Abstract
Objective
TCF7L2 variant rs7903146 is associated with increased risk for Type 2 diabetes. We investigated the effect of TCF7L2 variant rs7903146 and glucose tolerance on free fatty acid (FFA) metabolism.
Research design and methods
We recruited 120 individuals, half homozygous for the major CC allele and half homozygous for the minor TT allele at rs7903146; each underwent a 2-hour, 75g oral glucose tolerance test (OGTT). Plasma glucose, insulin and free fatty acid concentrations were measured on blood collected before and during the OGTT.
Results
Total FFA concentrations and percent FA species during OGTT were not different in CC and TT carriers when males and females were considered together. However, monounsaturated fatty acid (MUFA) concentrations and percentages were greater in TT than CC females during the OGTT. TT carriers with high HOMA-IR had significantly greater fasting FFA concentrations, lower disposition index (DI) and greater AUC of glucose than high HOMAIR CC carriers, whereas no such differences were observed in the low HOMA-IR group. We found that fasting (826 ± 25 vs. 634 ± 22 µmol/L, P < 0.0001) and OGTT plasma FFA concentrations were greater in IGT than NGT subjects, and the difference remained after adjusting for sex, age, BMI, and genotype. Finally, IGT subjects had greater MUFA concentrations and percentages than NGT subjects during OGTT.
Conclusions
Despite similar fasting insulin and glucose, fasting plasma FFA are greater in IGT than NGT adults. Insulin resistance and sex influence plasma FFA responses amongst carriers of the minor T allele of TCF7L2 rs7903146.
Keywords: prediabetes, insulin secretion, insulin action, fatty acids, free fatty acids
INTRODUCTION
Genome-wide association studies have established the TCF7L2 variant rs7903146, which is located in a noncoding region, as the strongest association with type 2 diabetes of all known single nucleotide polymorphisms (SNP) [1]. At the individual level, carrying the TCF7L2 risk allele increases the likelihood of type 2 diabetes by 50% and the population attributable risk is between 10–25% [2]. The mechanism by which this non-coding variant increases the risk of type 2 diabetes is still under investigation. Some studies indicate that the risk T allele is associated with impaired β-cell function. Indeed, cell culture studies, animal models and investigation of humans support a role of TCF7L2 in β-cell function and islet morphology [3–5].
In addition to pancreatic islets, TCF7L2 is highly expressed in a variety of glucose sensing and metabolizing tissues in human, including liver, brain, omental and subcutaneous adipose tissue [6–8]. It is a prominent transcription factor involved in Wnt signaling pathway. The Wnt pathway plays a crucial role in cell proliferation, differentiation, apoptosis, as well as in the maintenance of tissue homeostasis and metabolic processes [9]. In adipose tissue, TCF7L2 is known to have an effect on preadipocyte differentiation and inflammatory status [10]. The link between TCF7L2 and adipocyte metabolism was highlighted by a report that TCF7L2 expression decreases in subcutaneous adipose tissue from NGT obese T/T carriers under calorie restriction and that its effect on type 2 diabetes risk is modulated by obesity [11]. However few studies have evaluated the influence of TCF7L2 variant rs7903146 on lipid metabolism.
Greater plasma free fatty acid (FFA) concentrations are an important risk factor for the development of insulin resistance, beta-cell dysfunction, and type 2 diabetes. Increased FFA can inhibit glucose uptake and oxidation in muscle in healthy humans [12] and impair the suppression of hepatic glucose production by insulin [13]. Also, FFA are lipotoxic to beta cells and when exposed to prolonged elevation of FFA exhibit impaired insulin secretion [14]. Because saturated, mono- and polyunsaturated FFA may have different effects on insulin sensitivity [15], insulin secretion [16] and other metabolic pathways, and because interactions between the TCF7L2 genotype and plasma concentrations of saturated fatty acids with regards to impaired insulin secretion and action have been reported [17], we tested whether the fatty acid species differ between TCF7L2 genotypes. In this study we examined the relationship between the TCF7L2 variant rs7903146 in humans and FFA concentrations in response to an oral glucose tolerance test.
RESEARCH DESIGN AND METHODS
Subjects
The design of this study has been previously reported [18]. In brief, this Mayo Clinic IRB approved protocol utilized the Mayo Clinic Biobank repository to genotype 4000 randomly selected individuals at rs7903146. Samples were selected from adults 20–70 years old with no history of diabetes and who resided within a 100 mile radius of Mayo Clinic, Rochester, MN. Individuals homozygous for the major TT allele and homozygous for the minor CC allele who were matched for age, sex, fasting glucose and bodyweight were invited in writing to participate in the study. Persons taking medications that could affect glucose metabolism, who had a history of chronic illness or who had undergone upper gastrointestinal surgery were excluded. After obtaining written, informed consent we performed a 2–hour 75g oral glucose tolerance test (OGTT) after an overnight fast to define their glucose tolerance status. Fat free mass (FFM) and fat mass were measured using dual-energy X-ray absorptiometry (iDXA, GE, Wauwatosa, WI).
Analytical techniques
Genotyping of the rs7903146 SNP was done using Taqman (Applied Biosystems Inc., Foster City, CA). Blood samples from the OGTT were placed on ice, centrifuged at 4°C to separate plasma and stored at −20°C until assayed. Glucose and insulin concentrations were measured using a glucose oxidase method (Yellow Springs Instruments, Yellow Springs, OH) and a chemiluminescence assay (Access Assay; Beckman, Chaska, MN), respectively. Plasma free fatty acid (FFA) concentrations were measured as previously described [19]. We measured the concentrations of the C14:0, C16:0, C18:0 saturated fatty acids (SFA), the C16:1n-7, C18:1n-9 cis-monounsaturated fatty acids (MUFA), the C18:2n-6, C18:3n-3, C20:5n-3, C20:4n-6, C22:6n-3 polyunsaturated fatty acids (PUFA) and C16:1 trans-9 and C18:1 trans-9 trans-fatty acids. The percentages of fatty acids subgroups (%SFA, %MUFA, %PUFA) were also calculated.
Calculations
Area under curve above basal (AAB) was calculated using the trapezoidal rule. Homeostasis model assessment of insulin resistance (HOMA-IR) was derived from fasting glucose and insulin levels [(fasting plasma glucose × fasting serum insulin)/22.5]. Net insulin action (SI) was measured using the oral minimal model [20]. To estimate the stearoyl-CoA desaturation -1 (SCD1) activity we used product: precursor ratio (18:1 n-9/18:0 or 16:1 n-7/16:0) as previously described [21].
Statistical analyses
Biochemical variables were assessed for normality of distribution to determine whether parametric or non-parametric analyses were appropriate. Anthropometric data and clinical chemical parameters of two groups were compared using a non-paired t-test if normally distributed and a Wilcoxon test if not normally distributed. Age, sex, BMI and genotype were used as covariates in ANOVA models as indicated. Glucose, insulin and FFA concentrations were compared using repeated-measures ANOVA for the overall differences during the OGTT. A p-value of less than 0.05 was considered significant. Statistical tests were performed with SPSS version 19.0 for Windows (SPSS Inc., Chicago, IL). Data are presented as Means ± SD unless otherwise stated.
RESULTS
Subject characteristics
The subjects’ characteristics are provided in Table 1. The subjects in each genotype group were well-matched for sex, age, BMI and FFM. There were no significant differences in fasting plasma glucose or insulin concentrations between CC and TT carriers.
Table 1.
Subjects characteristics, plasma glucose and insulin concentrations in TCF7L2 rs7903146 SNP participants
| total subjects | males | females | ||||
|---|---|---|---|---|---|---|
| CC (n=60) |
TT (n=60) |
CC (n=22) |
TT (n=23) |
CC (n=38) |
TT (n=37) |
|
| Sex (M/F) | 22/38 | 23/37 | / | / | / | / |
| Age (years) | 41±14 | 42±15 | 39±14 | 41±16 | 42±14 | 43±14 |
| BMI (kg/m2) | 27.4±3.9 | 27.3±4.4 | 27.4±2.7 | 27.3±3.4 | 27.3±4.5 | 27.3±4.9 |
| FFM (kg) | 46.7±10.8 | 47.1±13.8 | 55.6±8.1 | 56.4±15.2 | 41.5±8.5 | 41.4±8.9 |
| Fasting glucose (mmol/l) |
5.4±0.4 | 5.4±0.5 | 5.5±0.5 | 5.5±0.7 | 5.3±0.4 | 5.4±0.5 |
| 60min glucose (mmol/l) |
9.1±1.8 | 10.3±2.1** | 9.1±2.0 | 10.2±2.7 | 9.2±1.7 | 10.3±1.8** |
| 120min glucose (mmol/l) |
7.6±1.4 | 8.2±2.0 | 7.0±1.2 | 7.6±2.0 | 8.0±1.4 | 8.5±1.9 |
| Fasting insulin (pmol/l) |
35±22 | 30±16 | 31±18 | 27±17 | 38±25 | 32±16 |
| 120min insulin (pmol/l) |
225±187 | 222±154 | 152±110 | 146±98 | 267±209 | 270±164 |
| AAB glucose (mmol/l·120min) |
357±119 | 432±148** | 328±124 | 410±154 | 373±115 | 445±145* |
| AAB insulin (nmol/l·120min) |
27.7±15.7 | 26.4±13.1 | 24.5±15.5 | 21.6±11.5 | 29.5±15.7 | 29.3±13.3 |
BMI - body mass index; FFM – fat free mass; AAB – area under curve above basal.
CC and TT refer to genotypes at rs7903146 SNP of TCF7L2.
Note:
- TT vs CC P<0.05,
- TT vs CC P<0.01.
Influence of CC and TT genotype on plasma glucose, insulin and FFA concentrations
As previously reported (18), in response to the OGTT, the 60 min plasma glucose concentrations and plasma glucose AAB (both P < 0.01 adjusted by age, sex, BMI) were greater in TT than CC carriers. The OGTT-stimulated insulin concentrations at 30, 60 and 120 min and the AAB (Table1) of insulin did not differ between CC and TT carriers. The 60 min plasma glucose and plasma glucose AAB concentrations were greater in both female and male TT than CC carriers (Table 1), although the difference was statistically significant only for females (Table 1). Twenty-five of the 60 CC genotype participants and 30 of the 60 TT genotype participants had impaired glucose tolerance (IGT).
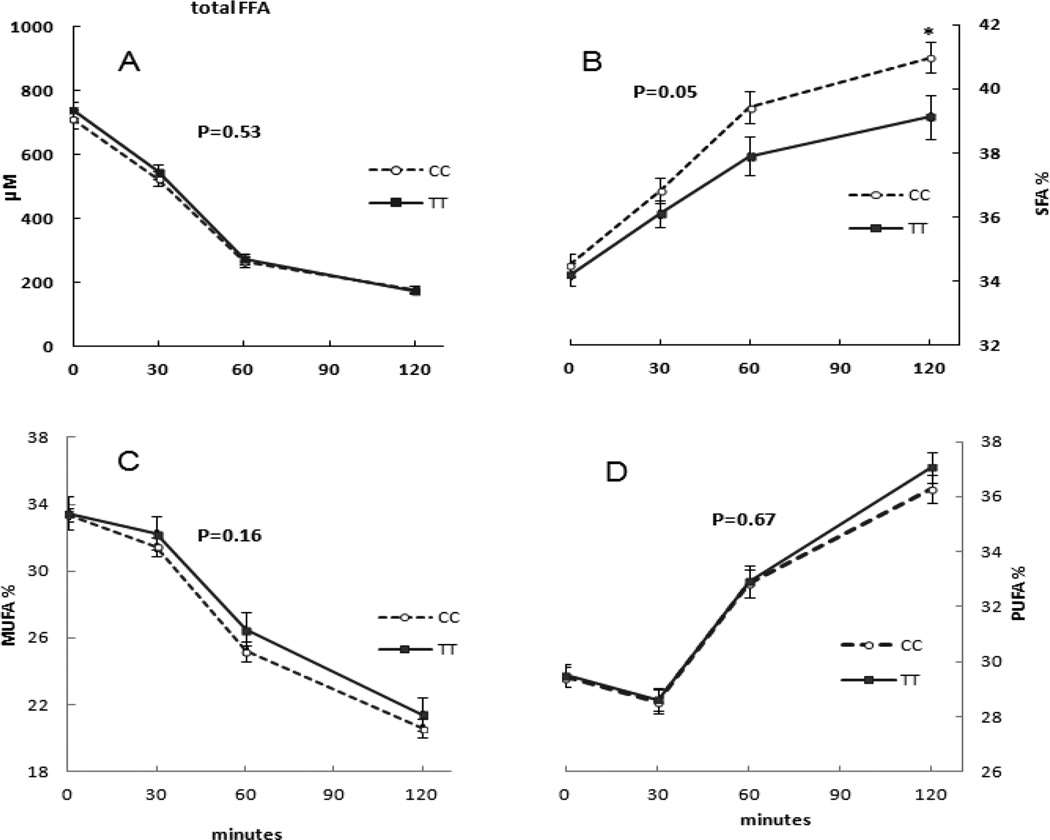
The baseline total FFA concentrations and the %SFA, %MUFA, %PUFA were not different between CC and TT carriers. Furthermore, there were no statistically significant differences in the patterns of total FFA concentrations between the two groups during the OGTT (Figure 1, panel A).
Figure 1.
The influence of TCF7L2 rs7903146 genotype on plasma FFA concentrations and fatty acid composition during the OGTT. There was not a statistically significant difference of total FFA concentrations and fatty acids composition between CC and TT carriers (P values by repeated-measures ANOVA).
For all subjects combined, the % SFA increased from 35 ± 3% (0 min) to 41 ± 5 (120 min) after glucose ingestion, the % MUFA decreased from 34 ± 3% (0 min) to 22 ± 4% (120 min), and the % PUFA increased from 30 ± 3% (0 min) to 37 ± 4% (120 min), (all P<0.001). There were no significant differences in these % changes in FA species during OGTT between CC and TT carriers when males and females were considered together (Figure 1).
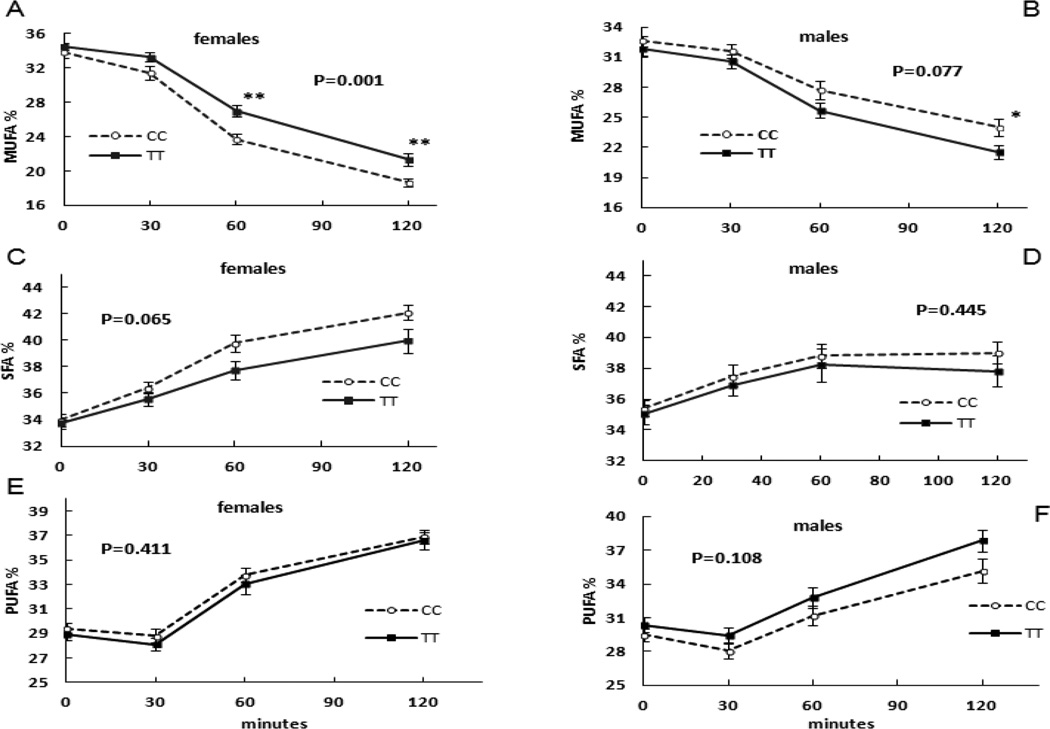
However, there were significant differences in % MUFA (P=0.001, Figure 2, panel A) and MUFA concentrations (P=0.02) between CC and TT females during OGTT. Repeated-measures ANOVA indicated that TT women had greater % MUFA and greater MUFA concentrations than CC women during OGTT. The % MUFA was subtly, but significantly greater in TT than CC females at 30 min (33 ± 3% vs. 31 ± 5%, P=0.05), 60 min (27 ± 4% vs. 24 ± 4%, P<0.001) and at 120 min (21 ± 5% vs. 19 ± 3%, P=0.004) by non-paired t-test. The MUFA concentrations at min 30 and 60 were significantly greater in TT than CC females. By repeated-measures ANOVA the difference in % MUFA between TT and CC males during OGTT was not statistically significant (Figure 2, panel B). The differences in % SFA between TT and CC genotype in females or males did not reach statistical significance by repeated-measures ANOVA (Figure 2, Panel C and D). Differences in % plasma MUFA between CC and TT women were due to the differences in oleic acid; plasma palmitoleic acid was not different between CC and TT females. The C18:1/C18:0 ratio in TT women was greater than that of CC women at 60,120 min of OGTT, but no differences in C16:1/C16:0 ratio.
Figure 2.
The influence of TCF7L2 rs7903146 genotype on plasma fatty acids concentrations during OGTT after stratification by sex. Panels A and B depict the changes in % MUFA during OGTT in females and males, respectively. Panels C and D depict the changes in % SFA during OGTT in females and males, respectively. Panels E and F depict the changes in % PUFA during OGTT in females and males, respectively. P values are for the repeated-measures ANOVA; P=0.001 in Panel A was statistically different % MUFA during OGTT between female CC and TT carriers. * P<0.05, ** P<0.01 by non-paired t-test.
There were no significant differences in % PUFA between CC and TT women (P=0.411, Figure 2, panel E), or between CC and TT women (P=0.108, Figure 2, panel F) during the OGTT by repeated-measures ANOVA.
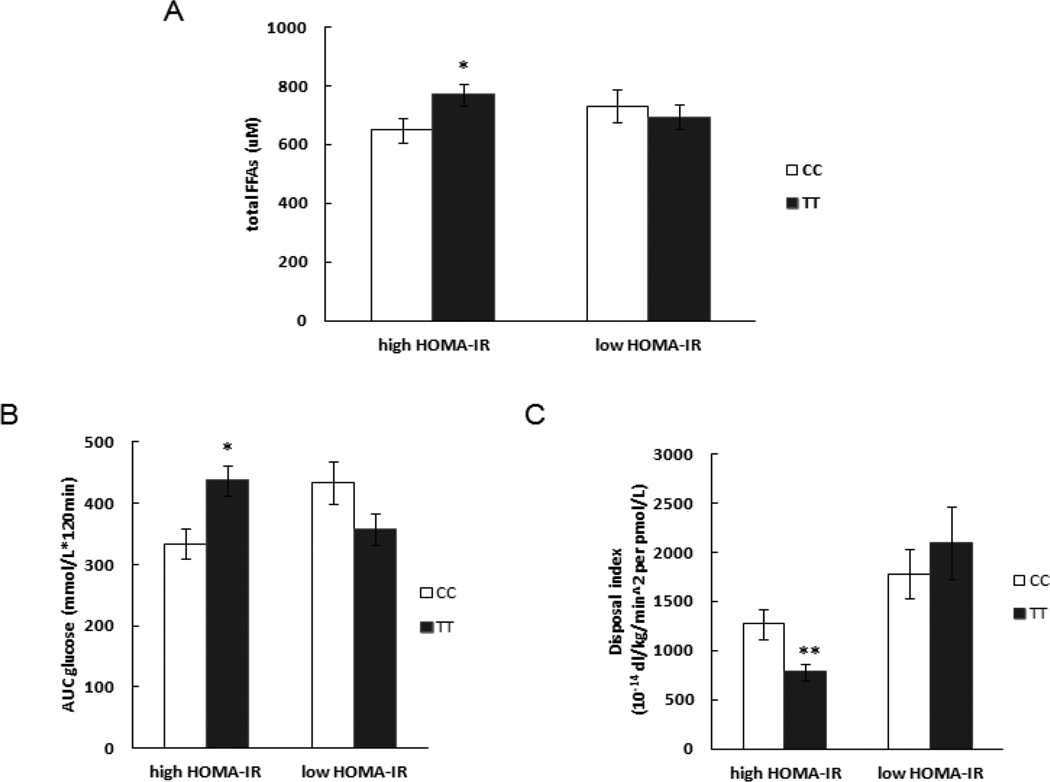
Modulation of genotype effects on FFA and DI by insulin sensitivity
Because of previous reports that insulin sensitivity modulates the metabolic effect of the TCF7L2 genotype [17], we assessed whether HOMA-IR influenced the effects of TCF7L2 genotype on FFA concentrations. We stratified the participants into tertiles of HOMA-IR and compared those in the high (HOMA-IR≥1.23) and low HOMA-IR (HOMA-IR <0.76) groups. Subjects with the TT genotype in the high HOMA-IR group had significantly higher fasting total FFA concentrations (770±166 vs. 649±194µM, P=0.04), as well as lower DI (781±348 vs. 1268±712 10−14dl/kg/min2 per pmol/l, P=0.008) and greater AAB of glucose (437±101 vs. 334±116 mmol/l·120min, P=0.005) than CC carriers in this group. However, there were no such differences in low HOMA-IR group (Figure 3). The differences of DI and AAB of glucose between CC and TT carriers in high HOMA-IR group were still significant after adjusting for BMI (P=0.019 for DI and P=0.008 for AAB of glucose). However the difference in fasting total FFA between CC and TT carriers in high HOMA-IR group did not reach statistical significance when BMI was adjusted for (P=0.066).
Figure 3.
The influence of TCF7L2 rs7903146 genotype on glucose disposal and total fatty acids concentration during OGTT in low and high HOMA-IR groups.
Plasma glucose, insulin and FFA concentrations in NGT and IGT participants
The characteristic of subjects grouped by IGT are provided in Table 2. Compared with NGT participants, there were more females in the IGT group; IGT participants were also 10 years older and 2 BMI units heavier. However, fasting plasma glucose and insulin concentrations were virtually identical in the two groups. Subjects with IGT had significantly greater total plasma FFA concentrations than NGT subjects, even after adjusting for sex, age, BMI, and genotype.
Table. 2.
Characteristics, plasma glucose and insulin concentrations of NGT and IGT subjects
| NGT (n=64) | IGT (n=56) | P | |
|---|---|---|---|
| Sex (M/F) | 30/34 | 15/41 | 0.02 |
| Age (years) | 37±13 | 47±14 | 0.0002 |
| BMI (kg/m2) | 26.5±3.8 | 28.3±4.4 | 0.02 |
| FFM (kg) | 48.3±11.8 | 45.2±12.8 | 0.17 |
| Fasting glucose (mmol/L) | 5.4±0.5 | 5.4±0.5 | 0.55 |
| 60min glucose (mmol/L) | 9.1±1.9 | 10.5±2.0 | * |
| 120min glucose (mmol/L) | 6.7±0.7 | 9.3±1.6 | * |
| Fasting insulin (pmol/L) | 33±23 | 33±16 | 0.97 |
| 120min insulin (pmol/L) | 149±99 | 308±194 | <0.0001 |
| AAB glucose (mmol/L*120min) | 324±104 | 475±131 | * |
| AAB insulin (nmol/L*120min) | 25.3±14.3 | 29.0±14.4 | 0.16 |
Because participants were selected for glucose intolerance, the plasma glucose concentrations
By definition, during the OGTT, IGT subjects had greater plasma glucose concentrations than NGT subjects, but there were no significant differences in plasma insulin concentrations at 30 and 60 min. By 120 min, plasma insulin concentrations were significantly less in NGT than IGT subjects. The AAB of insulin did not differ between NGT and IGT subjects.
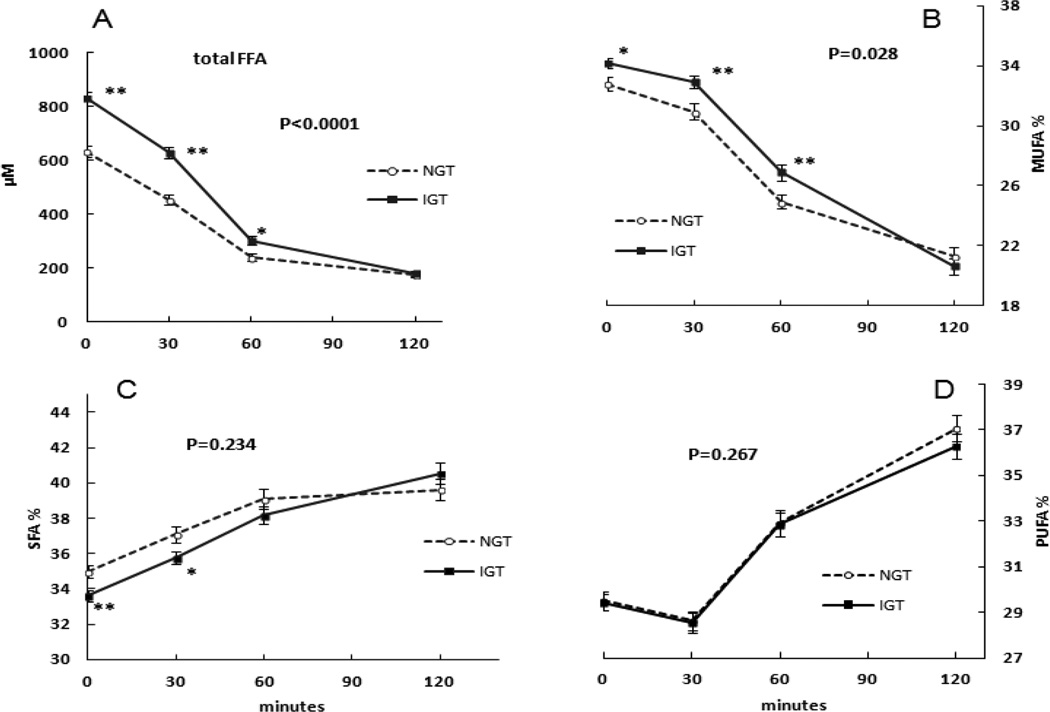
Plasma FFA concentrations were significantly greater in IGT than NGT subjects during OGTT (Figure 4, panel A) at 0, 30 and 60 min of the OGTT; by 120 min the FFA concentrations were no longer different between IGT and NGT. The concentrations of SFA, MUFA, and PUFA followed the same pattern.
Figure 4.
The differences of plasma fatty acids concentrations during OGTT between NGT and IGT subjects. The Mean ± SEM plasma total FFA (P<0.0001, panel A) and % MUFA (P=0.028, panel B) during the OGTT in NGT and IGT subjects were significantly different by repeated-measures ANOVA. There were no significant differences in % SFA (P=0.234, panel C) or % PUFA (P=0.267, panel D) between NGT and IGT subjects during the OGTT by repeated-measures ANOVA. * P<0.05, ** P<0.01 by non-paired t-test.
Plasma FFA composition differences between NGT and IGT
There were no significant differences in % SFA (P=0.234) and % PUFA (P=0.267), but % MUFA (P=0.028) were greater in IGT than NGT during the OGTT by repeated-measures ANOVA (Figure 4, panel B). By nonpaired t-tests, the % MUFA at 0 min (32.8 ± 3.4% vs. 34.1±2.6%, P=0.017), 30 min (30.9±4.3% vs. 32.9±3.2%, P=0.004) and 60 min (24.9±3.9% vs. 26.9±4.2%, P=0.010) were greater in IGT subjects than NGT subjects. At 0 min the C18:1/C18:0 and C16:1/C16:0 ratios were greater IGT than NGT subjects; this difference persisted for the C16:1/C16:0 ratios, but not C18:1/C18:0 ratios at 30 and 60 min of OGTT.
FFA concentrations in CC and TT carriers by IGT and NGT stratification
We found no significant differences in FFA concentrations or % SFA, MUFA or PUFA by genotype between NGT and IGT participants.
DISCUSSION
Given our previous report that the TCF7L2 variant rs7903146 has significant effects on glucose homeostasis, we elected to examine whether this might be mirrored by differences in plasma FFA concentrations. Contrary to our expectations, there were no differences in fasting plasma FFA concentrations between CC and TT carriers. Vcelak et al. [22] reported that women carrying the risk haplotype including TCF7L2 rs7903146 genotype had a greater percentage of fasting MUFA concentration and decreased percentage of fasting PUFA concentration in comparison with the low-risk carriers, in spite of the similar fasting FFA concentration. Our data is consistent with this publication [22] showing sex differences in plasma FFA composition between CC and TT carriers; we noted that the % MUFA were greater in female TT than CC carriers of TCF7L2 rs7903146 genotype during the OGTT, however the % MUFA were less in TT than CC males. This suggests that sex influence plasma FFA responses of TCF7L2 rs7903146 genotype.
In line with previous observations of the effects of insulin on the relative contribution of different FFA species to total FFA concentrations, there were significant changes in fatty acid composition of FFA during the OGTT. For all subjects, the percentage of SFA increased and the percentage of MUFA decreased. This most likely reflects differences in clearance between SFA, MUFA and PUFA when concentrations decrease. There may be differences in the preference of some enzymes in TG synthetic pathways for unsaturated fatty acid substrates [23, 24] that contribute to more rapid clearance of MUFAs from the circulating FFA pool.
It is also possible that the lipolytic pathway contributes to different FFA species; insulin inhibits hormone-sensitive lipase activity on TG and cholesterol esters, but not against diglycerides [25]. Triglycerides contain substantially more MUFA and less SFA than diglycerides [25], which could partially contribute to the selective decrease in plasma MUFAs during OGTT. Thus, possible explanations for higher % MUFA in female TT carriers during OGTT could be a reduced MUFA clearance compared with CC carriers or less of an antilipolytic effect of insulin on adipocyte TG containing MUFA.
Previous studies indicate that the impairment in insulin secretion associated with the TT genotype is magnified by impaired insulin action [17]. To understand whether this is true for fatty acid metabolism, we compared high and low HOMA-IR groups between genotypes. We found higher fasting FFA concentrations in TT than CC carriers in the high HOMA-IR subgroup. The TT carriers in the high HOMA-IR group also had a greater plasma glucose AUC and lower DI than the CC carriers. Whether glucose metabolism differences are related to the greater FFA concentrations cannot be determined from this study, but increasing plasma FFA by a lipid emulsion infusion has been shown to reduce insulin-stimulated total body glucose uptake in healthy humans [26]. Furthermore, increased plasma FFA concentrations are associated with a reduced ability to lower plasma glucose concentrations after an oral glucose load [27]. This is consistent with the greater plasma glucose AUC and fasting FFA concentrations in TT carriers in the high HOMA-IR sub-group.
More impressive than the modest differences in FFA composition between CC and TT carriers of the TCF7L2 variant rs7903146 was the greater fasting plasma FFA concentrations in IGT subjects than NGT subjects, independent of genotype. This held true even after adjusting for BMI, age, sex and genotype, and even in the context of identical fasting insulin and glucose concentrations. This finding is consistent with the suggestion that adipose tissue lipolysis is insulin resistant in adults with IGT [28].
Meantime, we found significantly greater % MUFA in IGT than NGT subjects. Given that IGT is associated with greater insulin resistance and metabolic risk, and that the T allele is the diabetes-related TCF7L2allele, it is possible the similar trend of MUFA concentrations in female TT carriers as in IGT subjects indicates a similar metabolic response in IGT subjects and TT women. It has been reported that the estimated stearoyl-CoA desaturase (SCD) enzyme activities predict an increase in the glucose AUC in follow-up of a metabolic syndrome population [29]. SCD converts palmitic acid to palmitoleic acid (C16:1n-7) and stearic acid to oleic acid (C18:1n-9), which are the two most abundant MUFA. The C18:1/C18:0 ratio in TT women was greater than that of CC women at 60,120 min of OGTT and a similar fasting FFA composition (both C18:1/C18:0 and C16:1/C16:0 ratios) was present in IGT subjects as in female TT carriers. It may indicate a similar metabolic response to convert more SFA to MUFA in IGT subjects and TT women. However, the MUFA changes were somewhat different in our female TT carriers and the IGT subjects in that MUFA differences in IGT subjects were seen in the fasting state and were due to both palmitoleic acid and oleic acid; the MUFA differences in TT women were restricted to oleic acid and seen only after glucose ingestion. The latter may indicate an effect on dietary trafficking of fatty acids rather than an SCD effect.
We also found a different effect of TCF7L2 rs7903146 genotype on fatty acid composition between men and women. The concentration and percentage MUFA in male TT carriers were lower than male CC carriers at 120 min of OGTT, which was the opposite in female subjects. This may be related to the low SCD1 expression in men; greater adipose SCD1 mRNA expression in present in women [30].
There are some limitations in our study. The sample size is relatively small relative to population studies for genotype effect analysis and we may have missed more subtle differences in FFA concentrations or composition between the two genotype groups. Although it was not a large population, we were able to find significant differences in FA between two genotype (CC and TT) carriers of TCF7L2 rs7903146 that were concordant with previous studies, thus establishing novel data to guide future studies if needed. In this study, we used an oral glucose loading to observe the effect of TCF7L2 rs7903146 on plasma FFA concentrations, but didn’t measure the adipose FA composition, which is heavily influenced by dietary FA intake as well as the endogenous FA metabolism [24]. Unfortunately, we don’t have detailed information regarding the dietary intake of FA in our participants.
CONCLUSIONS
In summary, we found that adults with IGT have significantly greater fasting plasma FFA concentrations and greater % MUFA. We also noted that insulin resistance and sex influence FFA responses amongst carriers of the minor T allele of TCF7L2 rs7903146. Insulin resistant TT carriers have the higher fasting FFA concentrations than CC carriers and, during the OGTT, female TT carriers have higher concentration and % MUFA due to higher oleic acid concentrations. Future studies directed towards adipose tissue handling of fatty acids and nutrient intake should be able to elucidate the underlying mechanisms by which the risk allele of TCF7L2 influences the fatty acids metabolism and to the role of diet in gene-associated diabetes risk.
Supplementary Material
Acknowledgments
Funding support: This work was supported by the National Institutes of Health (grant numbers RR024150, DK45343, DK40484, DK50456 and DK78646). Dr. Lu is sponsored by Natural Science Foundation of Shanghai (15ZR1413100).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: No potential conflicts of interest relevant to this article were reported.
Author contributions: J. L. ran the studies, researched the data and wrote the manuscript; L. Z. and R.T.V ran the studies; A.V. and M. D. J. designed the study, oversaw its conduct, researched data; M. D. J. reviewed/edited the manuscript; Dr. Michael D. Jensen is the guarantor of this work, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have approved the final article.
REFERENCES
- 1.Helgason A, Palsson S, Thorleifsson G, Grant SF, Emilsson V, Gunnarsdottir S, et al. Refining the impact of TCF7L2 gene variants on type 2 diabetes and adaptive evolution. Nat Genet. 2007;39:218–225. doi: 10.1038/ng1960. [DOI] [PubMed] [Google Scholar]
- 2.Cauchi S, Froguel P. TCF7L2 genetic defect and type 2 diabetes. Curr Diab Rep. 2008;8:149–155. doi: 10.1007/s11892-008-0026-x. [DOI] [PubMed] [Google Scholar]
- 3.Renstrom E. Impact of transcription factor 7-like 2 (TCF7L2) on pancreatic islet function and morphology in mice and men. Diabetologia. 2012;55:2559–2561. doi: 10.1007/s00125-012-2659-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shu L, Sauter NS, Schulthess FT, Matveyenko AV, Oberholzer J, Maedler K. Transcription factor 7-like 2 regulates beta-cell survival and function in human pancreatic islets. Diabetes. 2008;57:645–653. doi: 10.2337/db07-0847. [DOI] [PubMed] [Google Scholar]
- 5.Le Bacquer O, Kerr-Conte J, Gargani S, Delalleau N, Huyvaert M, Gmyr V, et al. TCF7L2 rs7903146 impairs islet function and morphology in non-diabetic individuals. Diabetologia. 2012;55:2677–2681. doi: 10.1007/s00125-012-2660-8. [DOI] [PubMed] [Google Scholar]
- 6.Cauchi S, Meyre D, Dina C, Choquet H, Samson C, Gallina S, et al. Transcription factor TCF7L2 genetic study in the French population: expression in human beta-cells and adipose tissue and strong association with type 2 diabetes. Diabetes. 2006;55:2903–2908. doi: 10.2337/db06-0474. [DOI] [PubMed] [Google Scholar]
- 7.Boj SF, van Es JH, Huch M, Li VS, Jose A, Hatzis P, et al. Diabetes risk gene and Wnt effector Tcf7l2/TCF4 controls hepatic response to perinatal and adult metabolic demand. Cell. 2012;151:1595–1607. doi: 10.1016/j.cell.2012.10.053. [DOI] [PubMed] [Google Scholar]
- 8.Shao W, Wang D, Chiang YT, Ip W, Zhu L, Xu F, et al. The Wnt signaling pathway effector TCF7L2 controls gut and brain proglucagon gene expression and glucose homeostasis. Diabetes. 2013;62:789–800. doi: 10.2337/db12-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith U. TCF7L2 and type 2 diabetes--we WNT to know. Diabetologia. 2007;50:5–7. doi: 10.1007/s00125-006-0521-z. [DOI] [PubMed] [Google Scholar]
- 10.Schinner S. Wnt-signalling and the metabolic syndrome. Horm Metab Res. 2009;41:159–163. doi: 10.1055/s-0028-1119408. [DOI] [PubMed] [Google Scholar]
- 11.Cauchi S, Choquet H, Gutierrez-Aguilar R, Capel F, Grau K, Proenca C, et al. Effects of TCF7L2 polymorphisms on obesity in European populations. Obesity (Silver Spring) 2008;16:476–482. doi: 10.1038/oby.2007.77. [DOI] [PubMed] [Google Scholar]
- 12.Boden G, Chen X. Effects of fat on glucose uptake and utilization in patients with noninsulin-dependent diabetes. J Clin Invest. 1995;96:1261–1268. doi: 10.1172/JCI118160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saloranta C, Koivisto V, Widen E, Falholt K, DeFronzo RA, Harkonen M, et al. Contribution of muscle and liver to glucose-fatty acid cycle in humans. Am J Physiol. 1993;264:E599–E605. doi: 10.1152/ajpendo.1993.264.4.E599. [DOI] [PubMed] [Google Scholar]
- 14.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Diabetes. 1995;44:863–869. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 15.Bhaswant M, Poudyal H, Brown L. Mechanisms of enhanced insulin secretion and sensitivity with n-3 unsaturated fatty acids. J Nutr Biochem. 2015;26:571–584. doi: 10.1016/j.jnutbio.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Lopez S, Bermudez B, Pacheco YM, Villar J, Abia R, Muriana FJ. Distinctive postprandial modulation of beta cell function and insulin sensitivity by dietary fats: monounsaturated compared with saturated fatty acids. Am J Clin Nutr. 2008;88:638–644. doi: 10.1093/ajcn/88.3.638. [DOI] [PubMed] [Google Scholar]
- 17.Delgado-Lista J, Perez-Martinez P, Garcia-Rios A, Phillips CM, Williams CM, Gulseth HL, et al. Pleiotropic effects of TCF7L2 gene variants and its modulation in the metabolic syndrome: from the LIPGENE study. Atherosclerosis. 2011;214:110–116. doi: 10.1016/j.atherosclerosis.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 18.Shah M, Varghese RT, Miles JM, Piccinini F, Dalla Man C, Cobelli C, et al. TCF7L2 Genotype and alpha-Cell Function in Humans Without Diabetes. Diabetes. 2016;65:371–380. doi: 10.2337/db15-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Persson X-MT, Blachnio-Zabielska AU, Jensen MD. Rapid measurement of plasma free fatty acid concentration and isotopic enrichment using LC/MS. J Lipid Res. 2010;51:2761–2765. doi: 10.1194/jlr.M008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab. 2004;287:E637–E643. doi: 10.1152/ajpendo.00319.2003. [DOI] [PubMed] [Google Scholar]
- 21.Warensjo E, Rosell M, Hellenius ML, Vessby B, De Faire U, Riserus U. Associations between estimated fatty acid desaturase activities in serum lipids and adipose tissue in humans: links to obesity and insulin resistance. Lipids Health Dis. 2009;8:37. doi: 10.1186/1476-511X-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vcelak J, Vejrazkova D, Vankova M, Lukasova P, Bradnova O, Halkova T, et al. T2D risk haplotypes of the TCF7L2 gene in the Czech population sample: the association with free fatty acids composition. Physiol Res. 2012;61:229–240. doi: 10.33549/physiolres.932272. [DOI] [PubMed] [Google Scholar]
- 23.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47:348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di-) acylglycerol and cholesteryl ester hydrolysis. J Lipid Res. 2002;43:1585–1594. doi: 10.1194/jlr.r200009-jlr200. [DOI] [PubMed] [Google Scholar]
- 26.Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994;93:2438–2446. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charles MA, Eschwege E, Thibult N, Claude JR, Warnet JM, Rosselin GE, et al. The role of non-esterified fatty acids in the deterioration of glucose tolerance in Caucasian subjects: results of the Paris Prospective Study. Diabetologia. 1997;40:1101–1106. doi: 10.1007/s001250050793. [DOI] [PubMed] [Google Scholar]
- 28.Abdul-Ghani MA, Molina-Carrion M, Jani R, Jenkinson C, Defronzo RA. Adipocytes in subjects with impaired fasting glucose and impaired glucose tolerance are resistant to the anti-lipolytic effect of insulin. Acta Diabetol. 2008;45:147–150. doi: 10.1007/s00592-008-0033-z. [DOI] [PubMed] [Google Scholar]
- 29.Lankinen MA, Stancakova A, Uusitupa M, Agren J, Pihlajamaki J, Kuusisto J, et al. Plasma fatty acids as predictors of glycaemia and type 2 diabetes. Diabetologia. 2015;58:2533–2544. doi: 10.1007/s00125-015-3730-5. [DOI] [PubMed] [Google Scholar]
- 30.Pinnick KE, Neville MJ, Fielding BA, Frayn KN, Karpe F, Hodson L. Gluteofemoral adipose tissue plays a major role in production of the lipokine palmitoleate in humans. Diabetes. 2012;61:1399–1403. doi: 10.2337/db11-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.