Abstract
We conducted a cross-sectional analysis to assess the distribution of human papillomavirus (HPV) types and explored an acceptable strategy for cervical screening in Shenzhen, China. A total of 2717 individuals ranging in age from 30–59 years were recruited. Clinical sensitivity and specificity as well as positive (PPV) and negative (NPV) predictive values were estimated. A triage strategy was regarded as acceptable when the NPV was at least 98.0%. 432 (15.9%) participants presented HPV positive. The five most prevalent HPV types were HPV52 (22.9%), HPV16 (12.7%), HPV53 (10.0%), HPV51 (8.6%), and HPV58 (8.1%). The CIN2+ risks for each HPV type were 40.0% for HPV33, 32.4% for HPV16, 18.2% for HPV58, 13.3% for HPV56, and 11.1% for HPV68 in descending order. Baseline cytology testing combined with HPV16/33/52/58 genotyping met the NPV thresholds at 98.6% with a PPV of 17.9%, demonstrating excellent clinical performance for detecting HPV types in CIN2+ patients. In conclusion, triaging HPV-positive women by baseline cytology combined with HPV16/58/33/52 genotyping is an acceptable strategy for cervical cancer screening in Shenzhen, China.
Keywords: human papillomavirus, prevalence, genotype, cervical screening, China
INTRODUCTION
Human papillomavirus (HPV) infection is the primary risk factor for cervical cancer [1–3]. Several longitudinal studies have demonstrated that being positive for high-risk types of HPV is a predictor of cervical intraepithelial neoplasia [4]. And the results of several randomised clinical trials have demonstrated that the effectiveness of cervical cancer screening can be improved by detecting high-risk HPV DNA as a primary screening method for cervical cancer [5–10]. Because HPV subtypes vary in their carcinogenic potential, genotyping is necessary for the triage of HPV-positive women. In addition to the most common types, HPV16 and HPV18, other high-risk HPV types must be accurately detected. Therefore, the information regarding the HPV prevalence and type distribution in a given population is necessary for the cancer prevention with prophylactic HPV vaccines and the development and evaluation of HPV screening tests.
Several studies have shown that HPV testing is more sensitive than cytology for identifying women with high grade disease [9, 11–16], and these results support the use of HPV detection as a single primary screening test. Many countries, including the United States, are introducing HPV DNA detection for primary screening [17]. However, HPV testing detects more transient infection than cytology, which may result in over referral for colposcopy and ultimately overtreatment [14, 18, 19]. Therefore, management of HPV-positive women is of major concern, especially in regions with an inefficient cytology-based screening program [18].
Shenzhen, a newly-emerging economic developed city in China, is short of medical professionals for its short history and low experience. Since 2005, Shenzhen is among the first demonstrative bases of cervical cancer prevention and control in China. It offers a favourable environment for performing cervical screening, and is financially capable of covering the screening costs of the entire population. Thus, HPV testing is widely used as a primary screening method of cervical cancer in Shenzhen. However, information on prevalence and type distribution of HPV in Shenzhen is incomplete. In addition, Shenzhen has insufficient medical infrastructure for cytology-based screening and lack well trained cytology experts. Therefore, it is not clear which screening strategy is optimal in Shenzhen, co-testing or primary HPV detection followed by cytology for HPV-positive women. Furthermore, because HPV genotyping has been recommended for screening triage, it is uncertain which combinations of high-risk HPV types provide useful information for clinical practice in Shenzhen, China.
To address these concerns, we conducted a population-based cervical screening survey to assess the distribution of HPV genotypes in Shenzhen, China, and explore an acceptable triage strategy to reduce the burden of cytological examination.
RESULTS
Demographic and clinical characteristics of the studied population
Of the 3000 eligible women recruited to participate in the study, 283 exited for invalid questionnaire or unwilling participation of screening. We therefore based our analyses on the availability of complete data with HPV testing results for 2717 women (90.6%). The main characteristics of the study participants are shown in Table 1. The mean age was 40.6 years (standard deviation, SD 7.9; range 30–59), and the mean age of first sexual intercourse and primiparity was 22.9 years(SD 2.9; range 14–40) and 25.3 years (SD 4.1; range 14–46), respectively. The percentage of women using oral contraceptives was 2.7% (74 of 2717). The mean number of live births was 1.6 (SD 1.1; range 0–7). Married women accounted for 92.4% (2510 of 2717) of participants and 74.5% (2023 of 2717) had various forms of medical insurance (Table 1).
Table 1. Demographic information of the study population.
| Characteristics | n (%) | |
|---|---|---|
| Marital status | ||
| Married | 2510(92.4) | |
| Other | 207(7.6) | |
| Age | 40.6 ± 7.9 | |
| Age of first sexual intercourse | 22.9 ± 2.9 | |
| ≤ 16 | 13(0.5) | |
| 17 ~ 20 | 581(22.8) | |
| ≥ 21 | 1957(76.7) | |
| Age of primiparity | 25.3 ± 4.1 | |
| ≤ 20 | 154(6.2) | |
| 21 ~ 25 | 1228(49.3) | |
| 26 ~ 30 | 911(36.6) | |
| ≥ 31 | 198(7.9) | |
| Number of live-births | 1.6 ± 1.1 | |
| 0 | 69(2.7) | |
| 1 | 1287(50.6) | |
| 2 | 857(33.7) | |
| ≥ 3 | 328(12.9) | |
| Contraceptive measures | ||
| Oral contraceptive | 74(2.7) | |
| Intrauterine device | 795(39.3) | |
| Tubal ligation | 62(2.3) | |
| Condom | 929(34.2) | |
| Rhythm method | 86(3.2) | |
| Coitus interruptus | 410(15.1) | |
| Other | 361(13.3) | |
| Medical insurance | ||
| Yes | 2023 (74.5) | |
| No | 694(25.5) | |
| HPV test | ||
| Positive | 432(15.9) | |
| Negative | 2285(84.1) | |
| Liquid-based cytology | ||
| NILM | 1127(94.6) | |
| ASCUS+ | 64 (5.4) | |
| Histological diagnosis | ||
| NILM | 797(86.4) | |
| CIN1 | 95(10.3) | |
| CIN2+ | 30(3.3) |
HPV: Human papillomavirus; NILM: No intraepithelial lesion or malignant cells; ASCUS: Atypical squamous cells of undermined significance; CIN: Cervical intraepithelial neoplasia
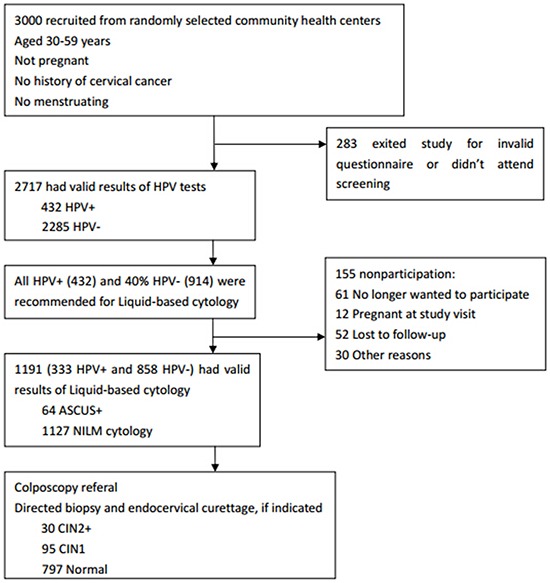
All study participants had valid HPV tests results, and 432 (15.9%) presented HPV positive. 333 HPV positive and 858 negative participants further underwent the cytology examination. The prevalence of abnormal cervical cytology (ASCUS+) was 5.4%. Among women who underwent liquid-based cytology testing, 922 were selected or randomly assigned for colposcopy. 30 women were diagnosed as CIN2+, and 95 were diagnosed as CIN1. (Table 1 and Figure 1)
Figure 1. Flow diagram of procedures at every step of the study protocol.
Prevalence and genotypes of HPV infections
Positive HPV detection results were obtained for 15.9% (432) of the study participants. A total of 23 genotypes were detected among the HPV positive women, and the five most prevalent HPV types were HPV 52 (99/432=22.9% of HPV infections), HPV16 (55/432=12.7% of HPV infections), HPV53 (43/432=10.0% of HPV infections), HPV51 (37/432=8.6% of HPV infections), and HPV58 (35/432=8.1% of HPV infections). Women demonstrating positivity for a single HPV genotype accounted for 75.2% (325/432), whereas 24.8% (107/432) of women were positive for multiple types. Among the latter, 18.1% (78/432) had dual infections, 5.1% (22/432) had triple infections, and 1.6% (7/432) had four or more infections (Figure 2a and Figure 2b).
Figure 2. Prevalence and genotypes of HPV infections.
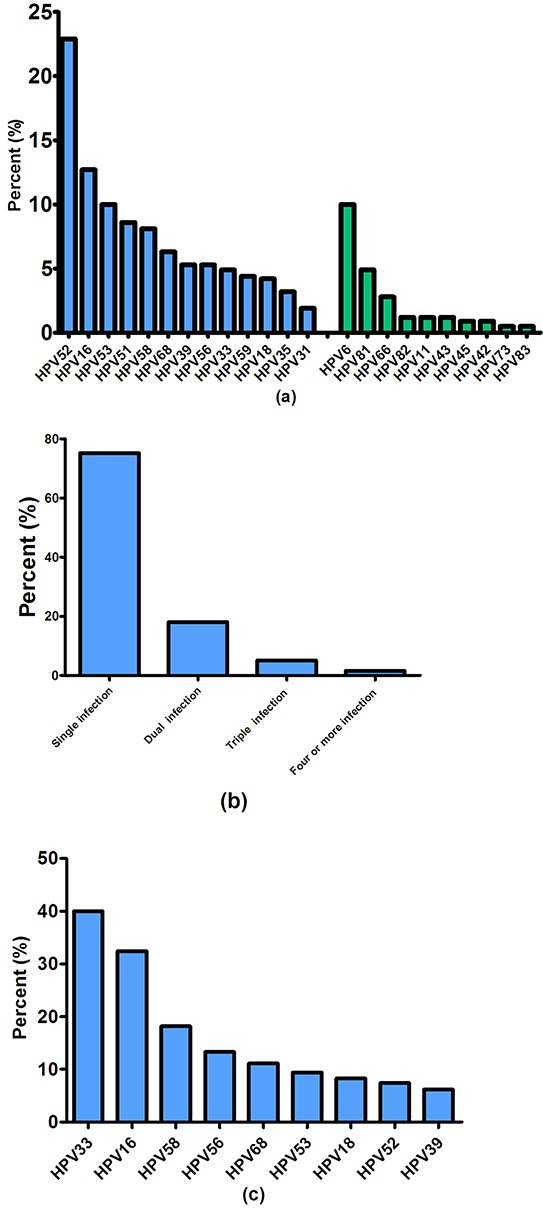
Figure 2a. Distribution of HPV genotypes in Shenzhen, China. The blue bar stands for the distribution of 13 high-risk HPV types, green bars stand for the distribution of other 10 HPV types; Figure 2b. Prevalence of single and multiple HPV infection; Figure 2c. Risk of CIN2+ for HPV genotypes in Shenzhen, China.
We also estimated the CIN2+ risks (positive predictive values and negative predictive values) for each individual HPV type. In Figure 2c, we showed these risks in descending order, from greatest to least. For HPV infected women, the risks of CIN2+ were 40.0% (95% CI: 15.2 - 64.8) for HPV33, 32.4% (95% CI: 18.0 - 49.8) for HPV16, 18.2% (95%CI: 5.2 - 40.3) for HPV58, 13.3% (95% CI: 1.7 - 40.5) for HPV56, and 11.1% (95% CI: 1.4 - 34.7) for HPV68.
Distribution of HPV genotypes by sociodemographic, cytological, and histological diagnosis
Table 2 explored the relationship between sociodemographic, cytological, and histologic diagnosis and the distribution of HPV genotypes. We observed that the prevalence of HPV positivity was significantly lower in married women (385/2510,15.3%) than in single/divorced women(47/207,22.7%)(P=0.005). For the age of first sexual intercourse, the prevalence of HPV infection was significantly different among ≤16 years, 17~20 years, and ≥21 years (P=0.047), with the highest prevalence in women aged 17~20 years(18.2%) then followed by women aged≥21 years (14.3%) and ≤16 years(7.7%). HPV infection prevalence was significantly lower among women who have medical insurance (14.9%) than among those without (18.7%) (P=0.018). Table 2 also shows the prevalence of HPV genotypes according to cervical cytology and histological diagnosis. The HPV positive prevalence was 25.3% in NILM cases, whereas the prevalence was 75.0% in women with abnormal cervical cytology (P<0.001). HPV 16/58 prevalence was 2.9%/1.8% in NILM cases, whereas the prevalence was 17.2%/10.9% among cases of abnormal cervical cytology (P<0.001). Infection with single and multiple HPV types was noted in 62.8%/37.2% of NILM cases and 43.8%/56.2% of abnormal cervical cytology cases (P=0.013). As expected, HPV positivity increased with the severity of the pathological result(P<0.001). Moreover, the prevalence of HPV16/58 also exerted a similar increasing trend with the development of pathology abnormalities(P<0.001). The prevalence of single and multiple HPV infections was statistically different among CIN2+, CIN1, and NILM groups (P=0.029).
Table 2. Distribution of HPV genotypes by sociodemographic, cytological and histologic diagnosis.
| Characteristic | HPV (n, %) | HPV16 (n, %) | HPV58 (n, %) | Infection with single HPV type (n, %) | Co-infection with multiple types (n, %) | |
|---|---|---|---|---|---|---|
| HPV positive | HPV negative | |||||
| Marital | ||||||
| Married | 385(15.3) | 2125(84.7) | 48(1.9) | 32(1.3) | 246(63.9) | 139(36.1) |
| Single/divorced | 47(22.7) | 160(77.3) | 7(3.4) | 3(1.4) | 24(51.1) | 23(48.9) |
| χ2 | 7.761 | 2.082 | 0.046 | 2.943 | ||
| P value | 0.005 | 0.149 | 0.831 | 0.086 | ||
| Age of first sexual intercourse | ||||||
| ≤16 | 1 (7.7) | 12(92.3) | 0(0.0) | 0(0.0) | 1(100) | 0(0.0) |
| 17 ~ 20 | 106(18.2) | 475(81.8) | 15(2.6) | 7(1.2) | 64(60.4) | 42(39.6) |
| ≥21 | 279(14.3) | 1678(85.7) | 34(1.7) | 24(1.2) | 180(64.5) | 99(35.5) |
| χ2 | 6.111 | 1.951 | 0.162 | 1.145 | ||
| P value | 0.047 | 0.377 | 0.922 | 0.564 | ||
| Age of primiparity | ||||||
| ≤20 | 20(13.0) | 134(87.0) | 3(1.9) | 2(1.3) | 15(75.0) | 5(25.0) |
| 21~25 | 193(15.7) | 1035(84.3) | 22(1.8) | 10(0.8) | 121(62.7) | 72(37.3) |
| 26~30 | 122(13.4) | 789(86.6) | 13(1.4) | 13(1.4) | 77(63.1) | 45(36.9) |
| ≥31 | 27(13.6) | 171(86.4) | 3(1.5) | 4(2.0) | 20(74.1) | 7(25.9) |
| χ2 | 2.765 | 0.538 | 3.138 | 2.414 | ||
| P value | 0.429 | 0.910 | 0.371 | 0.491 | ||
| Parity | ||||||
| Nullipara | 11(15.9) | 58(84.1) | 1(1.4) | 1(1.4) | 5(45.5) | 6(54.4) |
| Multipara | 367(14.8) | 2105(85.2) | 45(1.8) | 29(1.2) | 236(64.3) | 131(35.7) |
| χ2 | 0.064 | 0.052 | 0.044 | 1.642 | ||
| P value | 0.801 | 0.820 | 0.834 | 0.200 | ||
| Contraceptive measures | ||||||
| Oral contraceptive | 14(18.9) | 60(81.1) | 2(2.7) | 1(1.4) | 10(71.4) | 4(28.6) |
| Other contraceptive measures | 418(15.8) | 2225(84.2) | 53(2.0) | 34(1.3) | 260(62.2) | 158(37.8) |
| χ2 | 0.519 | 0.177 | 0.002 | 0.492 | ||
| P value | 0.471 | 0.674 | 0.961 | 0.483 | ||
| Medical insurance | ||||||
| Yes | 302(14.9) | 1721(85.1) | 38(1.9) | 27(1.3) | 189(62.6) | 113(37.4) |
| No | 130(18.7) | 564(81.3) | 17(2.4) | 8(1.2) | 81(62.3) | 49(37.7) |
| χ2 | 5.591 | 0.850 | 0.134 | 0.003 | ||
| P value | 0.018 | 0.357 | 0.714 | 0.957 | ||
| Cytological | ||||||
| NILM | 285(25.3) | 842(74.7) | 33(2.9) | 20(1.8) | 179(62.8) | 106(37.2) |
| ASCUS+ | 48(75.0) | 16(25.0) | 11(17.2) | 7(10.9) | 21(43.8) | 27(56.2) |
| χ2 | 74.302 | 34.610 | 22.949 | 6.220 | ||
| P value | <0.001 | <0.001 | <0.001 | 0.013 | ||
| Histological diagnosis | ||||||
| NILM | 184(23.1) | 613(76.9) | 12(1.5) | 9(1.1) | 105(57.1) | 79(42.9) |
| CIN1 | 83((87.4) | 12(12.6) | 14(14.7) | 9(9.5) | 59(71.1) | 24(28.9) |
| CIN2+ | 28(93.3) | 2(6.7) | 11(36.7) | 4(13.3) | 13(46.4) | 15(53.6) |
| χ2 | 214.819/194.228* | 124.413/121.720* | 41.330/40.160* | 7.058 | ||
| P value | <0.001/<0.001* | <0.001/<0.001* | <0.001/<0.001* | 0.029 | ||
HPV 16 and HPV 58 were both among the five most prevalent HPV types (HPV52, 16, 53, 51, and 58) and the five most risk HPV types (HPV33, 16, 58, 56, and 68) in Shenzhen. Therefore, we explored the relationship between the distribution of HPV16 and HPV58 and sociodemographic, cytological, and histologic diagnosis in Table 2.
HPV: Human papillomavirus; NILM: No intraepithelial lesion or malignant cells; ASCUS: Atypical squamous cells of undermined significance; CIN: Cervical intraepithelial neoplasia
Linear-by-Linear test
Evaluation of triage strategies for high-risk HPV positive women
The performance of the eleven triage strategies for HPV positive women at baseline is shown in Table 3. The inclusion of HPV16 and HPV16/58, yielded an NPV for CIN2+ cases of 93.8% and 94.5%, respectively, and a PPV of 32.4% and 25.9%, respectively. Triage of HPV-positive women based on cytology testing yielded an NPV for CIN2+ of 95.9% and a high PPV of 39.1%. The inclusion of HPV16/33, HPV16/58/33, and HPV16/33/52/58 genotyping at baseline provided a slightly higher NPV (95.5%, 95.6%, and 96.4%) and a decreased PPV (33.3%, 25.7%, and 17.1%). The NPV of the above triage strategies were well below our threshold of 98.0%, and therefore these strategies were deemed unacceptable. The triage strategy of baseline cytology testing combined with HPV16, HPV16/58, HPV16/33, or HPV16/58/33 genotyping demonstrated an NPV of 97.0%-97.6%, which was slightly below threshold, and a PPV of 24.5%-31.0%. Only the baseline cytology testing combined with HPV16/33/52/58 genotyping met the NPV thresholds, with an NPV of 98.6% and a PPV of 17.9%. The sensitivity and specificity for detection of CIN2+ were 92.9% and 54.2%, respectively.
Table 3. Different triage strategies for high-risk HPV positive women.
| Triage strategy | HPV positive women | |||
|---|---|---|---|---|
| Endpoint CIN2+ | ||||
| NPV (95%CI),% | PPV (95%CI),% | Sensitivity (95%CI),% | Specificity (95%CI),% | |
| Cytology | 95.9(92.5,98.0) | 39.1(25.0,53.2) | 64.3(46.5,82.0) | 89.2(84.8,92.7) |
| HPV16 | 93.8(90.1,96.4) | 32.4(18.0,49.8) | 42.9(24.5,62.8) | 90.6(86.5,93.9) |
| HPV16/58 | 94.5(90.8,97.1) | 25.9(15.3,39.0) | 53.6(33.9,72.5) | 83.9(78.9,88.1) |
| HPV16/33 | 95.5(92.1,97.7) | 33.3(20.8,46.3) | 60.7(42.6,78.8) | 87.3(82.7,91.0) |
| HPV16/58/33 | 95.6(92.0,97.9) | 25.7(16.0,37.6) | 64.3(46.5,82.0) | 80.5(75.3,85.1) |
| HPV16/58/33/52 | 96.4(92.3,98.7) | 17.1(11.0,24.7) | 78.6(59.1,91.7) | 59.9(53.8,65.9) |
| HPV16 & Cytology | 97.2(94.1,99.0) | 31.0(20.5,43.1) | 78.6(59.1,91.7) | 81.2(75.9,85.8) |
| HPV16/58 & Cytology | 97.0(93.7,98.9) | 25.9(17.0,36.6) | 78.6(59.1,91.7) | 75.8(70.1,80.9) |
| HPV16/33 & Cytology | 97.6(94.5,99.2) | 28.8(19.2,40.0) | 82.1(63.1,93.9) | 78.1(72.6,83.0) |
| HPV16/58/33 & Cytology | 97.4(94.1,99.2) | 24.5(16.2,34.4) | 82.1(63.1,93.9) | 72.7(66.9,78.0) |
| HPV16/58/33/52 & Cytology | 98.6(95.1,99.8) | 17.9(12.1,25.2) | 92.9(76.5,99.1) | 54.2(48.2,60.3) |
HPV: Human papillomavirus; CIN: Cervical intraepithelial neoplasia; NPV: Negative predictive value; PPV: Positive predictive value.
DISCUSSION
This independent study evaluated the prevalence of HPV genotypes in Shenzhen, a developed coastal city in China. A total of 15.9% of the study participants presented HPV positive, which was lower when compared to some western countries such as America, Italy, and Canada (18.1%-39.0%) [20–23], and higher than that in India and other regions of China (6.1%-12.9%) [20, 24]. This indicates the cultural, ethnic and regional differences in HPV prevalence [25]. And various detection methods may also contributed to the difference [26].
Our present study showed that the prevalence of HPV positivity was significantly lower in married women (15.3%) than in single/divorced women (22.7%), which was in agreement with a study conducted in Italy [27]. The low prevalence of HPV infection among married women may be due to the protective effect against HPV infection generated by living with a partner [27]. For the age of first sexual intercourse, the prevalence of HPV positivity was higher among the group aged 17~20 years(18.2%) than among that aged≥21 years (14.3%) and ≤16 years(7.7%). In some regions, an age at first sexual encounter of younger than 17 years is generally associated with increased risk of HPV infection, and the same conclusion was draw in our study. Early age of sexual debut was a risk factor for HPV infection. It is reported that the immature cervix during adolescence is more likely to acquire HPV infection and therefore have a greater risk of precancerous lesions. Recent international studies describe a higher risk of acquiring new HPV infections at younger ages. These findings suggest that younger women and the associated risk of early sexual debut should be identified as potential targets for the prevention of HPV infection [28–31]. HPV infection prevalence was significantly lower among women with medical insurance (14.9%) than among those without (18.7%). This can be attributed to the fact that having medical insurance is associated with individual economic conditions, education level, and employment situation in China, and these factors are important determinants for HPV infection [27]. It is reported that oral contraceptives use was significantly associated with HPV infection, while some other studies have failed to confirm such an association [32, 33]. In our study, we didn't found the significant association between the use of oral contraceptives and HPV positivity. Considering that HPV infections are closely linked with the sexual habit, and the adopted sexual behaviours are in turn closely related to contraception use of individual women, it is of great necessity to consider the effect of HPV infection, sexual habits, and other confounding factors(such as barrier contraception and socio-economic factors) in the future research so as to promote the research process in this field [33]. The HPV positive prevalence was 25.3% in NILM cases, and 75.0% among ASCUS+ women. Moreover, HPV positivity increased with the severity of the pathological result (P<0.001). Sellors et al. reported similar results, demonstrating that the presence of squamous intraepithelial lesions was strongly associated with HPV infection [22]. In addition, Ozturk and colleagues reported that HPV infection together with high rates of precancerous lesions leading to malignancy supported the association between HPV and cervical cancer [20]. In our current study, the high prevalence of HPV infection in precancerous lesions indicates the need for cervical screening and follow-up in women who are HPV positive.
Our results showed that HPV 52, HPV16, HPV53, HPV51, and HPV58 were the five most prevalent types in this population, which is similar to studies of HPV prevalence in other countries in Asia [34], and in other regions in China [24, 35]. A meta-analysis of HPV prevalence in 5 continents displayed that HPV16 and HPV18 were the most frequent types worldwide, with HPV16 being the most common type everywhere, and HPV18 was among the most common HPV types after HPV16 [34, 36]. However, HPV 52 and HPV58 are more common in the Asian population [37, 38]. In China, HPV 52 and HPV 16 are more prevalent, although variation also exists among different regions: HPV52, 16, 58, 31 were the most common types in Shanghai, HPV52, 16, 58, 18, 56 in Yunnan province, HPV16, 58, 52, 33, 11 in Zhejiang province and HPV16, 52, 58, 18, 45 in Guangdong province [24, 39–41]. These differences in HPV type prevalence may be linked to geographic location and complex immune/genetics factors which would influence the biological interactions between host immune system and different HPV subtypes [42, 43]. In addition, HPV genotype distribution may also vary by ethnicity/race, which is related to socioeconomic status [44, 45].
We also examined the risks of CIN2+ for each individual HPV type. Long-term observational studies show that HPV16 and HPV18 have an elevated risk of cervical lesions compared to other high-risk HPV types [46–49]. However, genotyping triage for HPV16 and HPV18 is not suitable for China because of the varying HPV genotype distribution between China and other countries. Thus, there is a need to distinguish additional HPV types to guide cervical screening. For the HPV infected women in our study, the CIN2+ risks for each HPV type were 40.0% for HPV33, 32.4% for HPV16, 18.2% for HPV58, 13.3% for HPV56, and 11.1% for HPV68 in descending order. The prevalence of HPV genotypes in our study indicated that compared to bivalent (HPV16, 18) and quadrivalent (HPV6, 11, 16, 18) HPV vaccines, the 9vHPV (HPV6, 11, 16, 18, 31, 33, 45, 52, 58) vaccine is more suitable for the population of Shenzhen, China. Our results also allude to the direction of future vaccines most suitable for the Chinese population. We also observed that the proportion of HPV16/58 in ASCUS+ cases was significantly higher than that in NILM cases, and the prevalence of HPV16/58 exerted an increasing trend with the development of pathology abnormalities. Similar findings showed that infection with HPV16/58/31/33 may increase the risk for progression of cervical lesions in the study population [50]. Another study also reported a strong association between abnormal cytology and HPV16/18 infection [51].
The proportion of women with multiple infections among HPV-positive participants was 24.8%, and our results correlate with the findings of similar studies conducted in China, in cities such as Beijing (27.7%), Shanxi (24.3%), and Henan (19.8%) [52]. Studies show that the prevalence of multiple HPV infection ranges from 9%-50%[51], and the ratio mainly depended on the individual immune status of the study population and the method used for HPV genotype detection [53]. In addition, the prevalence of single HPV infection was significantly higher in married women than in single/divorced women, and this ratio is consistent with the proportion of overall single infections in our study. In the current study, the prevalence of multiple HPV infections was more common in ASCUS+ (56.2%) and CIN2+(53.6%) cases than in NILM cases (37.2% and 42.9%). In addition, the prevalence of single HPV infection was more prevalent in NILM (57.1%) and CIN1(71.1%) cases compared to CIN2+(46.4%) cases. Several studies have shown that multiple HPV infections increase the severity of cervical lesions, and may influence the oncogenic potential of HPV [54–59]. Therefore, the survey of multiple infections in our study enhances our understanding of the role of multiple HPV infections in cervical cancer development. Among the women infected with multiple HPV types, double and triple HPV infection were more common, and our results are in agreement with the findings of similar studies [53].
Implementation of HPV detection has been recommended as a primary screening test for cervical screening. However, the specificity of HPV testing is far below that of cytology examination, and results in over-diagnosis and over-treatment of HR HPV-positive women [9]. Therefore, triage strategies for HPV positive women are necessary. Based on the prevalence of HPV types in Shenzhen, we estimated different combinations of HPV types in the study to find an acceptable triage strategy for HPV positive women. We also evaluated the performance of eleven triage strategies for HPV positive women. The acceptable triage strategy was baseline cytology testing combined with HPV16/33/52/58 genotyping, which met the thresholds for NPV of 98.6% and had an acceptable PPV of 17.9%[18]. This can best balance the safety (NPV) and the burden on patients for an acceptable strategy [60]. In our study, readouts of all HPV types for the reverse dot blot HPV test are provided concurrently, and testing for HPV16/33/52/58 in the study to triage HPV positive patients was very efficient and greatly reduced the manpower requirements in the clinical. The triage strategy of our study also indicated that cytology could be applied to HPV16/33/52/58 positive women, which could relieve the burden of cytological examination. In addition, this strategy will increase the sensitivity for identification of CIN2+ in HPV positive women, while maintaining acceptable PPV. Similar studies in the United States showed that HPV genotyping (HPV16/18) with or without cytology can provide safe and cost-effective cervical screening and the trade-offs in sensitivity for detection of CIN3+ versus the poor PPV [15]. Another study found that baseline and repeat cytology testing for HPV positive patients demonstrates the highest PPV(34%) and a low referral rate. However, this triage does not apply to cities with less efficient cytology screening, such as Shenzhen [60].
Our study has the advantage of examining a wide age range of participants (30-59 years), which is encouraged for HPV testing. Furthermore, our study was conducted in a population subset, in which the results can be extrapolated to the larger Chinese population. This study also has limitations. For instance, as a baseline strategy, an important disadvantage is the limited PPV, which although in an acceptable range, suggests a considerable risk for overtreatment [60]. Further studies will need to evaluate the performance of prospective studies to identify best practices.
In summary, HPV52, HPV16, HPV53, HPV51, and HPV58 were the most prevalent HPV types, and HPV33, HPV16, HPV58, HPV56 and HPV68 were the most risk HPV types in Shenzhen. The ability to identify HPV16, HPV58, and the other 21 HPV types make the reverse dot blot HPV test a very attractive option for HPV detection, and potentially contribute to improve patient management. In the management of HPV-positive women, triaging HPV-positive patients by baseline cytology testing combined with HPV16/33/52/58 genotyping seems safe and yields an acceptable PPV. The weights placed on the quality of cytology and the safety of the screening ultimately determines the management effectiveness of HPV-positive women.
MATERIALS AND METHODS
Study population and procedures
For this study, we enrolled women who were able to give independent informed consent, aged 30 to 59 years, living in Shenzhen, Guangdong Province, China for more than six months, and screened them for cervical cancer from 1 March to 15 June 2015. Eight community health centres in Luohu district and ten community health centres in Nanshan district were selected using the randomised cluster sampling method. All eligible women from the eighteen selected community health centres were invited to participate. Non-pregnant women with no history of cervical cancer, precancerous lesions, hysterectomy, pelvic radiation, and other screening contraindications were eligible for enrolment. All participants provided written informed consent. The study was approved by the Ethics Committee of Huazhong University of Science and Technology. The methods of this study were carried out in accordance with the approved guidelines expressed in the Declaration of Helsinki.
Screening was done at the community health centres of the two districts. The sequence of events from recruitment to study exit is shown in Figure 1. On screening day, sociodemographic information and cervical cancer-related risk factors including reproductive and behavioural data were collected by trained health workers in confidential settings after informed written consent was obtained. Then cervical specimens for HPV test were collected from each participant by gynaecologists. One month later, all HPV positive women and 40% HPV negative women were telephonically informed to come back to undergo liquid-based cytology. Eligible women were randomly referred for colposcopy and a biopsy was performed if indicated. Health workers in each community health centre filled the clinical part of the questionnaire with the results of biopsy.
Reverse dot blot HPV test
The cervical brushes were taken and immediately placed into a tube containing specimen transport medium (1mL). The samples were stored at −20°C until required for testing. The reverse dot blot HPV test can detect 13 high-risk HPV types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, and other 10 HPV types: 6, 11, 42, 43, 53, 66, 73, 81, 82 and 83 in cervical specimens. All detection procedures were performed according to the manufacturer's instructions for the reverse dot blot HPV test by the laboratory of Yaneng BIOscience (Shenzhen Co., Ltd. China). The performance of the reverse dot blot HPV test was approved by the China Food and Drug Administration.
Liquid based cytology
Liquid-based cytology samples were collected by cervical cytobrush sampling, and placed into tube with 2 ml PreservCyt solution (U.S. Cyt Company) along with the cytobrush. The samples were stored at 4°C, and then sent to the laboratory for tests. ThinPrep slides were doubled-screened by cytotechnologists. Cytological results were reported using the 2001 Bethesda classification system and were classifies as [61, 62]: 1) negative; 2) atypical squamous cells of undetermined significance(ASCUS); 3) low-grade squamous intraepithelial lesion(LSIL); 4) atypical squamous cells that cannot exclude HSIL (ASC-H); 5) high-grade squamous intraepithelial lesion(HSIL).
Colposcopy and histology
Colposcopy-guided tissue biopsies were taken from suspicious lesions by gynaecologists. Histology was examined and classified according to international criteria as follows [63]: normal, Cervical intraepithelial neoplasia (CIN) grade 1 (CIN 1), grade 2 (CIN 2), grade 3 (CIN 3), and invasive Squamous cell carcinoma (SCC).
Statistical analysis
Clinical sensitivity, clinical specificity, positive predictive value (PPV), and negative predictive value (NPV) were computed using conventional contingency tables. The PPV means the risk of CIN2+ with a positive test. 1-NPV signifies the risk of CIN2+ with a negative test [64]. Thus, a low PPV would indicate the unnecessary procedures induced by cervical screening, and a low NPV reflects that the detection was not sufficient to exclude the potential disease. The 95% confidence intervals (95% CI) were calculated for proportions of sensitivity, specificity, PPV and NPV using the exact binomial method. Comparison of the HPV genotype representation between the different groups regarding categorical variables was done using the Chi-square test. Linear-by-linear association test was used to analyse trend in distribution of HPV infection according to grade of pathology abnormalities. All statistical tests were two-sided, P values <0.05 were considered statistically significant.
The gold standard in our study was histologically confirmed CIN2 or more severe diagnosis (CIN2, CIN3 and cancer). Cytology in our study was dichotomized, and a positive result was regarded as ASCUS or worse (ASCUS, LSIL, ASC-H, HSIL). To evaluate the triage strategies, we considered the NPV for CIN2+ of at least 98.0% to be acceptable [65] (corresponding with a <2% risk of CIN2+ within the next 2-3 years). Analyses were done using the SAS (version 9.1.3) and SPSS (version 12.0) software.
Acknowledgments
We wish to thank the health workers of the 18 community health centres and the gynaecologists of the Women and Children's Hospital in the Nanshan and Luohu districts. We also thank the many citizens of Shenzhen City who participated in this study.
Abbreviations
- HPV
Human papillomavirus
- PPV
Positive predictive value
- NPV
Negative predictive value
- CIN
Cervical intraepithelial neoplasia
- SCC
Squamous cell carcinoma
- NILM
No intraepithelial lesion or malignant cells
- ASCUS
Atypical squamous cells of undetermined significance
- LSIL
Low-grade squamous intraepithelial lesion
- ASC-H
Atypical squamous cells that cannot exclude HSIL
- HSIL
High-grade squamous intraepithelial lesion
- CI
Confidence interval
- SD
Standard deviation
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
GRANT SUPPORT
This study was supported by the Health and Family Planning Commission of Shenzhen, China (grant number 201302110).
Author Contributions
Y.Y.W. designed the study, negotiated with the other researchers, and was responsible for study implementation. Data collection and analysis was done by Y.R.Z. and C.G.. Z.H.L. and L.L. edited and reviewed the paper. Y.R.Z. had full access to all data in the study and designed the analytic approach. S.F.N. had the final responsibility to submit the paper for publication.
REFERENCES
- 1.Mitra A, MacIntyre DA, Lee YS, Smith A, Marchesi JR, Lehne B, Bhatia R, Lyons D, Paraskevaidis E, Li JV, Holmes E, Nicholson JK, Bennett PR, Kyrgiou M. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Scientific reports. 2015;5:16865. doi: 10.1038/srep16865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ying H, Jing F, Fanghui Z, Youlin Q, Yali H. High-risk HPV nucleic acid detection kit-the careHPV test -a new detection method for screening. Scientific reports. 2014;4:4704. doi: 10.1038/srep04704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. The Journal of pathology. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Gu Y, Ma C, Zou J, Zhu Y, Yang R, Xu Y, Zhang Y. Prevalence characteristics of high-risk human papillomaviruses in women living in Shanghai with cervical precancerous lesions and cancer. Oncotarget. 2016;7:24656–63. doi: 10.18632/oncotarget.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding S, Qian SY, Zhang Y, Wu W, Lu G, Lu Y, Feng X, Li L, Shen P. Establishment of immunoassay for detecting HPV16 E6 and E7 RNA. Scientific reports. 2015;5:13686. doi: 10.1038/srep13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbyn M, Ronco G, Cuzick J, Wentzensen N, Castle PE. How to evaluate emerging technologies in cervical cancer screening? International journal of cancer Journal international du cancer. 2009;125:2489–2496. doi: 10.1002/ijc.24774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szarewski A, Ambroisine L, Cadman L, Austin J, Ho L, Terry G, Liddle S, Dina R, McCarthy J, Buckley H, Bergeron C, Soutter P, Lyons D, Cuzick J. Comparison of predictors for high-grade cervical intraepithelial neoplasia in women with abnormal smears. Cancer epidemiology, biomarkers & prevention. 2008;17:3033–3042. doi: 10.1158/1055-9965.EPI-08-0508. [DOI] [PubMed] [Google Scholar]
- 8.Cuzick J, Arbyn M, Sankaranarayanan R, Tsu V, Ronco G, Mayrand MH, Dillner J, Meijer CJ. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008;26:K29–41. doi: 10.1016/j.vaccine.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Bulkmans NW, Berkhof J, Rozendaal L, van Kemenade FJ, Boeke AJ, Bulk S, Voorhorst FJ, Verheijen RH, van Groningen K, Boon ME, Ruitinga W, van Ballegooijen M, Snijders PJ, Meijer CJ. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370:1764–1772. doi: 10.1016/S0140-6736(07)61450-0. [DOI] [PubMed] [Google Scholar]
- 10.Kjaer S, Hogdall E, Frederiksen K, Munk C, van den Brule A, Svare E, Meijer C, Lorincz A, Iftner T. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer research. 2006;66:10630–10636. doi: 10.1158/0008-5472.CAN-06-1057. [DOI] [PubMed] [Google Scholar]
- 11.Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, Ghiringhello B, Girlando S, Gillio-Tos A, De Marco L, Naldoni C, Pierotti P, Rizzolo R, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. The Lancet Oncology. 2010;11:249–257. doi: 10.1016/S1470-2045(09)70360-2. [DOI] [PubMed] [Google Scholar]
- 12.Naucler P, Ryd W, Tornberg S, Strand A, Wadell G, Elfgren K, Radberg T, Strander B, Johansson B, Forslund O, Hansson BG, Rylander E, Dillner J. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. The New England journal of medicine. 2007;357:1589–1597. doi: 10.1056/NEJMoa073204. [DOI] [PubMed] [Google Scholar]
- 13.Bulk S, Bulkmans NW, Berkhof J, Rozendaal L, Boeke AJ, Verheijen RH, Snijders PJ, Meijer CJ. Risk of high-grade cervical intra-epithelial neoplasia based on cytology and high-risk HPV testing at baseline and at 6-months. International journal of cancer Journal international du cancer. 2007;121:361–367. doi: 10.1002/ijc.22677. [DOI] [PubMed] [Google Scholar]
- 14.Cuzick J, Clavel C, Petry KU, Meijer CJ, Hoyer H, Ratnam S, Szarewski A, Birembaut P, Kulasingam S, Sasieni P, Iftner T. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. International journal of cancer Journal international du cancer. 2006;119:1095–1101. doi: 10.1002/ijc.21955. [DOI] [PubMed] [Google Scholar]
- 15.Castle PE, Stoler MH, Wright TC, Jr, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. The Lancet Oncology. 2011;12:880–890. doi: 10.1016/S1470-2045(11)70188-7. [DOI] [PubMed] [Google Scholar]
- 16.Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, Ratnam S, Coutlee F, Franco EL, Canadian Cervical Cancer Screening Trial Study G Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. The New England journal of medicine. 2007;357:1579–1588. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 17.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, Garcia FA, Moriarty AT, Waxman AG, Wilbur DC, Wentzensen N, Downs LS, Jr, Spitzer M, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. American journal of clinical pathology. 2012;137:516–542. doi: 10.1309/AJCPTGD94EVRSJCG. [DOI] [PubMed] [Google Scholar]
- 18.Rijkaart DC, Berkhof J, van Kemenade FJ, Coupe VM, Hesselink AT, Rozendaal L, Heideman DA, Verheijen RH, Bulk S, Verweij WM, Snijders PJ, Meijer CJ. Evaluation of 14 triage strategies for HPV DNA-positive women in population-based cervical screening. International journal of cancer Journal international du cancer. 2012;130:602–610. doi: 10.1002/ijc.26056. [DOI] [PubMed] [Google Scholar]
- 19.Arbyn M, Sasieni P, Meijer CJ, Clavel C, Koliopoulos G, Dillner J. Chapter 9: Clinical applications of HPV testing: a summary of meta-analyses. Vaccine. 2006;24:S3/78-89. doi: 10.1016/j.vaccine.2006.05.117. [DOI] [PubMed] [Google Scholar]
- 20.Youssef MA, Abdelsalam L, Harfoush RA, Talaat IM, Elkattan E, Mohey A, Abdella RM, Farhan MS, Foad HA, Elsayed AM, Elkinaai NA, Ghaith D, Rashed ME, Ghafar MA, Khamis Y, Hosni AN. Prevalence of human papilloma virus (HPV) and its genotypes in cervical specimens of Egyptian women by linear array HPV genotyping test. Infectious agents and cancer. 2016;11:6. doi: 10.1186/s13027-016-0053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camporiondo MP, Farchi F, Ciccozzi M, Denaro A, Gallone D, Maracchioni F, Favalli C, Ciotti M. Detection of HPV and co-infecting pathogens in healthy Italian women by multiplex real-time PCR. Le infezioni in medicina. 2016;24:12–17. [PubMed] [Google Scholar]
- 22.Sellors JW, Mahony JB, Kaczorowski J, Lytwyn A, Bangura H, Chong S, Lorincz A, Dalby DM, Janjusevic V, Keller JL. Prevalence and predictors of human papillomavirus infection in women in Ontario, Canada. Survey of HPV in Ontario Women (SHOW) Group. CMAJ. 2000;163:503–508. [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhn L, Denny L, Pollack A, Lorincz A, Richart RM, Wright TC. Human papillomavirus DNA testing for cervical cancer screening in low-resource settings. Journal of the National Cancer Institute. 2000;92:818–825. doi: 10.1093/jnci/92.10.818. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Liu F, Cheng S, Shi L, Yan Z, Yang J, Shi L, Yao Y, Ma Y. Prevalence of HPV infection among 28,457 Chinese women in Yunnan Province, southwest China. Scientific reports. 2016;6:21039. doi: 10.1038/srep21039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becker TM, Wheeler CM, McGough NS, Parmenter CA, Jordan SW, Stidley CA, McPherson RS, Dorin MH. Sexually transmitted diseases and other risk factors for cervical dysplasia among southwestern Hispanic and non-Hispanic white women. Jama. 1994;271:1181–1188. [PubMed] [Google Scholar]
- 26.Wang YY, Li L, Wei S, Peng J, Yuan SX, Xie JS, Liu ZH. Human Papillomavirus (HPV) infection in women participating in cervical cancer screening from 2006 to 2010 in Shenzhen City, South China. Asian Pacific journal of cancer prevention. 2013;14:7483–7487. doi: 10.7314/apjcp.2013.14.12.7483. [DOI] [PubMed] [Google Scholar]
- 27.Giambi C, Donati S, Carozzi F, Salmaso S, Declich S, Atti ML, Ronco G, Alibrandi MP, Brezzi S, Collina N, Franchi D, Lattanzi A, Minna MC, et al. A cross-sectional study to estimate high-risk human papillomavirus prevalence and type distribution in Italian women aged 18-26 years. BMC infectious diseases. 2013;13:74. doi: 10.1186/1471-2334-13-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, Tortolero-Luna G, Kjaer SK, Munoz N. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26:K1–16. doi: 10.1016/j.vaccine.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 29.Ripabelli G, Grasso GM, Del Riccio I, Tamburro M, Sammarco ML. Prevalence and genotype identification of human papillomavirus in women undergoing voluntary cervical cancer screening in Molise, central Italy. Cancer epidemiology. 2010;34:162–167. doi: 10.1016/j.canep.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Masia G, Mazzoleni AP, Contu G, Laconi S, Minerba L, Montixi S, Montis F, Onano A, Porcedda E, Coppola RC. Epidemiology and genotype distribution of human papillomavirus (HPV) in women of Sardinia (Italy) Vaccine. 2009;27:A11–16. doi: 10.1016/j.vaccine.2008.10.095. [DOI] [PubMed] [Google Scholar]
- 31.Agarossi A, Ferrazzi E, Parazzini F, Perno CF, Ghisoni L. Prevalence and type distribution of high-risk human papillomavirus infection in women undergoing voluntary cervical cancer screening in Italy. Journal of medical virology. 2009;81:529–535. doi: 10.1002/jmv.21347. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell SM, Sekikubo M, Biryabarema C, Byamugisha JJ, Steinberg M, Jeronimo J, Money DM, Christilaw J, Ogilvie GS. Factors associated with high-risk HPV positivity in a low-resource setting in sub-Saharan Africa. American journal of obstetrics and gynecology. 2014;210:81. doi: 10.1016/j.ajog.2013.08.038. e81-87. [DOI] [PubMed] [Google Scholar]
- 33.Syrjanen K, Shabalova I, Petrovichev N, Kozachenko V, Zakharova T, Pajanidi J, Podistov J, Chemeris G, Sozaeva L, Lipova E, Tsidaeva I, Ivanchenko O, Pshepurko A, et al. Oral contraceptives are not an independent risk factor for cervical intraepithelial neoplasia or high-risk human papillomavirus infections. Anticancer research. 2006;26:4729–4740. [PubMed] [Google Scholar]
- 34.Crow JM. HPV: The global burden. Nature. 2012;488:S2–3. doi: 10.1038/488S2a. [DOI] [PubMed] [Google Scholar]
- 35.Xu QX, Zhang ZY. High-risk human papillomavirus genotypes in cervical lesions and vaccination challenges in China. Asian Pacific journal of cancer prevention. 2015;16:2193–2197. doi: 10.7314/apjcp.2015.16.6.2193. [DOI] [PubMed] [Google Scholar]
- 36.Bruni L, Diaz M, Castellsague X, Ferrer E, Bosch FX, de Sanjose S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. The Journal of infectious diseases. 2010;202:1789–1799. doi: 10.1086/657321. [DOI] [PubMed] [Google Scholar]
- 37.Kim MJ, Kim JJ, Kim S. Type-specific prevalence of high-risk human papillomavirus by cervical cytology and age: Data from the health check-ups of 7,014 Korean women. Obstetrics & gynecology science. 2013;56:110–120. doi: 10.5468/OGS.2013.56.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhatla N, Lal N, Bao YP, Ng T, Qiao YL. A meta-analysis of human papillomavirus type-distribution in women from South Asia: implications for vaccination. Vaccine. 2008;26:2811–2817. doi: 10.1016/j.vaccine.2008.03.047. [DOI] [PubMed] [Google Scholar]
- 39.Singh S, Zhou Q, Yu Y, Xu X, Huang X, Zhao J, Han L, Wang K, Sun J, Li F. Distribution of HPV genotypes in Shanghai women. International journal of clinical and experimental pathology. 2015;8:11901–11908. [PMC free article] [PubMed] [Google Scholar]
- 40.Liu XX, Fan XL, Yu YP, Ji L, Yan J, Sun AH. Human papillomavirus prevalence and type-distribution among women in Zhejiang Province, Southeast China: a cross-sectional study. BMC infectious diseases. 2014;14:708. doi: 10.1186/s12879-014-0708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jing L, Zhong X, Zhong Z, Huang W, Liu Y, Yang G, Zhang X, Zou J, Jing C, Wei X. Prevalence of human papillomavirus infection in Guangdong Province, China: a population-based survey of 78,355 women. Sexually transmitted diseases. 2014;41:732–738. doi: 10.1097/OLQ.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 42.So KA, Hong JH, Lee JK. Human Papillomavirus Prevalence and Type Distribution Among 968 Women in South Korea. Journal of cancer prevention. 2016;21:104–109. doi: 10.15430/JCP.2016.21.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pontillo A, Bricher P, Leal VN, Lima S, Souza PR, Crovella S. Role of inflammasome genetics in susceptibility to HPV infection and cervical cancer development. Journal of medical virology. 2016;88:1646–1651. doi: 10.1002/jmv.24514. [DOI] [PubMed] [Google Scholar]
- 44.Natphopsuk S, Settheetham-Ishida W, Pientong C, Sinawat S, Yuenyao P, Ishida T, Settheetham D. Human papillomavirus genotypes and cervical cancer in northeast Thailand. Asian Pacific journal of cancer prevention. 2013;14:6961–6964. doi: 10.7314/apjcp.2013.14.11.6961. [DOI] [PubMed] [Google Scholar]
- 45.Suthipintawong C, Siriaunkgul S, Tungsinmunkong K, Pientong C, Ekalaksananan T, Karalak A, Kleebkaow P, Vinyuvat S, Triratanachat S, Khunamornpong S, Chongsuwanich T. Human papilloma virus prevalence, genotype distribution, and pattern of infection in Thai women. Asian Pacific journal of cancer prevention. 2011;12:853–856. [PubMed] [Google Scholar]
- 46.Katki HA, Kinney WK, Fetterman B, Lorey T, Poitras NE, Cheung L, Demuth F, Schiffman M, Wacholder S, Castle PE. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. The Lancet Oncology. 2011;12:663–672. doi: 10.1016/S1470-2045(11)70145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen HC, Schiffman M, Lin CY, Pan MH, You SL, Chuang LC, Hsieh CY, Liaw KL, Hsing AW, Chen CJ, Group C-HS. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. Journal of the National Cancer Institute. 2011;103:1387–1396. doi: 10.1093/jnci/djr283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schiffman M, Wentzensen N. Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer epidemiology, biomarkers & prevention. 2013;22:553–560. doi: 10.1158/1055-9965.EPI-12-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wentzensen N, Schiffman M, Dunn T, Zuna RE, Gold MA, Allen RA, Zhang R, Sherman ME, Wacholder S, Walker J, Wang SS. Multiple human papillomavirus genotype infections in cervical cancer progression in the study to understand cervical cancer early endpoints and determinants. International journal of cancer Journal international du cancer. 2009;125:2151–2158. doi: 10.1002/ijc.24528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jing L, Zhong X, Huang W, Liu Y, Wang M, Miao Z, Zhang X, Zou J, Zheng B, Chen C, Liang X, Yang G, Jing C, Wei X. HPV genotypes and associated cervical cytological abnormalities in women from the Pearl River Delta region of Guangdong province, China: a cross-sectional study. BMC infectious diseases. 2014;14:388. doi: 10.1186/1471-2334-14-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moga MA, Irimie M, Oanta A, Pascu A, Burtea V. Type-specific prevalence of human papillomavirus by cervical cytology among women in Brasov, Romania. Asian Pacific journal of cancer prevention. 2014;15:6887–6892. doi: 10.7314/apjcp.2014.15.16.6887. [DOI] [PubMed] [Google Scholar]
- 52.Wu EQ, Liu B, Cui JF, Chen W, Wang JB, Lu L, Niyazi M, Zhao C, Ren SD, Li CQ, Gong XZ, Smith JS, Belinson JL, Liaw KL, Velicer C, Qiao YL. Prevalence of type-specific human papillomavirus and pap results in Chinese women: a multi-center, population-based cross-sectional study. Cancer causes & control. 2013;24:795–803. doi: 10.1007/s10552-013-0162-8. [DOI] [PubMed] [Google Scholar]
- 53.Luo ZY, Chen Q, Yang H, Lin M, Chen CY, Yang C, Yang LY. The Prevalence and Genotype of Human Papillomavirus from Patients with Genital Warts in Eastern Guangdong Province. Asian Pacific journal of cancer prevention. 2015;16:5675–5679. doi: 10.7314/apjcp.2015.16.14.5675. [DOI] [PubMed] [Google Scholar]
- 54.Fife KH, Cramer HM, Schroeder JM, Brown DR. Detection of multiple human papillomavirus types in the lower genital tract correlates with cervical dysplasia. Journal of medical virology. 2001;64:550–559. doi: 10.1002/jmv.1085. [DOI] [PubMed] [Google Scholar]
- 55.Trottier H, Mahmud S, Prado JC, Sobrinho JS, Costa MC, Rohan TE, Villa LL, Franco EL. Type-specific duration of human papillomavirus infection: implications for human papillomavirus screening and vaccination. The Journal of infectious diseases. 2008;197:1436–1447. doi: 10.1086/587698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perrons C, Jelley R, Kleter B, Quint W, Brink N. Detection of persistent high risk human papillomavirus infections with hybrid capture II and SPF10/LiPA. Journal of clinical virology. 2005;32:278–285. doi: 10.1016/j.jcv.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 57.Woodman CB, Collins S, Winter H, Bailey A, Ellis J, Prior P, Yates M, Rollason TP, Young LS. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet. 2001;357:1831–1836. doi: 10.1016/S0140-6736(00)04956-4. [DOI] [PubMed] [Google Scholar]
- 58.Trottier H, Mahmud S, Costa MC, Sobrinho JP, Duarte-Franco E, Rohan TE, Ferenczy A, Villa LL, Franco EL. Human papillomavirus infections with multiple types and risk of cervical neoplasia. Cancer epidemiology, biomarkers & prevention. 2006;15:1274–1280. doi: 10.1158/1055-9965.EPI-06-0129. [DOI] [PubMed] [Google Scholar]
- 59.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. The New England journal of medicine. 1998;338:423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 60.Dijkstra MG, van Niekerk D, Rijkaart DC, van Kemenade FJ, Heideman DA, Snijders PJ, Meijer CJ, Berkhof J. Primary hrHPV DNA testing in cervical cancer screening: how to manage screen-positive women? A POBASCAM trial substudy. Cancer epidemiology, biomarkers & prevention. 2014;23:55–63. doi: 10.1158/1055-9965.EPI-13-0173. [DOI] [PubMed] [Google Scholar]
- 61.Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T, Jr, Young N, Forum Group M, Bethesda W. The 2001 Bethesda System: terminology for reporting results of cervical cytology. Jama. 2002;287:2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 62.Wu RF, Dai M, Qiao YL, Clifford GM, Liu ZH, Arslan A, Li N, Shi JF, Snijders PJ, Meijer CJ, Franceschi S. Human papillomavirus infection in women in Shenzhen City, People's Republic of China, a population typical of recent Chinese urbanisation. International journal of cancer Journal international du cancer. 2007;121:1306–1311. doi: 10.1002/ijc.22726. [DOI] [PubMed] [Google Scholar]
- 63.FA T, P D. Tumours of the uterine cervix. In: World Health Organization Classification of Tumours, editor. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon: IARC Press; 2003. pp. 259–89. [Google Scholar]
- 64.Porras C, Hildesheim A, Gonzalez P, Schiffman M, Rodriguez AC, Wacholder S, Jimenez S, Quint W, Guillen D, Kreimer AR, Herrero R, Group CVTV. Performance of self-collected cervical samples in screening for future precancer using human papillomavirus DNA testing. Journal of the National Cancer Institute. 2015;107:400. doi: 10.1093/jnci/dju400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castle PE, Sideri M, Jeronimo J, Solomon D, Schiffman M. Risk assessment to guide the prevention of cervical cancer. American journal of obstetrics and gynecology. 2007;197:356. doi: 10.1016/j.ajog.2007.07.049. e351-356. [DOI] [PMC free article] [PubMed] [Google Scholar]


