, possibly explaining in part their protection from rUTI. In naive mice, the dense actin network in underlying immature urothelial cells is believed to preclude IBC formation in these cells32,33. The smaller size of the superficial cells in sensitized and resolved mice, perhaps in tandem with the differential expression of cytoskeletal genes observed in sensitized bladders, may hinder IBC development. However, a significant subset of sensitized mice were susceptible to acute and chronic rUTI, despite being resistant to IBC formation. The probable determinative factor of acute outcomes in this mouse model is severe acute inflammation driven by COX-2 expression during the first 24 h post-challenge in sensitized mice.
The significant changes in bacterial niche occupation and host immune and exfoliation responses in sensitized mice relative to naive mice influence the outcome of the disease and allow various clinical isolates to cause severe acute and chronic infections. However, the change in niche occupation and in particular the loss of the IBC pathway in sensitized mice leave the bacteria particularly vulnerable to therapeutic interventions for rUTI that target the host response, such as COX-2 inhibition. The efficacy of COX-2 inhibitors in sensitized mice supports the recent finding that ibuprofen, a non-specific COX-2 inhibitor, was comparable to antibiotics for the treatment of uncomplicated cystitis in the clinic34,35 and suggests that COX-2 inhibitors may also be efficacious in preventing rUTI in women.
Our findings highlight the importance of using clinically relevant models of recurrent infection to investigate the pathophysiology and treatment of recurrent infections in which disease history is a significant independent risk factor for recurrence, such as otitis media36, cellulitis37 and Clostridium difficile colitis38. To our knowledge, existing animal models of these and other recurrent infections generally do not incorporate the effects of a prior infection and thus may fail to capture the full spectrum of disease pathogenesis. Our studies indicate that mucosal remodelling as a consequence of prior infection can have long-lasting effects on host susceptibility to infection, and further work is needed to characterize the mechanism(s) by which chronic bladder inflammation may cause mucosal remodelling. Understanding the pathophysiology of recurrent infections in relevant model systems could also reveal vulnerabilities in disease pathogenesis that can be successfully targeted with prophylactic and therapeutic interventions, which is urgently needed in the era of multi-drug-resistant infections39.
Methods
Ethics statement
All animal experimentation was conducted according to the National Institutes of Health guidelines for the housing and care of laboratory animals. All experiments were performed in accordance with institutional regulations after review and approval by the Animal Studies Committee at Washington University School of Medicine in St Louis, Missouri.
Bacterial strains
The uropathogenic E. coli isolates used in this study were the human cystitis isolate UTI89 (ref. 11) and the following derivatives thereof: UTI89 attHK022::COMGFP (kanamycin-resistant) and UTI89 attl::PSSH10-1 (spectinomycin-resistant)40, the human urosepsis isolate CFT073 Hk::Cm (chloramphenicol-resistant)15, the multi-drug-resistant human cystitis isolate EC958 (sequence type 131)14 and the human asymptomatic bacteriuria isolate 83972 (ref. 16). The non-uropathogenic E. coli isolates used were the laboratory strain MG1655 (ref. 19) and the gut commensal strain Nissle 1917 (ref. 20). Strains were cultured statically in lysogeny broth (LB) at 37 °C. The Klebsiella pneumoniae human cystitis isolate TOP52 1721 (ref. 17) was cultured statically in LB at 37 °C. Bacterial strains were cultured for two to three overnight passages to induce type 1 pilus expression. In experiments where mice had an initial infection and subsequent challenge infection (that is, challenge experiments in sensitized and resolved mice), UTI89 attHK022::COMGFP (kanamycin-resistant) was always used for the initial infection, whereas the challenge strain varied and was differently antibiotically marked.
Type 1 pilus expression
Type 1 pilus expression of bacterial strains was assessed by haemagglutination (HA) of guinea pig red blood cells as previously described41. Briefly, bacterial strains used in the ‘heterologous challenge’ experiments in Fig. 1 were normalized to an optical density at 600 nm (OD600) of 1 and guinea pig red blood cells were normalized to an OD640 of 2. Red blood cells were incubated overnight at 4 °C with a 1:2 dilution series of bacteria. The last dilution at which haemagglutination was observed is reported as the HA titre.
Mouse infections
C3H/HeN mice were obtained from Harlan Sprague Dawley (now Envigo). C57BL/6 mice were obtained from the Jackson Laboratory. All mice were female and were seven to eight weeks old (‘juvenile’) at the time of the first infection. E. coli inocula were prepared as previously described9 and a total of 107 or 108 c.f.u. of bacteria were inoculated into the bladders of C3H/HeN mice by transurethral catheterization as previously described42. For the initial infection, 108 c.f.u. UTI89 was always used, as C3H/HeN mice develop chronic cystitis in an infectious dose-dependent manner and this inoculum results in chronic cystitis in ~50% of mice. For challenge infections, 107 or 108 c.f.u. was used. We have found that adult naive and sensitized mice are susceptible to recurrent cystitis in an infectious dose-dependent manner, whereas resolved mice will resolve the challenge infection regardless of infectious dose9,10. A 108 dose was used for microscopy experiments (SEM images in Fig. 3 and immunofluorescence in Supplementary Fig. 11) to increase the likelihood of observing bacteria in sensitized and resolved bladders, in RNA-seq experiments to induce a more uniform response in sensitized bladders, and for the six-month convalescent experiment (Fig. 1b) because of the increased resistance to UPEC infection in aged nulliparous mice18. All other experiments used a 107 dose. To monitor infection outcomes, urine was collected and urine, bladder and kidney bacterial burden was determined as previously described9. We previously found that 104 c.f.u. ml−1 persistent bacteriuria was a highly specific and sensitive cutoff for detecting chronic cystitis9. Chronic cystitis during the initial infection was defined as persistent high-titre bacteriuria (>104 c.f.u. per ml urine) at every time point collected (1, 3, 7, 10, 14, 21 and 28 days post-infection). Resolution of cystitis during the initial infection was defined as urine bacterial titre dropping below 104 c.f.u. per ml urine during at least one time point. At four weeks post-infection, all mice were treated with trimethoprim and sulfamethoxazole in the drinking water (54 and 270 µg per ml water, respectively) for ten days. Urine samples were collected weekly after the initiation of antibiotics to confirm sterile urine, and any mice with treatment failure were excluded from subsequent analysis according to pre-established criteria. Four to five weeks after the initiation of antibiotics, mice were challenged with 107 or 108 c.f.u. of bacteria inoculated into the bladders by transurethral catheterization. To assess acute outcomes, mice were humanely euthanized 3 to 24 h post-challenge and bacterial burdens were determined. To assess chronic outcomes, mice were monitored for 28–30 days and then humanely euthanized. Chronic cystitis during the challenge infection was defined as persistent high-titre bacteriuria (>104 c.f.u. per ml urine) at 1, 3, 7, 10, 14, 21 and 28 or 30 days post-infection coupled with high bladder bacterial burden (>104 c.f.u. per bladder) and visibly enlarged, inflamed bladder at the time of euthanasia. Resolution of cystitis during the challenge infection was defined as urine bacterial titre dropping below 104 c.f.u. per ml urine during at least one time point, and/or bladder bacterial burden <104 c.f.u. at the time of euthanasia. For vaccine studies, the initial infection was shortened to two weeks (chronic infection lasting two weeks was previously shown to be sufficient to result in sensitization to rUTI; ref. 9), with urine collected at days 1, 3, 7, 10 and 14 post-infection. For experiments in C57BL/6 mice, the challenge infection was shortened to 14 days, with urine collected at days 1, 3, 7, 10 and 14 post-challenge. To study how long the sensitization phenotype lasts, the ‘convalescent’ period after the initiation of antibiotics was extended from four weeks to six months.
Experimental design of mouse infections
Our experience with this model shows that five mice per replicate (two replicates minimum) is the minimum number necessary to overcome any biological and/or technical variability and provide reliable and interpretable results within an experiment. A statistical analysis was not used to determine the number of mice per experiment. For vaccine and COX-2 inhibitor experiments, mice were randomly assigned to receive intervention or sham intervention. Investigators were not blinded to the infection history of the animals, except for Masson’s trichrome staining to assess collagen deposition.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
Mice were infected with 108 c.f.u. UTI89 attl::PSSH10-1 (spectinomycin-resistant) and humanely euthanized at indicated time points, or mock-infected with PBS and humanely euthanized at 3.5 h.p.i. Bladders were aseptically collected and flash-frozen in liquid nitrogen. RNA was extracted using the RNeasy Plus Mini kit (Qiagen) and reverse-transcribed with the iScript Reverse Transcription Supermix (BioRad). qRT-PCR for Ptgs2 (COX-2) expression was performed as previously described10. Briefly, 1 µl of 12.5 ng µl−1 cDNA was used with mPtgs2-specific intron-spanning primers: F:5′-gatgctcttccgagctgtg-3′, R:5′-ggattggaacagcaaggattt-3′. Expression values were normalized to 18S expression levels (F:5′-cggctaccacatccaaggaa-3′, R:5′-gctggaattaccgcggct-3′) and the fold change in expression relative to mock-infected adult naive bladders was determined by the 2−ΔΔCt method43. Each sample was tested in triplicate in N = 1 experiment.
RNA-seq
Illumina cDNA libraries were generated using the RNAtag-seq protocol as described in ref. 44. Briefly, 1 ug of total RNA was fragmented, depleted of genomic DNA, and dephosphorylated before its ligation to DNA adaptors carrying 5′-AN8-3′ barcodes with a 5′ phosphate and a 3′ blocking group. Barcoded RNAs were pooled and depleted of rRNA using the Ribo-Zero Gold rRNA depletion kit (Illumina). These pools of barcoded RNAs were converted to Illumina cDNA libraries in three main steps: (1) reverse transcription of the RNA using a primer designed to the constant region of the barcoded adaptor; (2) degradation of the RNA and ligation of a second adaptor to the single-stranded cDNA; (3) PCR amplification using primers that target the constant regions of the 3′ and 5′ ligated adaptors and contain the full sequence of the Illumina sequencing adaptors. cDNA libraries were sequenced on the Illumina HiSeq 2500 platform. Fastq files were trimmed by cutadapt twice (cutadapt-v1.6), once by base quality and once by polyA or polyT repeats. Trimmed reads were then aligned to the Mus musculus mm10 genome using tophat2 (ref. 45) (tophat2-v2.0.11, bowtie2-2.2.2). Gene counts were conducted by HTSeq (ref. 46) (HTSeq-v0.6.0, options: −format = bam −order = name −stranded = no −idattr = gene_id −mode = union) and the output was used for principal component analysis using R stats (R-v3.2.2) and subsequent statistical analyses. We sequenced n = 8 sensitized and n = 8 resolved mice; after eliminating outliers due to low read depth (<15,000,000 reads), n = 7 sensitized and n = 6 resolved mice were available for subsequent analyses. An average of 4.65% of reads mapped to rRNA, confirming good depletion. RNA-seq data have been deposited at NCBI under BioProject ID no. PRJNA327807.
Statistical analysis (RNA-seq)
DESeq2 (DESeq2-v1.8.1)47 was used to assess differential gene expression (Supplementary Table 1) due to its consistency and precise performance across a subset of varying numbers of replicates, its tolerance for a small number of replicates, its low proportion of false detections and its superior integration with R. It assumes a negative binomial distribution for gene counts, normalizes for read depth and fits a generalized linear model. Statistically significant differences in gene expression were assessed by the Wald test and adjusted for multiple comparisons using the Benjamini–Hochberg false-discovery rate correction. Padjusted < 0.05 was deemed significantly differentially expressed. Pathway analysis of differentially expressed genes was performed with Qiagen’s Ingenuity Pathway Analysis (IPA, www.qiagen.com/ingenuity). Significantly enriched pathways were determined by a right-tailed Fisher’s exact test, with Padjusted < 0.05 deemed significantly enriched. Enriched pathways were categorized into canonical pathways (for example, Supplementary Table 3) and also used for a broader analysis of ‘meta-pathways’ (Fig. 2e and Supplementary Table 4). For comparison to the previously published proteomics data set10 (Supplementary Table 2 and Supplementary Fig. 8), a stringent significance cutoff of Padjusted < 0.01 was used to determine the most significantly enriched or depleted proteins in the urothelial proteome of convalescent sensitized mice relative to convalescent resolved mice (n = 156 proteins). The corresponding genes were assessed by RNA-seq and deemed significantly differentially expressed (n = 22) if Padjusted < 0.05 in convalescent sensitized mice relative to convalescent resolved mice.
Histopathology and immunofluorescence
Bladders were aseptically collected and fixed overnight in methacarn (60% methanol, 30% chloroform, 10% glacial acetic acid), bisected to give two halves per bladder, paraffin-embedded and sectioned. Slides were stained with Masson’s trichrome and imaged with a Zeiss Axio Scan Z.1 bright-field slide scanner for a blinded investigator to assess collagen deposition and fibrosis23,48. For immunofluorescence experiments, slides were deparaffinized, hydrated, blocked with 10% heat-inactivated horse serum (HIHS) and 0.3% triton X-100 in PBS, incubated with primary antibody in 1% HIHS and PBS overnight at 4 °C and secondary antibody in PBS for 30–60 min at room temperature. The primary antibodies used were uroplakin IIIa (mouse monoclonal, 10R-U103a, Fitzgerald), Trp63 (rabbit polyclonal, GTX102425, GeneTex), E-cadherin (goat polyclonal IgG, AF748, R&D Systems), cytokeratin 5 (chicken polyclonal, 905901, BioLegend) and cytokeratin 20 (mouse monoclonal, M7019, DAKO). Samples were mounted in DAKO glycergel mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) (Life Technologies), and fluorescence was visualized on a Zeiss Axioskop Observer. Z1 microscope. COX-2 expression was detected as previously described10. Primary antibodies used were specific for COX-2 (mouse monoclonal, 610204, BD Transduction Laboratories) and E. coli O, K serotype (rabbit polyclonal, E3500-06, US Biological). Fluorescence was visualized on a Zeiss Axio Imager M2 microscope. Antibodies are verified at 1DegreeBio (http://1degreebio.org/), except cytokeratin 5 and cytokeratin 20 (verified at manufacturers’ websites).
Binucleate superficial cells
Binucleate superficial cells in convalescent bladders were examined by two methods. First, paraffin-embedded sections were stained and visualized on a Zeiss Axioskop Observer.Z1 microscope as described above. To determine the percentage of binucleate superficial cells, nuclei were enumerated in all superficial cells (TRP63−, keratin 5−, uroplakin IIIa+, keratin 20+ (weak/patchy staining observed in sensitized mice), with a basolateral band of E-cadherin). Adult naive bladders (n = 3) had 69–77 superficial cells, sensitized bladders (n = 6) had 135–202 superficial cells, and resolved bladders (n = 3) had 81–94 superficial cells, reflecting the smaller superficial cell size in sensitized and resolved mice. Next, binucleate cells were imaged in bladder whole mounts as described by Blango et al.49 with the following modifications: bladders were aseptically collected from adult naive, sensitized and resolved mice (n = 3 bladders per group), bisected, splayed, fixed for 20 min in 4% paraformaldehyde and washed 3 × 5 min in 1× PBS. Bladders were incubated for 10 min in 1:5,000 DAPI in 1× PBS with 0.002% saponin, rinsed 3 × 5 min in PBS and mounted in ProLong Gold antifade reagent (Thermo Fisher) and imaged on a Zeiss Axio Imager M2 microscope.
Scanning electron microscopy
Bladders were aseptically collected before challenge or at 6 or 24 h post-challenge with 108 c.f.u. UTI89 and bisected, splayed and fixed in EM fixative (2% paraformaldehyde, 2% glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.4). Samples were prepared by critical point drying. Briefly, samples were post-fixed in 1.0% osmium tetroxide, dehydrated in increasing concentrations of ethanol, then dehydrated at 31.1 °C and 1,072 p.s.i. for 16 min in a critical point dryer. Samples were mounted on carbon tape-coated stubs and sputter-coated with gold/palladium under argon. The bladders shown in Figs 2 and 3 and Supplementary Fig. 9 were imaged on a Zeiss Crossbeam 540 FIB-SEM. For preliminary analysis, samples were imaged on a Hitachi S-2600H SEM. ImageJ 1.47v (National Institutes of Health) was used to calculate epithelial cell surface area in five 500× magnification fields per bladder half (ten per mouse). Adobe Photoshop CS5.1 was used to slightly decrease the brightness and increase the contrast of the SEM images in Fig. 3 to enhance visibility.
Acute pathogenesis
Mice were challenged with 107 c.f.u. UTI89 attl::PSSH10-1 (spectinomycin-resistant)40 and humanely euthanized at acute time points. Urine sedimentation to determine neutrophil influx was performed as previously described9. Briefly, 80 µl of a 1:10 dilution of urine was centrifuged onto poly-l-lysine-coated glass slides and stained with a Hema 3 kit (Fisher Scientific). Slides were examined by light microscopy and the average number of polymorphonuclear leukocytes (PMN) per high-powered field (h.p.f.; 400× magnification) was calculated from counting five fields. A semi-quantitative scoring system was created to facilitate analysis: 0 = less than 1 PMN per h.p.f.; 1 = 1–5 PMN per h.p.f.; 2 = 6–10 PMN per h.p.f.; 3 = 11–20 PMN per h.p.f. and 4 = >20 PMN per h.p.f. Bladder burden was determined at 6, 12 and 24 h post-challenge as previously described9. Briefly, bladders were aseptically collected, homogenized in PBS, serially diluted and spotted onto LB agar plates containing appropriate antibiotics. Intracellular bacterial communities were enumerated at 6 and 24 h post-challenge by LacZ staining and fluorescent microscopy as previously described7,50. Bladder invasion assays were performed at 3 h post-challenge as previously described6 with the following modifications: after washing three times in PBS, bladders were incubated for 75 min in gentamicin in RPMI cell culture medium without added serum, rather than in PBS.
Inhibitor treatments
To test the effect of COX-2 inhibition on acute cystitis, mice were pretreated with SC-236 (Sigma) as previously described10. The drug was solvated in 1% Tween 80 and mice received 100 µl drug, or buffer alone, per 20 g body weight by oral gavage. Mice were humanely euthanized at 24 h.p.i. with 107 c.f.u. UTI89 and bladder bacterial burden was determined.
FimH vaccination
C3H/HeN mice were initially infected with 108 c.f.u. UTI89 attHK022::COMGFP (kanamycin-resistant) and antibiotic therapy was initiated at two weeks post-infection, which is sufficient to cause sensitization9. Four to five weeks after the initiation of antibiotics, mice were vaccinated with the type 1 pilus adhesin FimH coupled with its periplasmic chaperone FimC, with FimC alone (previously shown not to be protective against UTI; ref. 29), or with buffer alone. Five weeks post-vaccination, mice were boosted. Vaccination was performed as previously described29 with the following modifications: 15 µg of FimCH, FimC or buffer were emulsified 1:1 with Complete Freund’s Adjuvant for the primary vaccination or Incomplete Freund’s Adjuvant for the boost. Mice were injected subcutaneously with 50 µl per hind flank for a total of 100 µl. Five weeks post-boost, mice were challenged with 107 c.f.u. UTI89 attl::PSSH10-1 (spectinomycin-resistant) and infection outcomes were monitored over four weeks.
Statistical analysis (mouse experiments)
Statistics were performed in GraphPad Prism v6.07. A two-tailed Fisher’s exact test was used to test for significant differences in the incidence of chronic cystitis and percentage of binucleate superficial cells. For acute pathogenesis (bacterial titres, pyuria, IBC formation), surface area differences in superficial cells and qRT-PCR, the D’Agostino and Pearson omnibus normality test was used to test for normality of distribution, Kruskal–Wallis tests were used to assess statistical significance, and subsequent pairwise comparisons were performed with a two-tailed Mann–Whitney U test. For XY correlations, the D’Agostino and Pearson Omnibus normality test was used to test for normality of distribution, and Spearman correlations were performed to test for statistical significance. P < 0.05 was considered statistically significant. Multiple test corrections were not performed.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NIH) and the Office of Research on Women’s Health Specialized Center of Research (P50 DK64540 and R01 DK51406 to S.J.H; AI95542 to S.J.H. and M.C.; Mucosal Immunology Studies Team consortium U01 AI095776 Young Investigator Award and Mentored Clinical Scientist Research Career Development Award K08 AI083746 to T.J.H. and F30 DK096751 to D.J.S.) and by the National Science Foundation (Graduate Research Fellowship DGE-1143954 to V.P.O.). RNA-seq analysis design and support was provided by the Rheumatic Disease Core Center at Washington University (P30-AR048335, to E.D.O.R). This publication was made possible by grant no. U19 AI110818 from NIAID. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. SEM studies and sample preparation were performed by the Research Center for Auditory and Vestibular Studies, which is supported by the NIH NIDCD grant P30DC04665, and by the Washington University Center for Cellular Imaging (WUCCI), which is supported by the Washington University School of Medicine, The Children’s Discovery Institute of Washington University and St Louis Children’s Hospital, the Foundation for Barnes-Jewish Hospital and the National Institute for Neurological Disorders and Stroke (NS086741). The authors thank K. Dodson and D.J. Frank for editorial assistance and D. Liu, J. Lett, M. Joens and J. Fitzpatrick for technical assistance.
S.J.H. may receive royalty income based on the FimHvaccine technology that he developed, which was licensed by Washington University to Sequoia Sciences.
Footnotes
Data availability. The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information and are also available from the corresponding author upon request. RNA-seq data that support the findings of this study have been deposited at NCBI under BioProject ID no. PRJNA327807.
Author contributions
V.P.O. and T.J.H. conceived, designed and performed experiments, analysed data and wrote the manuscript. L.Y. and J.L. performed experiments and analysed data. D.J.S. and S.S. performed experiments. E.D.O.R. analysed data. M.C. conceived experiments. C.L.M., A.L.L. and S.J.H. conceived experiments, analysed data and wrote the manuscript.
Supplementary information is available for this paper.
Competing interests
The other authors declare no competing financial interests.
References
- 1.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 2.Foxman B, Barlow R, D’Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann. Epidemiol. 2000;10:509–515. doi: 10.1016/s1047-2797(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 3.Foxman B. The epidemiology of urinary tract infection. Nat. Rev. Urol. 2010;7:653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 4.Hooton TM, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N. Engl. J. Med. 1996;335:468–474. doi: 10.1056/NEJM199608153350703. [DOI] [PubMed] [Google Scholar]
- 5.Scholes D, et al. Risk factors associated with acute pyelonephritis in healthy women. Ann. Intern. Med. 2005;142:20–27. doi: 10.7326/0003-4819-142-1-200501040-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulvey MA, et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 7.Anderson GG, et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 8.Carey AJ, et al. Urinary tract infection of mice to model human disease: practicalities, implications and limitations. Crit. Rev. Microbiol. 2016;42:780–799. doi: 10.3109/1040841X.2015.1028885. [DOI] [PubMed] [Google Scholar]
- 9.Hannan TJ, Mysorekar IU, Hung CS, Isaacson-Schmid ML, Hultgren SJ. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathogens. 2010;6:e1001042. doi: 10.1371/journal.ppat.1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hannan TJ, et al. Inhibition of cyclooxygenase-2 prevents chronic and recurrent cystitis. EBioMedicine. 2014;1:46–57. doi: 10.1016/j.ebiom.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 2001;69:4572–4579. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz DJ, Conover MS, Hannan TJ, Hultgren SJ. Uropathogenic Escherichia coli superinfection enhances the severity of mouse bladder infection. PLoS Pathogens. 2015;11:e1004599. doi: 10.1371/journal.ppat.1004599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marrs CF, Zhang L, Foxman B. Escherichia coli mediated urinary tract infections: are there distinct uropathogenic E. coli (UPEC) pathotypes? FEMS Microbiol. Lett. 2005;252:183–190. doi: 10.1016/j.femsle.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 14.Totsika M, et al. Insights into a multidrug resistant Escherichia coli pathogen of the globally disseminated ST131 lineage: genome analysis and virulence mechanisms. PLoS ONE. 2011;6:e26578. doi: 10.1371/journal.pone.0026578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mobley HL, et al. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 1990;58:1281–1289. doi: 10.1128/iai.58.5.1281-1289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersson P, et al. Persistence of Escherichia coli bacteriuria is not determined by bacterial adherence. Infect. Immun. 1991;59:2915–2921. doi: 10.1128/iai.59.9.2915-2921.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosen DA, et al. Utilization of an intracellular bacterial community pathway in Klebsiella pneumoniae urinary tract infection and the effects of FimK on type 1 pilus expression. Infect. Immun. 2008;76:3337–3345. doi: 10.1128/IAI.00090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kline KA, Schwartz DJ, Gilbert NM, Lewis AL. Impact of host age and parity on susceptibility to severe urinary tract infection in a murine model. PLoS ONE. 2014;9:e97798. doi: 10.1371/journal.pone.0097798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerdes SY, et al. Experimental determination and system level analysis of essential genes in Escherichia coli MG1655. J. Bacteriol. 2003;185:5673–5684. doi: 10.1128/JB.185.19.5673-5684.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nissle A. Über die Grundlagen einer neuen ursächlichen Bekämpfung der pathologischen Darmflora. Dtsch. Med. Wochenschr. 1916;42:1181–1184. [Google Scholar]
- 21.Hansson S, et al. Follicular cystitis in girls with untreated asymptomatic or covert bacteriuria. J. Urol. 1990;143:330–332. doi: 10.1016/s0022-5347(17)39950-0. [DOI] [PubMed] [Google Scholar]
- 22.Schlager TA, LeGallo R, Innes D, Hendley JO, Peters CA. B cell infiltration and lymphonodular hyperplasia in bladder submucosa of patients with persistent bacteriuria and recurrent urinary tract infections. J. Urol. 2011;186:2359–2364. doi: 10.1016/j.juro.2011.07.114. [DOI] [PubMed] [Google Scholar]
- 23.Ray D, et al. Transcriptional profiling of the bladder in urogenital schistosomiasis reveals pathways of inflammatory fibrosis and urothelial compromise. PLoS Negl. Trop. Dis. 2012;6:e1912. doi: 10.1371/journal.pntd.0001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leigh R, et al. Dysfunction and remodeling of the mouse airway persist after resolution of acute allergen-induced airway inflammation. Am. J. Respir. Cell Mol. Biol. 2002;27:526–535. doi: 10.1165/rcmb.2002-0048OC. [DOI] [PubMed] [Google Scholar]
- 25.Hannan TJ, et al. Host–pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol. Rev. 2012;36:616–648. doi: 10.1111/j.1574-6976.2012.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007;4:e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robino L, et al. Intracellular bacteria in the pathogenesis of Escherichia coli urinary tract infection in children. Clin. Infect. Dis. 2014;59:e158–e164. doi: 10.1093/cid/ciu634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright KJ, Seed PC, Hultgren SJ. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell. Microbiol. 2007;9:2230–2241. doi: 10.1111/j.1462-5822.2007.00952.x. [DOI] [PubMed] [Google Scholar]
- 29.Langermann S, et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science. 1997;276:607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 30.Langermann S, et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J. Infect. Dis. 2000;181:774–778. doi: 10.1086/315258. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien VP, Hannan TJ, Nielsen HV, Hultgren SJ. Drug and vaccine development for the treatment and prevention of urinary tract infections. Microbiol. Spectrum. 2016;4 doi: 10.1128/microbiolspec.UTI-0013-2012. http://dx.doi.org/10.1128/microbiolspec.UTI-0013-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eto DS, Sundsbak JL, Mulvey MA. Actin-gated intracellular growth and resurgence of uropathogenic Escherichia coli. Cell. Microbiol. 2006;8:704–717. doi: 10.1111/j.1462-5822.2006.00691.x. [DOI] [PubMed] [Google Scholar]
- 33.Berry RE, Klumpp DJ, Schaeffer AJ. Urothelial cultures support intracellular bacterial community formation by uropathogenic Escherichia coli. Infect. Immun. 2009;77:2762–2772. doi: 10.1128/IAI.00323-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bleidorn J, Gagyor I, Kochen MM, Wegscheider K, Hummers-Pradier E. Symptomatic treatment (ibuprofen) or antibiotics (ciprofloxacin) for uncomplicated urinary tract infection? Results of a randomized controlled pilot trial. BMC Med. 2010;8:30. doi: 10.1186/1741-7015-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gágyor I, et al. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: randomised controlled trial. Br. Med. J. 2015;351:h6544. doi: 10.1136/bmj.h6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Froom J, et al. A cross-national study of acute otitis media: risk factors, severity, and treatment at initial visit. Report from the international primary care network (IPCN) and the ambulatory sentinel practice network (ASPN) J. Am. Board. Fam. Pract. 2001;14:406–417. [PubMed] [Google Scholar]
- 37.Bjornsdottir S, et al. Risk factors for acute cellulitis of the lower limb: a prospective case–control study. Clin. Infect. Dis. 2005;41:1416–1422. doi: 10.1086/497127. [DOI] [PubMed] [Google Scholar]
- 38.Fekety R, et al. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin. Infect. Dis. 1997;24:324–333. doi: 10.1093/clinids/24.3.324. [DOI] [PubMed] [Google Scholar]
- 39.Rasko DA, Sperandio V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 2010;9:117–128. doi: 10.1038/nrd3013. [DOI] [PubMed] [Google Scholar]
- 40.Wright KJ, Seed PC, Hultgren SJ. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect. Immun. 2005;73:7657–7668. doi: 10.1128/IAI.73.11.7657-7668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hultgren SJ, Porter TN, Schaeffer AJ, Duncan JL. Role of type 1 pili and effects of phase variation on lower urinary tract infections produced by Escherichia coli. Infect. Immun. 1985;50:370–377. doi: 10.1128/iai.50.2.370-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hung CS, Dodson KW, Hultgren SJ. A murine model of urinary tract infection. Nat. Protoc. 2009;4:1230–1243. doi: 10.1038/nprot.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shishkin AA, et al. Simultaneous generation of many RNA-seq libraries in a single reaction. Nat. Methods. 2015;12:323–325. doi: 10.1038/nmeth.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trapnell C, Pachter L, Salzberg SL. Tophat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Metcalfe PD, et al. Bladder outlet obstruction: progression from inflammation to fibrosis. BJU Int. 2010;106:1686–1694. doi: 10.1111/j.1464-410X.2010.09445.x. [DOI] [PubMed] [Google Scholar]
- 49.Blango MG, Ott EM, Erman A, Veranic P, Mulvey MA. Forced resurgence and targeting of intracellular uropathogenic Escherichia coli reservoirs. PLoS ONE. 2014;9:e93327. doi: 10.1371/journal.pone.0093327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Justice SS, Lauer SR, Hultgren SJ, Hunstad DA. Maturation of intracellular Escherichia coli communities requires SurA. Infect. Immun. 2006;74:4793–4800. doi: 10.1128/IAI.00355-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.