Abstract
Background
Cardiomyocyte hypertrophy is a critical precursor to the development of heart failure. Methods to phenotype cellular hypertrophy non-invasively are limited. The goal was to validate a CMR-based approach for the combined assessment of extracellular matrix expansion and cardiomyocyte hypertrophy.
Methods and Results
Two murine models of a) hypertension (N=18, with N=15 controls) induced by L-NG-Nitroarginine Methyl Ester (L-NAME) and b) pressure-overload (N=11) from transaortic constriction (TAC), were imaged by CMR at baseline and 7-weeks after L-NAME treatment, or up to 7 weeks following TAC. T1 relaxation times were measured before and after gadolinium contrast. The intracellular lifetime of water (τic), a cell size dependent parameter, and extracellular volume fraction (ECV), a marker of interstitial fibrosis, were determined with a model for transcytolemmal water exchange. Cardiomyocyte diameter and length were measured on FITC-wheat germ agglutinin stained sections. τic correlated strongly with histologic cardiomyocyte volume-to-surface ratio (r=0.78, P<0.001) and cell volume (r=0.75; P<0.001). Histological cardiomyocyte diameters and cell volume were higher in mice treated with L-NAME compared to controls (P<0.001). In the TAC model, CMR and histology showed an cell hypertrophy at two weeks post TAC, without significant fibrosis at this early time point. Mice exposed to TAC demonstrated a significant, longitudinal, and parallel increase in histological cell volume, volume-to-surface ratio, and τic,between 2 and 7 weeks after TAC.
Conclusion
The intracellular lifetime (τic) measured by contrast-enhanced CMR provides a non-invasive measure of cardiomyocyte hypertrophy. ECV and τic can track myocardial tissue remodeling from pressure overload.
Keywords: hypertrophy, cardiac magnetic resonance imaging
INTRODUCTION
Cardiomyocyte hypertrophy is an early response of the heart to stress, preceding the expansion of the extra-cellular matrix, interstitial fibrosis, and overt clinical heart failure (HF).1, 2 Clinically, cardiomyocyte hypertrophy represents a conserved, plastic, and prognostically important response to a variety of physiologic (e.g., exercise) and pathophysiologic triggers (e.g., hypertension, aortic stenosis).3-5 Although pathologic left ventricular hypertrophy (LVH) may be reversible, the presence of LVH already confers a significantly higher risk of stroke, incident HF and mortality,6-9 and accelerates the transition to HF.10 Therefore, methods to detect and more precisely phenotype hypertrophy at the level of the cardiomyocyte may facilitate earlier detection and intervention, and consequently inform preventative therapy for HF.
In animal models, an increase in cardiomyocyte volume is an early marker of remodeling, occurring in response to mechanical stretch during the transition to HF.11-13 At a macroscopic level, increased LV thickness is a manifestation of cardiomyocyte hypertrophy, as well as expansion of the extracellular matrix,14 both of which play an integral role in the transition from compensated cardiac hypertrophy to clinical HF.15 The lack of methods to quantify serially changes in cardiomyocyte volume in vivo in response to either therapy or varying physiologic conditions has limited the value of cardiomyocyte hypertrophy as a biomarker of pre-clinical disease.
The goal of this study was to establish and validate a novel cardiac magnetic resonance (CMR) technique to quantify cardiomyocyte hypertrophy at a cellular level, based on the concept that the lifetime of water within a cell changes with cell-size or cell-volume. We used two well-validated murine models of pressure-overload HF (hypertension and transverse aortic constriction) to validate the technique and establish its suitability for tracking longitudinal changes in cellular hypertrophy using CMR in vivo.
METHODS
Murine model of hypertensive heart disease via L-nitro-w-methyl ester (L-NAME)
To validate the intracellular lifetime of water as a surrogate marker of cell size, we first studied mice treated with L-NAME, a well-described model in which myocardial fibrosis, hypertrophy, and failure occur simultaneously.16-18 Thirty-three 8 week old, male, wild-type mice (mean body weight 37.4±2.3 grams (Taconic, Germantown, NY, USA) were randomly assigned to one of two experimental groups: (1) placebo (control group; n=15; tap water alone for 7 weeks) versus (2) L-NAME-treated (L-NAME group; n=18; L-NAME 3mg/ml in drinking water; Sigma, USA) for 7 weeks. Animals were kept under standard conditions and had normal food and water ad libitum. Non-invasive blood pressures were obtained at baseline, and weekly after treatment started, using a volume–pressure recording tail-cuff technique (CODA-1, Kent Scientific, Torrington, CT).13 Mice in the control (placebo) and L-NAME groups were imaged at baseline and after 7 weeks of treatment (placebo versus L-NAME) using a 4.7T MRI system (Bruker Biospin MRI, Billerica, MA). Blood samples were collected by retro-orbital puncture immediately after each CMR study for blood hematocrit determination (i-STAT, Abbott Point-of-Care, Princeton, NJ). Mice treated with L-NAME, or placebo, were euthanized following the second CMR study. Hearts were excised and fixed in formalin-solution for histological analysis.
Murine model of pressure overload by transverse aortic constriction
To study longitudinal changes in cellular hypertrophy and fibrosis under pressure overload, we selected the transverse aortic constriction model, in which early changes in tissue structure and LV mass are dominated by cardiomyocyte hypertrophy, and a later build-up of interstitial fibrosis. Eleven mice (mean body weight 26.9±2.4 g, C57BL/6, Jackson Laboratory, Bar Harbor, Maine, USA) were subjected to transverse aortic constriction (TAC) at 3 months of age, as previously described.19 Mice were anesthetized with a ketamine/xylazine mix (80-100 mg/kg/12 mg/kg) and a thoracotomy was performed. The exposed transverse aortic arch was then ligated with a 27-gauge needle between the innominate artery and left common carotid artery. The general rate of loss of animals in the few days after TAC is ~5-10%. Due to restriction on moving animals out of a barrier facility before surgery, MRI’s were limited to a post-surgery period. Two weeks after surgery, all mice with TAC underwent CMR to assess early development of cardiomyocyte hypertrophy. To investigate longitudinal changes in hypertrophy and fibrosis, mice were imaged at 4 weeks (n=2) and 7 weeks (n=4) post TAC. Animals were imaged in a 9.8T MRI system (Bruker Biospin MRI, Billerica, MA). Euthanasia and histologic analysis were performed at 2 weeks (n=5), 4 week (n=2) and 7 weeks (n=4). Our study protocol and animal care conformed with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication Number 85-23, Revised1996), and was approved by the Standing Committee on Animal Care and Use at Harvard Medical School.
Histopathologic analysis
Approximately 1 mm thick, short axis myocardial sections of the mouse hearts were fixed with buffered 10% formalin solution (Fisher Scientific, Pittsburgh, PA), and embedded in paraffin. To quantify cardiomyocyte size, sections were stained with fluoresceinisothiocyanate-conjugated (FITC-) wheat germ agglutinin to delineate the cell membrane.20 All sections were scanned with ScanScope scanners (Aperio Technologies, Inc; Vista, CA), and whole-slide images were sampled to a final resolution of 1.0 μm/pixel. Measurements of (minor) cell diameter (Dmin), and major cell-diameter (Dmaj), equivalent to the cardiomyocyte length, were obtained by image analysis of FITC-wheat germ agglutinin stained sections. From a mid-ventricular short-axis slice, we measured Dmin and Dmaj. The mid-ventricular short axis level was confirmed based on location and orientation of papillary muscles. Ten measurements of Dmaj were made in each of the anterior, septal, lateral, and inferior wall sections of the left ventricle in fields with longitudinally oriented cardiomyocytes. Only cells with well-defined cell membranes, and visible cell nuclei at mid-wall depth were selected. For Dmin,, we selected cardiomyocytes with a short axis orientation at all transmural depths. Criteria for the short axis of the cardiomyocytes were a circular shape and the visibility of the cell nucleus. Cardiomyocyte volume and surface area were calculated assuming a cell shape in the form of a prolate ellipsoid,21-24 using the median Dmin and Dmaj (see Figure 1). Connective tissue volume fraction, the histologic equivalent of extra-cellular volume fraction from CMR25-27, was quantified on sections stained with Masson’s trichrome stain, using a semi-automatic pixel color intensity algorithm in the Aperio Spectrum software to quantify pixels stained in blue.
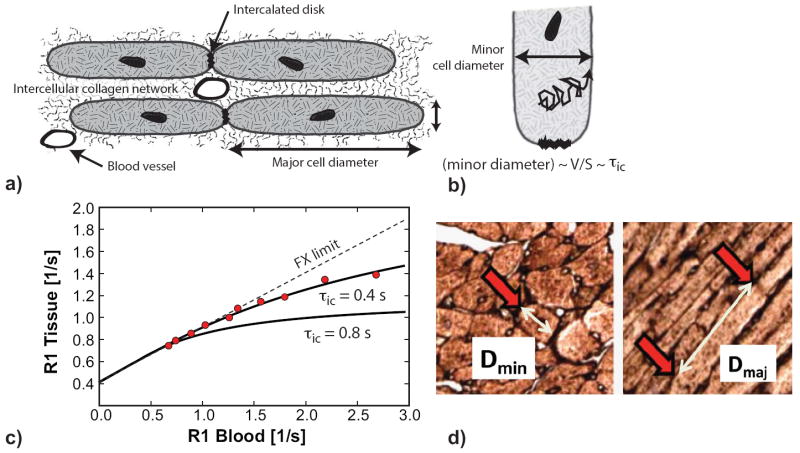
Figure 1.
Illustration of cardiomyocyte hypertrophy determination. (A) Cardiomyocytes have an elongated shape with the ratio of the major-to-minor cell-diameter on the order of 4:1. (B) The intracellular lifetime (τic) of a water molecule undergoing diffusional motion within the cell is proportional to the volume-to-surface ratio (V/S), a measure of cell size. (C) CMR measurements of the longitudinal relaxation rate constant (R1) before and after administration of gadolinium contrast were used to determine τic. The relation between R1 in myocardial tissue and R1 of blood starts out linear, with a slope proportional to the extracellular volume fraction. With increasing R1 in blood, τic ceases to be sufficiently short relative to the extra-to-intracellular R1 difference. The degree of deviation from linear dependence is sensitive to τic. Red data circles are from measurements in a mouse at 7 weeks after transverse aortic constriction. The solid line is the fit to the data with a two-space water-exchange model, using orthogonal distance regression. The τic for the best fit was 0.4 s. The line with long dashes illustrates how the model fit changes with an increase of τic to 0.8 s, causing a larger deviation from the straight line. (D) Cardiomyocyte length (Dmaj) and diameter (Dmin) were measured on digitized images of tissue slices stained with fluorescein isothiocyanate-conjugated (FITC-) wheat germ agglutinin. For Dmin a cell cross-section with approximately circular shape was chosen (highlighted by red arrow). Dmaj corresponded to the distance between intercalated discs, highlighted by the red arrows.
Cardiac magnetic resonance imaging
Mice were first anesthetized with isofluorane (induction 4-5%; maintenance 1-2.5% in oxygen from a precision vaporizer) in an induction chamber, and then positioned supine on a water-heated bed in the MRI scanner. CMR images were acquired with electrocardiographic and respiratory gating (model 1025L, SAII, Stony Brook, NY). For left ventricular (LV) size and function, short-axis cine gradient-echo images were obtained with full LV coverage (repetition time TR 5.9 ms; echo time TE 2.2ms; temporal resolution 20-30ms; in-plane spatial resolution 100-120 μm × 180-210 μm; 1 mm slice thickness, no gap). Manually traced epicardial and endocardial contours at end-systole and end-diastole were used to determine the LV end-diastolic volume, LV end-systolic volume, and LV myocardial mass, using Simpson’s rule, and LV ejection fraction (LVEF).
Gadolinium diethylenetriaminepentacetic acid (Magnevist, Berlex, Wayne, NJ) was injected subcutaneously in multiple steps up to a cumulative dose of 0.5mmol/kg. Myocardial T1 was measured in a mid-LV slice, once pre-contrast, and at least 4 times post-contrast, using a Look-Locker technique, no earlier than 6 minutes after contrast administration as described previously25 (TR 2.5 ms; TE 1.8 ms; flip angle = 10°, in-plane resolution 190 μm, 1 mm slice thickness). Each Look-Locker acquisition was made approximately 6-8 minutes after a subcutaneous injection of contrast. Due to the slow clearance of contrast with the subcutaneous injections (t1/2~70 min),25 this amounted to an essentially stepwise increase of R1 in blood over the course of the MRI study (< 2 hours).
For 6 myocardial segments, and the blood pool, signal intensity (SI) was plotted versus time after inversion (TI). T1 values were obtained by non-linear least-squares fitting of the SI vs. TI curves to an analytical expression for the magnitude signal measured during the inversion recovery,25 and correction for the radiofrequency pulse effects on the inversion recovery.28 The reciprocal of T1 (R1=1/T1) was used to plot the myocardial R1 against the R1 in the blood pool.
Determination of Intracellular Lifetime of Water
Water molecules exchange mostly by diffusion between the interstitial (extracellular) and intracellular spaces. The average intracellular lifetime (τic) depends on the mean time for diffusion to the cell membrane (Figure 1). Specifically, τic is proportional to the volume-to-surface ratio (V/S),29, 30 with V/S being on the order of the minor cell diameter in the case of cardiomyocytes, which normally have a diameter-to-length ratio cells of approximately 4:1. The myocardial T1 after administration of extra-cellular gadolinium contrast can be used to probe τic.29 The relaxation recovery rate (i.e. R1) of myocardial water changes linearly with the blood R1, as long as the rate of exchange of water (~1/τic) between the extra- and intracellular spaces does not constitute a bottleneck (relative to the R1 difference between intra- and extra-cellular spaces). One moves away from this fast exchange regime, when the concentration of gadolinium-contrast in blood and the extracellular space is increased. The R1 of tissue shows a sub-linear dependence on the R1 of blood when the exchange of water between intra- and extracellular spaces starts to constrain the rate of relaxation in the intracellular space. The R1 for myocardial tissue and blood data were fit with a 2-space water-exchange (2SX) model of equilibrium transcytolemmal water-exchange, originally developed by Landis et al.29, 31 The myocardial extracellular volume fraction (ECV) and τic are adjustable parameters of the model, determined by fitting the model to the observed R1 data using a non-linear orthogonal distance regression algorithm (URL http://www.netlib.org/odrpack/). The measured blood hematocrit was a fixed parameter. All R1 measurements, and in minimum at least five, were used for fitting to the model, and determining ECV and τic.
An increase of τic, e.g. as result of increasing cell dimensions, increases the curvature in the relation between the R1 in myocardial tissue and the R1 of blood, which provides the basis for detecting cardiomyocytesize changes. This is illustrated in Figure 1. Expansion of the myocardial interstitial space primarily affects the slope of the initially linear myocardial R1 curve, without direct effect on its curvature at higher R1 values. In the low R1 range, the relation between R1 in tissue and blood is linear because conditions of fast (transcytolemmal) exchange conditions prevail - the initial linear slope agrees with the widely used formula for calculation ECV from the change of R1 in tissue, divided by the corresponding change of R1 in blood.26, 32, 33.
Statistical Analyses
Continuous data were expressed as means ± standard deviation. Continuous variables were compared between groups of animals (e.g. L-NAME vs. placebo) by independent t-test, applied at each time point (baseline and 7-weeks). A paired t-test was used to compare measurements at baseline and follow-up within groups. Correlations were assessed by Pearson’s product moment correlation coefficient. For the TAC model, longitudinal changes of the CMR parameters, including τic and ECV, were analyzed with linear mixed-effects regression models (package “lme4”; URL http://lme4.r-forge.r-project.org/), with time since TAC as single predictor in each model, and including a random intercept component for each animal. The P-values for the fixed effects in the linear mixed-effects models were calculated by Markov chain Monte Carlo sampling (function pvals.fnc in package “languageR”, available at http://lib.stat.cmu.edu/R/CRAN/). Standard regression analysis was used to test the association of each histologic parameter with the time since TAC. Analyses were performed using SAS 9.3 (SAS Institute, Cary, NC) or R (version 2.15.1, R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/).
RESULTS
L-NAME and TAC induce significant LV hypertrophy
At baseline, control and L-NAME groups did not show any significant differences in body weight, blood pressure, heart rate, or CMR indices of LV structure and function (Table 1). Consistent with prior reports,16-18 the chronic administration of L-NAME led to a significant increase in the mean blood pressure (88±7 mmHg at baseline to 127±6mmHg at follow-up, P<0.001). After 7 weeks of treatment, the L-NAME and placebo-treated mice showed significant differences: LV mass increased in the L-NAME group (164±22mg vs. 96±14mg for placebo group, P<0.001; P<0.001 when indexed to body weight), and a systolic function decreased (LVEF = 61±3% vs. 50±8%, P<0.001).
Table 1.
Baseline and follow-up hemodynamic and CMR characteristics of control and L-NAME treated mice.
| Baseline | 7-week | |||||
|---|---|---|---|---|---|---|
| Characteristics (hemodynamic and CMR data) | Control (n=15) | L-NAME (n=18) | P-value* | Control (n=13) | L-NAME (n=17) | P-value* |
| Bodyweight [g] | 37.5 ± 2.7 | 37.7 ± 2.4 | 0.865 | 44.8 ± 4.5& | 40.0 ± 3.2& | 0.002 |
| Heart rate [bpm] | 492 ± 108 | 500 ± 74 | 0.827 | 403 ± 94 | 474 ± 70 | 0.41 |
| Mean blood pressure [mmHg] | 92 ± 7.3 | 88 ± 7.4 | 0.209 | 91 ± 8 | 126.7 ± 6.2& | <0.001 |
| LVEF [%] | 58 ± 3.9 | 58 ± 3.0 | 0.629 | 60.8 ± 3.1 | 50.1 ± 7.7$ | <0.001 |
| LVEDV [μl] | 130 ± 32 | 147 ± 32 | 0.187 | 111 ± 26 | 123 ± 37& | 0.23 |
| LVESV [μl] | 55 ± 14 | 61 ± 14 | 0.255 | 42 ± 11& | 61 ± 20 | 0.009 |
| LVmass [mg] | 97 ± 17 | 94 ± 12 | 0.615 | 96 ± 14 | 164 ± 22& | <0.001 |
| LVmass indexed to body weight [mg/g] | 2.5 ± 0.5 | 2.5 ± 0.4 | 0.592 | 2.2 ± 0.1& | 4.1 ± 0.5& | <0.001 |
| Myocardial extracellular volume fraction (MRI) | 0.26 ± 0.02 | 0.27 ± 0.03 | 0.677 | 0.25 ± 0.03 | 0.42 ± 0.08& | <0.001 |
| Intracellular life time of water [1/s] | 0.15 ± 0.07 | 0.17 ± 0.05 | 0.791 | 0.19 ± 0.07 | 0.44 ± 0.12& | <0.001 |
| Connective tissue fraction (histology), % | - | - | - | 2.6 ± 0.6 | 8.5 ± 1.6 | <0.001 |
| Dmaj [μm; major cell diameter] | - | - | - | 84.2 ± 3.6 | 90.4 ± 5.1 | 0.0012 |
| Dmin [μm; minor cell diameter] | - | - | - | 19.8 ± 0.9 | 26.2 ± 1.6 | <0.001 |
| Dmaj /Dmin | - | - | - | 4.26 ± 0.22 | 3.46 ± 0.15 | <0.001 |
| Cardiomyocyte volume by histology [104 × μm3] | - | - | - | 42.3 ± 6.0 | 78.1 ± 12.9 | <0.001 |
| Cell Surface Area [104μ m2] | - | - | - | 1.70 ± 0.14 | 2.41 ± 0.26 | <0.001 |
| Volume-to-Surface ratio (V/S) [μm] | - | - | - | 24.7 ± 1.4 | 32.2 ± 1.9 | <0.001 |
Abbreviations: LVEF=left ventricular ejection fraction, LVEDV=left ventricular end-diastolic volume, LVESV=left ventricular end-systolic volume, RVEF=right ventricular ejection fraction, RVEDV=right ventricular end-diastolic volume, RVESV=right ventricular end-systolic volume.
P-values for unpaired t-test of control vs. L-NAME mice;
P<0.05 for paired t-test of baseline vs. 7-week mice (control and L-NAME).
Table 2 summarizes the changes in cardiac structure and function between weeks 2 and 7 after TAC: TAC led to significant longitudinal changes for LV volumes, mass, function, ECV, and τic. Weight-indexed LV mass was significantly higher after TAC (P<0.001 for unpaired comparisons), and LV ejection fraction was lower (P<0.001 for unpaired comparisons), compared to the controls shown in Table 1, though these groups were not age-matched.
Table 2.
Hemodynamic and CMR characteristics of TAC mice at at 2 and 7 weeks post transverse aortic constriction.
| Hemodynamics and CMR data | 2 weeks post TAC | 7 weeks post TAC | P –value for effect of time post TAC |
|---|---|---|---|
| N=11; 14±1 days post TAC | N=4; 46 days post TAC | ||
| Weight [g] | 25.4±1.6 | 29.8±0.33 | <0.005 |
| LVEF [%] | 37±6 | 29±6 | <0.005 |
| LVEDV [μl] | 93±24 | 79±18 | <0.005 |
| LVESV [μl] | 59±20 | 57±13 | <0.005 |
| LV mass [mg] | 121±12 | 145±9 | <0.005 |
| LV mass index (body weight) [mg/g] | 4.7±0.3 | 4.9±0.4 | <0.005 |
| Myocardial extracellular volume fraction | 0.25±0.03 | 0.30±0.04 | <0.005 |
| Intracellular life time of water [1/s] | 0.22±0.05 | 0.49±0.18 | <0.005 |
| Histologic Measurements | (n=5) | (n=4) | |
| Dmaj [μm] | 87.1±1.1 | 88.5±1.0 | 0.155 |
| Dmin [μm] | 22.8±1.4 | 25.9±0.9 | 0.002 |
| Dmaj /Dmin | 3.83±0.24 | 3.43±0.13 | 0.008 |
| Connective tissue fraction (histology) | 0.023±0.007 | 0.032±0.009 | 0.0012 |
| Cardiomyocyte volume by histology[104 × μm3] | 56.7±7.4 | 73.4±5.1 | 0.0025 |
| Volume-to-Surface ratio (V/S) [μm] | 28.3±1.6 | 31.8±1.0 | 0.0014 |
Abbreviations: LVEF=left ventricular ejection fraction, LVEDV=left ventricular end-diastolic volume, LVESV=left ventricular end-systolic volume, RVEF=right ventricular ejection fraction, RVEDV=right ventricular end-diastolic volume, RVESV=right ventricular end-systolic volume.
The p-value refers to the coefficient for the linear trend of the displayed CMR or histologic index over time after TAC. For CMR measures, this involves repeated measurements for 11 mice at two time points, and a linear mixed effects model was used for each variable. For histology, the effect of time post TAC was tested by standard linear regression, as it only involved one measurement per mouse. (Mice necessarily had to be sacrificed for histologic assessment.)
Histologic assessment of cardiomyocyte hypertrophy
We measured the major (Dmaj) and minor (Dmin) cardiomyocyte dimensions and calculated cardiomyocyte volume, assuming a cell shape in the form of prolate ellipsoid (Figure 2).21-24 L-NAME induced a significant, but relatively modest change of Dmaj (P<0.001 vs. placebo), averaging 7%, and a mean 32% change for Dmin (P<0.001 vs. placebo; Table 1). The cardiomyocyte volume was 84% higher in mice treated with L-NAME versus placebo (78±13 × 104 μmm3 for L-NAME treated mice and 42±6 × 104 μmm3 for placebo, P<0.001). V/S was also higher in L-NAME vs. placebo-treated mice (Table 1).
Figure 2.

Cellular hypertrophy and interstitial fibrosis in L-NAME and TAC mice. Short-axis, mid-level LV sections of cardiac tissue from mice treated with placebo (panel A), L-NAME (panel B), and TAC (panel C). L-NAME and TAC are representative tissues after 7 weeks of exposure. Panels were stained with fluorescein isothiocyanate-conjugated (FITC-) wheat germ agglutinin to delineate cell membranes and scanned at a resolution equivalent to 1.0 μm/pixel to measure minor and major cell diameters in 15 fields within 4 myocardial segments. (A)-(B) are representative illustrations of larger short-axis diameters in the L-NAME and TAC groups relative to controls. Adjacent short-axis sections from the same mice, stained with Masson’s trichrome stains in (D)-(F) illustrate the absence of interstitial fibrosis in the control group and higher levels of interstitial fibrosis (blue) in L-NAME compared to TAC mice.
The histological measurements for mice with TAC are summarized in Table 2. The mice exposed to TAC had a significantly higher Dmin compared to controls, with Dmin reaching values similar to L-NAME treated mice.
In a combined analysis of cell sizes from all experimental groups, Dmin, but not Dmaj, was significantly different after TAC and treatment with L-NAME, compared to controls(P<0.001 for both TAC and L-NAME after 7 weeks as compared to controls). The histologic cardiomyocyte volume was significantly higher with longer exposure to TAC (57±7.4 104 × μmm3 at 2 weeks afterTAC vs. 73±2.5 104 × μmm3 for 7 weeks, P=0.006 for unpaired t-test), driven mostly by changes in Dmin. The histologic measurements showed that cardiomyocyte diameter (Dmin), cardiomyocyte volume, and volume-to-surface ratio were each significantly associated with time after TAC (Table 2).
Interstitial fibrosis and extracellular space expansion in L-NAME and TAC models
After 7 weeks of L-NAME treatment, the histologic connective tissue fraction was significantly higher than in placebo-treated mice (8.5±1.6% vs. 2.6±0.6%, P<0.001). In parallel, the myocardial ECV measured by CMR was also significantly higher for L-NAME versus placebo (0.42±0.08 vs, 0.25±0.03, P<0.001). Although mice exposed to TAC showed a significant increase in LV mass, they did not exhibit as marked an increase in histologic connective tissue fraction (2.3±0.1% for TAC at 7 weeks vs. 8.5±1.6% for L-NAME treated, P<0.001), or myocardial ECV (0.30±0.04 for TAC at 7 weeks vs. 0.42±0.08 for L-NAME, P=0.01) by 7 weeks (Table 2 and Figure 3).
Figure 3.

Intracellular lifetime of water and ECV in L-NAME mice. (A) The intracellular lifetime of water (τic) increased significantly in mice treated with L-NAME, and was significantly higher than in placebo-treated controls. (B) In mice exposed for 7 weeks to L-NAME the extracellular volume fraction increased also significantly, compared to baseline, and was also significantly higher than in placebo-treated controls.
Intracellular lifetime of water as a marker of cardiomyocyte hypertrophy
The τic determined by CMR, was significantly higher after 7 weeks of L-NAME, compared to placebo treatment (0.19±0.07 vs. 0.44±0.12, P<0.001; Table 1, Figure 3). In the TAC-group, τic increased significantly between 2 and 7 weeks after exposure to TAC (Table 2, and Figure 4). The rate of change of τic with time after TAC surgery was estimated to be 0.0581 s/week (P<0.002), using a linear mixed effects model for the repeated measurements of τic ranging from 2, to approximately 7 weeks post TAC. There was no significant difference for τic in mice after 7 weeks of L-NAME versus mice after 7 weeks of TAC (P=0.58)
Figure 4.

Longitudinal assessment of intracellular lifetime of water and cell size in mice exposed to TAC. A) Mice were imaged at approximately 2 weeks after transverse aortic constriction, and followed-up for up to 46 days before harvesting of the heart. Over this time the intracellular lifetime increased significantly (0.0581 s/week; P=0.0002) as illustrated by the solid line, calculated with a linear mixed effects (LME) model. Dashed lines connect repeated measurements within the same mouse. B) The volume-to-surface ratio calculated from the histologic major and minor cell diameters showed a significant association with the time between TAC and histological examination (0.79±0.18 μm/week; P=0.001). The solid line represents the linear regression line estimate for all mice, based on the fixed effects in the LME model.
When pooling all τic values from CMR with time-matched histologic data, τic demonstrated a strong positive association with cardiomyocyte volume-to-surface ratio (r=0.78, P<0.001; Figure 5). The correlation of τic with cell-volume was r=0.75 (P<0.001), with median minor cell diameter r=0.79 (P<0.001), and with the median major cell diameter r=0.43 (P=0.006). τic also demonstrated an inverse association with LVEF (r=-0.36 P=0.002).
Figure 5.

Association between intracellular lifetime of water and cell volume-to-surface ratio. For a diffusion-based exchange the intracellular lifetime of water is expected to be proportional to the cellular volume-to-surface ratio. The volume-to-surface ratio (V/S) was highly correlated (r=0.78; P<0.001) with the intracellular lifetime of water obtained from the T1 measurements before and after administration of an extracellular gadolinium contrast agent.
DISCUSSION
This study validates a novel T1-based CMR technique to detect and quantify changes in cardiomyocyte hypertrophy. Serial measurements of τic and myocardial ECV allowed the non-invasive identification of distinct but complementary aspects of myocardial remodeling at the cellular level. Specifically, τic was strongly associated with the histological volume-to-surface ratio, a measure of the characteristic cell size, and minor cell diameter. Not unexpectedly, the correlation of τic with the major cell diameter was much weaker, and this parameter was also not well suited to differentiate between normal and hypertrophied cardiomyocytes on histology. Our results suggest that development of interstitial fibrosis and cardiomyocyte hypertrophy can be temporally distinct and followed non-invasively. This is the first demonstration of the ability to track cardiomyocyte hypertrophy in vivo non-invasively. It could be used in conjunction with more established applications of T1 mapping by CMR to facilitate earlier detection of pathologic hypertrophy and assess myocardial remodeling in response to therapeutic interventions.
Post-contrast T1 relaxation time measurements have been used in both animals27, 34 and patients35 with hypertension as an index of pathologic diffuse interstitial expansion. In aortic stenosis, (1-myocardial ECV fraction) was reported as an index of cell volume fraction:36 it represents a combination of cell volume and density, rather than a direct measure of cellular hypertrophy. Our results suggest that myocardial interstitial expansion (e.g., myocardial ECV) and cardiomyocyte hypertrophy represent distinct characteristics of tissue structure, which can be measured serially by CMR. In mice after TAC, we demonstrate that cardiomyocyte hypertrophy may precede interstitial fibrosis, underlining the distinction between ECV and τic.
From a biological perspective, increases in cardiac mass can result from interstitial matrix expansion (e.g., fibrosis or aberrant protein deposition) and/or increases in cardiomyocyte volume.37 A differentiation between “physiologic” and “pathologic” hypertrophy may therefore require characterization of not only wall thickness and LV mass, but more specifically interstitial matrix expansion and cardiomyocyte cell size. In states of pathologic hypertrophy in response to pressure overload, an increase in cardiomyocyte cell size has been considered an early and conserved hallmark, putatively occurring before the onset of irreversible myocardial fibrosis and subsequent ventricular dysfunction, remodeling, and HF.11-13 In fact, mechanical stress has been associated with activation of a pro-fibrotic, pro-hypertrophic genetic program that may reinforce subsequent HF.38-41 In turn, increases in interstitial fibrosis appear to mark the transition from compensated cellular and organ-level pathologic hypertrophy to HF.11, 42, 43 In patients with LVH at risk for HF, alterations in the balance of collagen metabolism (as reflected by increases in matrix metalloproteinases and pro-collagen fragments) identify patients with clinical HF.44-46 Early intervention prior to development of overt fibrosis and myocardial dysfunction, may ameliorate the progression to overt HF.47
The proposed method has potential limitations: It is assumed that the cytolemmal permeability coefficient remains constant with the development of cell hypertrophy. Ischemic conditions could alter cell membrane permeability, and decrease active, ATP-dependent water transport across the plasma membrane.48 However it is unlikely that ischemia was a confounding factor in this study, based on the absence of apparent infarction by late-gadolinium enhancement.48 Other cardiac resident cell types (e.g. fibroblasts) may bias or impair the detection of cardiomyocyte hypertrophy, but in viable myocardium the volume fraction of connective tissue and the percentage of fibroblast volume are relatively small compared to the cardiomyocyte volume. The analysis of myocardial T1 and τic were based on transmural myocardial signal intensity averages. Because of the transmural variation of wall stress, it is conceivable that cardiomyocyte hypertrophy varies between endo- and epi-cardial layers. The spatial resolution of our T1 measurements in mice, and the use of a cine technique were not suitable for investigating any transmural variation of cell hypertrophy in this study. Mice in the TAC group were not imaged before TAC surgery due to restrictions on taking mice out of the animal facility before surgery. Our results, though limited to models of pressure overload, may also be applicable to eccentric hypertrophy. In human heart samples the cell diameter changes significantly in both concentric and eccentric hypertrophy (by 80% and 40%, respectively)49. Similarly, in animal models of pressure overload (concentric remodeling) and volume overload (predominantly eccentric remodeling), the cell diameter changed to a similar degree 50. We empirically observe that τic is most sensitive to changes in minor cell dimension (cell diameter), suggesting that τic may be able to track changes in cell size in cases of eccentric hypertrophy. These empirical observations are in agreement with the theoretical prediction that τic changes in proportion to the volume-to-surface ratio (V/S)29. The V/S for a cardiomyocyte with a length-to-diameter ratio ~ 4:1 has a higher sensitivity to changes in cell diameter, than to changes in cell length, consistent with the stronger correlation of τic with Dmin than with Dmaj. An expected limitation is a lack of sensitivity to changes in cell length, expected with the development of eccentric hypertrophy, or variations in cardiomyocyte shape, such as cylindrical versus ellipsoidal. The application of τic to eccentric hypertrophy will require further validation.
In conclusion, this study validates a non-invasive, T1-based CMR method for the assessment of cardiomyocyte hypertrophy. In models of pressure-overload HF, CMR distinguished cellular hypertrophy, characterizing the early tissue phenotype in TAC, and extracellular space expansion, a hallmark of chronic pressure overload. Ultimately, these results suggest a role for CMR as a non-invasive tool to quantify two critical aspects of early myocardial remodeling at the transition between compensated hypertrophy and clinical HF.
Acknowledgments
We would like to thank Deborah Burstein, PhD, director of the Beth Israel Deaconess Small Animal Imaging Facility, and Reza Akhavan, MS, for their support.
Funding Sources: The Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R01HL090634. Drs. Coelho-Filho and Shah are supported by Post-Doctoral Fellowships from the American Heart Association (AHA 11POST5550053 to OCF; AHA 11POST110033 to RVS). Dr. Neilan is supported by an American Heart Association Fellow-to-Faculty grant (12FTF12060588).
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Berk BC, Fujiwara K, Lehoux S. Ecm remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327–334. doi: 10.1161/CIRCULATIONAHA.108.845792. [DOI] [PubMed] [Google Scholar]
- 3.Devereux RB, Dahlof B, Levy D, Pfeffer MA. Comparison of enalapril versus nifedipine to decrease left ventricular hypertrophy in systemic hypertension (the preserve trial) Am J Cardiol. 1996;78:61–65. doi: 10.1016/s0002-9149(96)00228-7. [DOI] [PubMed] [Google Scholar]
- 4.Dweck MR, Joshi S, Murigu T, Gulati A, Alpendurada F, Jabbour A, Maceira A, Roussin I, Northridge DB, Kilner PJ, Cook SA, Boon NA, Pepper JR, Mohiaddin RH, Newby DE, Pennell DJ, Prasad SK. Left ventricular remodelling and hypertrophy in patients with aortic stenosis: Insights from cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:50. doi: 10.1186/1532-429X-14-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma S, Maron BJ, Whyte G, Firoozi S, Elliott PM, McKenna WJ. Physiologic limits of left ventricular hypertrophy in elite junior athletes: Relevance to differential diagnosis of athlete’s heart and hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40:1431–1436. doi: 10.1016/s0735-1097(02)02270-2. [DOI] [PubMed] [Google Scholar]
- 6.Devereux RB, Dahlof B, Gerdts E, Boman K, Nieminen MS, Papademetriou V, Rokkedal J, Harris KE, Edelman JM, Wachtell K. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: The losartan intervention for endpoint reduction in hypertension (life) trial. Circulation. 2004;110:1456–1462. doi: 10.1161/01.CIR.0000141573.44737.5A. [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB, Gordon T, Offutt D. Left ventricular hypertrophy by electrocardiogram. Prevalence, incidence, and mortality in the framingham study. Ann Intern Med. 1969;71:89–105. doi: 10.7326/0003-4819-71-1-89. [DOI] [PubMed] [Google Scholar]
- 8.Larstorp AC, Okin PM, Devereux RB, Olsen MH, Ibsen H, Dahlof B, Kjeldsen SE, Wachtell K. Regression of ecg-lvh is associated with lower risk of new-onset heart failure and mortality in patients with isolated systolic hypertension; the life study. Am J Hypertens. 2012;25:1101–9. doi: 10.1038/ajh.2012.86. [DOI] [PubMed] [Google Scholar]
- 9.Lindholm LH, Ibsen H, Dahlof B, Devereux RB, Beevers G, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Kristiansson K, Lederballe-Pedersen O, Nieminen MS, Omvik P, Oparil S, Wedel H, Aurup P, Edelman J, Snapinn S. Cardiovascular morbidity and mortality in patients with diabetes in the losartan intervention for endpoint reduction in hypertension study (life): A randomised trial against atenolol. Lancet. 2002;359:1004–1010. doi: 10.1016/S0140-6736(02)08090-X. [DOI] [PubMed] [Google Scholar]
- 10.Olivetti G, Melissari M, Balbi T, Quaini F, Cigola E, Sonnenblick EH, Anversa P. Myocyte cellular hypertrophy is responsible for ventricular remodelling in the hypertrophied heart of middle aged individuals in the absence of cardiac failure. Cardiovasc Res. 1994;28:1199–1208. doi: 10.1093/cvr/28.8.1199. [DOI] [PubMed] [Google Scholar]
- 11.Boluyt MO, O’Neill L, Meredith AL, Bing OH, Brooks WW, Conrad CH, Crow MT, Lakatta EG. Alterations in cardiac gene expression during the transition from stable hypertrophy to heart failure. Marked upregulation of genes encoding extracellular matrix components. Circ Res. 1994;75:23–32. doi: 10.1161/01.res.75.1.23. [DOI] [PubMed] [Google Scholar]
- 12.Lorell BH, Carabello BA. Left ventricular hypertrophy: Pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. doi: 10.1161/01.cir.102.4.470. [DOI] [PubMed] [Google Scholar]
- 13.Scimia MC, Hurtado C, Ray S, Metzler S, Wei K, Wang J, Woods CE, Purcell NH, Catalucci D, Akasaka T, Bueno OF, Vlasuk GP, Kaliman P, Bodmer R, Smith LH, Ashley E, Mercola M, Brown JH, Ruiz-Lozano P. Apj acts as a dual receptor in cardiac hypertrophy. Nature. 2012;488:394–8. doi: 10.1038/nature11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Simone G, de Divitiis O. Extracellular matrix and left ventricular mechanics in overload hypertrophy. Adv Clin Path. 2002;6:3–10. [PubMed] [Google Scholar]
- 15.Iwanaga Y, Aoyama T, Kihara Y, Onozawa Y, Yoneda T, Sasayama S. Excessive activation of matrix metalloproteinases coincides with left ventricular remodeling during transition from hypertrophy to heart failure in hypertensive rats. J Am Coll Cardiol. 2002;39:1384–1391. doi: 10.1016/s0735-1097(02)01756-4. [DOI] [PubMed] [Google Scholar]
- 16.Arnal JF, el Amrani AI, Chatellier G, Menard J, Michel JB. Cardiac weight in hypertension induced by nitric oxide synthase blockade. Hypertension. 1993;22:380–387. doi: 10.1161/01.hyp.22.3.380. [DOI] [PubMed] [Google Scholar]
- 17.Moreno H, Jr, Metze K, Bento AC, Antunes E, Zatz R, de Nucci G. Chronic nitric oxide inhibition as a model of hypertensive heart muscle disease. Basic Res Cardiol. 1996;91:248–255. doi: 10.1007/BF00788911. [DOI] [PubMed] [Google Scholar]
- 18.Pechanova O, Bernatova I, Pelouch V, Babal P. L-name-induced protein remodeling and fibrosis in the rat heart. Physiol Res. 1999;48:353–362. [PubMed] [Google Scholar]
- 19.Xiao CY, Chen M, Zsengeller Z, Li H, Kiss L, Kollai M, Szabo C. Poly(adp-ribose) polymerase promotes cardiac remodeling, contractile failure, and translocation of apoptosis-inducing factor in a murine experimental model of aortic banding and heart failure. J Pharmacol Exp Ther. 2005;312:891–898. doi: 10.1124/jpet.104.077164. [DOI] [PubMed] [Google Scholar]
- 20.Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, Hewett TE, Breit SN, Molkentin JD. Gdf15/mic-1 functions as a protective and antihypertrophic factor released from the myocardium in association with smad protein activation. Circ Res. 2006;98:342–350. doi: 10.1161/01.RES.0000202804.84885.d0. [DOI] [PubMed] [Google Scholar]
- 21.Yaniv Y, Juhaszova M, Wang S, Fishbein KW, Zorov DB, Sollott SJ. Analysis of mitochondrial 3d-deformation in cardiomyocytes during active contraction reveals passive structural anisotropy of orthogonal short axes. PLoS One. 2011;6:e21985. doi: 10.1371/journal.pone.0021985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas TA, Kuzman JA, Anderson BE, Andersen SM, Schlenker EH, Holder MS, Gerdes AM. Thyroid hormones induce unique and potentially beneficial changes in cardiac myocyte shape in hypertensive rats near heart failure. Am J Physiol Heart Circ Physiol. 2005;288:H2118–2122. doi: 10.1152/ajpheart.01000.2004. [DOI] [PubMed] [Google Scholar]
- 23.Boyett MR, Frampton JE, Kirby MS. The length, width and volume of isolated rat and ferret ventricular myocytes during twitch contractions and changes in osmotic strength. Exp Physiol. 1991;76:259–270. doi: 10.1113/expphysiol.1991.sp003492. [DOI] [PubMed] [Google Scholar]
- 24.Sorenson AL, Tepper D, Sonnenblick EH, Robinson TF, Capasso JM. Size and shape of enzymatically isolated ventricular myocytes from rats and cardiomyopathic hamsters. Cardiovasc Res. 1985;19:793–799. doi: 10.1093/cvr/19.12.793. [DOI] [PubMed] [Google Scholar]
- 25.Coelho-Filho OR, Mongeon FP, Mitchell R, Moreno H, Jr, Nadruz W, Jr, Kwong R, Jerosch-Herold M. The role of transcytolemmal water exchange in magnetic resonance measurements of diffuse myocardial fibrosis in hypertensive heart disease. Circ Cardiovasc Imaging. 2013;6:134–41. doi: 10.1161/CIRCIMAGING.112.979815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: Preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 27.Messroghli DR, Nordmeyer S, Buehrer M, Kozerke S, Dietrich T, Kaschina E, Becher PM, Hucko T, Berger F, Klein C, Kuehne T. Small animal look-locker inversion recovery (salli) for simultaneous generation of cardiac t1 maps and cine and inversion recovery-prepared images at high heart rates: Initial experience. Radiology. 2011;261:258–265. doi: 10.1148/radiol.11101943. [DOI] [PubMed] [Google Scholar]
- 28.Deichmann R, Haase A. Quantification of t1 values by snapshot-flash nmr imaging. J Magn Reson. 1992;96:608–612. [Google Scholar]
- 29.Landis CS, Li X, Telang FW, Molina PE, Palyka I, Vetek G, Springer CS., Jr Equilibrium transcytolemmal water-exchange kinetics in skeletal muscle in vivo. Magn Reson Med. 1999;42:467–478. doi: 10.1002/(sici)1522-2594(199909)42:3<467::aid-mrm9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 30.Yankeelov TE, Rooney WD, Huang W, Dyke JP, Li X, Tudorica A, Lee JH, Koutcher JA, Springer CS., Jr Evidence for shutter-speed variation in cr bolus-tracking studies of human pathology. NMR Biomed. 2005;18:173–185. doi: 10.1002/nbm.938. [DOI] [PubMed] [Google Scholar]
- 31.Landis CS, Li X, Telang FW, Coderre JA, Micca PL, Rooney WD, Latour LL, Vetek G, Palyka I, Springer CS., Jr Determination of the mri contrast agent concentration time course in vivo following bolus injection: Effect of equilibrium transcytolemmal water exchange. Magn Reson Med. 2000;44:563–574. doi: 10.1002/1522-2594(200010)44:4<563::aid-mrm10>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 32.Jerosch-Herold M, Sheridan D, Kushner JD, Nauman DJ, Burgess D, Dutton D, Hershberger RE. Cardiac magnetic resonance contrast enhancement differentiates patients affected with familial dilated cardiomyopathy from asymptomatic relatives. J Cardiovasc Magn Reson. 2006;8:154–155. [Google Scholar]
- 33.Schelbert EB, Testa SM, Meier CG, Ceyrolles WJ, Levenson JE, Blair AJ, Kellman P, Jones BL, Ludwig DR, Schwartzman D, Shroff SG, Wong TC. Myocardial extravascular extracellular volume fraction measurement by gadolinium cardiovascular magnetic resonance in humans: Slow infusion versus bolus. J Cardiovasc Magn Reson. 2011;13:16. doi: 10.1186/1532-429X-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Messroghli DR, Nordmeyer S, Dietrich T, Dirsch O, Kaschina E, Savvatis K, Oh-I D, Klein C, Berger F, Kuehne T. Assessment of diffuse myocardial fibrosis in rats using small-animal look-locker inversion recovery t1 mapping. Circ Cardiovasc Imaging. 2011;4:636–640. doi: 10.1161/CIRCIMAGING.111.966796. [DOI] [PubMed] [Google Scholar]
- 35.Ugander M, Oki AJ, Hsu LY, Kellman P, Greiser A, Aletras AH, Sibley CT, Chen MY, Bandettini WP, Arai AE. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J. 2012;33:1268–1278. doi: 10.1093/eurheartj/ehr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flett AS, Sado DM, Quarta G, Mirabel M, Pellerin D, Herrey AS, Hausenloy DJ, Ariti C, Yap J, Kolvekar S, Taylor AM, Moon JC. Diffuse myocardial fibrosis in severe aortic stenosis: An equilibrium contrast cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging. 2012;13:819–826. doi: 10.1093/ehjci/jes102. [DOI] [PubMed] [Google Scholar]
- 37.Dorn GW, 2nd, Robbins J, Sugden PH. Phenotyping hypertrophy: Eschew obfuscation. Circ Res. 2003;92:1171–1175. doi: 10.1161/01.RES.0000077012.11088.BC. [DOI] [PubMed] [Google Scholar]
- 38.Adams JW, Sakata Y, Davis MG, Sah VP, Wang Y, Liggett SB, Chien KR, Brown JH, Dorn GW., 2nd Enhanced galphaq signaling: A common pathway mediates cardiac hypertrophy and apoptotic heart failure. PNAS. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hein S, Arnon E, Kostin S, Schonburg M, Elsasser A, Polyakova V, Bauer EP, Klovekorn WP, Schaper J. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: Structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–991. doi: 10.1161/01.cir.0000051865.66123.b7. [DOI] [PubMed] [Google Scholar]
- 40.Krayenbuehl HP, Hess OM, Monrad ES, Schneider J, Mall G, Turina M. Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation. 1989;79:744–755. doi: 10.1161/01.cir.79.4.744. [DOI] [PubMed] [Google Scholar]
- 41.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. Il-33 and st2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohammed SF, Ohtani T, Korinek J, Lam CS, Larsen K, Simari RD, Valencik ML, Burnett JC, Jr, Redfield MM. Mineralocorticoid accelerates transition to heart failure with preserved ejection fraction via “nongenomic effects”. Circulation. 2010;122:370–378. doi: 10.1161/CIRCULATIONAHA.109.915215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shapiro BP, Owan TE, Mohammed S, Kruger M, Linke WA, Burnett JC, Jr, Redfield MM. Mineralocorticoid signaling in transition to heart failure with normal ejection fraction. Hypertension. 2008;51:289–295. doi: 10.1161/HYPERTENSIONAHA.107.099010. [DOI] [PubMed] [Google Scholar]
- 44.Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: Relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–2096. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 45.Martos R, Baugh J, Ledwidge M, O’Loughlin C, Murphy NF, Conlon C, Patle A, Donnelly SC, McDonald K. Diagnosis of heart failure with preserved ejection fraction: Improved accuracy with the use of markers of collagen turnover. Eur J Heart Fail. 2009;11:191–197. doi: 10.1093/eurjhf/hfn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Post WS, Larson MG, Myers RH, Galderisi M, Levy D. Heritability of left ventricular mass: The framingham heart study. Hypertension. 1997;30:1025–1028. doi: 10.1161/01.hyp.30.5.1025. [DOI] [PubMed] [Google Scholar]
- 47.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. [Google Scholar]
- 48.Zhang Y, Poirier-Quinot M, Springer CS, Jr, Balschi JA. Active trans-plasma membrane water cycling in yeast is revealed by nmr. Biophys J. 2011;101:2833–2842. doi: 10.1016/j.bpj.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sawada K, Kawamura K. Architecture of myocardial cells in human cardiac ventricles with concentric and eccentric hypertrophy as demonstrated by quantitative scanning electron microscopy. Heart Vessels. 1991;6:129–142. doi: 10.1007/BF02058278. [DOI] [PubMed] [Google Scholar]
- 50.Toischer K, Rokita AG, Unsold B, Zhu W, Kararigas G, Sossalla S, Reuter SP, Becker A, Teucher N, Seidler T, Grebe C, Preuss L, Gupta SN, Schmidt K, Lehnart SE, Kruger M, Linke WA, Backs J, Regitz-Zagrosek V, Schafer K, Field LJ, Maier LS, Hasenfuss G. Differential cardiac remodeling in preload versus afterload. Circulation. 2010;122:993–1003. doi: 10.1161/CIRCULATIONAHA.110.943431. [DOI] [PMC free article] [PubMed] [Google Scholar]